Abstract
The pharmacokinetics, pharmacodynamics, efficacy, and safety of a new recom-binant Escherichia coli–asparaginase preparation was compared with Asparaginase medac. Thirty-two children with acute lymphoblastic leukemia were randomized to receive one of both agents at a dose of 5000 U/m2 every 3 days, for a total of 8 doses during induction treatment. The serum activity-time profile after the first dose of recombinant asparaginase was similar to that of Asparaginase medac. The trough serum activities were greater than the desired threshold of 100 U/L in both treatment groups. Asparagine was completely depleted in serum and in cerebrospinal fluid, whereas glutamine levels were only moderately influenced. No significant difference between the 2 treatments regarding the degree of asparagine depletion, duration of depletion, complete remission rate, and minimal residual disease status at the end of induction, overall frequency or intensity of adverse events was seen. Observed adverse reactions are known as possible and labeled side effects of asparaginase treatment and chemotherapy. We conclude that the new recombinant asparaginase and other native Asparaginase medac are bioequivalent and have the same pharmacodynamic effects and the same direct toxicity profile in children with acute lymphoblastic leukemia. This trial was registered at http://www.controlled-trials.com as no. ISRCTN 75734403.
Introduction
Asparaginase breaks down extracellular asparagine to aspartic acid and ammonia, thereby inhibiting the growth of lymphatic leukemic cells. For many years, asparaginase has represented a central component of treatment protocols for children and adults with de novo or relapsed acute lymphoblastic leukemia (ALL). Several trials have clearly demonstrated that this drug contributes to the total treatment outcome of children with ALL by at least 10% to 20%.1-6 Furthermore, it has been shown that treatment with a suboptimal asparaginase regimen that does not result in sufficient asparaginase trough activity levels in serum (ie, < 100 U/L) leads to an inferior outcome of patients.2,3,7
Several asparaginase preparations are currently on the market either derived from Escherichia coli (Asparaginase medac, Kidrolase, Paronal; Leunase, Elspar) or Erwinia chrysanthemi (Erwinase), in its native form or as a pegylated enzyme (Oncaspar). However, these preparations differ more or less in their pharmacokinetic, pharmacodynamic, and immunogenic properties. Therefore, asparaginase dose regimens differ from country to country to some degree in respective ALL protocols, and treatment results are not easily comparable.
Currently available native E coli asparaginase preparations were developed 20 to 30 years ago. Their production process is inefficient, overaged, and has never been updated. These “old” preparations contain certain amounts of higher aggregates (up to 20%). In preclinical investigations, it has been demonstrated that these aggregates are less active than the tetramers (medac, H.-J.K., unpublished data). Furthermore, these aggregates may be more immunogenic than the tetramers. During the last decades, the inefficient production process has led to several periods of a shortage of supply of this essential drug in several countries. Furthermore, the contamination with aggregates has prevented the approval of these old asparaginase preparations in various countries. In a large number of European and other countries, no asparaginase is currently approved. Therefore, asparaginase in its native as well as pegylated form is on the pediatric need list of the European Medicines Agency.8
A new asparaginase preparation was developed with the aim to improve the production process and purification procedures according to the most recent guidelines for biotechnology-derived proteins as well as to ensure the long-term supply of this essential drug. The amino acid sequence and enzymatic activity of the new recombinant asparaginase preparation were engineered to be identical to that of Asparaginase medac.
The asparaginase enzyme is a tetramer composed of 4 identical subunits with one active site per subunit. Besides tetramers, commercially available asparaginase preparations contain up to 20% monomers and higher aggregates (octamers, dodecamers among others). Higher aggregates have less enzymatic activity than tetramers and may express new antigens that are not present on tetramers. A new formulation was developed with the recombinant asparaginase that contains less than 1% aggregates.
Within an intensive preclinical testing program, it has been demonstrated that the enzyme kinetics (Michelis-Menten rate constant for the substrates asparagine and glutamine), acute and subacute toxicity in rats and dogs, the in vitro antileukemic activity in 2 cell lines, the in vivo antileukemic activity in an ALL-severe combined immunodeficiency mouse model, and the pharmacokinetics in beagle dogs are similar for both recombinant asparaginase and Asparaginase medac (H.-J.K., unpublished data). Therefore, it could be administered within the clinical study program at the same dose schedules that were developed for Asparaginase medac.
Within a pilot phase 1 trial, 7 adult patients with relapsed/chemorefractory hematologic neoplasias received recombinant asparaginase intravenously as a single agent at a dose of 5000 U/m2 every third day for a maximum of 8 doses. Pharmacokinetic parameters (half-life, volume of distribution, peak and trough activity levels in serum) were in the expected range that is known for other native E coli asparaginase preparations. At all measured time points, the asparaginase serum activity levels were above the demanded threshold of 100 U/L, which correlated with a complete disappearance of asparagine in serum below the lower limit of quantification. The only mentionable adverse event seen in this trial was a significant decline of antithrombin III levels, which is a well-known and frequently observed side effect of asparaginase treatment (medac, H.-J.K., unpublished results).
The present randomized, double-blind trial aimed to demonstrate that the new recombinant asparaginase can safely replace Asparaginase medac within a treatment protocol for children with de novo ALL. The study was performed at a single institution in The Netherlands based on the Dutch ALL treatment protocol DCOG-ALL10.
Although asparaginase has been used for the treatment of ALL for more than 3 decades, this is the first prospective trial that directly compared the pharmacology, efficacy, and safety of 2 E coli asparaginases from different producers.
Methods
Asparaginase preparations
Recombinant asparaginase was developed by medac and Wacker Biotech (formerly ProThera, Jena, Germany). The final formulation of this investigational drug product was designed by medac.
Asparaginase medac is produced by Kyowa-Hakko (Tokyo, Japan). It is approved in some European countries for the treatment of patients with lymphoblastic leukemia/lymphoma. Both preparations are available in vials with 10 000 U as a lyophilisate.
Patients and treatment
Altogether, 32 of 37 consecutive children with previously untreated ALL participated in the study between January 4, 2005 (first patient signed informed consent) and October 17, 2006 (last patient last data) at the Erasmus MC–Sophia Children's Hospital (Rotterdam, The Netherlands). The hospital review board approved this study in accordance with the Declaration of Helsinki. Three children (or their guardians) did not give informed consent. In one case, informed consent was withdrawn before start of treatment, and one child was not eligible because of impaired liver function. All children participated in the induction therapy of trial DCOG ALL-10 and received within protocol IA, a typical ALL combination chemotherapy treatment consisting of prednisolone (60 mg/m2 per day; days 1-36), vincristine (1.5 mg/m2; days 8, 15, 22, and 29), daunorubicin (30 mg/m2; days 8, 15, 22, and 29), E coli–asparaginase (5000 U/m2; days 12, 15, 18, 21, 24, 27, 30, and 33), intrathecal injections with methotrexate at day 1, and triple intrathecal injections with methotrexate, cytosine arabinoside, and prednisolone (days 15 and 33). All patients had received a Port-a-Cath device before the first infusion of asparaginase.
Patients were randomized to receive either recombinant asparaginase or Asparaginase medac in a double-blind fashion. Asparaginase is usually infused over a period of 0.5 to 2 hours. In daily practice, there is no need to use an exact infusion time with the help of an electronic pump. The primary endpoint of the present study was a comparison of the area under the curve (AUC) of asparaginase in serum after the first dose. The exact measurement of this parameter required a constant and short infusion rate of the study drug. Therefore, the first dose of asparaginase was administered in a volume of 20 mL (solvent: normal saline) as intravenous infusion over 30 minutes by use of an electronic pump that guaranteed a constant infusion rate. All subsequent doses of asparaginase were administered in a volume of 50 to 250 mL (volume depending on the age and size of the child) over approximately 1 hour using conventional infusion equipment. After administration of the first dose, serial blood samplings (1-2 mL) were performed within 72 hours and analyzed for asparaginase serum levels. The resulting data were used for calculation of pharmacokinetic parameters and for demonstrating bioequivalence of both asparaginase preparations.
All subsequent doses of asparaginase were administered in a volume of 50 to 250 mL over 1 hour using conventional infusion equipments. For the determination of asparaginase trough levels and amino acids in serum, additional blood samples were drawn just before asparaginase infusions 2 to 8. Further blood samples were drawn after the last asparaginase infusion on protocol days 39, 45, 52, 59, and 64.
Before intrathecal chemotherapy instillation at days 1, 15, and 33 (and during treatment phase B at days 45 and 59), cerebrospinal fluid (CSF) samples (> 0.5 mL) were drawn for determination of amino acid levels.
Blood and CSF sample processing guidelines
Blood samples were drawn from the infusion line in all patients but one (fingertip). After first infusion (over 30 minutes) of study medication, blood samples were collected at the following time points: 0 hour (immediately after infusion), 0.25, 0.5, 1, 2, 4, 6, 24, 48, and 72 hours.
The infusion line was flushed with 5 to 10 mL normal saline to avoid drug remnants. After 3 seconds, 5 mL blood was taken from the infusion line and discarded. The next 1 to 2 mL blood was collected into a tube that contained no additives. Thereafter, the infusion line was flushed again with 5 mL normal saline.
The tubes with the blood samples were immediately put on ice and transferred to the laboratory for serum processing. The blood sample was centrifuged not later than 15 minutes after blood sampling at 600g to 800g and 4°C for 10 minutes. Serum was harvested and divided into 2 aliquots (> 200 μL each) for determination of asparaginase activity or amino acid concentrations. The samples for amino acid determination were then deproteinated (to avoid further enzymatic activity) by adding 1 part of 10% sulphosalicylic acid to 4 parts of serum, thoroughly mixed and centrifuged for 5 minutes at 10 000g to 13 000g, and the supernatant harvested.
CSF samples were immediately put on ice and transferred to the laboratory within 15 minutes. The samples were deproteinized as described for the blood samples and the supernatant harvested.
The tubes with the processed samples were put into a special box prepared for each patient and frozen at −80°C. Samples were stored at this temperature not longer than 2 months until shipping and 3 months until analysis. In preliminary experiments, the stability of the analytes (asparaginase, amino acids) in serum and CSF samples had been demonstrated for at least 3 months.
Analytical assays
The analytical assays of the serum and CSF samples were carried out by CRS Clinical Research Services Mannheim (Grünstadt, Germany). Before starting measurements, all analytical methods had been validated and documented in a validation report.
Serum levels of asparaginase were determined with a sensitive microplate reader-based method developed by Lanvers et al.9 The assay is more sensitive than the usually used Nessler assay and allows determination of asparaginase trough levels as low as 2.5 U/L. This method has already routinely been used for the therapeutic drug monitoring of asparaginase in human serum by the German ALL study groups during the last few years.
Serum and CSF levels of the amino acids asparagine, aspartic acid, glutamine, and glutamic acid were analyzed by use of a reverse-phase high-performance liquid chromatography method. The lower limit of quantification of this assay for asparagine in serum and CSF samples was 0.5 μM.
Efficacy assessment
Efficacy of treatment was determined by evaluating the complete remission (CR) rate and minimal residual disease (MRD) status at protocol day 33 (after induction phase 1A).
CR was defined on morphologic grounds by the presence of less than 5% leukemic blasts in bone marrow (M1 marrow), no leukemic blasts in peripheral blood and CSF, no other documented extramedullary leukemia with the exception of testicular enlargement, and regenerating hematopoiesis.
MRD status was determined by determination of clonal immunoglobulin H (IgH), T-cell receptor-δ (TCR-δ), and TCR-γ rearrangements with polymerase chain reaction (PCR) on day 33. MRD negativity was defined as MRD < 10−4 with 2 MRD-PCR targets, which reach sufficient sensitivity: at least one target with a reproducible sensitivity ≤ 10−4 and one target with a reproducible sensitivity ≤ 10−3. If the 2 used MRD-PCR targets led to different MRD levels, then the highest level was used because the lower one might be because of a leukemic subclone.
For exploratory comparison of treatment-specific CR and MRD rates at day 33, exact 95% confidence intervals for the difference of the 2 rates were calculated.
Objectives
The primary objective was to determine the ratio of the population geometric means of the 72-hour serum concentration vs time curves (AUC) for the first administration of recombinant ASNase and Asparaginase medac.
Secondary objectives were as follows: (1) trough levels of ASNase activity in serum during subsequent ASNase infusions (just before each ASNase administration); (2) serum and CSF levels of the following amino acids: asparagine, aspartic acid, glutamine, and glutamic acid; (3) CR rate and MRD status at the end of induction therapy; and (4) adverse events.
Statistics
The primary objective of this trial was to show that the 90% confidence interval (CI) of the ratio of the population geometric means of the 72-hour asparaginase serum activity versus time curves (AUC0-72h) for the first administration of recombinant asparaginase (test product) and Asparaginase medac (reference product) is within the equivalence limit 0.75 to 1.33. This wider acceptance range (compared with traditional bioequivalence trials with “chemical” pharmaceuticals) was chosen because of an expected substantial variance of individual asparaginase serum activities. Because the primary goal was to demonstrate bioequivalence, which is usually determined by comparison of AUC, the AUC after the first dose and not, for instance, the trough asparaginase activity level, was used as the primary endpoint.
A total of 32 patients were randomized in a 1:1 ratio to show equivalence with a power of 80% using 2 one-sided t tests at the 5% significance level on the log-transformed data. This sample size assumed treatment-specific coefficient of variations of 25%.
All pharmacokinetic parameters were calculated with noncompartmental procedures based on SAS software version 9.1 (SAS Institute, Cary, NC).
Results
Patient characteristics
Altogether, 32 children (17 male and 15 female) with a diagnosis of ALL participated in this study. Two patients receiving recombinant asparaginase treatment were excluded from the pharmacokinetic analysis because of missing serum samples; however, both patients could be analyzed for efficacy and safety. The children were between 1 and 14 years of age at diagnosis, with a median of 4.5 years in both groups. Patients in the recombinant asparaginase group had a higher median white blood cell count and more blast cells in peripheral blood. Additional patient characteristics are shown in Table 1.
Patient characteristics
| Parameter . | Recombinant asparaginase . | Asparaginase medac . |
|---|---|---|
| Median age, y; range | 4.5; 2-14 | 4.5; 1-11 |
| Median body surface area, m2; range | 0.79; 0.54-1.88 | 0.72; 0.48-1.22 |
| Sex: male/female | 9/7 | 8/8 |
| Median WBC (×109/L); range | 9.8; 0.7-578.0 | 4.0; 0.6-109.0 |
| Median peripheral blasts (%); range | 50.5; 0-96 | 28.0; 0-79 |
| Median marrow blasts (%); range | 92.8; 59.2-97.0 | 90.1; 33.8-98.6 |
| Immunophenotype (number of patients) | ||
| Pro–B-ALL | 1 | 1 |
| Common ALL | 8 | 10 |
| Pre–B-ALL | 4 | 3 |
| T-ALL | 3 | 2 |
| Genetics (number of patients) | ||
| BCR-ABL | 0 | 1 |
| TEL-AML | 5 | 5 |
| MLL-AF4 | 0 | 1 |
| Other | 9 | 9 |
| No aberrations | 2 | 0 |
| Parameter . | Recombinant asparaginase . | Asparaginase medac . |
|---|---|---|
| Median age, y; range | 4.5; 2-14 | 4.5; 1-11 |
| Median body surface area, m2; range | 0.79; 0.54-1.88 | 0.72; 0.48-1.22 |
| Sex: male/female | 9/7 | 8/8 |
| Median WBC (×109/L); range | 9.8; 0.7-578.0 | 4.0; 0.6-109.0 |
| Median peripheral blasts (%); range | 50.5; 0-96 | 28.0; 0-79 |
| Median marrow blasts (%); range | 92.8; 59.2-97.0 | 90.1; 33.8-98.6 |
| Immunophenotype (number of patients) | ||
| Pro–B-ALL | 1 | 1 |
| Common ALL | 8 | 10 |
| Pre–B-ALL | 4 | 3 |
| T-ALL | 3 | 2 |
| Genetics (number of patients) | ||
| BCR-ABL | 0 | 1 |
| TEL-AML | 5 | 5 |
| MLL-AF4 | 0 | 1 |
| Other | 9 | 9 |
| No aberrations | 2 | 0 |
Asparaginase pharmacokinetics after first dose
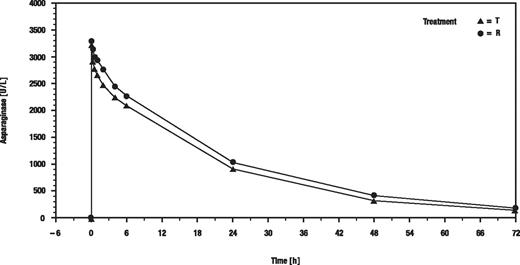
Figure 1 shows the geometric means of serum asparaginase activity versus time (hours) after the first of the 8 infusions.
Time course of asparaginase activity after first administration of MC1003. T indicates recombinant asparaginase; and R, Asparaginase medac.
Time course of asparaginase activity after first administration of MC1003. T indicates recombinant asparaginase; and R, Asparaginase medac.
The point estimate of AUC0-72h for the treatment ratio recombinant asparaginase/Asparaginase medac was 86.01, and the 90% CI (77.52-95.43) was contained within the predefined acceptance range for equivalence of 75% to 133%. Therefore, the primary study goal was fulfilled. However, the difference between AUC values was statistically significant (P = .02) but too small to be considered as clinically meaningful.
Maximum serum activity (Cmax), half-life, total clearance, and volume of distribution were not significantly different in both groups. Cmax was reached immediately after infusion for most patients. Table 2 shows the pharmacokinetic parameters calculated after the first dose of asparaginase.
Pharmacokinetic parameters of serum activities of asparaginase
| Parameter . | Recombinant asparaginase, N = 14 . | Asparaginase medac, N = 16 . | P . |
|---|---|---|---|
| AUC0-72h (U · h/L) | |||
| Median | 60 164.5 | 69 135.6 | .02 |
| Range | 38 626.8-80 764.3 | 49 243.8-83 850.1 | |
| Cmax (U/L) | |||
| Median | 3526.7 | 3699.8 | .21 |
| Range | 2231.3-4525.5 | 2898.2-4968.0 | |
| t1/2 λz (h) | |||
| Median | 17.3295 | 18.5499 | .19 |
| Range | 12.5392-22.9148 | 12.7322-27.3761 | |
| Cltot (L/h) | |||
| Median | 0.053 | 0.050 | .12 |
| Range | 0.043-0.178 | 0.027-0.117 | |
| Vdss (L) | |||
| Median | 0.948 | 0.966 | .24 |
| Range | 0.691-2.770 | 0.413-2.327 | |
| Parameter . | Recombinant asparaginase, N = 14 . | Asparaginase medac, N = 16 . | P . |
|---|---|---|---|
| AUC0-72h (U · h/L) | |||
| Median | 60 164.5 | 69 135.6 | .02 |
| Range | 38 626.8-80 764.3 | 49 243.8-83 850.1 | |
| Cmax (U/L) | |||
| Median | 3526.7 | 3699.8 | .21 |
| Range | 2231.3-4525.5 | 2898.2-4968.0 | |
| t1/2 λz (h) | |||
| Median | 17.3295 | 18.5499 | .19 |
| Range | 12.5392-22.9148 | 12.7322-27.3761 | |
| Cltot (L/h) | |||
| Median | 0.053 | 0.050 | .12 |
| Range | 0.043-0.178 | 0.027-0.117 | |
| Vdss (L) | |||
| Median | 0.948 | 0.966 | .24 |
| Range | 0.691-2.770 | 0.413-2.327 | |
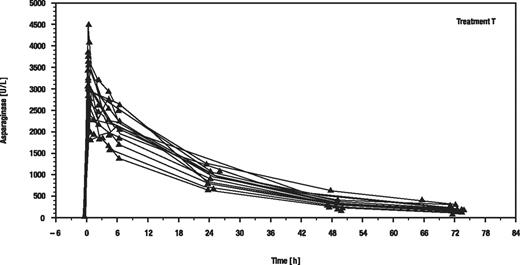
Surprisingly, the individual serum asparaginase activity time courses were very similar with a lower than expected variability in both groups (exemplarily shown for recombinant asparaginase in Figure 2). This might be because of the strict application of the predefined guidelines for sample withdrawal and processing in a single institution.
Individual serum asparaginase activity versus time (hours) after the first of the 8 infusions of recombinant asparaginase.
Individual serum asparaginase activity versus time (hours) after the first of the 8 infusions of recombinant asparaginase.
Asparaginase trough levels during ALL induction treatment
The goal of asparaginase treatment is to achieve serum activities larger than 100 U/L for a desired period. In numerous investigations, it had been shown that such levels guarantee a complete asparagine depletion.10,11 Therefore, serum trough levels of asparaginase activity were measured just before asparaginase infusions 2 to 8 (Table 3).
Descriptive of serum trough activities of asparaginase (median; range)
| Day of induction treatment . | Recombinant asparaginase, N = 14 . | Asparaginase medac, N = 16 . |
|---|---|---|
| 15 | 137.80; 37.5-279.6 | 195.55; 37.5-345.8 |
| 18 | 164.75 84.9-282.2 | 204.20; 115.8-488.5 |
| 21 | 121.60; 37.5-275.8 | 163.90; 81.8-496.0 |
| 24 | 150.05; 102.7-369.4 | 207.60; 37.5-398.1 |
| 27 | 145.20; 76.7-291.3 | 189.30; 37.5-507.3 |
| 30 | 162.20; 37.5-324.8 | 193.60; 106.3-499.5 |
| 33 | 141.45; 37.5-394.5 | 179.25; 37.5-423.8 |
| 39 | 21.10; 1.3-177.0 | 16.30; 9.5-161.8 |
| Day of induction treatment . | Recombinant asparaginase, N = 14 . | Asparaginase medac, N = 16 . |
|---|---|---|
| 15 | 137.80; 37.5-279.6 | 195.55; 37.5-345.8 |
| 18 | 164.75 84.9-282.2 | 204.20; 115.8-488.5 |
| 21 | 121.60; 37.5-275.8 | 163.90; 81.8-496.0 |
| 24 | 150.05; 102.7-369.4 | 207.60; 37.5-398.1 |
| 27 | 145.20; 76.7-291.3 | 189.30; 37.5-507.3 |
| 30 | 162.20; 37.5-324.8 | 193.60; 106.3-499.5 |
| 33 | 141.45; 37.5-394.5 | 179.25; 37.5-423.8 |
| 39 | 21.10; 1.3-177.0 | 16.30; 9.5-161.8 |
Asparaginase infusions were administered on days 12, 15, 18, 21, 24, 27, 30, and 33.
The observed average trough activities were above the desired threshold of more than 100 U/L in both groups but slightly lower under the recombinant asparaginase. At each day of induction, the responder rates (response = asparaginase trough activity> 100 U/L) were equal in both treatment arms (difference = 0%; 95% CI, −24.5% to 24.6%, unconditional exact Röhmel-Mansmann test, P = 1.0).
Pharmacodynamic results
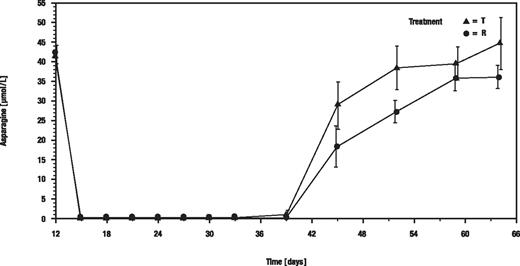
Mean asparagine concentrations in serum dropped from the predose concentrations of 41.83 μM (recombinant asparaginase group) and 42.52 μM (Asparaginase medac group) to below the lower limit of quantification of the bioanalytical method (< 0.5 μM) under both treatments. The mean depletion of asparagine in serum remained greater than 99% under treatment from immediately after the first infusion on day 12 until the last infusion on day 33 under both treatments (Figure 3).
Arithmetic means of asparagine concentrations in serum. T indicates recombinant asparaginase; and R, Asparaginase medac.
Arithmetic means of asparagine concentrations in serum. T indicates recombinant asparaginase; and R, Asparaginase medac.
The asparagine concentrations in serum correlated significantly (Spearman r = −0.7084 for test and r = −0.6813 for reference; P < .001) to asparaginase serum activities under both treatments, that is, the higher the concentrations of asparaginase, the lower the asparagine concentrations.
The mean (SD) duration of depletion after end of asparaginase treatment was 7.6 (3.2) days under the recombinant asparaginase and 9.0 (3.5) days under the Asparaginase medac treatment (P = .21; log-rank test).
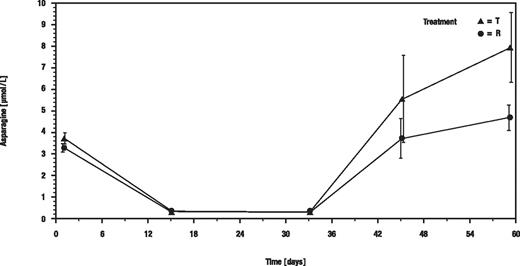
In CSF, the mean asparagine concentrations dropped from 3.71 μM (test) and 3.26 μM (reference) to below the limit of quantification under both treatments on days 15 and 33 in nearly all samples (Figure 4).
Arithmetic means of asparagine concentrations in CSF. T indicates recombinant asparaginase; and R, Asparaginase medac.
Arithmetic means of asparagine concentrations in CSF. T indicates recombinant asparaginase; and R, Asparaginase medac.
In conclusion, both drugs were highly efficient in depleting serum and CSF from asparagine and can be considered as equally effective with respect to this important parameter.
Influence of asparaginase treatment on glutamine levels
Besides asparagine, asparaginase is also able to cleave the amino acid glutamine to glutamic acid and ammonia, but with much less efficiency. The Michaelis-Menten constants of recombinant asparaginase and Asparaginase medac are very similar (2900 ± 800 for the substrate glutamine but 5.0 ± 0.7 for asparagine).
Whereas both asparaginase preparations completely depleted serum and CSF of asparagine, glutamine levels were only moderately affected with a very high interindividual variability (Table 4). However, a continuous turnover of glutamine occurred during treatment as indicated by a significant rise of the cleavage product glutamic acid.
Serum levels of glutamine and glutamic acid (median/range)
| Protocol day . | Glutamine (μmol/L) . | Glutamic acid (μmol/L) . | ||
|---|---|---|---|---|
| Recombinant asparaginase . | Asparaginase medac . | Recombinant asparaginase . | Asparaginase medac . | |
| 12 | 405(289-1050) | 418(285-767) | 36(11-114) | 39(22-155) |
| 15 | 371(226-834) | 379(286-983) | 43(19-114) | 51(24-138) |
| 21 | 469(13-921) | 419(254-536) | 50(1-71) | 56(36-112) |
| 27 | 443(204-1021) | 351(43-489) | 47(30-80) | 58(4-97) |
| 33 | 537(112-1180) | 384(304-683) | 50(25-96) | 67(31-173) |
| 39 | 493(277-642) | 427(220-632) | 63(49-106) | 70(39-98) |
| 64 | 469(351-1379) | 502(196-766) | 32(12-67) | 31(9-738) |
| Protocol day . | Glutamine (μmol/L) . | Glutamic acid (μmol/L) . | ||
|---|---|---|---|---|
| Recombinant asparaginase . | Asparaginase medac . | Recombinant asparaginase . | Asparaginase medac . | |
| 12 | 405(289-1050) | 418(285-767) | 36(11-114) | 39(22-155) |
| 15 | 371(226-834) | 379(286-983) | 43(19-114) | 51(24-138) |
| 21 | 469(13-921) | 419(254-536) | 50(1-71) | 56(36-112) |
| 27 | 443(204-1021) | 351(43-489) | 47(30-80) | 58(4-97) |
| 33 | 537(112-1180) | 384(304-683) | 50(25-96) | 67(31-173) |
| 39 | 493(277-642) | 427(220-632) | 63(49-106) | 70(39-98) |
| 64 | 469(351-1379) | 502(196-766) | 32(12-67) | 31(9-738) |
Serum levels were measured before asparaginase infusions 1 (day 12 = baseline), 2, 4, 6, and 8, as well as 6 days (day 39) and 1 month (day 64) after last asparaginase infusion.
Remission status
All patients except one achieved a CR. The patient who did not reach a CR was in very bad condition before study entry and had several bad prognostic factors (high tumor load, extreme leukocytosis, T-ALL, poor response to 1 week of prednisone).
A high percentage of patients had MRD levels less than 10−4 on day 33 of induction treatment (Table 5). This suggests that the induction treatment regimen used in protocol DCOG-ALL10 is very effective with both asparaginase preparations.
MRD status at protocol day 33
| MRD status . | Recombinant asparaginase . | Asparaginase medac . |
|---|---|---|
| MRD negative (< 10−4) | 13 (81.3%) | 12 (75.0%) |
| MRD positive (≥ 10−4) | 3 (18.8%) | 3 (18.8%) |
| Not known | 1 (6.3%) |
| MRD status . | Recombinant asparaginase . | Asparaginase medac . |
|---|---|---|
| MRD negative (< 10−4) | 13 (81.3%) | 12 (75.0%) |
| MRD positive (≥ 10−4) | 3 (18.8%) | 3 (18.8%) |
| Not known | 1 (6.3%) |
Safety
All drug-related serious adverse events were in line with the currently known side effects of asparaginase. The underlying disease also may have contributed to their occurrence. A difference in severity or frequency between recombinant asparaginase and Asparaginase medac treatment could not be seen. No deaths occurred during the study. Two patients in each group experienced a severe adverse reaction: 2× deep venous thrombosis (one in each group), 1× severe neutropenia (Asparaginase medac treatment), 1× severe hyperglycemia (recombinant ASNase treatment). All events resolved completely after respective treatment. No allergic reaction was observed during induction treatment.
Safety laboratory parameters showed a wide variability; changes in liver and kidney function parameters, blood cell count, and coagulation parameters were observed because of the underlying diseases, general condition, and chemotherapy; a difference between both asparaginase preparations was not evident.
Changes in bilirubin, transaminases (serum glutamicoxalacetic transaminase/serum glutamic pyruvic transaminase), and creatinine during induction treatment with recombinant asparaginase are shown in Table 6.
Influence of recombinant asparaginase treatment on laboratory parameters (mean ± SD)
| Parameter . | Normal value . | Baseline (< day 1) . | Week 1 (days 1-11) . | Week 2 (days 12-17) . | Week 3 (days 18-23) . | Week 4 (days 24-32) . | Day 33 . |
|---|---|---|---|---|---|---|---|
| Bilirubin (μmol/L) | < 17 | 7.8 ± 3.9 | 9.1 ± 5.2 | 14.9 ± 6.5 | 16.9 ± 7.1 | 21.7 ± 14.1 | 29.4 ± 16.3 |
| SGOT (U/L) | < 56 | 37 ± 25 | 138 ± 235 | 75 ± 141 | 30 ± 23 | 28 ± 14 | 40 ± 22 |
| SGPT (U/L) | < 39 | 23 ± 29 | 284 ± 514 | 222 ± 333 | 98 ± 105 | 82 ± 54 | 80 ± 37 |
| Creatinine (μmol/L) | 18-88 | 38 ± 21 | 32 ± 15 | 29 ± 13 | 29 ± 15 | 29 ± 11 | 26 ± 13 |
| Parameter . | Normal value . | Baseline (< day 1) . | Week 1 (days 1-11) . | Week 2 (days 12-17) . | Week 3 (days 18-23) . | Week 4 (days 24-32) . | Day 33 . |
|---|---|---|---|---|---|---|---|
| Bilirubin (μmol/L) | < 17 | 7.8 ± 3.9 | 9.1 ± 5.2 | 14.9 ± 6.5 | 16.9 ± 7.1 | 21.7 ± 14.1 | 29.4 ± 16.3 |
| SGOT (U/L) | < 56 | 37 ± 25 | 138 ± 235 | 75 ± 141 | 30 ± 23 | 28 ± 14 | 40 ± 22 |
| SGPT (U/L) | < 39 | 23 ± 29 | 284 ± 514 | 222 ± 333 | 98 ± 105 | 82 ± 54 | 80 ± 37 |
| Creatinine (μmol/L) | 18-88 | 38 ± 21 | 32 ± 15 | 29 ± 13 | 29 ± 15 | 29 ± 11 | 26 ± 13 |
Because the blood coagulation system is known to be very sensitive to asparaginase treatment, the levels of fibrinogen, antithrombin III, D-dimers, prothrombin time, and activated partial thromboplastin time were measured during induction treatment. Fibrinogen and antithrombin III levels dropped significantly below baseline values during asparaginase treatment (days 12-33) but recovered within a week after last administration. D-dimer levels (normal range, < 0.25 mg/L) were elevated at baseline but improved during induction treatment to normal values. Data for recombinant asparaginase are displayed in Table 7. Similar results were obtained with the reference product.
Influence of recombinant asparaginase treatment on coagulation parameters (mean ± SD)
| Parameter . | Baseline . | Day 15 . | Day 21 . | Day 27 . | Day 33 . | Day 39 . |
|---|---|---|---|---|---|---|
| PT (s) | 13.5 ± 2.1 | 12.1 ± 1.4 | 13.4 ± 2.4 | 14.3 ± 3.7 | 18.9 ± 23.4 | 11.0 ± 1.1 |
| PTT (s) | 27.2 ± 4.5 | 43.3 ± 44.0 | 61.5 ± 45.1 | 80.5 ± 57.2 | 50.3 ± 42.2 | 37.5 ± 19.1 |
| Fibrinogen (g/L) | 2.77 ± 1.06 | 0.99 ± 0.32 | 0.83 ± 0.53 | 0.74 ± 0.42 | 1.13 ± 1.05 | 2.37 ± 1.36 |
| AT III (U/mL) | 0.98 ± 0.15 | 0.94 ± 0.13 | 0.74 ± 0.14 | 0.65 ± 0.18 | 0.62 ± 0.21 | 0.89 ± 0.22 |
| D dimer (mg/L) | 0.82 ± 0.79 | 0.30 ± 0.39 | 0.17 ± 0.13 | 0.14 ± 0.08 | 0.23 ± 0.28 | 0.18 ± 0.14 |
| Parameter . | Baseline . | Day 15 . | Day 21 . | Day 27 . | Day 33 . | Day 39 . |
|---|---|---|---|---|---|---|
| PT (s) | 13.5 ± 2.1 | 12.1 ± 1.4 | 13.4 ± 2.4 | 14.3 ± 3.7 | 18.9 ± 23.4 | 11.0 ± 1.1 |
| PTT (s) | 27.2 ± 4.5 | 43.3 ± 44.0 | 61.5 ± 45.1 | 80.5 ± 57.2 | 50.3 ± 42.2 | 37.5 ± 19.1 |
| Fibrinogen (g/L) | 2.77 ± 1.06 | 0.99 ± 0.32 | 0.83 ± 0.53 | 0.74 ± 0.42 | 1.13 ± 1.05 | 2.37 ± 1.36 |
| AT III (U/mL) | 0.98 ± 0.15 | 0.94 ± 0.13 | 0.74 ± 0.14 | 0.65 ± 0.18 | 0.62 ± 0.21 | 0.89 ± 0.22 |
| D dimer (mg/L) | 0.82 ± 0.79 | 0.30 ± 0.39 | 0.17 ± 0.13 | 0.14 ± 0.08 | 0.23 ± 0.28 | 0.18 ± 0.14 |
Discussion
This trial shows that recombinant asparaginase and Asparaginase medac are bioequivalent and have the same pharmacodynamic effects and the same direct toxicity profile in children with de novo ALL.
For both compounds, the dose regimen used (5000 U/m2, every third day for 8 doses) leads to sufficient asparaginase trough activity levels in serum that guarantee a complete asparagine depletion in serum and CSF, which is the desired pharmacologic effect of treatment with this enzyme. Asparagine depletion in serum below the limit of detection can be observed already at the end of the first asparaginase infusion and lasts up to 7 to 9 days after the last asparaginase dose. Thereafter, asparaginase levels drop below 20 U/L and asparagine rises again within a few days to normal levels.
The direct toxicity profile of recombinant asparaginase and Asparaginase medac was compared against the background of a standard combination chemotherapy, including a sterpoid, vincristine, and an anthracycline. The toxicity profile appeared not to be different, and one case of trombosis occurred in each arm. Long-term safety and especially the occurrence of hypersensitivity reactions and silent inactivation in a later phase of treatment were not evaluated because the study was not designed for this. This will be done in a nationwide study that is planned.
Furthermore, our data show that asparaginase levels as low as 20 U/L correlate with a complete asparagine depletion. This challenges the traditional but never proven view that asparaginase trough levels should exceed 100 U/L.10,11 Based on these results, it can be concluded that asparaginase serum activity levels are a suitable and easy to measure parameter for the monitoring of the treatment effect of this enzyme.
Glutamine is a second possible substrate for asparaginase. There are conflicting results in the literature on the degree and role of cleavage of this amino acid during asparaginase treatment.10,12,13 Within this trial, glutamine levels were only moderately influenced by both asparaginase preparations. In the majority of patients, glutamine levels dropped not below 50% of baseline levels, even in patients with asparaginase trough levels of more than 300 U/L. This is understandable because normal glutamine serum levels are significantly lower (∼450 μM) than the Michaelis-Menten constant of E coli–asparaginase for this substrate (2900 μM), making it doubtful that asparaginase is able to compete with the continuous endogenous production and exogenous supply of this amino acid. Mean glutamic acid levels were slightly increased vs baseline during treatment with both asparaginase preparations, suggesting a constant but low cleavage of glutamine by the enzyme. In contrast, normal serum levels of asparagine (∼45 μM) exceed 9-fold the Km value of asparaginase for this substrate (5 μM), enabling asparaginase to work at full speed.
All pharmacokinetic parameters on various asparaginase preparations published so far are based on a few serum samples taken from patients after repeated infusions of this enzyme. The most frequently cited paper is that of Asselin et al from 1993.14 They reported a half-life of approximately 30 hours after repeated intramuscular injections of 2500 or 25 000 U/m2 of a native E coli–asparaginase (Elspar) calculated by regression analysis from individual serum activity data that were collected once daily for 4 to 6 days after asparaginase injection.
Within our trial, 10 blood samples were drawn from each patient at predefined time points from end of infusion up to 72 hours after first dose of asparaginase, which allowed the calculation of 30 individual pharmacokinetic datasets in asparaginase-naive patients with noncompartmental procedures.
Maximum serum concentration and half-life were not significantly different between both patient groups but numerically lower after recombinant asparaginase, resulting in a statistically significant lower AUC0-72h of asparaginase serum activity. However, this difference is relatively small and has no pharmacodynamic or clinical relevance. Both agents can therefore be applied in the same dose schedule, namely, 5000 U/m2.
The calculated median half-life (17.3 hours for recombinant asparaginase and 18.5 hours for Asparaginase medac) is significantly shorter than that reported by Asselin et al.14 We suppose that this difference may be attributed to a sustained release of asparaginase after intramuscular injection.
As expected, the volume of distribution equaled the plasma volume, confirming the assumption that asparaginase is not able to penetrate into extravascular spaces.
Both asparaginase preparations were very well tolerated. The observed adverse drug reactions are well-known side effects of asparaginase treatment.
In conclusion, recombinant asparaginase was shown to possess comparable pharmacologic properties, efficacy, and safety as Asparaginase medac and therefore can safely replace the latter agent in respective treatment protocols for children with ALL. Another study is currently in the planning phase that will compare the efficacy and safety of both preparations in a larger patient group, including antibody formation and pharmacokinetics when using asparaginase in the postinduction phase. Finally, a pegylated version of this product is currently under preclinical development.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Manfred Birkel (CRS Clinical Research Services Mannheim GmbH, Grünstadt, Germany) for the measurement and analysis of pharmacokinetic/pharmacodynamic data.
This work was supported by a grant of medac GmbH (Hamburg, Germany), the sponsor of this clinical protocol.
Authorship
Contribution: R.P., I.A., R.S., and H.-J.K. designed the study protocol and wrote the paper; I.T.-F. monitored the data at the study site; U.P. was the statistician; I.v.d.V. and E.V. were responsible for the preparation of blood and CSF samples as well as documentation of efficacy and safety in the individual patients.
Conflict-of-interest disclosure: H.-J.K., I.T.-F., and U.P. are employees of medac GmbH. R.P., I.A., R.S., I.v.d.V., and E.V. declare no competing financial interests.
Correspondence: Rob Pieters, Department Pediatric Oncology/Hematology, Erasmus MC–Sophia Children's Hospital, Molewaterplein 60, 3015 GJ Rotterdam, The Netherlands; e-mail: rob.pieters@erasmusmc.nl.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal