Abstract
Our studies focus on the pathways that restrict homing of specific subsets of immune cells, and thereby fine-tune the immune response at specific lymphoid and peripheral tissues. Here, we report that CCL2 (at picomolar [pM] levels) renders both murine and human T cells defective in their ability to develop CCR7-triggered activation of LFA-1– and LFA-1–mediated adhesion strengthening to endothelial ICAM-1 both in vitro and in vivo. CCL2 also attenuated lymphocyte chemotaxis toward lymph node chemokines. Consequently, low-dose CCL2 inhibited lymphocyte homing to peripheral lymph nodes but did not affect lymphocyte trafficking through the spleen. Impaired homing of lymphocytes to peripheral lymph nodes resulted in attenuated progression of both asthma and adjuvant arthritis. Thus, pM levels of circulating CCL2 can exert global suppressive effects on T-cell trafficking and differentiation within peripheral lymph nodes, and may be clinically beneficial as an anti-inflammatory agent.
Introduction
The surveillance of the body for foreign antigens is a critical function of the immune system. Lymphocytes migrate from the blood into tissues and secondary lymphoid organs, and return to the blood via lymph vessels and the thoracic duct. The majority of lymphocytes are capable of tissue-selective trafficking (homing), recognizing organ-specific adhesion molecules on specialized endothelial cells. Previous studies focused on the specific recruitment of leukocytes to the lymph nodes (LNs) or to sites of inflammation. However, little is known about the molecular mechanisms that negatively control or prevent homing of cells to these sites. Our studies focus on the pathways that restrict homing of specific subsets of immune cells and thereby fine-tune the immune response at specific lymphoid and peripheral tissues.
Despite our detailed understanding of the steps involved in lymphocyte entry to LNs, relatively little is known about how the cells enter the spleen and the white pulp of this compartment. The entry of T and B cells into the spleen was regarded, until recently, as a process for which integrin activation was not required, but recent results showed that integrin activation also has a role in the entry of lymphocytes into the splenic white pulp. However, although integrin inhibition causes a decrease in lymphocyte entry into the white pulp, it has no influence on the total B-cell number in the spleen. In addition, although inhibition of a single integrin (LFA-1 or α4β7) results in a dramatic inhibition of homing of T and B cells to the LN, it has only a minor effect on their entrance into the splenic white pulp.1
Previously, we characterized pathways that negatively regulate homing of B cells into the LN. We demonstrated that immature B cells can down regulate their own integrin-mediated adhesion to the extracellular matrix, and thereby suppress their migration into nonsplenic sites.2 This inhibition is mediated by 2 independent pathways. The first one involves the secretion of IFN-γ, which is transcribed and secreted at low levels by immature B cells.3,4 The second pathway is regulated by the chemokine receptor, CCR2, which is expressed on murine immature B cells and whose expression is down-regulated after differentiation to the mature stage. CCR2-deficient (CCR2−/−) B cells exhibit a remarkable elevation in their response to CXCL12 stimulation, as demonstrated by their cytoskeletal rearrangement and migration in a transwell assay in vitro and homing to the LN in vivo, independent of the negative regulation by IFN-γ.5
Naive T lymphocytes traffic through the T-cell areas of secondary lymphoid organs in search of antigen presented by dendritic cells.6,7 Upon antigen recognition, specific T cells proliferate and, in the presence of polarizing cytokines, differentiate toward Th1 or Th2 cells that produce distinct patterns of cytokines and mediate different types of protective and pathologic responses.8,9 To enter lymph nodes, T lymphocytes need to firmly interact with and extravasate across specialized lymph node venules, the high endothelial venules (HEVs). Naive and central memory T cells use surface expressed L-selectin (CD62L) for their capture and rolling on these venules, while they depend on CC chemokine receptor 7 (CCR7) for integrin-mediated arrest on HEVs.10-13 After T-cell differentiation to Th1 or Th2, the LN homing receptors are down-regulated, while tissue homing receptors are acquired,14-17 enabling the Th1 and Th2 cells to exhibit specific migratory capacities in vivo.18,19
Because low doses of CCL2 strongly impair B-lymphocyte homing to LN, we hypothesized that this chemokine may also interfere with T lymphocyte homing to these tissues. In this study, we show that picomolar (pM) levels of CCL2 down-regulate migration of T cells in vitro and in vivo and interfere with the ability of T-cell LFA-1 to undergo conformational activation in response to CCR7 signals and to develop CCR7-dependent lymphocyte adhesion strengthening both in vitro and in vivo on HEVs. This results in a dramatic effect on T-cell homing to the LN, and in the consequent attenuation of the inflammatory responses.
Methods
Animals
C57BL/6, Balb/c, CCR2 deficient (CCR2−/−)20 mice (6-8 weeks of age) and female Lewis rats were raised and maintained under pathogen-free conditions. All animal procedures and experiments were approved by the Animal Research Committee at the Weizmann Institute and were in accordance with French procedural guidelines for animal handling.
Cells
Spleen cells were obtained from mice as previously described.21 Spleen cells were incubated with anti-CD45R (B220; BD Biosciences, San Jose, CA) magnetic beads and CD45-negative cells were collected. CD4+ and CD8+ T cells were purified using anti-CD4 or anti-CD8 magnetic beads (Miltenyi Biotec, Bergisch Gladbach, Germany).
RNA isolation and reverse transcription
RNA isolation and reverse transcriptions were performed as previously described.5
Preparation of cell extract
Stimulated cells were lysed in lysis buffer that was previously described.5
ERK detection
Detection of ERK phosphorylation was performed as previously described.5
Vav detection
Naive T cells were pretreated with or without CCL2 (0.1 ng/mL) for 30 minutes. The cells were then stimulated with 100 ng/mL CCL21 for time ranging from 0-2.5 minutes and lysed immediately. Protein-G Sepharose beads (Pharmacia; Amersham, Uppsala, Sweden) were conjugated to mAb p-Tyr (pTyr99; Santa Cruz Biotechnology, Santa Cruz, CA) and added to the cell lysates. Phosphorylated proteins were immunoprecipitated overnight and washed. The proteins were transferred into nitrocellulose membrane and probed with anti-Vav (UBI, Hauppauge, NY) followed by horseradish peroxidase–conjugated anti–mouse or rabbit IgG (Jackson ImmunoResearch Labs, West Grove, PA).
Flow cytometric analysis of LFA-1 activation
PBL were either pretreated with CCL2 (0.1 ng/mL) or incubated in medium for 30 minutes at 37°C, and were then either left intact or stimulated for either 1 or 5 minutes with 10 nM CCL21, in the presence of the high-affinity LFA-1 reporter mAb, 327C. Cells were then washed at 4°C, stained with PE-conjugated secondary Ab (Jackson ImmunoResearch Labs), and analyzed by FACScan (Becton Dickinson, Erembodegem, Belgium).
Transwell migration
T cells were incubated with either 0.1 ng/mL CCL2, 0.1 ng/mL (5 μM) of the MEK inhibitor, U0126 (Calbiochem, Gibbstown, NJ), which prevents ERK phosphorylation, or left untreated. Chemotaxis was assayed using transwell chambers as previously described.3
Cytoskeleton rearrangement
T cells were pretreated in the presence or absence of CCL2 0.1 ng/mL (5 μM) of the MEK inhibitor, U0126 (Calbiochem) or left untreated. Cytoskeleton rearrangement was analyzed as previously described by staining with fluorescein isothiocyanate (FITC)–phalloidin.3
Tracking of cells in vivo
Cells were stained with 5 μM carboxyfluorescein diacetate succinimidyl ester (CFDA-SE; Invitrogen, Carlsbad, CA) for 15 minutes. Homing of T cells to the spleen and the LN was analyzed as previously described.22
Laminar flow adhesion assays
Purified VCAM-1 or ICAM-1-Fc were coated alone or with CCL21 (R&D Systems, Minneapolis, MN) as previously described.23,24 All flow experiments were conducted at 37°C. Cells were perfused through the flow chamber at low shear stress (0.5-0.75 dyn/cm2) for 2 minutes. Tethers were defined as transient or rolling if cells attached briefly (< 2 seconds) to the substrate, and as arrests if cells immediately arrested and remained stationary for at least 5 seconds of continuous flow. To assess adhesion strengthening post arrest resistance to detachment over time was determined by subjecting cells to a shear stress of 5 dyn/cm2 for 5 minutes. More than 90% of lymphocyte arrests on VCAM-1 or ICAM-1 were blocked by pretreating cells with VLA-4 or LFA-1 blockers, respectively.
Intravital microscopy of mouse subiliac LN
Mice were anesthetized by intraperitoneal injection of 1 mg/mL xylazine and 5 mg/mL ketamine. The right femoral artery was catheterized. The left subiliac LN was prepared for IVM as previously described, and the mouse was then transferred to a customized intravital video microscopy setup.25,26 For visualization of naive lymphocyte interactions with LN vascular endothelium, naive lymphocytes obtained from single T- and B-cell suspensions of a pool of C57BL/6 subiliac, axillary, brachial, and mesenteric LNs (C57BL/6 or CCR2−/− mice) were incubated with CCL2 (R&D Systems) or left untreated, fluorescently labeled by calcein AM (Invitrogen, 0.25 μM, 5 minutes, 37°C), and then injected into the right femoral artery. Cell behavior in LN venules was assessed as previously described.25,26 The rolling fraction was determined as the percentage of cells that rolled along the vascular lining out of the total flux per venule. The sticking fraction was defined as the percentage of cells firmly adherent for at least 10 seconds, 30 seconds, or 1 minute.
OVA sensitization and challenge
BALB/c mice were immunized and challenged with OVA to induce a model of asthma as described previously.4
Lung histology
Lungs histology was performed as previously described.4
Bronchoalveolar lavage
Bronchoalveolar lavage (BAL) was performed on day 19, 4 hours after the last airway hyperresponsiveness (AHR) evaluation, as previously described.27
Rodent model for rheumatoid arthritis induction and assessment
Rheumatoid arthritis (RA) was induced as described previously,28 using 1 mg per rat of heat-killed Mt strain H37Ra (Difco). Each experimental and control group contained at least 8 rats. The day of adjuvant arthritis (AA) induction was designated as day 0, and disease severity was assessed by direct observation of all 4 limbs in each animal. A relative score between 0 and 4 was assigned to each limb based on the degree of joint inflammation, redness, and deformity; thus, the maximum possible score for an individual animal was 16.28 Mice were injected daily with CCL2 (240 ng in 300 μL phosphate-buffered saline [PBS]) or with PBS as control from day 0 to day 5. The person who scored the disease was blinded to the identity of the groups. Experiments were repeated at least 3 times and produced similar results.
Statistical analysis
For statistical comparison of paired samples, a 2-tailed Student t test was used.
Results
CCL2 inhibits naive T-cell cytoskeleton rearrangements necessary for optimal transwell migration toward lymph node chemokines
To determine whether CCR2 and its ligand, CCL2, at pM levels control T-cell migration, and whether they can regulate the homing of these cells to the LN and to sites of inflammation in vivo, we began by analyzing CCR2 transcription in T-cell subsets. As shown previously29 and in Figure 1A, CCR2 mRNA was present in both CD4+ and CD8+ T cells. We then studied the inhibitory effect of pM range CCL2 on naive T cells in vitro. The migratory response of naive T cells to CCL21 (0.1 μg/mL) was analyzed in a transwell assay. Naive T cells were suspended with or without CCL2 (0.1 ng/mL) placed in the upper chamber of a transwell, and cells were allowed to migrate toward CCL21 placed in the lower chamber. While CCL2 treatment did not modify CCR7 cell surface expression levels (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article), pM levels of CCL2 inhibited the migration of control naive T cells toward CCL21 by 40% to 50% (Figure 1B). These low levels of CCL2 had no significant effect on the migratory response of CCR2-deficient naive T cells (Figure 1C).
CCR2 is expressed on naive T cells and inhibits their cytoskeleton rearrangement and migration. (A) CD4+ and CD8+ T cells were separated. RNA was isolated and levels of CCR2 and HPRT mRNA were analyzed by reverse transcription–polymerase chain reaction. The results presented are representative of 3 independent experiments. (B,C) Transwell migration. Control or CCR2−/− naive T cells were pretreated for 30 minutes with medium or CCL2 (0.1 ng/mL). The cells were then placed in the upper well of a 24-well transwell plate in the presence or absence of CCL21 (0.1 μg/mL). After 3 hours, the number of migrating cells found in the lower chamber was evaluated by fluorescence-activated cell sorting (FACS) analysis. Percent migration was calculated as the number of migrating cells in the lower chamber as a fraction of the input cell number in the upper chamber. The results presented are representative of 3 different experiments. (D-F) Cytoskeleton rearrangement. Naive T cells from control (D-F) or CCR2−/− mice (D) were stimulated with CCL21 (0.1 μg/mL) for 15 seconds in the presence or absence of CCL2 (0.1 ng/mL; D-F), various concentrations of CCL2 (E), or Rantes, Mip1b, or Eotoxin (0.1 ng/mL; F). The cells were fixed and permeabilized, and their intracellular F-actin was stained with FITC-phalloidin. The change in polymerized actin was analyzed by FACS. Percentage increase in actin polymerization was calculated as the polymerization of actin in the presence of chemokine stimulation/polymerization of actin without chemokine ×100. The results presented are representative of 3 separate experiments. (G) Naive T cells were pretreated with or without CCL2 (0.1 ng/mL) for 30 minutes. The cells were then stimulated with CCL21 and lysed immediately. Lysates were separated on reducing 10% (wt/vol) sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotted with anti-phosphospecific ERK 1/2 (p-ERK). Immunoblots were stripped and reprobed with anti-[total] ERK 1/2. (H) Cytoskeleton rearrangement. Naive T cells were stimulated with medium or CCL21 (0.1 μg/mL) for 15 seconds in the presence or absence of CCL2 (0.1 ng/mL) or the MEK inhibitor, U0126 (5 μM). The cells were fixed and permeabilized, and their intracellular F-actin was stained with FITC-phalloidin. The change in polymerized actin was analyzed by FACS and calculated as described above. (I) Transwell migration. Naive T cells were pretreated for 30 minutes with medium or CCL2 (0.1 ng/mL) in the presence or absence of the MEK inhibitor, U0126 (5 μM). After 3 hours, the number of migrating cells found in the lower chamber was evaluated by FACS analysis. Percentage migration was calculated as described above.
CCR2 is expressed on naive T cells and inhibits their cytoskeleton rearrangement and migration. (A) CD4+ and CD8+ T cells were separated. RNA was isolated and levels of CCR2 and HPRT mRNA were analyzed by reverse transcription–polymerase chain reaction. The results presented are representative of 3 independent experiments. (B,C) Transwell migration. Control or CCR2−/− naive T cells were pretreated for 30 minutes with medium or CCL2 (0.1 ng/mL). The cells were then placed in the upper well of a 24-well transwell plate in the presence or absence of CCL21 (0.1 μg/mL). After 3 hours, the number of migrating cells found in the lower chamber was evaluated by fluorescence-activated cell sorting (FACS) analysis. Percent migration was calculated as the number of migrating cells in the lower chamber as a fraction of the input cell number in the upper chamber. The results presented are representative of 3 different experiments. (D-F) Cytoskeleton rearrangement. Naive T cells from control (D-F) or CCR2−/− mice (D) were stimulated with CCL21 (0.1 μg/mL) for 15 seconds in the presence or absence of CCL2 (0.1 ng/mL; D-F), various concentrations of CCL2 (E), or Rantes, Mip1b, or Eotoxin (0.1 ng/mL; F). The cells were fixed and permeabilized, and their intracellular F-actin was stained with FITC-phalloidin. The change in polymerized actin was analyzed by FACS. Percentage increase in actin polymerization was calculated as the polymerization of actin in the presence of chemokine stimulation/polymerization of actin without chemokine ×100. The results presented are representative of 3 separate experiments. (G) Naive T cells were pretreated with or without CCL2 (0.1 ng/mL) for 30 minutes. The cells were then stimulated with CCL21 and lysed immediately. Lysates were separated on reducing 10% (wt/vol) sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotted with anti-phosphospecific ERK 1/2 (p-ERK). Immunoblots were stripped and reprobed with anti-[total] ERK 1/2. (H) Cytoskeleton rearrangement. Naive T cells were stimulated with medium or CCL21 (0.1 μg/mL) for 15 seconds in the presence or absence of CCL2 (0.1 ng/mL) or the MEK inhibitor, U0126 (5 μM). The cells were fixed and permeabilized, and their intracellular F-actin was stained with FITC-phalloidin. The change in polymerized actin was analyzed by FACS and calculated as described above. (I) Transwell migration. Naive T cells were pretreated for 30 minutes with medium or CCL2 (0.1 ng/mL) in the presence or absence of the MEK inhibitor, U0126 (5 μM). After 3 hours, the number of migrating cells found in the lower chamber was evaluated by FACS analysis. Percentage migration was calculated as described above.
Among the requirements for integrin-mediated migration are an increased rate of actin polymerization, and extensive reorganization of the actin-based cytoskeleton. To determine whether CCL2 regulates the cytoskeletal rearrangement of T cells, we followed the actin polymerization of naive T cells, which were preincubated with or without CCL2 (0.1 ng/mL), then stimulated with CCL21. The cells were immediately fixed with paraformaldehyde, permeabilized and stained with FITC-phalloidin, and then analyzed by flow cytometry to determine the state of their cytoskeleton. As can be seen in Figure 1D, CCL21 stimulation induced actin polymerization, which was inhibited by about 40% to 50% in control cells pre-treated with CCL2 with almost no effect on CCR2-deficient cells. The inhibitory effect of CCL2 occurred only at pM levels (Figure 1E), demonstrating that the CCR2 receptor is involved in the cytoskeleton rearrangement induced by pM levels of CCL2. To determine whether additional chemokines at pM levels have a similar inhibitory effect on T cells, we analyzed the effect of several chemokines at low dose on the actin polymerization of naive T cells. As can be seen in Figure 1F, low levels of Rantes, Mip1β, Eotaxin and CXCL12 had no inhibitory effect on the actin polymerization of naive T cells. Importantly, the effect of CCL2 was not specific to CCL21. A dramatic inhibition in the ability of T cells to rearrange their actin was found in CXCL12 or CCL19 stimulated cells as well (Figure S2). Thus, CCL2 appears to act as a specific inhibitor of the migration response of T cells by down-regulating their ability to polymerize their actin, similar to its effect on B cells.5
We have previously shown that activation of ERK1/2 is inhibited when immature B cells are pretreated with CCL2.5 We therefore wished to determine whether CCL2 similarly inhibits ERK phosphorylation in T cells. As can be seen in Figure 1G, CCL21 induced phosphorylation of ERK1/2, and this activation was inhibited in cells treated with CCL2 for 0.5 to 1 minute. Thus, CCL2 regulates a signaling cascade that involves ERK1/2, resulting in inhibition of cytoskeleton rearrangement and migration. To address the functional relationships between ERK activation levels and T-cell migration and actin polymerization, naive T cells were incubated in the presence or absence of the MEK inhibitor, U0126. As shown in Figure 1H and I, blocking ERK phosphorylation significantly suppressed the ability of T cells to polymerize their actin and migrate, similarly to the effect of CCL2, suggesting that inhibition of ERK phosphorylation by CCL2 is involved in the down-regulation of T-cell migration.
CCL2 inhibits the postarrest integrin-dependent adhesion strengthening of T lymphocytes on ICAM-1 and VCAM-1 triggered by coimmobilized CCL21
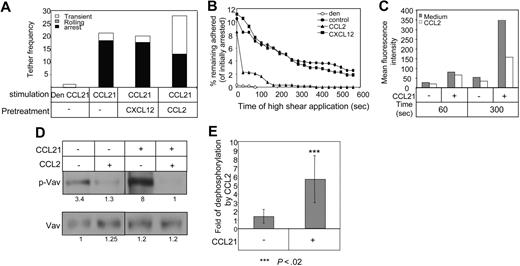
To provide additional mechanistic data regarding the inhibitory effects of CCL2 on lymphocyte migration, and to extend our observations to human T cells, we next performed in vitro flow chamber–based assays to assess the activation of 2 major integrins, VLA-4 and LFA-1, by CCL21. We tested whether short exposure of human T lymphocytes to low-dose CCL2 can impair the ability of their LFA-1 and VLA-4 integrins to undergo activation by in situ signals from surface-bound CCL21 under shear stress conditions. CCL21, when coimmobilized with either ICAM-1 or VCAM-1, triggers robust Gi-mediated LFA-1 or VLA-4–mediated lymphocyte sticking under shear flow conditions.23,30 Treatment of human T cells with pM levels of CCL2 had a minor effect on LFA-1 (Figure 2A) or VLA-4 (Figure S3) activation by surface-bound CCL21, as evident from the almost normal capacity of CCL2 treated T cells to arrest (stick) on the LFA-1 or the VLA-4 ligands, ICAM-1 (Figure 2A) and VCAM-1 (Figure S3), respectively. Nevertheless, once arrested on these ligands, CCL2 pretreated T cells failed to remain firmly adhered to the integrin ligands for prolonged periods and readily detached from these ligands when exposed to continuous application of shear stresses for several minutes after initial sticking (Figures 2B, S3). The increased detachment of CCL2 pretreated T cells from ICAM-1 or VCAM-1 coimmobilized with CCL21 was not associated, however, with reduction in cell spreading. On the other hand, similar pretreatment of T lymphocytes with low doses of CXCL12 retained, both initial integrin mediated sticking and subsequent resistance to detachment when exposed to similar application of continuous shear flow (Figures 2B, S3). We conclude that low-dose CCL2, but not CXCL12, interferes with crucial cytoskeletal rearrangements required for initially activated integrins to maintain their firm adhesion and resist detachment in the presence of prolonged exposure to shear stress. In addition, analysis of LFA-1 activation, using the 327C mAb, a reporter of high-affinity LFA-1 conformational state in cells treated with pM levels of CCL2, revealed that while CCL2 had no effect on LFA-1 activation triggered by CCL21 in a short-term assay (1 minute), CCL2-pretreated T cells failed to undergo further LFA-1 activation during prolonged (5 minutes) exposure to CCL21, in contrast to sham-treated T cells (Figure 2C). Thus, CCL2 induces a time-dependent suppression of LFA-1 activation by the lymph node chemokine CCL21.
Low-dose CCL2 does not interfere with rapid LFA-1 or VLA-4 activation by CCL21 but impairs adhesion persistence in vitro. (A) Frequency and strength of adhesive tethers between human T cells (intact or CCL2-pretreated) interacting with ICAM-1 coated alone or coimmobilized with CCL21 or denaturated (den) CCL21 (control). Experiments were performed at a shear stress of 0.5 dyn/cm2. (B) Human T cells (intact or CCL2 pre treated) were allowed to accumulate for 2 minutes at a shear stress of 0.5 dyn/cm2 on ICAM-1/CCL21 or denaturated CCL21 (den) and their adhesion persistence (ability to resist detachment by a constant application of high shear stress (5 dyn/cm2) was assessed at the indicated time points. Results are shown as percentage of cells initially accumulated on the integrin ligands. The experiment shown is representative of 4 independent experiments. (C) Effect of pre-exposure of lymphocytes to CCL2 (0.1 ng/mL) on the CCL21-triggered induction of the high-affinity LFA-1 epitope 327C in PBL. FACS staining showing spontaneous and CCL21-triggered expression of the 327C epitope at the indicated time points is presented as mean fluorescence intensity (MFI). The experiment shown is representative of 3 independent experiments. (D) Naive T cells were pretreated with or without CCL2 (0.1 ng/mL) for 30 minutes. The cells were then stimulated with CCL21 and lysed immediately. Immunoprecipitates were separated by 10% (wt/vol) SDS-PAGE and p-Vav and Vav were analyzed as described in “Vav detection.” (E) Graph summarizing the inhibition of Vav phosphorylation by CCL2 in CCL21-treated or untreated cells from 4 different experiments.
Low-dose CCL2 does not interfere with rapid LFA-1 or VLA-4 activation by CCL21 but impairs adhesion persistence in vitro. (A) Frequency and strength of adhesive tethers between human T cells (intact or CCL2-pretreated) interacting with ICAM-1 coated alone or coimmobilized with CCL21 or denaturated (den) CCL21 (control). Experiments were performed at a shear stress of 0.5 dyn/cm2. (B) Human T cells (intact or CCL2 pre treated) were allowed to accumulate for 2 minutes at a shear stress of 0.5 dyn/cm2 on ICAM-1/CCL21 or denaturated CCL21 (den) and their adhesion persistence (ability to resist detachment by a constant application of high shear stress (5 dyn/cm2) was assessed at the indicated time points. Results are shown as percentage of cells initially accumulated on the integrin ligands. The experiment shown is representative of 4 independent experiments. (C) Effect of pre-exposure of lymphocytes to CCL2 (0.1 ng/mL) on the CCL21-triggered induction of the high-affinity LFA-1 epitope 327C in PBL. FACS staining showing spontaneous and CCL21-triggered expression of the 327C epitope at the indicated time points is presented as mean fluorescence intensity (MFI). The experiment shown is representative of 3 independent experiments. (D) Naive T cells were pretreated with or without CCL2 (0.1 ng/mL) for 30 minutes. The cells were then stimulated with CCL21 and lysed immediately. Immunoprecipitates were separated by 10% (wt/vol) SDS-PAGE and p-Vav and Vav were analyzed as described in “Vav detection.” (E) Graph summarizing the inhibition of Vav phosphorylation by CCL2 in CCL21-treated or untreated cells from 4 different experiments.
The small GTPases of the Rho subfamily, including Rho, Rac, and Cdc42 proteins,31,32 are key players in the regulation of the dynamics of actin cytoskeleton as well as in conformational integrin activation and post ligand–binding events such as integrin clustering and adhesion strengthening. Guanine-nucleotide exchange factors (GEF) stimulate the exchange of GDP for GTP to generate active Rho GTPases.33 Activation of Rho regulates the assembly of actin filaments, whereas Rac and Cdc42 regulate actin polymerization to generate lamellipodia and filopodia protrusions, respectively.31 Vav1 is a key GEF for Rac.34-36 Activation of Vav1-Rac signaling pathway was recently shown to control efficient up-regulation of integrin-dependent T lymphocyte adhesion.37 Thus, to determine whether CCL2-mediated inhibition involves interference with Vav1 function, we analyzed Vav phosphorylation in cells stimulated with CCL21 in the presence or absence of CCL2. As shown in Figure 2D and E, CCL2 inhibited both spontaneous and CCL21-induced Vav phosphorylation, suggesting that inhibition by CCL2 of Vav phosphorylation may represent a mechanism accounting for impairment in integrin-mediated adhesion strengthening and actin polymerization.
CCL2 inhibits firm LFA-1–mediated lymphocyte adhesion but not initial sticking to HEVs in mouse peripheral lymph nodes
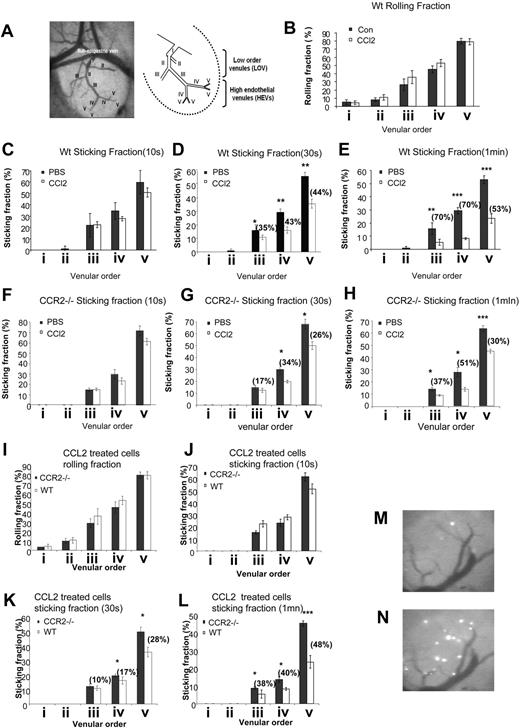
To corroborate our in vitro findings in a more physiologic setting, we next investigated the effects of low-dose CCL2 on lymphocyte adhesion to HEVs in the peripheral LN (PLN) microcirculation by using intravital microscopy (IVM). Previous IVM observations of normal murine subiliac LNs established that the LN venular tree consists of up to 5 branching orders,25,26,38 in which the low-order venules (LOVs) comprise a large collecting venule in the hilus (order I), and upstream branches in the medulla (order II and certain order III). The higher order branches (most order III and all order IV and V venules) are HEVs directly connected to the LN capillaries and flow toward the LN sub- and paracortex (Figure 3A). We analyzed the behavior (rolling, sticking) of control and CCL2 (low-dose)–treated lymphocytes in the LOVs (order II) and HEVs (orders III, IV, and V). Lymphocyte arrest on these HEVs is mediated by their LFA-1 integrin and requires in situ lymphocyte activation by CCL21 signals presented on the luminal aspects of these venules.12 Treatment with low-dose CCL2 did not significantly alter the ability of lymphocytes to establish L-selectin–mediated rolling on high- or low-order HEVs (Figure 3B). Similarly, the percentage of naive lymphocytes that arrested for at least 10 seconds after rolling inside the HEVs (sticking fraction 10s) was comparable for both control and CCL2-treated lymphocytes (Figure 3C). Thus, similar to our findings in vitro, CCL2 pretreatment did not affect the earliest events of CCL21-triggered LFA-1–mediated lymphocyte adhesions in vivo. However, lymphocytes treated with low-dose CCL2 remained arrested for only several seconds on the HEV walls before detaching, thus leading to a severe reduction in the percentage of cells remaining adherent 30 seconds or 1 minute after their initial arrest (Figure 3, D and E, respectively) when compared with control lymphocytes. The effect of pM levels of CCL2 on adhesion strengthening has both CCR2-dependent and -independent components. Although we observed some effects of CCL2 on CCR2−/− lymphocyte adhesion to HEV walls in vivo (Figure 3F-H), these effects were significantly reduced compared with wild-type lymphocytes (Figure 3I-L). It remains to be established through which receptor and pathway CCL2 can exert a limited effect when CCR2 is absent. This impairment in the capacity of CCL2-treated lymphocytes to resist shear-mediated detachment while attached to HEVs resulted in a striking reduction in the number of firmly bound lymphocytes in the PLN venular tree, 30 minutes after injection of the fluorescent cells (Figure 3M,N). Because without the capacity to remain adherent minutes after initial sticking on the HEV target vessels, lymphocytes would fail to extravasate these vessels, we conclude that low dose of CCL2 inhibits integrin-dependent lymphocyte firm adhesion to and extravasation across HEV walls in the mouse PLN microcirculation.
CCL2 inhibits firm integrin-mediated lymphocyte adhesion to HEV walls in mouse PLNs. (A) Intravital micrograph and sketch showing a typical subiliac LN at low (5×/0.12 NA, N Plan, water immersion) magnification. LN venous blood drains into an extralymphoid side branch of the superficial epigastric vein via the LOVs (orders I, II, and some order III venules) and the HEVs (orders III, IV and V venules). (B) Rolling and (C-H) sticking fractions of calcein-labeled naive control or CCL2-treated lymphocytes (C-E) or CCR2−/− control or CCL-2 treated lymphocytes (F-H) in the venular tree of subiliac LNs. Lymphocytes were pretreated with CCL2 (1 ng/mL, 30 minutes) or left untreated and injected into the femoral artery. Rolling fraction = 100 × number of rolling cells/number of fast cells. Sticking fraction = 100 × number of arrested cells (more than 10 seconds or 30 seconds or 1 minute) / number of rolling cells. Data shown are means plus or minus SEM of 3 to 5 venules per mouse (n = 3 animals analyzed). (I-L) Rolling (I) and sticking fractions (J-L) of calcein-labeled naive CCL2-treated WT or CCR2−/− lymphocytes in the venular tree of subiliac LNs. Wild-type or CCR2−/− lymphocytes were pretreated with CCL2 (1 ng/mL, 30 minutes) and injected into the femoral artery of mice. Rolling fraction = 100 × number of rolling cells / number of fast cells. Sticking fraction = 100 × number of arrested cells (more than 10 seconds or 30 seconds or 1 minute) / number of rolling cells. Data shown are means plus or minus SEM of 3 to 9 venules per mouse (n = 3 animals analyzed for wild-type lymphocytes and n = 4 animals analyzed for CCR2−/− lymphocytes). Percentages of inhibition are indicated. (M,N) Intravital micrographs (10×/0.3 NA, HCX APO, water immersion) of lymphocyte arrest in the PLN venular tree. The accumulation of CCL2-treated (M) or control (N) lymphocytes in PLN venules was analyzed 30 minutes after intravenous injection of the fluorescently labeled cells. All photomicrograph images (A,M,N) were obtained with a Leica INM 100 microscope (Leica Microsystems SA, Rueil-Malmaison, France) coupled to a silicon-intensified target camera (Hamamatsu Photonics, Massy, France); videos were recorded on DVCAM video tapes (DSR-11 Sony; IEC-ASV, Toulouse, France) and images were obtained with an image processing unit (Argus 20; Hamamatsu Photonics) and numerized with DPS velocity software (Digital Processing System, Hicksville, NY).
CCL2 inhibits firm integrin-mediated lymphocyte adhesion to HEV walls in mouse PLNs. (A) Intravital micrograph and sketch showing a typical subiliac LN at low (5×/0.12 NA, N Plan, water immersion) magnification. LN venous blood drains into an extralymphoid side branch of the superficial epigastric vein via the LOVs (orders I, II, and some order III venules) and the HEVs (orders III, IV and V venules). (B) Rolling and (C-H) sticking fractions of calcein-labeled naive control or CCL2-treated lymphocytes (C-E) or CCR2−/− control or CCL-2 treated lymphocytes (F-H) in the venular tree of subiliac LNs. Lymphocytes were pretreated with CCL2 (1 ng/mL, 30 minutes) or left untreated and injected into the femoral artery. Rolling fraction = 100 × number of rolling cells/number of fast cells. Sticking fraction = 100 × number of arrested cells (more than 10 seconds or 30 seconds or 1 minute) / number of rolling cells. Data shown are means plus or minus SEM of 3 to 5 venules per mouse (n = 3 animals analyzed). (I-L) Rolling (I) and sticking fractions (J-L) of calcein-labeled naive CCL2-treated WT or CCR2−/− lymphocytes in the venular tree of subiliac LNs. Wild-type or CCR2−/− lymphocytes were pretreated with CCL2 (1 ng/mL, 30 minutes) and injected into the femoral artery of mice. Rolling fraction = 100 × number of rolling cells / number of fast cells. Sticking fraction = 100 × number of arrested cells (more than 10 seconds or 30 seconds or 1 minute) / number of rolling cells. Data shown are means plus or minus SEM of 3 to 9 venules per mouse (n = 3 animals analyzed for wild-type lymphocytes and n = 4 animals analyzed for CCR2−/− lymphocytes). Percentages of inhibition are indicated. (M,N) Intravital micrographs (10×/0.3 NA, HCX APO, water immersion) of lymphocyte arrest in the PLN venular tree. The accumulation of CCL2-treated (M) or control (N) lymphocytes in PLN venules was analyzed 30 minutes after intravenous injection of the fluorescently labeled cells. All photomicrograph images (A,M,N) were obtained with a Leica INM 100 microscope (Leica Microsystems SA, Rueil-Malmaison, France) coupled to a silicon-intensified target camera (Hamamatsu Photonics, Massy, France); videos were recorded on DVCAM video tapes (DSR-11 Sony; IEC-ASV, Toulouse, France) and images were obtained with an image processing unit (Argus 20; Hamamatsu Photonics) and numerized with DPS velocity software (Digital Processing System, Hicksville, NY).
CCL2 inhibits naive T-cell homing into lymph nodes
The inhibitory effect of CCL2 on CCL21 chemokine-triggered integrin adhesions in vitro and in vivo and on chemotaxis of T lymphocytes in vitro suggested that this cytokine could also interfere with the homing of naive T cells into LNs, a process critically dependent on CCL21-triggered adhesion and motility. We therefore analyzed the ability of CCL2-treated naive T cells to enter into peripheral LNs (PLNs).
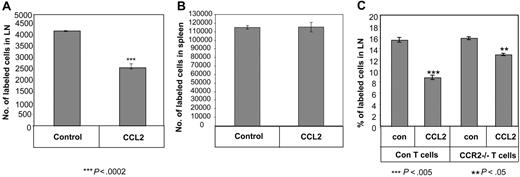
Naive T cells preincubated in the presence or absence of CCL2 (0.1 ng/mL) were labeled with the fluorescent dye, CFDA-SE, and injected intravenously into C57BL/6 mice. The proportion of labeled cells recovered in PLNs was determined 3 hours after injection. Compared with the control population (treated with PBS), there was a significant reduction (∼50%) in the homing of CCL2-treated naive T cells into the LN, while its effect on the homing of CCR2−/− T cells to this compartment was significantly lower (Figure 4A,C). Interestingly, there was no difference in the migration of the treated versus untreated control T cells to the spleen (Figure 4B), further supporting our view that entry into the spleen is different from trafficking to the LN and does not require intact integrin-mediated adhesion strengthening. Our results demonstrate, therefore, that CCL2 inhibits the migration of naive T cells into LNs but not to spleen in vivo.
CCL2 inhibits homing of naive T cells into LNs. (A,B) FITC-labeled T cells were incubated in the presence or absence of CCL2 (0.1 ng/mL) and injected into mice. After 3 hours, the PLNs (A) or spleen (B) were collected and the FITC-positive population was analyzed by FACS. (C) FITC-labeled control or CCR2−/− T cells were incubated in the presence or absence of CCL2 (0.1 ng/mL) and injected into mice. After 3 hours, the PLNs were collected and the FITC-positive population was analyzed by FACS. The results presented are representative of 4 separate experiments.
CCL2 inhibits homing of naive T cells into LNs. (A,B) FITC-labeled T cells were incubated in the presence or absence of CCL2 (0.1 ng/mL) and injected into mice. After 3 hours, the PLNs (A) or spleen (B) were collected and the FITC-positive population was analyzed by FACS. (C) FITC-labeled control or CCR2−/− T cells were incubated in the presence or absence of CCL2 (0.1 ng/mL) and injected into mice. After 3 hours, the PLNs were collected and the FITC-positive population was analyzed by FACS. The results presented are representative of 4 separate experiments.
CCL2 exerts antiinflammatory effects in vivo
The powerful inhibitory effect of CCL2 on chemokine triggered migration and integrin-dependent adhesion of T lymphocytes in vitro and in vivo suggests that this cytokine might serve as an antiinflammatory mediator at pM levels.
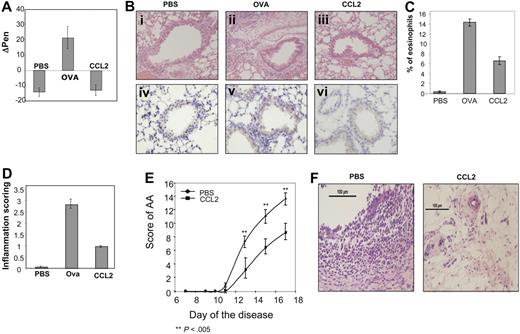
We therefore analyzed the influence of this chemokine in 2 prototypic inflammatory models, AA, and asthma. Asthma is a chronic inflammatory disorder of the bronchial airways characterized by intermittent episodes of airway obstruction and wheezing. Specific symptoms include variable airflow obstruction, AHR, and airway inflammation. It has been suggested that T lymphocytes, and in particular CD4+ T cells producing a Th2 pattern of cytokines, have a prominent effect on the pathogenesis of this disease.39-42 To examine the possible antiinflammatory effect of CCL2 on allergic airway reactivity, we used a treatment protocol that was described previously,4 involving intraperitoneal injection of 60 ng/day (180 ng/mL) of CCL2, beginning on the first day of ovalbumin (OVA) inhalation. The airway responsiveness of anesthetized, spontaneously breathing mice was evaluated after 5 days of OVA inhalations. The OVA-challenged mice significantly increased their airway responsiveness to the antigen challenge. However, no airway hyperactivity was observed in control (PBS-challenged) mice or in OVA-challenged mice treated with CCL2 (Figure 5A).
CCL2 mediates an anti-inflammatory role in vivo. (A) Asthma model: control (PBS-treated), OVA-primed mice (OVA), and OVA-primed mice injected intraperitoneally with 60 ng of CCL2 (CCL2) as described in “Methods” were analyzed for airway responsiveness on day 19. Values shown represent Δ Penn, which was calculated by subtracting control Penn measurements before antigen challenge from the Penn measurements after late or early antigen challenge. Baseline Penn levels were comparable among PBS-treated control, OVA-primed mice, and OVA-primed mice treated with CCL2. The results represent an average of 9 animals per treatment. (Bi-iii) Lung histology in mice that received CCL2 treatment. Histologic features of representative control (PBS), OVA-primed (OVA), and OVA-primed treated with low-dose CCL2 (CCL2) animals are shown. (Biv-vi) Immunohistochemical staining of the T cells in lung sections. (C) Bronchoalveolar lavage cell recovery in mice after treatment with CCL2. Percentage eosinophil recovery in BAL fluids in the various mice. Values represent the mean of 5 animals. (D) The peribronchial and perivascular inflammatory infiltrates were given an inflammatory score between 1 and 4 by a pathologist. The graph represents the average scores of 9 animals from each treatment group. (E,F) Arthritis model: AA was induced as described in “Rodent model for rheumatoid arthritis induction and assessment,” and rats immediately divided into 2 groups that were injected with low-dose CCL2 (240 ng in 300 μL PBS) or PBS. (E) A disease score between 0 and 4 was assigned to each limb, based on the degree of joint inflammation, redness, and deformity; the maximum possible score for an individual animal was 16. The graph represents the scores of 9 animals in each group that were measured every day. (F) Joint histology in PBS- or CCL2-treated mice. Results are presented as the mean plus or minus SEM of the difference between the 2 values for all the animals in each group. All photomicrograph images (B,F) were obtained with an Olympus AK 70 microscope (Center Valley, PA), Olympus Ach 20×/0.4 ∞/0.17. Camera make and model: Nikon Digital Camera DXM 1200F. Name and version number of image-acquisition software: Nikon ACT-l for DMX1200F.
CCL2 mediates an anti-inflammatory role in vivo. (A) Asthma model: control (PBS-treated), OVA-primed mice (OVA), and OVA-primed mice injected intraperitoneally with 60 ng of CCL2 (CCL2) as described in “Methods” were analyzed for airway responsiveness on day 19. Values shown represent Δ Penn, which was calculated by subtracting control Penn measurements before antigen challenge from the Penn measurements after late or early antigen challenge. Baseline Penn levels were comparable among PBS-treated control, OVA-primed mice, and OVA-primed mice treated with CCL2. The results represent an average of 9 animals per treatment. (Bi-iii) Lung histology in mice that received CCL2 treatment. Histologic features of representative control (PBS), OVA-primed (OVA), and OVA-primed treated with low-dose CCL2 (CCL2) animals are shown. (Biv-vi) Immunohistochemical staining of the T cells in lung sections. (C) Bronchoalveolar lavage cell recovery in mice after treatment with CCL2. Percentage eosinophil recovery in BAL fluids in the various mice. Values represent the mean of 5 animals. (D) The peribronchial and perivascular inflammatory infiltrates were given an inflammatory score between 1 and 4 by a pathologist. The graph represents the average scores of 9 animals from each treatment group. (E,F) Arthritis model: AA was induced as described in “Rodent model for rheumatoid arthritis induction and assessment,” and rats immediately divided into 2 groups that were injected with low-dose CCL2 (240 ng in 300 μL PBS) or PBS. (E) A disease score between 0 and 4 was assigned to each limb, based on the degree of joint inflammation, redness, and deformity; the maximum possible score for an individual animal was 16. The graph represents the scores of 9 animals in each group that were measured every day. (F) Joint histology in PBS- or CCL2-treated mice. Results are presented as the mean plus or minus SEM of the difference between the 2 values for all the animals in each group. All photomicrograph images (B,F) were obtained with an Olympus AK 70 microscope (Center Valley, PA), Olympus Ach 20×/0.4 ∞/0.17. Camera make and model: Nikon Digital Camera DXM 1200F. Name and version number of image-acquisition software: Nikon ACT-l for DMX1200F.
Histopathologic examination of lung tissue from OVA mice revealed a pleomorphic peribronchial and perivascular infiltrate, consisting mainly of eosinophils and lymphocytes, that was not seen in control (PBS-challenged) or in OVA-challenged CCL2-treated mice (Figure 5Bi-iii). Furthermore, immunohistochemical staining of lung tissue demonstrated an increased number of T cells (CD3+ cells) in OVA-challenged mice. Treatment of mice with low doses of CCL2 dramatically reduced this T-cell infiltration (Figure 5Biv-vi). Moreover, pM levels of CCL2 induced a significant effect on the eosinophil numbers in the BALs. While the percentage of this population was dramatically elevated in the BAL of OVA-challenged mice, a significant decrease was observed in the BAL of CCL2-treated mice (Figure 5C).
In addition, the peribronchial and perivascular inflammatory infiltrates were given inflammatory scores between 1 and 4 by a pathologist unaware of the treatment to which each mouse had been exposed (Figure 5D). The inflammatory infiltrate in the OVA-treated mice was significantly increased, as compared with those of control animals; in contrast, histologic changes in the lungs of OVA + CCL2-treated mice were barely detectable, and the peribronchial inflammatory infiltrate was significantly reduced in these mice (Figure 5B-D).
AA is an experimental autoimmune disease that models several features of human rheumatoid arthritis,28 an inflammatory disorder characterized by infiltration of leukocytes into the synovial tissue (ST) and synovial fluid (SF) of the joints. This model is associated with a Th1 response. AA was induced as described in “Rodent model for rheumatoid arthritis induction and assessment,” and rats were immediately divided into 2 groups that were injected daily with low-dose CCL2 (240 ng in 300 μL PBS) or PBS. Starting from day 10 after induction, the disease was scored. As can be seen in Figure 5E and F, injection of low-dose CCL2 significantly reduced the severity of the inflammation.
Taken together, our in vivo studies indicate that CCL2 displays significant anti-inflammatory activity in animal models for 2 major human inflammatory diseases, RA and asthma.
Discussion
Cell migration is controlled by multistep processes that include chemoattraction, cell-cell adhesion, and transmigration through cell layers.10,43 Chemotactic signals control leukocyte navigation by regulating migration from the blood into tissues as well as subsequent localization of cells within the tissue microenvironment. The CC chemokine receptor 2 (CCR2) serves as the receptor for CCL2 and is expressed on a variety of cell types of the immune system.44,45
In this study, we followed the effects of T-cell conditioning with low levels of CCL2 on T-cell responses to LN chemokines. Our results indicate that initial CCL21-triggered VLA-4– and LFA-1–dependent arrests are not perturbed by CCL2 treatment. Nevertheless, subsequent adhesion strengthening of the 2 integrins was dramatically defective in CCL2-treated T cells. Interestingly, this was correlated with a defect in integrin activation. In addition, reduced Vav phosphorylation was detected in CCL2 treated lymphocytes supporting the possibility that Vav may be one of several impaired effectors.
These results suggest a novel role for CCL2 in a peripheral downmodulation of a key integrin-mediated process necessary for initially arrested lymphocytes to remain adherent to blood vessel walls for sufficient periods to enable successful diapedesis and homing of T cells to multiple target sites. Our results also demonstrate the strong inhibition of the ability of lymphocytes to rearrange their cytoskeleton and migrate toward chemokine gradients. We suggest that together, these 2 inhibitory effects result in a strong effect on the ability of lymphocytes to enter into LN and consequently to expand into the effector lymphocyte subsets, Th1, Th2, and the interleukin-17 (IL-17)–producing cells (Th17 cells), that were shown to have a crucial role in the induction of both asthma46 and RA.47,48
CCL2 conditioning of T cells may also impair integrin activation by inflammatory chemokines and inhibit chemotaxis to these chemokines, and thereby further attenuate effector lymphocyte recruitment to the nonlymphoid sites of asthma (lung) and RA (joints). These questions, and the ability of CCL2 to block Vav1 activation by inflammatory chemokines in addition to the LN chemokines investigated in this study, remain open for further investigation.
T cells do not secrete CCL2, but, because circulating pools of CCL2 are elevated in some inflammatory settings, we believe that the CCL2-CCR2 axis may trigger a negative feedback mechanism that attenuates general LN homing, thereby restricting T-cell activation in different inflammatory processes. As only low (pM) levels of CCL2 trigger this suppressive effect, this negative feedback mechanism, may prevail at the end of the inflammatory response. When CCL2 levels in the tissue and in the serum are sufficiently low, naive and central memory T cells are prevented from entering the LN but normally home to the spleen. The pool of effector T cells generated in LN draining the inflamed tissue is then reduced, as is subsequent entry of effector cells into sites of late and resolved inflammatory processes.
The effect of CCR2 and CCL2 in inflammatory models is still not clear. At first CCR2 and its ligand, CCL2, were considered to have a clear proinflammatory role based on experiments in which their absence or inhibition led to a reduction in the development of inflammatory and autoimmune conditions such as asthma and RA.49-52 However, despite these findings, it seems that the immunoregulatory effect of CCL2 in asthma and RA might be more complex than initially believed, and that CCL2 might have an anti-inflammatory effect as well. In mice lacking CCL2 or CCR2, asthma could still be induced53 and it was shown that mice deficient in CCR2 exhibit a strong Th1 to Th2 switch in response to infection challenge, as characterized by a large increase in production of IL-4, IL-5, and anti–pathogen-specific Abs, including IgE.54,55 Moreover, in 2 models of asthma, the absence of CCR2 resulted in an exacerbated pulmonary response that was only partially explained by an increase in the levels of IL-5, IL-13, and CCL2.49,56 In addition, analysis of serum of asthmatic children revealed an unexpected decrease in the levels of serum CCL2, as compared with control children. CCR2 was also found to have a protective role in RA, because induction of collagen-induced arthritis in CCR2−/− mice caused a more severe disease with enhanced T-cell production and higher numbers of activated T and B cells in the LNs, as compared with control mice.57
Our study suggests that the anti-inflammatory effect of pM levels of CCL2 requires the CCR2 receptor for its inhibitory function. However, although no functional effect of CCL2 on CCR2-deficient cells was detected in our in vitro studies, in vivo we observed some effects of CCL2 on CCR2−/− lymphocyte adhesion to HEV walls in vivo; nevertheless, the effects observed were significantly reduced compared with wild-type lymphocytes. This CCR2-independent inhibition might result from the binding of CCL2 to a decoy receptor such as D6, a chemokine receptor with structural homology to signaling receptors, which binds ligands such as CCL2 with high affinity, but neither mediates cell migration nor activates conventional signaling pathways. CCL2 binding to this receptor has been suggested to regulate inflammation.58-61 Alternatively, CCL2 might down-regulate migration by forming a heterodimer with CCL21, affecting the binding of CCL21 to its receptor. It remains to be established through which receptor and pathway CCL2 can exert a limited effect when CCR2 is absent, and whether additional CCR2-binding chemokines can regulate its function.
Our results demonstrate that while CCL2 at nanomolar levels is a key inflammatory chemokine for monocytes and effector T-lymphocyte subsets, at pM levels it can exert global suppressive effects on T-cell trafficking into inflamed lymph nodes. Thus, this chemokine may have clinical application as a general anti-inflammatory agent.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This research was supported by MAIN (Migration and Inflammation) European Network of Excellence (FP6-502935), the Ligue Nationale contre le Cancer (Equipe labellisée to J.P.G.), the Minerva Foundation, and the Gurwin Foundation. I.S. holds the Dr Morton and Anne Kleiman Professorial Chair.
Authorship
Contribution: L.F., G.H., and E.Z. designed and performed some of the experiments, analyzed results, and wrote the paper; C.M. designed and performed the IVM experiments and analyzed results; V.G., G.L.-T., S.F., and R.M. performed experiments and analyzed results; A.H. and D.S. analyzed the results; T.A.-W. performed experiments and analyzed results; R.A. analyzed the results and participated in writing; J.P.-G. designed the IVM experiments, analyzed the results, and participated in writing; and I.S. designed and supervised the experiments, analyzed the results, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Idit Shachar, Weizmann Institute of Science, 2 Herzel Street, Rehovot, Israel 76100; e-mail: Idit.Shachar@weizmann.ac.il.
References
Author notes
*L.F., G.H., E.Z., and C.M. contributed equally to this study.

![Figure 1. CCR2 is expressed on naive T cells and inhibits their cytoskeleton rearrangement and migration. (A) CD4+ and CD8+ T cells were separated. RNA was isolated and levels of CCR2 and HPRT mRNA were analyzed by reverse transcription–polymerase chain reaction. The results presented are representative of 3 independent experiments. (B,C) Transwell migration. Control or CCR2−/− naive T cells were pretreated for 30 minutes with medium or CCL2 (0.1 ng/mL). The cells were then placed in the upper well of a 24-well transwell plate in the presence or absence of CCL21 (0.1 μg/mL). After 3 hours, the number of migrating cells found in the lower chamber was evaluated by fluorescence-activated cell sorting (FACS) analysis. Percent migration was calculated as the number of migrating cells in the lower chamber as a fraction of the input cell number in the upper chamber. The results presented are representative of 3 different experiments. (D-F) Cytoskeleton rearrangement. Naive T cells from control (D-F) or CCR2−/− mice (D) were stimulated with CCL21 (0.1 μg/mL) for 15 seconds in the presence or absence of CCL2 (0.1 ng/mL; D-F), various concentrations of CCL2 (E), or Rantes, Mip1b, or Eotoxin (0.1 ng/mL; F). The cells were fixed and permeabilized, and their intracellular F-actin was stained with FITC-phalloidin. The change in polymerized actin was analyzed by FACS. Percentage increase in actin polymerization was calculated as the polymerization of actin in the presence of chemokine stimulation/polymerization of actin without chemokine ×100. The results presented are representative of 3 separate experiments. (G) Naive T cells were pretreated with or without CCL2 (0.1 ng/mL) for 30 minutes. The cells were then stimulated with CCL21 and lysed immediately. Lysates were separated on reducing 10% (wt/vol) sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotted with anti-phosphospecific ERK 1/2 (p-ERK). Immunoblots were stripped and reprobed with anti-[total] ERK 1/2. (H) Cytoskeleton rearrangement. Naive T cells were stimulated with medium or CCL21 (0.1 μg/mL) for 15 seconds in the presence or absence of CCL2 (0.1 ng/mL) or the MEK inhibitor, U0126 (5 μM). The cells were fixed and permeabilized, and their intracellular F-actin was stained with FITC-phalloidin. The change in polymerized actin was analyzed by FACS and calculated as described above. (I) Transwell migration. Naive T cells were pretreated for 30 minutes with medium or CCL2 (0.1 ng/mL) in the presence or absence of the MEK inhibitor, U0126 (5 μM). After 3 hours, the number of migrating cells found in the lower chamber was evaluated by FACS analysis. Percentage migration was calculated as described above.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/112/13/10.1182_blood-2007-12-129122/4/m_zh80240827510001.jpeg?Expires=1765925286&Signature=d2BZwiH-2P1ufHEI3nl484S78doLRNuNDhDXHxhcpeoxNY2mcpvwrqeKGs469wvaoBqzCuDnhgv5~RZdLofX3xcGKqedPDVJBoR99nKUdOFZh8fP77ddXZG4Waxao4MQluvIUBJa3EouCUwtM-Xfww7o22oKl1sdK2-vEl5sHYiPeuWmCZlvZLMFMOJbQpqY~X44bbybuEngFG1KTnaclp2ww0IngmBtcQ~GmY4QKE6P16gfDGKfH8fw1rE4cVLbsHPWO-Np9ZmmN4n0hPdWlbxvfCZEk4DXfkgPBt0MLwnXnTNvmLeRYlzF90xP9DvJFy7oCm249-m5xve52Yo6gg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal