Abstract
Interleukin-3 (IL-3) released by infiltrating inflammatory cells in different pathologic settings contributes to organ and tumor angiogenesis. Here we demonstrate that IL-3 expands a subset of CD45+ circulating angiogenic cells clonally derived from the hemopoietic progenitors. Moreover, CD45+ cells exposed to IL-3 acquire arterial specification and contribute to the formation of vessels in vivo. Depletion of signal transducer and activator of transcription 5 (STAT5) provides evidence that IL-3–mediated cell expansion and arterial morphogenesis rely on STAT5 activation. In addition, by means of Tie2-transgenic mice, we demonstrate that STAT5 also regulates IL-3–induced expansion and arterial specification of bonemarrow–derived CD45+ cells. Thus, our data provide the first evidence that, in inflammatory microenvironments containing IL-3, angiogenic cells derived from hemopoietic precursors can act as adult vasculogenic cells. Moreover, the characterization of the signaling pathway regulating these events provides the rationale for therapeutically targeting STAT5 in these pathologic settings.
Introduction
In the past, it has been assumed that new blood vessels originate from sprouting cells and cooption of neighboring preexisting vessels.1 It has recently been proposed, however, that both vascular healing and tumor angiogenesis are also supported by mobilization and recruitment of other cells, including the bone marrow (BM)–derived cells denoted as endothelial progenitor cells (EPCs).2,3 The existence of a BM reservoir of EPCs has attracted interest, especially as target for therapeutic intervention, both in chronic inflammatory diseases and cancer. However, a consensus on the identity and biologic activity of EPCs is still controversial. In general, 2 types of EPCs have been described to date: early and late EPCs.4 Although they share common features, they have distinct features with respect to morphology, proliferative potential, and in vitro functional characteristics.5-10 Because early EPCs do not adopt a typical endothelial phenotype in vitro and enhance neovascularization in an indirect paracrine fashion in vivo, they have been redefined as circulating angiogenic cells (CAC).4 More recently, it has been reported that CD45+ CAC retain some myeloid progenitor activity and are unable to originate perfused vessels in vivo.10 Thus, the best way to distinguish between EPCs and hemopoietic-derived CAC seems by their function rather than by their phenotype.
The positive contribution of hemopoietic-derived cells in inflammatory and tumor neoangiogenesis has been extensively demonstrated.11-13 The release of angiogenic mediators, including IL-3, by activated T cells has been reported to contribute to this process.14-18 Early in vivo and in vitro studies have implicated interleukin (IL) in the survival, proliferation, and differentiation of hemopoietic progenitor/stem cells (HSC) as well as of mature cells.19,20 However, a clinical study revealed that administration of IL-3 also increased BM vascularization,21 and it has recently been demonstrated that IL-3 is involved in inflammatory and tumor angiogenesis.22,23 IL-3 exerts its biologic effects by activating the signal transducer and activator of transcription 5 (STAT5) signaling pathway, both in hemopoietic24 and vascular tissues22 as revealed by the intrinsic defective IL-3 responsiveness of STAT5-deficient HSC25 and endothelial cells (EC).26 The 2 highly homologous proteins STAT5A and STAT5B,27 activated by Janus tyrosine kinase 2 (JAK2)28 or c-Src kinases,29 act as signaling components between the plasma membrane and the nucleus, and as transcriptional factors, by regulating the expression of genes involved in different cell functions including proliferation, survival, and differentiation.27
During development, specification of EC into arterial or venous cells represents the main determinant of vascular diversity. Physiologic parameters were considered the main factors in establishing arterial and venous identity.30 However, more recently, it has been shown that the membrane ligand EphrinB2 and the receptors Notch1 and Notch4, together with their ligand Dll4, provide molecular signature of arterial diversification, whereas the EphrinB2 receptor (EphB4) is distinctive to the venous endothelium.31-34 These findings allude to the possibility that genetic programming of arterial and venous identity can be activated before the onset of circulation.
The plasticity of BM progenitors in adult life has been extensively documented. BM microenvironment is a milieu of soluble mediators that regulate programs involved in cell proliferation and differentiation as well as in lineage commitment and cell specification.3 The present study investigates the role of the inflammatory cytokine IL-3 in the commitment of BM-derived angiogenic cells. We provide evidence that when circulating or BM-resident CD45+ angiogenic cells are exposed to a microenvironment containing IL-3, they undergo cell proliferation and arterial specification via a STAT5-mediated pathway. Thus, these data demonstrate for the first time that IL-3 can provide a permissive environment enabling angiogenic cells to expand and directly contribute to neovascularization.
Methods
Reagents
Endothelial basal medium (EBM)–2 supplemented with 10% FBS and endothelial growth medium (EGM-2) containing 10% FBS, hydrocortisone, human fibroblast growth factor, vascular endothelial growth factor, insulin growth factor 1, ascorbic acid, human epidermal growth factor, gentamicin, and amphotericin-B were from Lonza Walkersville (Walkersville, MD). The other reagents used throughout the study are listed in Document S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article). The approval was obtained both from Servizio Immunoematologia e Medicina Trasfusionale (SIMT) and from the Institutional Review Board of S. Giovanni Battista Hospital, Turin. Informed consent was obtained in accordance with the Declaration of Helsinki. We also declare that for the present study, we had no direct contact with human subjects. Animal procedures conformed to the Guide for Care and Use of Laboratory Animals (National Institutes of Health publication no. 93-23, revised 1985).
Antibodies
The antibodies used are provided in Document S1.
Cell purification and cell sorting.
Peripheral-blood mononuclear cells isolated by Ficoll Histopaque 1077 (Sigma-Aldrich, St Louis, MO) were resuspended in 20% FBS 199 Medium and plated on fibronectin-coated dishes (BD BioCoat; BD Biosciences, San Jose, CA) as described by Hill et al.35 Sorting of CD45+, CD45−, CD14+, CD14−, CD34+, CD34−, CD133+, and CD133− cells from human peripheral blood and CD45+ cells from human or mouse BM samples was performed on MoFlo Cell Sorter using Summit software (MoFlo; Dako Colorado, Fort Collins, CO). The institutional review board of the hospital approved the study.9
SKOV-3 cells were cultured in Ham's F12 medium/10% FBS. Sorted cells were cultured for 12 days on 20 μg/mL fibronectin-coated dishes in EBM-2 with or without 10 ng/mL of IL-3. Parallel experiments were performed using EGM-2 medium. Human tumor endothelial cells (TECs) were grown as described previously.36
Isolation and culture of mature liver EC and of BM-derived cells.
BM-MNC, isolated from murine BM flushed from the femurs of FVB mice, were cultured on 20 μg/mL fibronectin-coated dishes in EGM-2 medium or in EBM-2, with or without IL-3 (10 ng/mL). Mature liver EC from wild-type (WT), Tie2-ΔSTAT5A, and Tie2-ΔSTAT5B transgenic mice (Tie2-Δ5A and Tie2-Δ5B) were isolated by positive magnetic cell sorting (anti-liver sinus endothelial cells magnetic beads; Miltenyi Biotec, Auburn, CA) and cultured in 20% BCS 199 Medium.
Isolation of vascular cells from human arterial and venous samples.
The institutional review board of the hospital approved the study. Experimental details are provided in Document S1.
Uptake of Dil-acLDL.
Cells were incubated with 2.5 μg/mL Dil-acetylated low-density lipoprotein (Dil-acLDL; Biomedical Technologies) at 37°C and subsequently fixed with 3% paraformaldehyde. The uptake of Dil-acLDL was analyzed by fluorescence microscopy.
Flow cytometry.
To analyze cell-cycle progression, fluorescence-activated cell sorting (FACS) analysis was performed as described previously.26 Cell-surface molecules were evaluated by flow cytometry as described previously.37 Frequency of marker-positive cells is expressed as mean plus or minus standard deviation (SD).
RNA isolation and quantitative real-time PCR.
CD45+ cells were cultured with or without IL-3 for different times. Human arterial and venous cells were used as positive controls. mRNA quantification was performed by quantitative real-time polymerase chain reaction (Q-RT-PCR), as described previously.37 The relative expression of Ephrin B1, Ephrin B2, Notch1, Notch4, and EphB4 were calculated by using comparative threshold cycle methods. The human and murine primer sequences used were described previously.38,39
Genotyping the JAK2 mutation.
Sorted CD45+ cells were from 5 patients with polycythemia vera (PV) harbouring JAK2 mutation 1849G>T. As control, sorted CD45+ cells were from patients not harboring the mutation. To detect the mutated or the WT JAK2 allele, a DNA allele-specific PCR was performed as described.40 The institutional review board of the hospital approved the study.
Western blot analysis and immunoprecipitation.
Cells were lysed and protein concentration was obtained as described previously.37 In selected experiments, AG-490 (100 μM; α-cyano-(3,4-dihydroxy)-N-benzylcinnamide), PP1 (10 μM; 4-amino-5-(4-methylphenyl)-7-(t-butyl)pyrazolo[3,4-d]-pyrimidine), PP2 (10 μM; PP2-4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazole[3,4-d]pyrimidine) and SU6656 (2 μM; 2-oxo-3-(4,5,6,7-tetrahydro-1H-indol-2-ylmethylene)-2,3-dihydro-1H-indole-5-sulfonic acid dimethylamide) were used. For immunoprecipitation analysis, cell extracts were processed as described previously.26
Endogenous depletion of STAT5A and STAT5B by siRNAs and transfection of DN STAT5 constructs (Δ5A and Δ5B).
To obtain inactivation of endogenous STAT5A or STAT5B, CD45+ cells were processed as described in detail in Document S1. Mouse BM-derived CD45+ cells were also transiently transfected with dominant-negative (DN) STAT5A and STAT5B constructs,41 alone or in combination as described previously.26
Tube-like structure formation assay.
The analysis of tube-like structures formation is described in Document S1.
Invasion assay.
Invasion assay was performed as described.42 Details are reported in Document S1. To characterize the incorporated cells, the cluster was recovered and subjected to 0.03% collagenase treatment. The recovered cells were cultured in 20% FBS 199 medium and analyzed for the uptake of Dil-acLDL.
Src activity assay.
CD45+ cells, cultured with or without IL-3 or SU6656, were washed on ice with 1 mM Na3VO4 in PBS and then lysed in radioimmunoprecipitation assay buffer. The c-Src kinase assay was performed as described previously.43
In vivo experiments.
To evaluate the ability of IL-3–cultured cells to be incorporated into neoformed vessels, 2 × 106 TECs were added to Matrigel and injected into SCID mice. Five days later, IL-3–cultured CD45+ cells were labeled with the fluorescent dye carboxyfluorescein diacetate succinimidyl ester (CSFE; Invitrogen, Carlsbad, CA) and injected in the tail vein of the mice and processed as described previously.37 The number of incorporated cells per vessel was counted in 10 randomly selected fields in 3 different samples.
For angiogenesis assay, transgenic mice were injected subcutaneously with growth factor-reduced Matrigel containing IL-3 (20 ng/mL) and processed as described previously.18,22
To evaluate the vasculogenic capability of the IL-3–cultured cells (Matrigel plug assay), SCID mice were injected subcutaneously with growth factor-reduced Matrigel containing 100 ng/mL IL-3 and 4 × 106 CSFE-labeled CD45+ cells. Five and 15 days after Matrigel plugs were removed and processed for immunohistochemistry and immunofluorescence.
Immunohistochemistry and immunofluorescence.
Sections from paraffin-embedded blocks of Matrigel plugs were collected onto poly-lysine–coated slides and processed as described previously.37 For immunofluorescence assay, the samples were processed as described previously37 with the use of human leukocyte antigen (HLA) I mouse major histocompatibility complex (MHC) II, CD31, EphrinB1, and EphB4 antibodies. To quantify the percentage of cells expressing arterial or venous markers, the number of positive cells in 10 randomly selected fields was scored and divided by the total number of cells. The number of MHC II or HLA I positive vessels was determined by counting 10 randomly selected fields in 3 different samples. Images were acquired with a Zeiss LSM 5 Pascal confocal laser-scanning microscope (Carl Zeiss, Jena, Germany) equipped with a helium/neon laser (543 mm), an argon laser (450-530 mm), and an EC planar Neofluar 40×/1.3 oil-immersion differential interference contrast objective lens. Images were analyzed using Zeiss LSM 5 version 3.2 software.
Generation and characterization of Tie2-ΔSTAT5A and Tie2-ΔSTAT5B transgenic mice.
Procedure for generation of the transgene constructs are detailed in Document S1. To generate transgenic mice, the purified SalI-SalI fragments of ΔStat5A and ΔStat5B Myc-tagged44 containing the Tie2 promoter were microinjected in the pronucleus of fertilized eggs from FVB female mice. The injected embryos were transferred to pseudopregnant female mice, and the offspring genotype was tested. DNA from founder mice positive for PCR was further subjected to Southern blot analysis to confirm the presence of the transgenes. Five independent transgenic lines for ΔStat5A and 5 for ΔStat5B were generated and used for analysis of the phenotype.
Enzyme-linked immunosorbent assay and radioimmunoassay.
The release of angiogenic factors upon IL-3 challenge was evaluated as reported in detail in Document S1.
Statistical analysis
Densitometric analysis was used to calculate the differences among experimental conditions (P < .05 indicates statistical significance between experimental and control values). Details are reported in Document S1.
Results
IL-3 induces expansion of circulating angiogenic cells derived from hemopoietic progenitors.
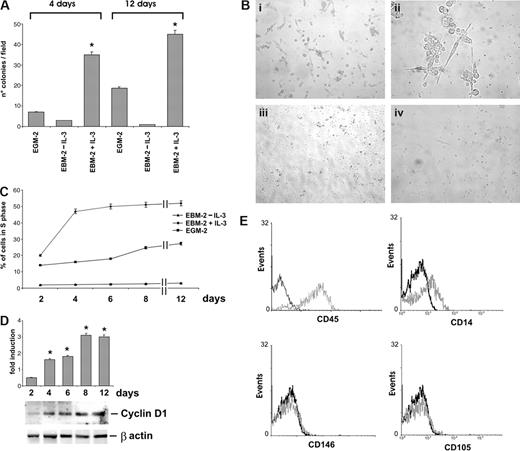
It has been shown that cells selected on fibronectin-coated dishes retain CAC phenotype.10 IL-3, released in inflammation sites, promotes vascular growth by acting on mature EC.18,22,23 To investigate whether IL-3 can also affect CAC biology, CAC were cultured in EBM-2 medium, with or without IL-3. EGM-2, the standard EPC culture medium, was used as control. The results shown in Figure 1A,B indicate that, after 4 and 12 days of culture, the number of colonies was remarkably higher in IL-3–cultured cells than in control cells (EGM-2). Moreover, FACS analysis revealed a different kinetic of CAC expansion in the 2 different culture conditions. In fact, exposure to IL-3 induced an earlier and higher proportion of cells in S phase compared with that of EGM-2 cultures (Figure 1C). The expression of S phase–related cyclin D1 was consistently already detectable after 2 days of IL-3 challenge (Figure 1D). Neither colony formation nor increase in the percentage of cells in the S phase could be detected when the cells were cultured in EBM-2 medium without IL-3 (Figure 1A-C).
IL-3 induces angiogenic cell expansion. (A) The number of colonies obtained by culturing angiogenic cells in EGM-2 or in EBM-2, with or without IL-3, for 4 or 12 days is reported. Data are the mean of 10 fields (± SD; *P < .05, EBM-2 + IL-3 vs EBM-2–IL-3 or vs EGM-2). Magnification, 10×. (B) Representative photomicrographs of colonies obtained by culturing angiogenic cells for 4 days in EBM-2 + IL-3 (panels i,ii), in EGM-2 (panel iii), or in EBM-2–IL-3 (panel iv). Magnification, 10× (panels i,iii,iv) and 40× (panel ii). (C) The percentage of angiogenic cells in the S phase was evaluated by FACS analysis in different culture conditions. (D) IL-3-cultured angiogenic cells were lysed and cell extracts were subjected to sodium dodecyl sulfate (SDS)–polyacrylamide gel electrophoresis (PAGE). The filter was immunoblotted (IB) with anti–cyclin D1 and anti–β-actin antibodies, as indicated. (E) FACS analysis was performed after 4 days of IL-3 culture. Black lines, preimmune IgG used as negative control; gray lines, CD45, CD14, CD146, and CD105 markers. Three different experiments were performed with comparable results. Photomicrographs of cells were acquired with a Leica DM IL inverted contrasting microscope (Leica Microsystems, Wetzlar, Germany) equipped with 10×/20×/40× hiPlan objective lenses. Images were analyzed using Leica QWin software, version 3.
IL-3 induces angiogenic cell expansion. (A) The number of colonies obtained by culturing angiogenic cells in EGM-2 or in EBM-2, with or without IL-3, for 4 or 12 days is reported. Data are the mean of 10 fields (± SD; *P < .05, EBM-2 + IL-3 vs EBM-2–IL-3 or vs EGM-2). Magnification, 10×. (B) Representative photomicrographs of colonies obtained by culturing angiogenic cells for 4 days in EBM-2 + IL-3 (panels i,ii), in EGM-2 (panel iii), or in EBM-2–IL-3 (panel iv). Magnification, 10× (panels i,iii,iv) and 40× (panel ii). (C) The percentage of angiogenic cells in the S phase was evaluated by FACS analysis in different culture conditions. (D) IL-3-cultured angiogenic cells were lysed and cell extracts were subjected to sodium dodecyl sulfate (SDS)–polyacrylamide gel electrophoresis (PAGE). The filter was immunoblotted (IB) with anti–cyclin D1 and anti–β-actin antibodies, as indicated. (E) FACS analysis was performed after 4 days of IL-3 culture. Black lines, preimmune IgG used as negative control; gray lines, CD45, CD14, CD146, and CD105 markers. Three different experiments were performed with comparable results. Photomicrographs of cells were acquired with a Leica DM IL inverted contrasting microscope (Leica Microsystems, Wetzlar, Germany) equipped with 10×/20×/40× hiPlan objective lenses. Images were analyzed using Leica QWin software, version 3.
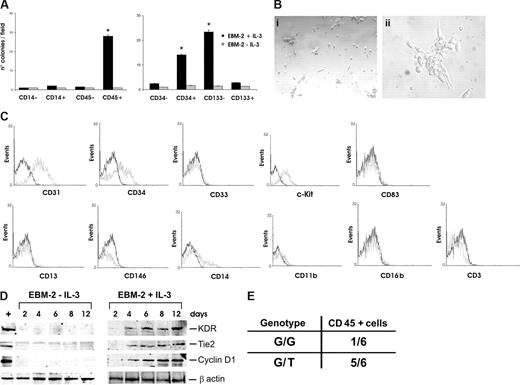
The characterization of IL-3–expanded cells was performed using specific surface markers for CAC, such as CD45, CD14, CD146, and CD105.10 As shown in Figure 1E, cells expanded in the presence of IL-3 mainly expressed CD45 (95%) and showed a low frequency of the monocyte-marker CD14 (10%) and no expression of the mature endothelial surface markers CD146 and CD105. These results suggest that IL-3 expands a subset of peripheral cells specifically bearing hemopoietic progenitor markers. To confirm these data, different cell populations, such as CD45+, CD45−, CD14+, and CD14− cells were sorted and cultured with or without IL-3. Consistent with the above data, the results reported in Figure 2A demonstrate that only cells positively sorted for CD45 were able to form colonies on fibronectin coated-dishes upon IL-3 treatment. In addition, morphologic features of such colonies were very similar to those obtained by culturing nonpurified cells (Figure 2Bi,ii). CD45+ sorted cells also expressed cyclin D1 early only after IL-3 challenge (Figure 2D). In addition, FACS analysis demonstrated that CD45+ expanded cells expressed CD31, CD34, and c-Kit but not the mature myeloid markers CD13 and CD33, as well as the dendritic marker CD83 the granulocyte marker CD16b and the lymphocyte marker CD3. Finally, consistent with the above data, a low frequency of CD14 and CD11b and no expression of CD146 were detected (Figure 2C). Time course analysis of VEGFR2/KDR and Tie2 expression revealed that both proteins appeared 4 days after IL-3 challenge, were still detectable after 12 days, but were undetectable when cells were cultured without IL-3 (Figure 2D). To further characterize the population expanded in the presence of IL-3, CD34- and CD133-positive and -negative cells were sorted. The results reported in Figure 2A demonstrate that only cells sorted for CD34 positivity and CD133 negativity could be expanded in the presence of IL-3, whereas, as expected, no cell expansion could be detected in the absence of IL-3. Thus, these results demonstrate that expansion and endothelial differentiation of a subset of CD45+/CD34+/CD133− angiogenic cells occurs in response to IL-3. This cell population is defined as CD45+ throughout the study.
IL-3 expands a CD45+ cell subset derived from hemopoietic progenitors. (A) The numbers of colonies obtained by culturing the indicated sorted cells for 4 days, with or without IL-3. Data are the mean of 10 fields (± SD; *P < .05, left panel, CD45+ vs CD14−, CD14+ and CD45−; right panel, CD34+ and CD133− vs CD34− and CD133+). Magnification, 10×. (B) Representative photomicrographs of colonies obtained from IL-3-cultured CD45+-sorted cells. Magnification, 10× (panel i) and 40× (panel ii). (C) FACS analysis was performed on IL-3–expanded CD45+-sorted cells. Black lines, preimmune IgG (negative control); gray lines, CD31, CD34, CD33, c-Kit, CD13, CD146, CD14, CD11b, CD83, CD16b, and CD3 markers. (D) Cell extracts from CD45+ cells, cultured with or without IL-3, were subjected to SDS-PAGE. The filter was IB with anti-KDR, anti-Tie2, anti–cyclin D1, and anti–β-actin antibodies. (E) Genotype of CD45+ cells isolated from patients harboring or not V617F JAK2 mutation. G/G represents WT and G/T represents heterozygous V617F JAK2 mutations, respectively. Three different experiments were performed with comparable results.
IL-3 expands a CD45+ cell subset derived from hemopoietic progenitors. (A) The numbers of colonies obtained by culturing the indicated sorted cells for 4 days, with or without IL-3. Data are the mean of 10 fields (± SD; *P < .05, left panel, CD45+ vs CD14−, CD14+ and CD45−; right panel, CD34+ and CD133− vs CD34− and CD133+). Magnification, 10×. (B) Representative photomicrographs of colonies obtained from IL-3-cultured CD45+-sorted cells. Magnification, 10× (panel i) and 40× (panel ii). (C) FACS analysis was performed on IL-3–expanded CD45+-sorted cells. Black lines, preimmune IgG (negative control); gray lines, CD31, CD34, CD33, c-Kit, CD13, CD146, CD14, CD11b, CD83, CD16b, and CD3 markers. (D) Cell extracts from CD45+ cells, cultured with or without IL-3, were subjected to SDS-PAGE. The filter was IB with anti-KDR, anti-Tie2, anti–cyclin D1, and anti–β-actin antibodies. (E) Genotype of CD45+ cells isolated from patients harboring or not V617F JAK2 mutation. G/G represents WT and G/T represents heterozygous V617F JAK2 mutations, respectively. Three different experiments were performed with comparable results.
To evaluate whether IL-3–expanded cells are clonally related or derived from the same parent cell, CD45+ cells were sorted from 5 patients with PV and cultured in the presence of IL-3. PV is a myeloproliferative disorder that develops from a somatic event in a single HSC clone.45,46 A gain-of-function mutation of JAK2, due to V617F JAK2 mutation, frequently occurs in patients with PV. Therefore, if IL-3-expanded cells are derived from the same parent cell, DNA isolated from these cells should harbor the same mutation. Indeed, the genotyped CD45+ expanded cells displayed the heterozygous JAK2 mutation demonstrating their derivation from the mutant HSC (Figure 2E).
CD45+ cells exposed to IL-3 acquire the ability to form vascular networks and become able to invade tumor in vitro.
To evaluate whether CD45+ cells exposed to IL-3 acquire endothelial cell features, the ability to form vascular networks and to invade a cluster of tumor cells was assayed. Figure 3A shows typical cord-like structures formed by cells exposed to IL-3, and Figure 3B shows their capability to invade a cluster of SKOV-3 tumor cells and to concentrate into the center of the cluster. Neither feature was shared with EGM-2–cultured cells that did not form cord-like structures and remained at the border of tumor cluster. Similar results were obtained by culturing the cells in EBM-2 without IL-3 (data not shown). Thus, these data indicate that only IL-3–cultured cells acquire functional features of vascular cells. The positive staining for Dil-acLDL additionally characterizes the vascular network inside the cluster of SKOV-3 cells (data not shown).
CD45+ cells exposed to IL-3 acquire the ability to form vascular networks and to invade tumor in vitro. (A) For in vitro angiogenesis assay, CD45+ cells cultured in EBM-2 + IL-3 or EGM-2 were plated on Matrigel-coated surface. Histogram represents the number of tube-like structures per field. Data are the mean of 10 fields (± SD; *P < .05, EBM-2 + IL-3 vs EGM-2). Magnification, 10×. (B) Labeled CD45+ cells, cultured in EBM-2 + IL-3 or EGM-2, were incubated with tumor cells. After 48 hours, bright field (left panels) and fluorescent images (right panels) were taken using an inverted phase microscope. Magnification, 40×.
CD45+ cells exposed to IL-3 acquire the ability to form vascular networks and to invade tumor in vitro. (A) For in vitro angiogenesis assay, CD45+ cells cultured in EBM-2 + IL-3 or EGM-2 were plated on Matrigel-coated surface. Histogram represents the number of tube-like structures per field. Data are the mean of 10 fields (± SD; *P < .05, EBM-2 + IL-3 vs EGM-2). Magnification, 10×. (B) Labeled CD45+ cells, cultured in EBM-2 + IL-3 or EGM-2, were incubated with tumor cells. After 48 hours, bright field (left panels) and fluorescent images (right panels) were taken using an inverted phase microscope. Magnification, 40×.
IL-3 induces arterial specification of CD45+ cells.
As shown by Yoder et al,10 CD45+ and CD14+ angiogenic cells differentiate into phagocyte-macrophages and fail to undergo endothelial specification and to form perfused vessels in vivo. Our findings that IL-3–cultured cells were able to form cord-like structures and invade tumor mass in vitro raised the possibility that IL-3–expanded cells could undergo endothelial specification. To investigate the ability of IL-3 to support venous or arterial morphogenesis, Q-RT-PCR and/or immunofluorescence for arterial markers (EphrinB2, EphrinB1, Notch1, and Notch4) or for venous marker (EphB4) were performed. Data reported in Figure 4A,B provide evidence that IL-3 induces EphrinB2, EphrinB1, Notch1, and Notch4 expression without affecting EphB4 expression. No expression of arterial markers was detected in cells cultured without IL-3. These data first demonstrate that CD45+ cells, when exposed to IL-3, undergo arterial specification.
IL-3 induces arterial specification of CD45+ cells acting as vasculogenic cells. (A) Q-RT-PCR was performed on CD45+ cells cultured with or without IL-3. The indicated arterial and venous markers were evaluated. Expression levels are presented as fold increase (logarithmic scale) in comparison with baseline levels and were normalized by using GAPDH as housekeeping gene. The mRNA isolated from arterial or venous samples were used as positive control (+; *P < .05, day 2 of culture vs days 4, 6, 8, and 12). (B) IL-3–expanded CD45+ cells were stained with CD31 (red), with EphrinB1 (green; top panel) or with EphB4 (green; bottom panel). Colocalization of CD31 and EphrinB1 or CD31 and EphB4 is reported in merge. DAPI staining was used as nuclear marker. Magnification, 20×. (C) Sections of Matrigel plugs containing TECs, recovered from SCID mice injected (panel ii) with prelabeled IL-3–cultured CD45+ cells, were analyzed by immunohistochemistry (IH). Panel i shows representative functional vessel formed by TECs. Black arrows indicate the incorporated CD45+ cells. The number of incorporated cells per vessel was reported on the right (4 nonsequential sections for each experiment were analyzed). Magnification, 40×. (D) IH of Matrigel plugs containing IL-3 and labeled CD45+ cells implanted subcutaneously into SCID mice. Panels i,iii, Matrigel recovered after 5 days; panels ii and iv, Matrigel recovered after 15 days. Prelabeled cells were detected by Fluorescein/Oregon Green Antibody (panels i,ii). Ephrin B1 staining was also performed (panels iii,iv). Black arrows indicate positive CD45+ cells. Magnification, 20× (panels i and iii) and 40× (panels ii and iv). (E) Matrigel plugs recovered after 15 days were stained with anti–human HLA I (red) or with anti–mouse MHC II (green) antibodies. DAPI staining was used as nuclear marker. Magnification, 20×. The number of human or host-derived vessels is reported in the histogram. Three different experiments were performed with comparable results.
IL-3 induces arterial specification of CD45+ cells acting as vasculogenic cells. (A) Q-RT-PCR was performed on CD45+ cells cultured with or without IL-3. The indicated arterial and venous markers were evaluated. Expression levels are presented as fold increase (logarithmic scale) in comparison with baseline levels and were normalized by using GAPDH as housekeeping gene. The mRNA isolated from arterial or venous samples were used as positive control (+; *P < .05, day 2 of culture vs days 4, 6, 8, and 12). (B) IL-3–expanded CD45+ cells were stained with CD31 (red), with EphrinB1 (green; top panel) or with EphB4 (green; bottom panel). Colocalization of CD31 and EphrinB1 or CD31 and EphB4 is reported in merge. DAPI staining was used as nuclear marker. Magnification, 20×. (C) Sections of Matrigel plugs containing TECs, recovered from SCID mice injected (panel ii) with prelabeled IL-3–cultured CD45+ cells, were analyzed by immunohistochemistry (IH). Panel i shows representative functional vessel formed by TECs. Black arrows indicate the incorporated CD45+ cells. The number of incorporated cells per vessel was reported on the right (4 nonsequential sections for each experiment were analyzed). Magnification, 40×. (D) IH of Matrigel plugs containing IL-3 and labeled CD45+ cells implanted subcutaneously into SCID mice. Panels i,iii, Matrigel recovered after 5 days; panels ii and iv, Matrigel recovered after 15 days. Prelabeled cells were detected by Fluorescein/Oregon Green Antibody (panels i,ii). Ephrin B1 staining was also performed (panels iii,iv). Black arrows indicate positive CD45+ cells. Magnification, 20× (panels i and iii) and 40× (panels ii and iv). (E) Matrigel plugs recovered after 15 days were stained with anti–human HLA I (red) or with anti–mouse MHC II (green) antibodies. DAPI staining was used as nuclear marker. Magnification, 20×. The number of human or host-derived vessels is reported in the histogram. Three different experiments were performed with comparable results.
IL-3–expanded cells are incorporated in tumor neovessels and undergo arterial morphogenesis in vivo.
In both organ and tumor angiogenesis, EPCs can contribute to neovessel formation.2,3 We thus evaluated whether IL-3–cultured cells could be recruited in vivo by tumor neovasculature. To this end, TECs were added to Matrigel and implanted subcutaneously into SCID mice.37 After 5 days, labeled IL-3–expanded CD45+ cells were injected in mice. Two days after, Matrigel plugs were recovered for immunostaining. As shown in Figure 4C, TECs were able to form a vascular network in Matrigel (panel i) and the injected cells could insert into newly formed vessels (panel ii). The number of incorporated cells is reported in the histogram.
Emerging data indicate that progenitor cells are able to incorporate into existing vascular structures to form new vessels and improve perfusion.47 De novo formation of vasculature by IL-3–cultured CD45+ cells was assayed in vivo. To this end, Matrigel plugs containing IL-3 and CSFE-labeled CD45+ cells were injected into SCID mice. Five and 15 days after injection, Matrigel plugs were recovered and analyzed by immunohistochemistry. The results demonstrated that after 5 days, the labeled cells formed cord structures, whereas after 15 days, many of these labeled cells formed functional vessels, as documented by erythrocytes in their lumen (Figure 4D, panels i and iii and panels ii and iv, respectively). To exclude the possibility that the neovessels derived from vasculogenic cells of host origin, immunofluorescence assay was performed using the anti–human HLA class I and the anti–mouse MHC II antibodies. We found that the majority of vessels contain human HLA class I–positive cells (Figure 4E). The occurrence of arterial morphogenesis is documented by the EphrinB1 staining (Figure 4D, panels iii,iv). In addition to explore the possibility that these effects could depend on angiogenic factors released in response to IL-3, the production of VEGF and of IGF-1 was evaluated in IL-3–cultured cells. This cultured medium was compared with that of EGM-2–cultured cells and with that of primary EC cultured with IL-3. Although both conditional media eventually contained similar amounts of both VEGF and IGF-1 (Figure S1), only cells cultured with IL-3 acquired arterial specification sustain the possibility that a VEGF/IGF-1–independent pathway may account for our results.
STAT5 phosphorylation, depending on c-Src-kinase activation, regulates IL3-induced ex vivo expansion of CD45+ cells.
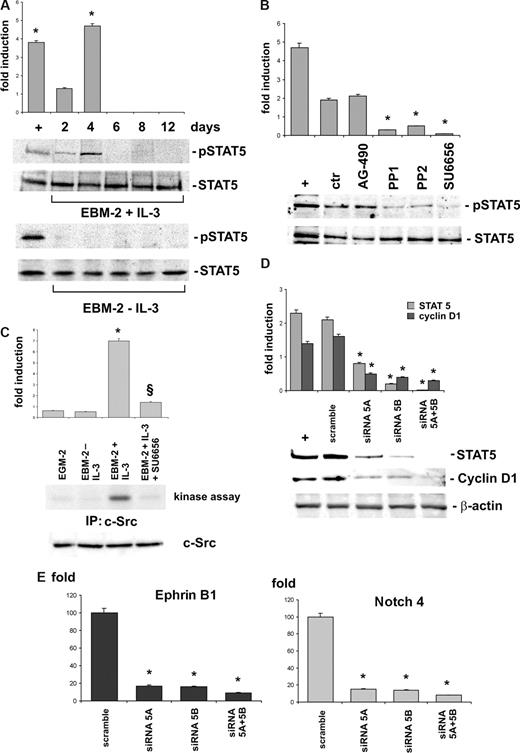
IL-3–mediated proliferation of both hemopoietic and endothelial cells rely on STAT5 signaling pathway.22,24,26 Thus, we investigated whether this signaling pathway also controlled CD45+ cell expansion and arterial morphogenesis. We thus assayed STAT5 activation at different time intervals of IL-3 cultures. Data reported in Figure 5A revealed that STAT5 underwent activation early after exposure to IL-3 and that its activation correlated with cell expansion corresponding to day 4. As expected, we failed to detect STAT5 activation in cells cultured in the absence of IL-3. Because JAK2 and c-Src have been reported to control STAT5 activation,28,29 we evaluated the effect on IL-3–mediated STAT5 activation of the JAK2 pharmacologic inhibitor AG-490 and of 3 different c-Src kinase pharmacologic inhibitors: PP1, PP2, and SU6656. As shown in Figure 5B, unlike AG-490, the c-Src kinase inhibitors prevented STAT5 activation. c-Src kinase activity consistently occurred only in IL-3–cultured cells, and SU6656 treatment prevented its activation (Figure 5C).
c-Src-kinase–dependent STAT5 activation regulates IL-3–induced ex vivo expansion of angiogenic cells. (A) CD45+ cells cultured with or without IL-3 were analyzed by Western blot (WB) using anti-p-STAT5 or anti-STAT5 antibodies. (B) Cell extracts from IL-3-cultured CD45+ cells, pretreated or not with AG-490, PP1, PP2, and SU6656, were subjected to SDS-PAGE. The filter was IB with anti-p-STAT5 and anti-STAT5 antibodies. (C) CD45+ cells, cultured as indicated, were evaluated for c-Src kinase activity as described in “Antibodies” (*P < .05, EBM-2 + IL-3 vs EBM-2 − IL-3 or vs EGM-2; §P < .05, EBM-2 + IL-3 + SU6656 vs EBM-2 + IL-3). The amount of c-Src in cell extracts was evaluated by WB using a specific antibody. (D) IL-3-cultured CD45+ cells transfected with STAT5A and/or STAT5B siRNA or with the scrambled sequence (scramble) were lysed. The filters were IB with anti-STAT5, anti–cyclin D1, or anti–β-actin antibodies. (E) Q-RT-PCR was performed to evaluate the expression of arterial markers in CD45+ depleted or not (scramble) of STAT5A and/or STAT5B as indicated. Expression levels are presented as above described. (*P < .05, experimental groups vs scramble.) Three independent experiments were performed with comparable results. IL-3–treated EC were used as positive control (+) throughout the figure.
c-Src-kinase–dependent STAT5 activation regulates IL-3–induced ex vivo expansion of angiogenic cells. (A) CD45+ cells cultured with or without IL-3 were analyzed by Western blot (WB) using anti-p-STAT5 or anti-STAT5 antibodies. (B) Cell extracts from IL-3-cultured CD45+ cells, pretreated or not with AG-490, PP1, PP2, and SU6656, were subjected to SDS-PAGE. The filter was IB with anti-p-STAT5 and anti-STAT5 antibodies. (C) CD45+ cells, cultured as indicated, were evaluated for c-Src kinase activity as described in “Antibodies” (*P < .05, EBM-2 + IL-3 vs EBM-2 − IL-3 or vs EGM-2; §P < .05, EBM-2 + IL-3 + SU6656 vs EBM-2 + IL-3). The amount of c-Src in cell extracts was evaluated by WB using a specific antibody. (D) IL-3-cultured CD45+ cells transfected with STAT5A and/or STAT5B siRNA or with the scrambled sequence (scramble) were lysed. The filters were IB with anti-STAT5, anti–cyclin D1, or anti–β-actin antibodies. (E) Q-RT-PCR was performed to evaluate the expression of arterial markers in CD45+ depleted or not (scramble) of STAT5A and/or STAT5B as indicated. Expression levels are presented as above described. (*P < .05, experimental groups vs scramble.) Three independent experiments were performed with comparable results. IL-3–treated EC were used as positive control (+) throughout the figure.
STAT5 consists of 2 highly homologous proteins, STAT5A an STAT5B, that we depleted by siRNA technology to validate their role in IL-3–mediated cell expansion (Figure 5D). FACS analysis performed on STAT5A- or -B-depleted cells revealed a reduction of proliferating cells. This effect was much more evident when both STAT5s were silenced (Table 1). Similar results were also obtained when the c-Src inhibitor SU6656 was added to the cell cultures (Table 1). Cyclin D1 expression consistently decreased in cells selectively depleted of STAT5A or STAT5B and was abrogated in cells silenced for both STAT5s (Figure 5D). Moreover, when EphrinB1 and Notch4 expression was assayed in STAT5s silenced cells by Q-RT-PCR, no increase of their expression occurred (Figure 5E). Thus, these results provide evidence for the role of STAT5s in controlling IL-3–mediated CD45+ cell expansion and arterial specification.
Effect of STAT5 isoforms on IL-3–cultured CD45+ cell proliferation
| Cell-cycle phases . | Circulating human CD45+ cells . | Mouse BM-derived CD45+ cells . | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Scramble . | siRNA STAT5A . | siRNA STAT5B . | SiRNA STAT5A+ STAT5B . | SU6656 . | Scramble . | siRNA STAT5A . | siRNA STAT5B . | siRNA STAT5A+ STAT5B . | |
| G0/G1 | 40 ± 2 | 55 ± 3 | 57 ± 1 | 64 ± 1 | 63 ± 2 | 52 ± 2 | 70 ± 3 | 69 ± 1 | 75 ± 2 |
| S | 50 ± 3 | 31 ± 3* | 33 ± 1* | 26 ± 2* | 28 ± 1* | 35 ± 2 | 17 ± 2* | 15 ± 2* | 13 ± 3* |
| G2/M | 10 ± 1 | 14 ± 1 | 10 ± 2 | 10 ± 3 | 9 ± 3 | 13 ± 3 | 13 ± 1 | 16 ± 3 | 12 ± 2 |
| Cell-cycle phases . | Circulating human CD45+ cells . | Mouse BM-derived CD45+ cells . | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Scramble . | siRNA STAT5A . | siRNA STAT5B . | SiRNA STAT5A+ STAT5B . | SU6656 . | Scramble . | siRNA STAT5A . | siRNA STAT5B . | siRNA STAT5A+ STAT5B . | |
| G0/G1 | 40 ± 2 | 55 ± 3 | 57 ± 1 | 64 ± 1 | 63 ± 2 | 52 ± 2 | 70 ± 3 | 69 ± 1 | 75 ± 2 |
| S | 50 ± 3 | 31 ± 3* | 33 ± 1* | 26 ± 2* | 28 ± 1* | 35 ± 2 | 17 ± 2* | 15 ± 2* | 13 ± 3* |
| G2/M | 10 ± 1 | 14 ± 1 | 10 ± 2 | 10 ± 3 | 9 ± 3 | 13 ± 3 | 13 ± 1 | 16 ± 3 | 12 ± 2 |
Circulating human CD45+ cells and mouse BM-derived CD45+ cells cultured in the presence of IL-3 were transiently transfected with scramble sequence or STAT5A and STAT5B siRNA, alone or in combination. After 48 hours of treatment, the cells were fixed with 70% ethanol. DNA was stained with propidium iodide and analyzed with a flow cytometer (FACScan; BD Immunocytometry Systems, San Jose, CA). The percentage of cells in each phase of the cell cycle was determined by ModFit LT software (Verity Software House, Topsham, ME). Human CD45+ cells were also pretreated with SU6656. The numbers are the means plus or minus SD of 3 different experiments done on separate days, each performed in duplicate. (*P < .05, experimental groups vs scramble.)
IL-3 induces proliferation of mouse BM CD45+ cells via the STAT5 signaling pathway.
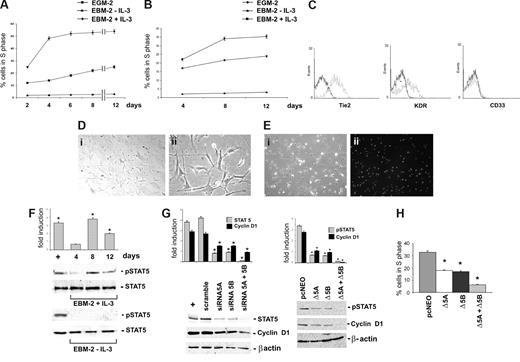
CD45+ cells physiologically home in BM and mobilize into circulation in response to microenvironment changes.3 We thus investigated the effects exerted by IL-3 on BM-derived CD45+ cells and, in particular, on their ability to proliferate and to acquire functional features of vascular cells. To this end, CD45+ cells obtained from human (Figure 6A) or from mouse BM-MNC (Figure 6B) were cultured with or without IL-3 for different time intervals. The reported data provide evidence that BM cells challenged with IL-3 share with their peripheral counterparts a similar kinetic of expansion and the same surface markers (Figure 6C). In addition, at day 8 of culture, the cells became spindle-shaped (Figure 6D) and able to uptake Dil-acLDL (Figure 6E). Likewise, biochemical analysis demonstrated a pick of STAT5 activation at day 8 of culture corresponding to the day of maximal expansion only when the cells were cultured in the presence of IL-3 (Figure 6F). The contribution of the 2 STAT5 isoforms in IL-3-mediated effects was investigated in STAT5A-and STAT5B-silenced cells by evaluating cyclin D1 expression (Figure 6G left panel) and cell-cycle progression (Table 1). Indeed, cyclin D1 expression and the number of cells into the cell cycle decreased in cells silenced for each of the 2 isoforms, and the effects were even more marked in cells depleted of both STAT5s. Similar results were obtained when CD45+ BM-cells were transfected with the DN STAT5A or STAT5B constructs, alone or in combination (Figure 6G right panel, H).
IL-3 induces BM-derived CD45+ cell proliferation via STAT5 activation. (A,B) The percentage of cells in the S phase was evaluated by FACS analysis on human (A) or mouse (B) BM-derived CD45+ cells cultured as indicated. (C) FACS analysis was performed on IL-3–cultured BM-derived CD45+ cells. Black lines, preimmune IgG (negative control); gray lines, Tie2, KDR, and CD33 markers. (D) Representative photomicrographs of 8 days IL-3–cultured cells. Magnification, 10× (panel i) and 40× (panel ii). (E) Representative sample of IL-3–cultured BM-derived CD45+ cells labeled with Dil-acLDL. Phase-contrast (panel i) and fluorescence (panel ii). Magnification, 10×. (F) Cell extracts from BM-derived CD45+ cells, cultured with or without IL-3 as indicated, were subjected to SDS-PAGE. The filter was IB with anti-p-STAT5 and anti-STAT5 antibodies. (G) IL-3–cultured BM-derived CD45+ cells transfected with STAT5A or STAT5B siRNA, alone or in combination, or with the scrambled sequence (scramble) were lysed. Cell extracts were analyzed by WB with anti–STAT5, anti–cyclin D1, and anti–β-actin antibodies (left panel). IL-3–treated ECs were used as positive control (+). In selected experiments, IL-3–cultured CD45+ cells, transfected with STAT5A and/or STAT5B DN constructs or with the empty vector (pcNEO), were assayed for the expression of p-STAT5, cyclin D1, and β-actin (right panel). (H) The percentage of cells in the S phase was evaluated by FACS analysis on BM-derived CD45+ cells transfected with STAT5A and/or STAT5B DN constructs or with the pcNEO vector (*P < .05, experimental groups vs pcNEO). Three different experiments were performed with comparable results.
IL-3 induces BM-derived CD45+ cell proliferation via STAT5 activation. (A,B) The percentage of cells in the S phase was evaluated by FACS analysis on human (A) or mouse (B) BM-derived CD45+ cells cultured as indicated. (C) FACS analysis was performed on IL-3–cultured BM-derived CD45+ cells. Black lines, preimmune IgG (negative control); gray lines, Tie2, KDR, and CD33 markers. (D) Representative photomicrographs of 8 days IL-3–cultured cells. Magnification, 10× (panel i) and 40× (panel ii). (E) Representative sample of IL-3–cultured BM-derived CD45+ cells labeled with Dil-acLDL. Phase-contrast (panel i) and fluorescence (panel ii). Magnification, 10×. (F) Cell extracts from BM-derived CD45+ cells, cultured with or without IL-3 as indicated, were subjected to SDS-PAGE. The filter was IB with anti-p-STAT5 and anti-STAT5 antibodies. (G) IL-3–cultured BM-derived CD45+ cells transfected with STAT5A or STAT5B siRNA, alone or in combination, or with the scrambled sequence (scramble) were lysed. Cell extracts were analyzed by WB with anti–STAT5, anti–cyclin D1, and anti–β-actin antibodies (left panel). IL-3–treated ECs were used as positive control (+). In selected experiments, IL-3–cultured CD45+ cells, transfected with STAT5A and/or STAT5B DN constructs or with the empty vector (pcNEO), were assayed for the expression of p-STAT5, cyclin D1, and β-actin (right panel). (H) The percentage of cells in the S phase was evaluated by FACS analysis on BM-derived CD45+ cells transfected with STAT5A and/or STAT5B DN constructs or with the pcNEO vector (*P < .05, experimental groups vs pcNEO). Three different experiments were performed with comparable results.
CD45+ BM cells isolated from Tie2-ΔSTAT5A and Tie2-ΔSTAT5B transgenic mice fail to be expanded by IL-3.
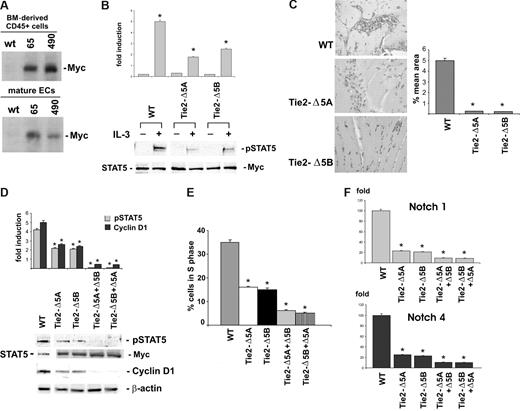
Data obtained in experiments using the Tie2 promoter demonstrated a close relationship between hemopoietic cells and EC.48,49 Thus, to gain further insight into the role of STAT5 in regulating IL-3–mediated CD45+ cell expansion, 2 independent lines of transgenic mice expressing the DN forms of STAT5A and STAT5B under the control of a Tie2 promoter were generated (Figure S2A-C). Both BM-derived CD45+ cells and mature ECs expressing the DN STAT5A or STAT5B were isolated (Figure 7A). Consistent with the inability of IL-3 to induce STAT5 activation in Tie2-Δ5A– and Tie2-Δ5B–derived EC (Figure 7B), we failed to detect neovessel formation in vivo in the 2 lines of transgenes (Figure 7C) when IL-3 was used as angiogenic mediator. STAT5 activation and cell-cycle progression were then evaluated in IL-3–treated BM-derived CD45+ cells isolated from these transgenic mice. As shown in Figure 7D, STAT5 phosphorylation was decreased both in Tie2-Δ5A and Tie2-Δ5B CD45+ cells, cell-cycle progression was inhibited (Figure 7E), and cyclin D1 expression was down-regulated (Figure 7D). All these events were completely prevented when we introduced the DN STAT5B or STAT5A isoforms in CD45+ Tie2-Δ5A and Tie2-Δ5B cells, respectively (Figure 7D,E). Consistent with data obtained when peripheral CD45+ cells were silenced for STAT5s, IL-3 failed to induce arterial specification in the 2 lines of transgenes (Figure 7F). Thus, these data first identify STAT5 as the signaling pathway involved in the control of IL-3–mediated BM-derived CD45+ cell expansion and arterial commitment.
BM-derived CD45+ cells isolated from ΔSTAT5A and ΔSTAT5B transgenic mice fail to undergo cell expansion when cultured with IL-3. (A) BM-derived CD45+ cells (top panel) and mature EC (bottom panel) were isolated and lysed from the independent founder lines (65 and 490) and from WT mice. Protein extracts were immunoprecipitated with the 9E10 anti-Myc antibody and subjected to SDS-PAGE, and the filters were IB with the 9E10 anti-Myc antibody. (B) Cell extracts from WT, Tie2-Δ5A–, and Tie2-Δ5B–derived ECs, treated or untreated with IL-3, were analyzed by WB using anti–p-STAT5, anti–STAT5, and 9E10 anti-Myc antibodies. (C) Hematoxylin-eosin–stained sections of Matrigel plugs containing IL-3 recovered from WT, Tie2-Δ5A, and Tie2-Δ5B mice are reported. Quantification of neovascularization was expressed as percentage (± SD) of the vessel area to the total Matrigel area. Each individual experimental group included 5 mice. (*P < .05, experimental groups vs WT.) (D) Cell extracts from IL-3–cultured-CD45+ cells obtained from WT, Tie2-Δ5A, or Tie2-Δ5B mice, transfected with the STAT5B or STAT5A DN constructs, respectively, were analyzed by WB using anti–p-STAT5, anti-STAT5, anti–cyclin D1, 9E10 anti-Myc, and anti–β-actin antibodies. (E) The percentage of cells in the S phase evaluated by FACS analysis on CD45+ cells from WT, Tie2-Δ5A, and Tie2-Δ5B mice and on Tie2-Δ5A and Tie2-Δ5B CD45+ cells, transfected with the STAT5B and STAT5A DN constructs, respectively (*P < .05, experimental groups vs WT). IL-3 was added in the culture media. (F) Q-RT-PCR was performed to evaluate the expression of arterial markers on CD45+ cells isolated from WT, Tie2-Δ5A, and Tie2-Δ5B mice. Expression levels are presented as described above. (*P < .05, experimental groups vs WT.) Three different experiments were performed with comparable results.
BM-derived CD45+ cells isolated from ΔSTAT5A and ΔSTAT5B transgenic mice fail to undergo cell expansion when cultured with IL-3. (A) BM-derived CD45+ cells (top panel) and mature EC (bottom panel) were isolated and lysed from the independent founder lines (65 and 490) and from WT mice. Protein extracts were immunoprecipitated with the 9E10 anti-Myc antibody and subjected to SDS-PAGE, and the filters were IB with the 9E10 anti-Myc antibody. (B) Cell extracts from WT, Tie2-Δ5A–, and Tie2-Δ5B–derived ECs, treated or untreated with IL-3, were analyzed by WB using anti–p-STAT5, anti–STAT5, and 9E10 anti-Myc antibodies. (C) Hematoxylin-eosin–stained sections of Matrigel plugs containing IL-3 recovered from WT, Tie2-Δ5A, and Tie2-Δ5B mice are reported. Quantification of neovascularization was expressed as percentage (± SD) of the vessel area to the total Matrigel area. Each individual experimental group included 5 mice. (*P < .05, experimental groups vs WT.) (D) Cell extracts from IL-3–cultured-CD45+ cells obtained from WT, Tie2-Δ5A, or Tie2-Δ5B mice, transfected with the STAT5B or STAT5A DN constructs, respectively, were analyzed by WB using anti–p-STAT5, anti-STAT5, anti–cyclin D1, 9E10 anti-Myc, and anti–β-actin antibodies. (E) The percentage of cells in the S phase evaluated by FACS analysis on CD45+ cells from WT, Tie2-Δ5A, and Tie2-Δ5B mice and on Tie2-Δ5A and Tie2-Δ5B CD45+ cells, transfected with the STAT5B and STAT5A DN constructs, respectively (*P < .05, experimental groups vs WT). IL-3 was added in the culture media. (F) Q-RT-PCR was performed to evaluate the expression of arterial markers on CD45+ cells isolated from WT, Tie2-Δ5A, and Tie2-Δ5B mice. Expression levels are presented as described above. (*P < .05, experimental groups vs WT.) Three different experiments were performed with comparable results.
Discussion
The data presented here lead to 2 major conclusions: (1) circulating CD45+ cells and their BM counterparts undergo cell proliferation, acquire arterial specification, and act as vasculogenic cells when exposed to IL-3, and (2) these effects rely on STAT5 signaling pathway.
Emerging data identify 2 distinct circulating progenitor cells able to sustain neoangiogenesis: EPCs, which are true progenitor cells with high proliferative capacity and the ability to form large colonies of mature ECs,10 and CACs or early EPCs, which are monocyte-like cells appearing early in culture.35 CACs do not proliferate after 4 days of culture,9 do not express the hemopoietic-specific cell-surface antigen CD45, the monocyte/macrophage cell-surface antigen CD14, or other surface markers specific to mature ECs, such as VE-cadherin, CD31, von Willebrand factor, and endothelial nitric-oxide synthase. Here, we showed that CAC cultured in the presence of IL-3 underwent proliferation after 4 days of culture. The expanded cells expressed CD45, CD34, low frequency of CD14, and no expression of myeloid differentiating markers (CD13 and CD33). However, the finding that these cells expressed KDR and Tie2 suggests that IL-3 specifically amplifies a subset of hemopoietic-derived progenitors not yet myeloid- or monocyte-committed. IL-3 was consistently found to expand sorted CD45+ and CD34+ but not sorted CD14+ cells. These cells maintained an endothelial phenotype and did not acquire myeloid commitment.
PV is a clonal myeloproliferative disorder.45,46 A valine-to-phenylalanine substitution at position 617 (V617F) of the JAK2 gene, leading to a gain-of-function mutation, has recently been identified in the majority of patients with PV.45,46 Herein, we demonstrate that CD45+ cells isolated from patients with PV and cultured with IL-3, bore the same JAK2 mutation as the pathologic BM-derived cells, confirming their origin from HSC.10
The involvement of hemopoietic cells in both normal and tumor angiogenesis is well established.11 However, whether these cells or CACs directly participate in the angiogenic process is still controversial. So far, rather than a direct contribution of these cells to neovessel formation, it seems that they have a broader function in promoting angiogenesis.9 Data presented herein demonstrate that circulating CD45+ cells and their BM counterparts, cultured on fibronectin in the presence of IL-3, acquired endothelial features, formed a vascular network in vitro, invaded a cluster of SKOV-3 tumor cells, and could be incorporated in neovessels originated in vivo. Thus, these data identify for the first time a hemopoietic-derived cell population that, when exposed to an inflammatory microenvironment containing IL-3, proliferates, acquires endothelial features and directly contributes to neovessel formation.
In the last decade, our knowledge of the molecular differences between arterial and venous EC and of the pathways underlying their developmental specification has rapidly increased.50,51 Early genetic approach identified the Shh/VEGF/Notch cross-talk in a central position for vascular morphogenesis. However, the evidence that arterial differentiation was not completely abrogated by Notch and/or Shh blocking could reflect the existence of additional VEGF/Notch-independent pathways.38 Indeed, several lines of evidence indicate that signal from outside vasculature are involved in arterial/venous fate decisions during development.52,53 Moreover, the occurrence of diseases restricted to arteries (eg, atherosclerosis) or veins (eg, varicose veins) suggests that such specification might be also recapitulated in adult life. Our results provide evidence that circulating and BM-derived CD45+ cells challenged with IL-3 recapitulate arterial specification, as shown by the expression of Ephrin B1, Ephrin B2, Notch1, and Notch4. In addition, arterial committed CD45+ cells were able to form vessels in vivo, providing further evidence that, indeed, these hemopoietic-derived cells participate as postnatal vasculogenic cells.
Accumulating evidence suggests that expansion and differentiation of stem cells and progenitors are dynamic multistage processes that require sequential activation of particular signaling molecules.3 However, despite several efforts, the exact molecular and biochemical pathways involved in these events are not well defined. Our study provides the first evidence that IL-3–mediated CD45+ expansion and arterial morphogenesis involve a c-Src–mediated activation of the STAT5 signaling pathway. STAT5s regulate the expression of genes that determine important cellular phenotypes. Moreover, STAT5 transcriptional activity also affects the expression of a variety of genes involved in cell-cycle control, including cyclin D1.27 The results of the present study demonstrate that, in circulating CD45+ cells and in their BM counterparts, STAT5 depletion prevents IL-3–mediated cyclin D1 expression and cell-cycle progression as well as arterial specification.
In Tie2-deficient mice, a severe impairment of both angiogenesis and definitive hemopoiesis occurred.54,55 We found that STAT5 inactivation in Tie2-transgenic mice prevented IL-3–sustained neovessel formation, IL-3–mediated cyclin D1 expression, and progression into the cell cycle of BM-derived CD45+ cells as well as their arterial specification. Two naturally occurring truncated STAT5 proteins lacking the transactivation domain have been described in immature myeloid cells, suggesting that they could act as regulatory proteins during myeloid differentiation.27 It is then possible that, besides affecting myeloid commitment, the expression of these naturally occurring STAT5s in BM-derived CD45+ cells might also control postnatal vasculogenic process.
The organ specific stem and progenitor cells reside in an adaptive BM microenvironment in which they readily sense and respond to demand for revascularization by undergoing differentiation. However, whether this process initiates in BM or at the neoangiogenic sites is not clear. The tissue-specific extracellular matrix, adhesion molecules and cytokines expressed at the neoangiogenic site are crucial for the selective homing and differentiation of stem and progenitor cells. Our data sustain the possibility that, in pathologic settings in which angiogenetic developmental process are recapitulated, IL-3, possibly by inducing the release of vascular growth factors, can provide a permissive environment for expansion and endothelial commitment of hemopoietic-derived CD45+ progenitors.
To inhibit or to induce vascular growth represents an attractive therapeutic strategy. Our results provide the first evidence that, in a permissive microenvironment containing IL-3, CD45+ angiogenic cells can act as postnatal vasculogenic cells. These data enable the possibility to develop strategies for their in vitro expansion and genetic modification, leading to tissue vascularization in clinical settings that require rapid angiogenesis. Alternatively, the identification of the STAT5 signaling pathway as the molecular signature of IL-3–mediated vasculogenic process provides the rational for novel therapeutic strategy that can hamper tumor angiogenesis.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr T. N. Sato for kindly providing the plasmid containing the Tie2 promoter and Dr A. Miyaijma for kindly providing the plasmids containing the dominant negative STAT5A and STAT5B. We also thank Prof Silvia Giordano and Prof Giovanni Camussi for revising the manuscript. Finally, we thank Dr Paola Bernabei for technical assistance.
This work was supported by grants from the Italian Association for Cancer Research (AIRC; M.F.B.), MIUR (Ministero dell'Università e Ricerca Scientifica), Cofinanziamento Ministero dell'Università e della Ricerca Scientifica e Tecnologica (MURST), and fondi ex-60% (M.F.B. and L.P.).
Authorship
Contribution: A.Z. performed isolation and characterization of CAC and in vivo experiments; P.D. performed biologic experiments; A.R. performed transfections; G.T. performed isolation and characterization of CAC.; A.T. performed biologic experiments; L.D. performed Src kinase assay; P.F.diC. performed JAK2 genotype; L.P. was senior advisor; F.A. performed transgenic mice generation; M.F.B. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Maria Felice Brizzi, MD, Department of Internal Medicine, University of Torino, Corso Dogliotti 14, 10126, Torino, Italy; e-mail: mariafelice.brizzi@unito.it.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal