Abstract
CD4+ T cells can differentiate into multiple effector subsets, but the potential roles of these subsets in anti-tumor immunity have not been fully explored. Seeking to study the impact of CD4+ T cell polarization on tumor rejection in a model mimicking human disease, we generated a new MHC class II-restricted, T-cell receptor (TCR) transgenic mouse model in which CD4+ T cells recognize a novel epitope in tyrosinase-related protein 1 (TRP-1), an antigen expressed by normal melanocytes and B16 murine melanoma. Cells could be robustly polarized into Th0, Th1, and Th17 subtypes in vitro, as evidenced by cytokine, chemokine, and adhesion molecule profiles and by surface markers, suggesting the potential for differential effector function in vivo. Contrary to the current view that Th1 cells are most important in tumor rejection, we found that Th17-polarized cells better mediated destruction of advanced B16 melanoma. Their therapeutic effect was critically dependent on interferon-γ (IFN-γ) production, whereas depletion of interleukin (IL)–17A and IL-23 had little impact. Taken together, these data indicate that the appropriate in vitro polarization of effector CD4+ T cells is decisive for successful tumor eradication. This principle should be considered in designing clinical trials involving adoptive transfer–based immunotherapy of human malignancies.
Introduction
The role of CD4+ cells in antitumor immunity remains controversial and poorly understood.1,2 They are known to mediate potent therapeutic effect in the setting of hematopoietic stem cell allotransplantation and donor lymphocyte infusion in hematologic malignancy,3,4 but antigen-specific T helper (Th) cells have been studied to much lesser extent. A lack of clarity regarding CD4+ cells is due, in no small part, to the complexity of their biology. CD4+ T cells can differentiate into diverse subsets with specific phenotypes that can have self-reinforcing and opposing functions, but these T-cell subsets have not been comprehensively studied in tumor-bearing mice.
Historically, CD4+ T lymphocytes have been thought of as mere providers of stimuli to help the putatively more important CD8+ effectors, which eliminate cancer by direct cytotoxicity.5-7 There are several studies showing that CD4+ T helper (Th) cells are capable of protecting the host against tumor challenge and even of mediating tumor regression on their own in the setting of either solid or hematopoietic disease.8-13 Furthermore, protection was maintained against MHC class II–negative multiple myeloma model and involved cross-presentation by professional antigen-presenting cells (APCs) and activation of tumoricidal activity mediated by macrophages secreting IFN-γ.14 A similar IFN-γ–dependent mechanism was involved in the rejection of MHC class II–negative tumor in severe combined immunodeficient (SCID) mice.15 In some cases, the ability to reject antigen-expressing tumor by specific naive Th cells was thought to be substantially better than the ability of CD8+ cells.16 Classically, effector CD4+ T cells have been categorized into T helper 1 (Th1) and T helper 2 (Th2) subsets.17,18 Limited studies indicate that both subtypes elicit antitumor effects,19-21 but the Th1-polarized cells, secreting IFN-γ and capable of enhancing activity of cytotoxic CD8+ lymphocytes, have traditionally been regarded as more efficient.22-25 However, it is also clear that CD4+ T regulatory cells (Tregs) can efficiently suppress the function of antitumor CD8+ T cells.5,26-28
Recently, the novel Th17 lineage, generated in the presence of TGF-β and IL-6 and expanded under the influence of IL-23,29-31 has been associated with responses against certain infections and implicated in the development of autoimmunity in animal models that had been previously linked to Th1-type responses (experimental autoimmune encephalitis, collagen-induced arthritis).32,33 They also seem to play an important role in the pathogenesis of graft-versus-host disease (GVHD).34,35 Th17 cells have been found in various tumors, including mycosis fungoides, Sézary syndrome, and prostate cancer.36,37 Kryczek et al reported the presence of naturally occurring Th17 cells and Tregs in the tumor microenvironment and tumor-draining lymph nodes in both human and mice tumors.38 Proinflammatory cytokines including IL-17A, IL-6, and IL-23 have been described to impair immune surveillance by CD8+ T cells, and promote de novo carcinogenesis and neovascularization of tumors via STAT3 signaling and other mechanisms.39-45 In contrast, some publications have reported antitumor activity of IL-17A or IL-2346,47 that can be T-cell dependent.48 Nevertheless, the role of cancer-specific Th17 cells in cancer immunity has not been elucidated, but their ability to cause inflamma-tion and destruction of tissues might be of interest in the therapy of malignancy.
To compare the antitumor efficacy of in vitro–polarized TCR transgenic Th1, Th17, and nonpolarized Th0 cells, we sought to develop a model that closely resembled human disease. Although several models have been previously described, one of the main flaws of currently available systems is that they usually involve using cancer cells modified to express potentially highly immunogenic foreign or surrogate antigens (eg, OVA or H-Y in female hosts). In other cases, overexpression of potent cytokines, including IFN-γ, by the tumor cells was required to observe antitumor effects. Frequently, only very early, small nonvascularized tumors or unrealistic microscopic hepatic or pulmonary “metastases” could be treated. In other existing tumor models, treatment is given before the tumor challenge. Tumor protection models may not mimic a clinically relevant scenario, and their relevance to human patients with established disease is uncertain.
In the clinical setting, solid tumors are large, vascularized, and poorly immunogenic. Immune responses against cancer can be regarded as an autoimmune process where the targets are often nonmutated self-proteins representing tissue differentiation antigens. As current models of CD4-based anticancer responses, even though valuable, have serious shortcomings and do not approximate a real-life scenario, we sought a novel, more realistic model that closely mimicked human disease. We created a transgenic mouse that expresses a MHC class II–restricted TCR recognizing an endogenous melanocyte differentiation antigen called tyrosinase-related protein 1 (TRP-1 or gp75). TRP-1 is present in normal melanocytes as well as in melanoma cells and therefore is a potential target for immunotherapy in humans.49 Using tumor-specific CD4+ effector cells, we characterized in vitro Th1- and Th17-polarized subsets and compared them with nonpolarized (Th0) cells. We then adoptively transferred these cells in an effort to treat large, established, unmanipulated B16 melanoma. Thus, we describe for the first time a CD4+, MHC class II–restricted immunotherapy model targeting naturally occurring self-antigen.
Methods
All animal experimental procedures have been approved by the National Cancer Institute Animal Use Committee.
Mice and tumor lines
C57BL/6 and RAG1−/−BW TRP-1 TCR transgenic mice were bred at the National Institutes of Health (NIH). RAG1−/−, IFN-γ−/−, IFN-γR−/−, and B6.PL (Thy1.1+) mice with C57BL/6 background were obtained from The Jackson Laboratory (Bar Harbor, ME). B16 (H-2b), a TRP-1+ spontaneous murine melanoma, MCA205, a TRP-1− murine methylcholanthrene-induced fibrosarcoma, and EL-4, a TRP-1− murine T-cell leukemia cell line, have been obtained from the National Cancer Institute tumor repository. We generated B16/CIITA cells, which overexpress MHC class II (data not shown) by transfecting B16 cells with a plasmid encoding the mouse MHC class II transactivator (CIITA) that was kindly provided by Peter Cresswell (Yale University School of Medicine, New Haven, CT). All cells were maintained in culture media (CM) composed of RPMI 1640 with 10% heat-inactivated fetal bovine serum (Invitrogen, Frederick, MD), 0.03% l-glutamine, 100 μg/mL streptomycin, 100 μg/mL penicillin, and 50 μg/mL gentamicin sulfate (NIH Media Center).
Generation of TRP-1 TCR transgenic mice
The TRP-1 TCR was generated from white-based brown mutant mice BW (cappuccino) mice. The BW white-based brown mutation is a spontaneous defect in synthesis of TRP-1 protein that arose in irradiated mice. The defect has been characterized as an inversion of exon 1 of the tyrp1 gene and results in a total absence of TRP-1 protein in melanocytes and other tissues. BW mice were backcrossed for 8 generations onto the C57BL/6 background using a speed congenic-based technique. They were immunized once with 107 plaque forming units (pfu) recombinant TRP-1 vaccinia virus (TRP-1 rVV),49 following by 100 μg murine TRP-1 protein 3 weeks later. Four days prior to harvesting organs, mice were boosted with Sindbis virus TRP-1 DNA by gene gun (4 μg/mouse). Lymphocytes were fused with PEG 1500 at a 1:1 ratio to the LacZ inducible hybridoma BWZ.36/CD8a fusion partner, and were plated in HAT selection media.50 Fourteen days later, wells were screened against B16/CIITA cell line for reactivity. Clone 7A6 was found the most reactive and further subcloned. Reactivity against TRP-1 protein and truncated TRP-1 peptides was tested using 3 Gy irradiated peptide-pulsed splenocytes as described below. Fluorescence-activated cell sorting (FACS) analysis identified that 7A6 hybridoma expressed Vα3.2 and Vβ14 TCR chains. The method for generating the TCR transgenics is identical to that described for the pmel-1 mice.48 Briefly, RNA was isolated from this clone and α and β TCR regions were amplified by 5′-rapid amplification of cDNA ends (5′-RACE; Life Technologies, Bethesda, MD) using constant region antisense primers α1 (5′-GGCTACTTTCAGCAGGAGGA-3′) and β1 (5′-AGGCCTCTGCACTGATGTTC-3′), respectively. 5′-RACE products were amplified with nested TCR α and β constant region primers α2 (5′-GGGAGTCAAAGTCGGTGAAC-3′) and β2 (5′-CCACGTGGTCAGGGAAGAAG-3′), respectively, and cloned into pCR4TOPO TA sequencing vectors (Invitrogen, Frederick, MD). TCR α and β transcripts were sequenced as Vα3.3Jα20 and Vβ14Dβ2Jβ2.5, respectively. The α and β genomic variable domains were PCR amplified (Perkin-Elmer) with primers gα1 (5′-TCTCCCGGGCTTCTCACTGCCTAGCCATGATGAAATCCTTGAGTGTTTC-3′) and gα2 (5′-GTAGCGGCCGCGTAAAATCTATCCTAGTGTTCCCCAGA-3′) or gβ1 (5′-GATCTCGAGAATCTGCCATGGGCACCAG-3′) and gβ2 (5′-GATACCGCGGTTCCTTTCCAAGACCAT-3′), respectively. The genomic variable domains were TA-cloned into pCR4TOPO (Invitrogen), validated by sequencing, subcloned into TCR cassette vectors, and coinjected into fertilized C57BL/6 embryos that yielded 13 founders. Founder number 9, in which the transgene insertion site was found on the Y chromosome, was successfully bred and crossed into RAG1−/− (black) mice and subsequently into RAG1−/−BW (cappuccino) background.
Histology
Eyes were enucleated 14 days after adoptive transfer, fixed in 10% formalin, embedded in methylacrylate, sectioned via papillary–optic nerve axis, and H&E stained.
Generation and functional characterization of Th0-, Th1-, or Th17-polarized cells from TRP-1 mice
Single-cell suspensions (106 cells/mL) of spleen cells from RAG1−/−BW TRP-1 TCR transgenic mice were seeded into 24-well plates in CM with C57BL/6 3000 rad irradiated splenocytes pulsed with TRP-1106-130 (SGHNCGTCRPGWRGAACNQKILTVR) peptide. Th0 cells were plated in the absence of exogenous cytokines. To obtain highly polarized cells, rmIL-12 (3.3 ng/mL; Peprotech, Rocky Hill, NJ) was added to Th1 cultures and rmIL-6 (5 ng/mL), rhTGF-β1 (10 ng/mL; R&D Systems, Minneapolis, MN), and anti–IFN-γ antibody (10 μg/mL; eBioscience, San Diego, CA) were added to Th17 cultures. Tregs were generated in the presence of rhTGF-β1 (10 ng/mL) and anti–IFN-γ antibody (10 μg/mL). On the third day of culture, CM containing 30 IU/mL rhIL-2 (Chiron, Emeryville, CA) was added to all culture conditions and polarizing cytokines were supplemented. Cells were cultured for 1 week before being used for experiments.
Images were acquired at 100× magnification using the National DC5-163 microscope with integrated digital camera (National Optical & Scientific Instruments, San Antonio, TX) and Motic (Richmond, BC) Image Plus 2.0 software. They were further cropped using PowerPoint software (Microsoft, Redwood, WA).
Cytokine release assays
T cells were tested for secretion of IFN-γ, TNF-α, IL-2, IL-6, IL-10, IL-17A, IL-21, and CCL20 in release assays using R&D Systems antibody pair according to manufacturer's protocol. Irradiated splenocytes (3000 rad) were pulsed with escalating doses of TRP-1106-130 or 1 μM of an irrelevant peptide (gp10025-33). In some experiments, different tumor cells lines (B16, B16 CIITA, MCA 205, and EL4) were used as target cells. Effector cells and target cells were incubated in a 0.2-mL culture volume in individual wells of 96-well plates for 24 hours at 37°C. Cytokine secretion was measured in culture supernatants diluted to be in the linear range of the assay.
Flow cytometry and antibodies
Antibodies against FoxP3 and IL-17A were purchased from eBioscience. All other antibodies used in this report were purchased from BD Pharmingen (San Diego, CA). For intracellular cytokine staining, cells were stimulated overnight with 50 ng/mL PMA (Sigma-Aldrich, St Louis, MO) and 750 ng/mL ionomycin (Calbiochem, San Diego, CA) or not at all. After 1 hour, GolgiStop (BD Pharmingen) was added to inhibit export of cytokines. Cells were surface stained for 15 to 30 minutes at 4°C with anti-CD4 and anti-Vβ14 antibodies in PBS supplemented with 1% BSA and 0.2% sodium azide. For intracellular staining T cells were fixed and permeabilized with IC Fixation/Permeabilization Buffers (eBioscience) according to the manufacturer's instruction and stained with anti–IL-17A and anti–IFN-γ antibodies. For Foxp3 staining, nonstimulated T cells were stained using the same protocol. Flow cytometry acquisition was performed on a FACS Canto II or FACSCalibur and analyzed with FlowJo software (TreeStar, Eugene, OR).
Microarray analysis
RNA was isolated from polarized Th0, Th1, and Th17 cells cultured in vitro for 7 to 8 days using RNeasy columns (QIAGEN, Valencia, CA). RNA was indirectly labeled via a single round of linear amplification with Amino Allyl MessageAmp II reagents (Ambion, Austin, TX) with the control Th0-polarized cells serving as a reference. The labeled experimental RNA was combined with labeled control RNA and hybridized overnight to 38 000-spot long oligonucleotide MEEBO arrays (Advanced Technology Center Microarray Facility NCI). Arrays were scanned using a GenePix 4000B scanner (Axon Instruments, Union City, CA) and data were acquired with GenePix Pro 5.1 (Axon Instruments). The data files were imported into GeneSpring GX 7.3.1 (Silicon Genetics, Redwood City, CA) for all analyses. GEO accession number is GSE10814 (http://www.ncbi.nlm.nih.gov/geo/).51
Adoptive cell transfer protocol and vitiligo score
Mice 6 to 12 weeks of age (n = 5-6 for all groups) were injected subcutaneously with 5 × 105 B16-F10 melanoma cells and treated 10 to 14 days later with adoptively transferred TRP-1–specific CD4+ T cells derived from TCR transgenic splenocytes polarized in vitro. Lymphopenia was induced by nonmyeloablative (5 Gy) total body irradiation (TBI) of tumor-bearing mice on the day of cell transfer. Where indicated, a single dose of recombinant TRP-1 vaccinia virus vaccine (TRP-1 rVV) and/or 6 doses of rhIL-2 (36 ng/dose; Chiron) given 12 hours apart were administered by intraperitoneal injection. Tumors were measured using calipers, and the products of the perpendicular diameters were recorded. All experiments were performed in a blinded, randomized fashion and repeated independently at least twice, with similar results. Vitiligo on treated mice was scored on a scale of 0 to 5 as follows: 0 indicates no vitiligo (wild type); 1, depigmentation detected; 2, more than 10% vitiligo; 3, more than 25% vitiligo; 4, more than 50% vitiligo; and 5, more than 75% vitiligo. Mice were evaluated and scored by 2 independent investigators blinded to group at approximately 3 months after adoptive cell transfer.
In vivo CFSE proliferation assay
Polarized TRP-1 cells (Thy1.2) were labeled with 1 μM CFSE (Invitrogen) and adoptively transferred into sublethally irradiated (500 R) B6PL mice (Thy1.1). Spleens were harvested on days 3 and 6 and analyzed by FACS for presence of CFSE fluorescent dye after gating on Thy1.2+ CD4+ population.
Enumeration of adoptively transferred cells
On the days indicated, mice were killed and their organs were harvested and homogenized into a single-cell suspension using the rubber end of a 3-cc syringe and a 40-μm filter cup. Cells were labeled with the following mAbs (BD Pharmingen): FITC-conjugated anti-Vβ14 and PE-conjugated anti-CD4. Samples were analyzed using a FACSCalibur flow cytometer and FlowJo 7.1 software. Samples were enumerated using trypan blue exclusion. The absolute number of TRP-1 T cells was calculated by multiplying the absolute cell count by the total percentage of Vβ14+CD4+ cells.
In vivo cytokine neutralization
Neutralizing antibodies against murine IL-17A and IL-23 were purchased from R&D Systems; antibodies against IFN-γ were obtained from eBioscience. Tumor-bearing C57/BL6 mice were treated with Th17 TRP-1 cells. Mice were injected intraperitoneally with 100 μg neutralizing antibodies starting 24 hours after adoptive cell transfer. Injections were repeated every other day for 5 cycles. The control group received isotype-matched antibody (R&D Systems).
Statistical analysis
Tumor graphs were compared using analysis of variance. P values less than .05 were considered significant. Statistical analyses comparing frequencies of vitiligo were performed using 1-way ANOVA with Bonferroni correction for multiple comparisons. A P value of .05 or lower was considered significant. Kaplan-Meyer survival curves were compared by Wilcoxon test. A P value of .05 or lower was considered significant. Cell numbers were compared by Student t test. A P value of .05 or lower was considered significant.
Results
Identification of TRP-1-specific MHC class II–restricted TCR and generation of TRP-1 transgenic mouse
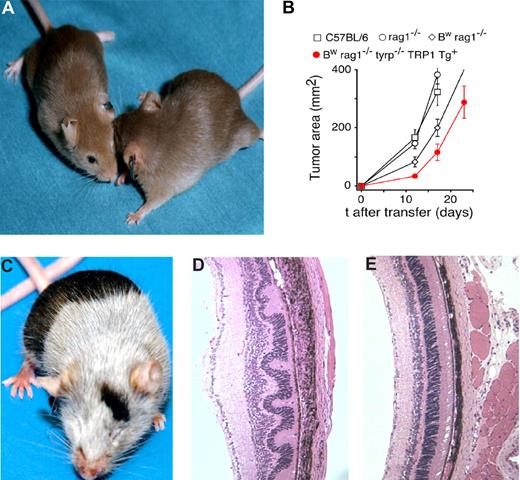
We sought to create a realistic model of MHC class II–restricted, CD4+ T cells specific for a self-antigen. We had indirect evidence49 that a recombinant vaccinia virus (rVV)–based vaccine encoding TRP-1 elicited a Th-dependent response in C57BL/6 mice resulting in antibody-mediated autoimmune vitiligo. We thus attempted to clone CD4+ cells reactive to the TRP-1 antigen. Identification of TRP-1–specific CD4+ T cells using immunized wild-type mice was not successful (data not shown), most likely due to tolerance-related mechanisms present in TRP-1–positive (black) animals. We thus sought to generate melanocyte-reactive CD4+ cells from antigen-negative animals, known as Bw or “white-based brown mutation mice” with characteristic “cappuccino” appearance (Figure 1A). The mutation occurred at the Oak Ridge National Laboratories (Oak Ridge, TN) in C3H male animals exposed to ionizing radiation. The defect has been characterized as an inversion of the first exon of the tyrp-1 gene,52 resulting in complete absence of the TRP-1 protein expression, even in truncated or mutated form, thus Bw mice represent a true immunologic knockout of TRP-1.52 Bw mice were backcrossed onto the C57BL/6n background for 8 generations using a speed congenic approach. After multiple rounds of vaccination, T-cell fusion was performed and a hybridoma strongly recognizing the MHC class II–overexpressing B16 melanoma cell line (B16/CIITA) was isolated as described in “Methods.” Further analysis of the recognition of truncated peptides established a minimal TRP-1 epitope corresponding to amino acids 113 to 127 (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). TCR was identified as Vβ14Vα3.2. It was cloned and expressed in transgenic C57BL/6 mice. A founder with Y-chromosome–linked TRP-1 TCR transgene was identified and bred onto a RAG1−/− background to eliminate rearrangement of endogenous TCR.
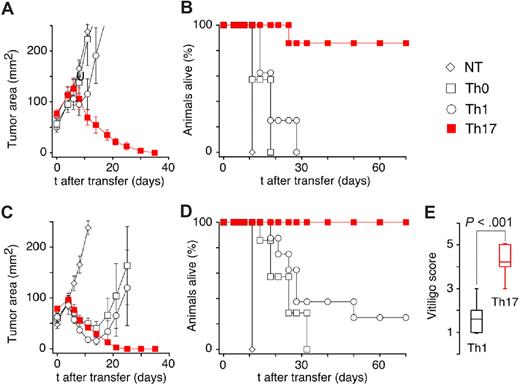
Characterization of the TRP-1 CD4+ model. (A) Characteristic cappuccino phenotype of white-based brown mutation (Bw) after 8 rounds of backcrossing onto a C57BL/6 background using “speed congenics.” The coat color appearance derives from a defect in exon 1 of tyrosinase-related protein-1 (tyrp1) gene. The MHC class II–restricted TCR used to create the TRP-1–specific transgenic mouse was isolated from Bw mice after multiple rounds of vaccination. (B) RAG1−/−Bw TRP-1 Tg+ animals are marginally protected against the B16 challenge. C57BL/6, RAG1−/−, RAG1−/−Bw, and their RAG1−/−BwTRP-1 TCR transgenic littermates were injected subcutaneously with 0.5 × 106 B16 melanoma cells. Results for tumor area are the mean of measurements from at least 5 mice per group (± SEM). Data shown are representative of 2 independent experiments. (C) Adoptive transfer of 0.25 × 106 naive purified TRP-1 CD4+ T cells into a tyrp1+/+ (wt, black) RAG1−/− mouse results in a rapid development of massive vitiligo. (D) H&E staining of ocular tissue from the mice that received adoptive transfer of naive TRP-1 cells revealed diffuse damage with edema, retinal folding, disruption of pigmented epithelium, and inflammatory infiltrate in the choroid. (E) An H&E stain of a normal eye at a similar magnification from an untreated RAG1−/− mouse is shown as a control.
Characterization of the TRP-1 CD4+ model. (A) Characteristic cappuccino phenotype of white-based brown mutation (Bw) after 8 rounds of backcrossing onto a C57BL/6 background using “speed congenics.” The coat color appearance derives from a defect in exon 1 of tyrosinase-related protein-1 (tyrp1) gene. The MHC class II–restricted TCR used to create the TRP-1–specific transgenic mouse was isolated from Bw mice after multiple rounds of vaccination. (B) RAG1−/−Bw TRP-1 Tg+ animals are marginally protected against the B16 challenge. C57BL/6, RAG1−/−, RAG1−/−Bw, and their RAG1−/−BwTRP-1 TCR transgenic littermates were injected subcutaneously with 0.5 × 106 B16 melanoma cells. Results for tumor area are the mean of measurements from at least 5 mice per group (± SEM). Data shown are representative of 2 independent experiments. (C) Adoptive transfer of 0.25 × 106 naive purified TRP-1 CD4+ T cells into a tyrp1+/+ (wt, black) RAG1−/− mouse results in a rapid development of massive vitiligo. (D) H&E staining of ocular tissue from the mice that received adoptive transfer of naive TRP-1 cells revealed diffuse damage with edema, retinal folding, disruption of pigmented epithelium, and inflammatory infiltrate in the choroid. (E) An H&E stain of a normal eye at a similar magnification from an untreated RAG1−/− mouse is shown as a control.
TRP-1–specific CD4+ T cells are found only in TRP-1 antigen–negative transgenic animals
TRP-1–specific TCR was generated from immunized Bw mice that do not have the gp75 protein, and it was thus possible that cells expressing this TCR would be subjected to the negative selection in antigen-positive (black, wild-type) animals. Therefore, transgenic TRP-1 mice were crossed into Bw (antigen negative, cappuccino) RAG−/− background. We have analyzed the repertoire of CD4+ T cells in TRP-1 antigen–positive and –negative RAG1−/− transgenic animals. Only transgenic animals devoid of TRP-1 antigen (Bw RAG1−/− TRP-1 TCR Tg+) had readily detectable CD4+Vβ14+ T cells in the peripheral lymphoid organs, while TRP-1 antigen–positive (black) transgenic mice were indistinguishable from their nontransgenic RAG1−/− counterparts (Figure S2). This suggested that highly avid TRP-1–reactive TCRs were deleted from the T-cell repertoire in C57BL/6 mice.
TRP-1–specific CD4+ T cells fail to protect transgenic host against tumor challenge but mediate autoimmunity after adoptive transfer
To evaluate the degree of protection against the growth of melanoma conveyed by TRP-1 cells, we inoculated BwRAG1−/−TRP-1 Tg mice with B16 cells. Despite the presence of a large population of melanoma-specific T cells, TCR transgenic animals were not protected against B16 challenge. Tumor take was 100% (7 of 7). The growth of the subcutaneously inoculated melanoma was only minimally delayed in those mice in comparison with nontransgenic C57BL/6, RAG1−/− (black, wild-type), or BwRAG1−/− (cappuccino) littermates (Figure 1B). All animals eventually had to be killed because of rapid disease progression. Similar lack of protection against the tumor has been demonstrated previously in other TCR transgenic models and attributed to immunologic ignorance and lack of costimulatory signaling.53-55
To evaluate if the CD4+Vβ14+ T cells found in transgenic animals could mediate biologic activity in vivo, we performed adoptive transfer of CD4-selected splenocytes from TCR transgenic mice into RAG1−/− black (wild-type) recipients. Rapid development of antigen-specific autoimmunity, as evidenced by extensive vitiligo, was observed in all recipients (Figure 1C). Treated mice also developed ocular injury with massive uveal infiltration and disruption of the retinal architecture (Figure 1D,E). Nearly complete vitiligo was also observed after the adoptive transfer of naive TRP-1 cells into sublethally irradiated C57BL/6 animals, however the degree of ocular damage was not as pronounced (data not shown), perhaps because of the presence of regulatory elements in the immunocompetent hosts. C57BL/6 and RAG1−/− recipients remained otherwise healthy and survived for more than 12 months in our animal facility.
Characterization of in vitro–generated polarized TRP-1–specific Th cells
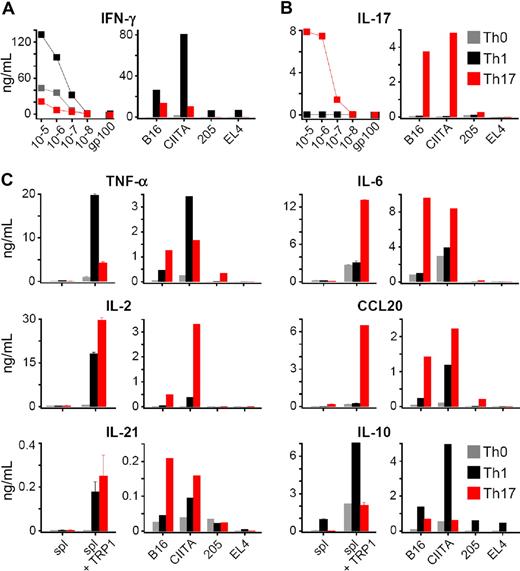
Our observations indicated that TRP-1 CD4+ T cells were able to cause massive autoimmunity, thus we sought to use them to treat tumors. Generation of anticancer T cells used in the real-life clinical settings usually involves stimulation and expansion in vitro, therefore culture conditions might be crucial for the therapeutic outcome of the adoptive cell transfer therapy. To investigate this question, we generated different TRP-1 T helper subsets (Th1, Th17, and Th0) by culturing the cells under strictly defined polarizing conditions. The degree of expansion of both Th1 and Th17 was similar and higher in comparison with the neutral (Th0) condition (data not shown). To confirm the subset commitment, we analyzed the cytokine secretion profile, phenotype, and gene expression patterns. Th1 cells produced high quantities of IFN-γ (Figure 2A) upon peptide stimulation. They also produced TNF-α, IL-10, and lower amounts of IL-2 (Figure 2C). As expected, only Th17-skewed cells secreted significant quantities of IL-17A (Figure 2B). They also produced smaller but significant quantities of IFN-γ and TNF-α, as well as high levels of IL-2, IL-6, IL-21, and CCL20 (MIP3-α), which has been implicated in mucosal and skin immunity, as well as in inflammatory-type pathology.56,57 Nonpolarized Th0 cells secreted IFN-γ at intermediate levels, but were not able to produce IL-17A. A similar cytokine profile was produced for each of the Th subtypes in response to stimulation with B16 melanoma. Recognition was stronger upon exposure to B16/CIITA cell line, engineered to express higher levels of MHC class II molecules. There was no release of cytokines upon incubation with TRP-1–negative tumor cell lines MCA205 and EL-4, confirming the specificity of transgenic T cells (Figure 2A-C). In addition, intracellular staining upon restimulation demonstrated that virtually all Th1 cells produced IFN-γ, while only those cells programmed in TGF-β and IL-6 contained a significant percentage of IL-17A–secreting lymphocytes and a small number of IFN-γ–producing cells (Figure S3) consistent with the other reports.38,58,59 Taken together, these data indicated that the population of cells polarized using Th17 conditions acquired the specific ability to secrete IL-17A.
TRP-1 cells expanded in vitro under polarizing conditions show highly different cytokine secretion patterns. (A) Release of INF-γ by Th0, Th1, and Th17 TRP-1 cells as measured by ELISA. Polarized TRP-1–specific TCR transgenic T cells were restimulated overnight with TRP-1106-130 peptide–pulsed splenocytes at escalating concentrations or gp100 control peptide (at a concentration of 10−5 mg/mL; left panel) or were incubated overnight with B16 and B16/CIITA melanoma cell lines. Tumor cell lines lacking the relevant antigen, MCA205 and EL-4, were used as specificity control (right panel). (B) Release of IL-17A by Th0, Th1, and Th17 TRP-1 cells upon stimulation with escalating concentrations of TRP-1106-130 peptide (left) or B16 and B16/CIITA melanoma cell lines (right). MCA205 and EL-4 were used as a negative control. (C) Secretion of TNF-α, IL-2, IL-6, IL-10, IL-17, IL-21, and CCL20 was measured by ELISA after overnight stimulation with TRP-1106-130 peptide–pulsed splenocytes (left panels) or B16 and B16/CIITA melanoma cells (right panels). Splenocytes pulsed with gp-100 peptide and MCA205 and EL-4 tumor cells were used as specificity control.
TRP-1 cells expanded in vitro under polarizing conditions show highly different cytokine secretion patterns. (A) Release of INF-γ by Th0, Th1, and Th17 TRP-1 cells as measured by ELISA. Polarized TRP-1–specific TCR transgenic T cells were restimulated overnight with TRP-1106-130 peptide–pulsed splenocytes at escalating concentrations or gp100 control peptide (at a concentration of 10−5 mg/mL; left panel) or were incubated overnight with B16 and B16/CIITA melanoma cell lines. Tumor cell lines lacking the relevant antigen, MCA205 and EL-4, were used as specificity control (right panel). (B) Release of IL-17A by Th0, Th1, and Th17 TRP-1 cells upon stimulation with escalating concentrations of TRP-1106-130 peptide (left) or B16 and B16/CIITA melanoma cell lines (right). MCA205 and EL-4 were used as a negative control. (C) Secretion of TNF-α, IL-2, IL-6, IL-10, IL-17, IL-21, and CCL20 was measured by ELISA after overnight stimulation with TRP-1106-130 peptide–pulsed splenocytes (left panels) or B16 and B16/CIITA melanoma cells (right panels). Splenocytes pulsed with gp-100 peptide and MCA205 and EL-4 tumor cells were used as specificity control.
Because culturing CD4+ T cells in IL-2 might expand the Tregs and their presence might negatively affect in vivo effectiveness of adoptive cell transfer therapy, we analyzed Foxp3 expression in our cells. Flow cytometry demonstrated that Tregs were absent among Th1-skewed TRP-1 cells, while a small population of Tregs was readily detectable in both Th17 and nonpolarized Th0 cell cultures (Figure S4). Cytofluorimetric analysis showed that Th1 T cells retained relatively more naive central memory–like characteristics, with the highest percentage of CD62Lhigh and CD45RBhigh cells. In contrast, virtually the entire population of Th17-skewed T cells was CD62Llow and CD45RBlow, markers that were consistent with an effector memory phenotype. The Th17 population showed higher expression of CD38 and lower levels of SCA-1 (Ly6A) and CD49d (integrin α4, VLA-4) (Figure 3A), suggesting that biologic differences between the subsets were deeper than just their cytokine profiles.
In vitro–polarized effector CD4+ T-cell subsets acquire distinct phenotypes and gene expression profiles. (A) In vitro polarizing conditions alter the phenotype of TRP-1 cells. Th0, Th1, and Th17 TRP-1 T cell were analyzed using flow cytometry for the expression of selected activation markers and adhesion molecules: CD62L, CD45RB, SCA1 (Ly6a), CD38, and CD49d (integrin α4, VLA-4). Percentage of positive cells is calculated based on the comparison with an isotype control antibody. (B) Relative alterations in mRNA quantities of selected genes are observed using microarray analysis. Th1 versus Th17 TRP-1 cells were compared, using Th0 mRNA as a reference. mRNAs are grouped by their function (integrins and adhesion molecules, matrix metalloproteinases [MMPs] and related molecules, cytokines and their receptors, chemokines, and chemokine receptors). A cutoff for significant difference in the level of mRNA message expression was set at 2-fold.
In vitro–polarized effector CD4+ T-cell subsets acquire distinct phenotypes and gene expression profiles. (A) In vitro polarizing conditions alter the phenotype of TRP-1 cells. Th0, Th1, and Th17 TRP-1 T cell were analyzed using flow cytometry for the expression of selected activation markers and adhesion molecules: CD62L, CD45RB, SCA1 (Ly6a), CD38, and CD49d (integrin α4, VLA-4). Percentage of positive cells is calculated based on the comparison with an isotype control antibody. (B) Relative alterations in mRNA quantities of selected genes are observed using microarray analysis. Th1 versus Th17 TRP-1 cells were compared, using Th0 mRNA as a reference. mRNAs are grouped by their function (integrins and adhesion molecules, matrix metalloproteinases [MMPs] and related molecules, cytokines and their receptors, chemokines, and chemokine receptors). A cutoff for significant difference in the level of mRNA message expression was set at 2-fold.
To further elucidate these differences, we performed a transcriptome analysis of in vitro–polarized cell populations. Messenger RNA from Th0 cells was used as a reference to compare Th1 and Th17 gene expression profiles (Figure 3B). The Th17-polarized population showed a striking up-regulation of IL-17A (105-fold difference) and CCL20/MIP3α (95-fold difference). mRNAs encoding IL-17F and IL-22, which are additional markers of Th17 polarization, were also elevated. As suggested by the results of the enzyme-linked immunosorbent assay (ELISA) (Figure 2), mRNA levels for IL-2 and IL-21 as well as another common γ-chain cytokine, IL-9, were higher in Th17-polarized population. As expected, the relative expression of IFN-γ and IFN-α was higher in Th1 cells. We also observed significant differences in mRNA levels of multiple chemokines and chemokine receptors, integrins, and other adhesion molecules, as well as matrix metalloproteinases (MMPs), suggesting important differences in the ability of the polarized cells to migrate and infiltrate target tissues. CD103 (integrin αE), which has been involved in tissue-restricted, anticancer cytotoxic responses, was relatively higher in the Th17 population. Consistent with flow cytometry, the mRNA levels for L-selectin (CD62L), important for the ability to migrate into lymph nodes, was predominant in Th1-polarized lymphocytes. Among the metalloproteinases genes, MMP-13 (collagenase-3) was the most overrepresented in Th1 cells, and MMP-19, a potent basement membrane–degrading enzyme, was the most active in Th17-skewed cells.
Thus, we concluded that in vitro culture conditions greatly modified the biology of the effector T cells. Each Th cell subset not only displayed dissimilar polarization-defining cytokine profiles as tested by ELISA and microarray, but also expressed different chemokines, surface phenotype, and adhesion molecules.
Th17-polarized TRP-1–specific T cells mediate highly efficient treatment of large established tumor
To determine whether the striking differences that we observed in vitro would translate into different efficacy after adoptive transfer in vivo, we treated B16-bearing C57BL/6 mice with adoptively transferred Th0, Th1, or Th17 cells. To mimic a clinically relevant scenario, we allowed the tumor to grow for 10 to 12 days before treatment. Surprisingly, only Th17-skewed cells mediated a significant (P = .001 vs Th0- and Th1-treated groups) tumor regression (Figure 4A) leading to a complete cure and the long-term survival (Figure 4B). In a parallel experiment, we added a potent adjunct regimen consisting of intraperitoneal dose of a recombinant vaccinia vaccine (rVV) encoding TRP-1 peptide given together with IL-2. Without coadministration of specific T cells, this combination does not cause any significant inhibition of melanoma growth. Despite initial tumor shrinkage (Figure 4C), all animals injected with Th0 cells relapsed and eventually had to be killed because of melanoma progression. Similarly, most of the mice treated with Th1 effectors had to be killed because they developed relapsing disease and only a minority survived long-term (Figure 4D). In contrast, animals treated with Th17-polarized TRP-1 T lymphocytes swiftly rejected tumors and remained tumor-free (Figure 4D). Long-term surviving mice developed vitiligo in both Th1- and Th17-treated groups, but the severity of this autoimmune manifestation was far greater in the Th17-treated animals (Figure 4E). Overall, the Th17-polarized TRP-1 CD4+ lymphocytes conferred the most effective response against large B16 tumor upon adoptive cell transfer, and more importantly, they did not require coadministration of exogenous IL-2 or antigen-specific vaccination. This result was unexpected and contrary to our initial speculation that Th1-polarized cells would mediate a more potent antitumor effect, as this subtype was able to secrete the highest quantities of IFN-γ, a molecule classically linked to tumor rejection.
Th17-polarized TRP-1 cells are highly efficient in mediating the rejection of established B16 melanoma tumor upon adoptive cell transfer. (A) C57BL/6 mice B16 tumors that were sublethally irradiated (5 Gy TBI) were left untreated as controls (NT) or received adoptive transfer of 1 × 106 Th0-, Th1-, or Th17-polarized TRP-1 T cells. Th17-treated animals displayed a statistically significant greater tumor regression compared with other groups (P = .001 vs Th0- and Th1-treated groups) that were not different from NT group (P > .05). Results for tumor area are the mean of measurements from at least 5 mice per group (± SEM). Data shown are representative of multiple independent experiments. (B) Percentage of animals alive following treatment described in panel A (n = 5-7, Th1 vs Th17 P < .001). (C) C57BL/6 mice B16 tumors were sublethally irradiated and left untreated as control (NT) or received adoptive transfer of 1 × 106 Th0-, Th1-, or Th17-polarized TRP-1 T cells. In addition, mice received intravenous dose of recombinant TRP-1 vaccinia virus vaccine (rVV) immediately following cell transfer. IL-2 (36 ng/dose) was injected intraperitoneally twice daily for 3 days. Statistically significant tumor regression compared with NT group was observed in all treatment groups. The group treated with Th17 cells had a response significantly better than the Th0-treated group (P < .01), while there was no statistical difference between Th1- and Th17-injected groups (P = .175, n = 5). (D) Percentage of animals alive following treatment described in panel C (combined data from 2 independent experiments (n = 7–14, Th1 vs Th17; P < .001). (E) Animals surviving treatment with Th1 TRP-1 cells, rVV TRP-1 vaccine, and IL-2 developed less vitiligo than mice treated with Th17 TRP-1 cells, vaccination, and IL-2. Vitiligo score: 0 indicates no vitiligo (wild type); 1, depigmentation detected; 2, more than 10% vitiligo; 3, more than 25% vitiligo; 4, more than 50% vitiligo; and 5, more than 75% vitiligo. Evaluation was performed approximately 3 to 4 months after adoptive cell transfer.
Th17-polarized TRP-1 cells are highly efficient in mediating the rejection of established B16 melanoma tumor upon adoptive cell transfer. (A) C57BL/6 mice B16 tumors that were sublethally irradiated (5 Gy TBI) were left untreated as controls (NT) or received adoptive transfer of 1 × 106 Th0-, Th1-, or Th17-polarized TRP-1 T cells. Th17-treated animals displayed a statistically significant greater tumor regression compared with other groups (P = .001 vs Th0- and Th1-treated groups) that were not different from NT group (P > .05). Results for tumor area are the mean of measurements from at least 5 mice per group (± SEM). Data shown are representative of multiple independent experiments. (B) Percentage of animals alive following treatment described in panel A (n = 5-7, Th1 vs Th17 P < .001). (C) C57BL/6 mice B16 tumors were sublethally irradiated and left untreated as control (NT) or received adoptive transfer of 1 × 106 Th0-, Th1-, or Th17-polarized TRP-1 T cells. In addition, mice received intravenous dose of recombinant TRP-1 vaccinia virus vaccine (rVV) immediately following cell transfer. IL-2 (36 ng/dose) was injected intraperitoneally twice daily for 3 days. Statistically significant tumor regression compared with NT group was observed in all treatment groups. The group treated with Th17 cells had a response significantly better than the Th0-treated group (P < .01), while there was no statistical difference between Th1- and Th17-injected groups (P = .175, n = 5). (D) Percentage of animals alive following treatment described in panel C (combined data from 2 independent experiments (n = 7–14, Th1 vs Th17; P < .001). (E) Animals surviving treatment with Th1 TRP-1 cells, rVV TRP-1 vaccine, and IL-2 developed less vitiligo than mice treated with Th17 TRP-1 cells, vaccination, and IL-2. Vitiligo score: 0 indicates no vitiligo (wild type); 1, depigmentation detected; 2, more than 10% vitiligo; 3, more than 25% vitiligo; 4, more than 50% vitiligo; and 5, more than 75% vitiligo. Evaluation was performed approximately 3 to 4 months after adoptive cell transfer.
Th17-polarized TRP-1 cells have a survival advantage after adoptive transfer into tumor-bearing hosts
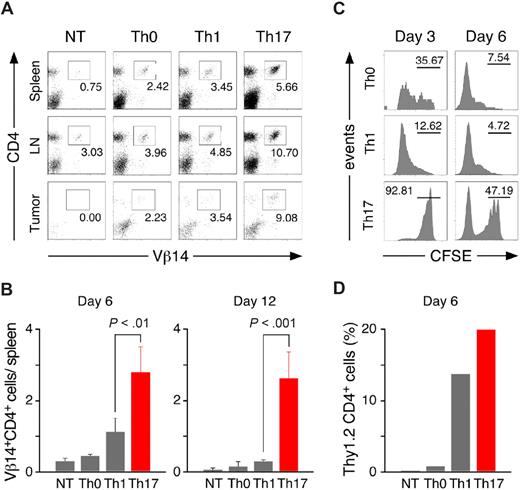
To better understand the differences in treatment outcomes between the groups, we analyzed spleens, lymph nodes, and tumors 6 and 12 days after treatment for the presence of adoptively transferred effector cells. Flow cytometry revealed the highest frequency of Vβ14+ CD4+ T cells in organs harvested from the Th17 group (Figure 5A). The absolute numbers of Vβ14+ CD4+ splenocytes recovered after transfer from Th17-treated animals were consistently the highest, indicating persistence and/or proliferation advantage of cells polarized with TGF-β and IL-6 over the other subtypes (Figure 5B). In animals treated with Th0 or Th1 cells, the numbers of Vβ14+ CD4+ T cells recovered on day 12 were at the level of the background found in untreated C57BL/6 animals. Even more pronounced differences in the persistence of Th0-, Th1-, and Th17-skewed TRP-1 cells were observed after transfer into tumor-bearing RAG1−/− mice devoid of other T cells with strikingly high frequency of Vβ14+ CD4+ cells in the tumor-draining lymph nodes (Figure S5).
Th17-polarized TRP-1 cells have a survival advantage after adoptive transfer into tumor-bearing hosts. (A) Spleens, lymph nodes, and tumors were harvested on day 6 from nontreated animals (NT) or animals treated with Th0, Th1, or Th17 cells and analyzed by flow cytometry for expression of Vβ14 and CD4. The panel is representative of 4 distinct experiments. (B) The total number of Vβ14+CD4+ cells recovered from spleens of treated animals on days 6 and 12 was calculated as described in “Methods” (± SD, n = 3-4). (C) In vivo proliferation of polarized TRP-1 cells. CFSE-labeled Th0, Th1, or Th17 (Thy1.2+) cells were adoptively transferred into 500 R irradiated B6.PL hosts (Thy1.1+). Splenocytes were harvested on days 3 and day 6 and analyzed by flow cytometry. Histograms show CFSE fluorescence after gating on Thy1.2+ population. Th0 and Th1 displayed a greater dilution of the florescent dye compared with Th17 cells. (D) Polarized TRP-1 T cells (Thy1.2+) were transferred into sublethally irradiated tumor-bearing B6.PL (Thy1.1+) hosts. The frequency of Th1.2+CD4+ cells in tumor-draining lymph nodes pooled from at least 3 animals/group was measured by flow cytometry 6 days after adoptive cell transfer.
Th17-polarized TRP-1 cells have a survival advantage after adoptive transfer into tumor-bearing hosts. (A) Spleens, lymph nodes, and tumors were harvested on day 6 from nontreated animals (NT) or animals treated with Th0, Th1, or Th17 cells and analyzed by flow cytometry for expression of Vβ14 and CD4. The panel is representative of 4 distinct experiments. (B) The total number of Vβ14+CD4+ cells recovered from spleens of treated animals on days 6 and 12 was calculated as described in “Methods” (± SD, n = 3-4). (C) In vivo proliferation of polarized TRP-1 cells. CFSE-labeled Th0, Th1, or Th17 (Thy1.2+) cells were adoptively transferred into 500 R irradiated B6.PL hosts (Thy1.1+). Splenocytes were harvested on days 3 and day 6 and analyzed by flow cytometry. Histograms show CFSE fluorescence after gating on Thy1.2+ population. Th0 and Th1 displayed a greater dilution of the florescent dye compared with Th17 cells. (D) Polarized TRP-1 T cells (Thy1.2+) were transferred into sublethally irradiated tumor-bearing B6.PL (Thy1.1+) hosts. The frequency of Th1.2+CD4+ cells in tumor-draining lymph nodes pooled from at least 3 animals/group was measured by flow cytometry 6 days after adoptive cell transfer.
To further evaluate this phenomenon, we transferred CFSE-labeled polarized TRP-1 cells (Thy 1.2) into B6.PL hosts (Thy1.1). By the third day, most of the Th1 TRP-1 cells found in the spleens have already divided as evidenced by a decrease in their fluorescence intensity, while a majority of Th17-polarized cells retained CFSE labeling (Figure 5C). This difference was even more pronounced by the sixth day, when almost the entire Th1 population was CFSElow. In contrast, Th17 cells retained more fluorescent dye, suggesting that their enhanced ability to persist was due to a survival advantage rather than to increased proliferation. Moreover, the frequency of Thy1.2 cells recovered from the tumor-draining lymph nodes was higher in mice treated with Th17-skewed lymphocytes than in other groups (Figure 5D). Therefore, improved persistence might be one of the key reasons for better in vivo antitumor efficacy of transferred Th17 population, and the failure of Th1 to control the disease might be due to inability to persist and expand despite the higher ability to secrete IFN-γ in vitro.
Tumor rejection by Th17-polarized TRP-1 T cells is critically dependent on IFN-γ
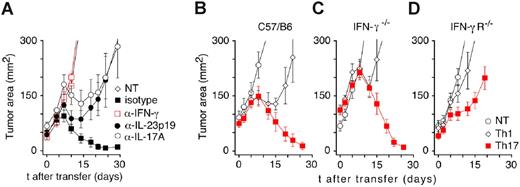
Because the Th17-polarized TRP-1 cells were the most effective in mediating tumor rejection, we hypothesized that proinflammatory IL-17A might be an important factor in this process. To test this assumption, we treated animals with Th17 T cells and subsequently injected them with neutralizing anti–IL-17A antibodies. In the same experiments, other groups received monoclonal neutralizing antibodies against IFN-γ or IL-23, which is known to support the survival of Th17 T lymphocytes. Unexpectedly, tumor rejection was completely inhibited only by anti–IFN-γ treatment. The effect of in vivo neutralization of IL-17A and IL-23 (Figure 6A) did not reach statistical significance (P > .05 vs Th17 isotype control). In other experiments, even the combination of anti–IL-17A and anti–IL-23 neutralizing antibodies did not consistently impair the treatment efficacy of Th17-skewed TRP-1 cells (data not shown).
Th17-polarized TRP-1 CD4+ T cells reject tumor in an IFN-γ–dependent mechanism. (A) In vivo neutralization of cytokines after adoptive transfer of Th17 TRP-1 cells. Sublethally irradiated (500 R) tumor-bearing C57BL/6 mice were treated with 1 × 106 Th17 TRP-1 cells and injected intraperitoneally every other day with 100 μg neutralizing antibodies directed against IFN-γ, IL-17A, IL-23, or isotype control antibody. Results for tumor area are the mean of measurements from at least 5 mice per group (± SEM). Data shown are representative of 3 independent experiments (NT vs Th17, P = .003; NT vs Th17 anti–IFN-γ, P = .558; Th17 isotype vs Th17 anti–IL-17A, P = .117; Th17 isotype vs Th17 anti–IL-23, P = .754). (B) Tumor-bearing C57BL/6, (C) IFN-γ−/−, and (D) IFN-γ receptor–deficient (IFN-γR−/−) mice were treated with 1 × 106 Th1- and Th17-polarized TRP-1 CD4+ T cells. Tumor growth was measured as described previously (± SEM, n = 5-8; NT vs Th17, P < .001 in C57/BL6 hosts, P < .05 in IFN-γ−/− hosts, and P < .05 in IFN-γR−/− hosts).
Th17-polarized TRP-1 CD4+ T cells reject tumor in an IFN-γ–dependent mechanism. (A) In vivo neutralization of cytokines after adoptive transfer of Th17 TRP-1 cells. Sublethally irradiated (500 R) tumor-bearing C57BL/6 mice were treated with 1 × 106 Th17 TRP-1 cells and injected intraperitoneally every other day with 100 μg neutralizing antibodies directed against IFN-γ, IL-17A, IL-23, or isotype control antibody. Results for tumor area are the mean of measurements from at least 5 mice per group (± SEM). Data shown are representative of 3 independent experiments (NT vs Th17, P = .003; NT vs Th17 anti–IFN-γ, P = .558; Th17 isotype vs Th17 anti–IL-17A, P = .117; Th17 isotype vs Th17 anti–IL-23, P = .754). (B) Tumor-bearing C57BL/6, (C) IFN-γ−/−, and (D) IFN-γ receptor–deficient (IFN-γR−/−) mice were treated with 1 × 106 Th1- and Th17-polarized TRP-1 CD4+ T cells. Tumor growth was measured as described previously (± SEM, n = 5-8; NT vs Th17, P < .001 in C57/BL6 hosts, P < .05 in IFN-γ−/− hosts, and P < .05 in IFN-γR−/− hosts).
To further evaluate the role of IFN-γ in our model, we compared treatment outcomes in tumor-bearing wild-type (C57BL/6), IFN-γ deficient (IFN-γ−/−) mice, and IFN-γ receptor–deficient (IFN-γR−/−) animals. Therapy with Th17-polarized cells was equally effective in both WT (C57BL/6) and IFN-γ−/− hosts (Figure 6B-C), while tumor growth in IFN-γR−/− mice was only minimally delayed, and all treated animals were killed due to the progressive disease (Figure 6E). The latter findings suggested that the IFN-γ secreted by transferred cells was sufficient to mediate treatment, as long as the tumor-bearing host was sensitive to IFN-γ.
Discussion
This paper describes a new model for the treatment of large poorly immunogenic melanoma based on the adoptive transfer of TCR transgenic CD4+ T cells specific for the shared self-/tumor antigen TRP1. Using this murine model, we assessed the therapeutic efficacy of various CD4+ T cell subsets—specifically Th1, Th17, and Th0—generated in vitro under well-established polarizing conditions.
Th1 cells are considered the most important CD4+ T-cell subset for tumor rejection because of their marked ability to release IFN-γ and to orchestrate type 1 innate and adaptive responses. IFN-γ is well established as a key factor determining rejection of solid tumors and is important for cytotoxic function of CD8+ T cells.60 It has direct proapoptotic and antiangiogenic effects, activates innate immunity, and up-regulates expression of MHC molecules on the tumor increasing their immunogenicity and susceptibility to immune-mediated lysis.23,61,62 In the case of the B16 melanoma, IFN-γ potently induces MHC class I and class II molecules, which are normally expressed at very low levels.61 Despite robust IFN-γ production, Th1-polarized TRP-1 cells were found to be less effective than Th17-skewed population in eliciting tumor rejection in our model. Surprisingly and somehow counterintuitively, we found that Th17-mediated tumor responses were highly dependent on IFN-γ–based mechanisms. Indeed, the effects of Th17-polarized cells were completely abrogated by the administration of IFN-γ–depleting antibodies.
The tumor-eradicating population we describe in the present paper is highly skewed toward Th17 cells. This is evidenced by the specific release of IL-17A, as well as other cytokines characteristic of Th17 including IL-21, IL-17F, IL-22, and CCL20. It should be noted that programming CD4+ T cells under the influences of IL-6 and TGF-β did not completely abolish the capacity to secrete IFN-γ by CD4+ T cells in our experiments, and the cells described here have some degree of heterogeneity. This is in accordance with other reports describing Th17 polarization.63-66 It is not clear whether the production of IFN-γ by Th17-skewed cells is part of the evolution of the T-cell subset in vivo or whether IFN-γ is produced by a preformed Th1-like component that subsequently expands and causes tumor rejection. What is clear is that polarization toward high IFN-γ–producing Th1-skewed T cells is less effective in this tumor treatment model. The experiment that would rigorously test the activities of pure IL-17–producing cells within the Th17-polarized population would likely involve a knockin mouse with a fusion protein composed of IL-17 and a fluorescent protein, such as those mice bred by Rudensky and colleagues (Fontenot et al67 ) for FoxP3 to identify living Tregs. Such a mouse would enable us to sort viable IL-17–producing cells for in vitro and in vivo experiments. Some clarity could theoretically be also brought to our model using ROR-γt– or T-bet–deficient effector cells, which by default are not able to polarize in a certain directions. Unfortunately, the genetic knockout approach not only eliminates any potential for plasticity in the polarized population, but more importantly can be associated with severe developmental abnormalities of the immune system (eg, ROR-γt deficiency).68,69 Moreover, initial efforts in the clinical/translational setting are likely to use polarized cells, which will likely be composed of an admixture of Th subtypes capable of evolving after transfer into the host.
The roles of Th1 and Th17 cells in mouse models of autoimmune disease have recently been reviewed.33 The genetic elimination of Th1-defining transcription factor T-bet appears to be protective against the development of autoimmunity in some models involving Th17 responses,70-73 while in some other models removal of this transcription factor exacerbated end-organ damage.74 In one report, in vivo silencing of T-bet caused inhibition of both Th1 and Th17 pathogenic cells, the latter due to down-regulation of IL-23 receptor.71 Furthermore, overexpression of IL-23 in a mouse tumor model led to the generation of CD4-dependent antitumor responses involving both IL-17 and IFN-γ.33,75 Emerging data indicate that Th17-mediated responses might undergo evolution, with the initial phase dominated by proinflammatory IL-17 secretion followed by a gradual increase of IFN-γ production.65,76 Moreover, recent reports indicate that various cytokines (IL-1α, IL-21, IL-23) might be used for generating Th17 cells in vitro,58,77-80 and the resulting populations might vary in their biologic properties, plasticity, and the ability to secrete cytokines, including IL-10 or IFN-γ. Further studies using the TRP-1 model described in the present paper might enable the delineation of the molecular components of Th1 and Th17 antitumor immunity.
Analysis of the expansion of adoptively transferred cells clearly showed that Th17-skewed population proliferated less intensely than Th1 cells, but Th17-programmed TRP-1 cells likely had a survival advantage as they were consistently recovered in larger numbers. Some publications have demonstrated that RORγt, a key transcription factor of Th17 differentiation,66 elicits an antiproliferative and antiapoptotic influence in thymocytes via bclXL up-regulation.68,69,81,82 Conversely, T-bet might impair the long-term persistence of T cells, while promoting their short-term proliferation ability.83 Another factor potentially contributing to the increased survival and functionality of Th17 population is their ability to consistently secrete higher quantities of IL-2, possibly making their therapeutic effect independent from the administration of exogenous cytokines. Th1-skewed T cells might be susceptible to self-suppression by high quantities of secreted IFN-γ and IL-10, which down-regulate type 1–related responses in an autocrine and paracrine negative feedback loop that limits excessive tissue damage during immune response against pathogens.84,85 Even though IL-10 had been initially described as a primarily Th2-type cytokine, it is now clear that it is produced by many types of cells (dendritic cells [DCs], macrophages, B cells, CD4+ and CD8+ T cells). Indeed, IL-12–induced Th1 lymphocytes may be the major source of IL-10 in vivo,86 and neutralization of IL-10 might be one of the strategies leading to improvement in efficacy of adoptive cell transfer therapy.
In addition to enhanced persistence, other features of Th17 cells may be responsible for their superior anticancer activity. These characteristics include alterations in trafficking patterns, as suggested by the expression of adhesion molecules and MMPs, as well as the ability to produce multiple other homeostatic and proinflammatory cytokines or chemokines, not evaluated in our work, such as IL-9, IL-17F, IL-21, CCL20 (MIP3α), or IL-22. Interestingly, IL-22 has been implied in mediating pathological changes in psoriasis,87 but in some other models it has been nonessential or even associated with protective effect against the autoimmune end-organ damage.88,89 The function of each of these factors in immunity against cancer remains to be elucidated.
The role of polarization-defining IL-17A also remains unclear in our model. IL-17A has been thought to impair immune surveillance and promote tumor growth by several mechanisms,42,43,90 and Th17 cells have been detected in both murine and human cancers.37,38 We did not observe tumor growth acceleration after transfer of large numbers of Th17-polarized cells with defined tumor specificity. On the other hand, our attempts at depleting IL-17A as well as IL-23 in vivo yielded only partial and inconsistent results, perhaps due to incomplete effect of the monoclonal antibodies used. IL-17A might deliver a secondary or redundant proinflammatory stimulus or might be a mere cytokine marker of cells programmed with TGF-β and IL-6, which acquire a distinct set of biologic abilities, leading to a greater antitumor potential. It is also quite possible that cancer-promoting and antitumor effects of proinflammatory environment may not necessarily be mutually exclusive, but perhaps might depend on the timing and context as clearly shown in the work of Kryczek et al where dynamic changes between Th17 and Foxp3+ T cells were found in the tumor microenvironment.38
In summary, we have demonstrated Th-mediated treatment of large, established solid tumors, where the target is an unmodified self-/tissue differentiation antigen relevant to human disease. For the first time, we compared the therapeutic potential of Th0-, Th1-, and Th17-polarized effectors and unexpectedly found the Th17-skewed cells to be the most effective, although Th1 cells secreted the highest quantities of IFN-γ, a molecule that was indispensable in this model. Our findings indicate that tumor-specific CD4+ T cells might be strikingly efficient in mediating the anticancer effect in the absence of antigen-specific vaccination and exogenous administration of IL-2. Importantly, the in vitro programming conditions were crucial for the successful function of the effector cells after adoptive transfer into tumor-bearing host. It is likely that our observations are relevant not only to solid tumors, but to hematologic malignancies as well. Clinical trials comparing various subtypes of tumor-specific T cells can be conducted using lymphocytes genetically engineered to express the appropriate TCR or chimeric receptor. Therefore, proper polarization might be of particular importance for the design of adoptive cell transfer–based immunotherapies of human tumors, where the requirements for the generation of Th17 cells have recently been elucidated.91-93
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Drs John J. O'Shea and Arian Laurence from the National Institute for Arthritis, Musculoskeletal and Skin Diseases (NIAMS, NIH, Bethesda, MD) for carefully reading the paper and Dr Peter Cresswell (Yale University School of Medicine, New Haven, CT) for providing a plasmid encoding the mouse MHC class II transactivator (CIITA).
D.C.P. is a PhD candidate at George Washington University and this work is submitted in partial fulfillment of the requirement for the PhD.
National Institutes of Health
Authorship
Contribution: P.M. and A.B. wrote the paper and designed and performed experiments; K.R.I. generated TRP-1 hybridoma and identified the epitope; P.A.A. generated TRP-1 transgenic mice and performed experiments; L.C., A.K., L.G., C.M.P., C.H., D.C.P., C.E.T., K.P., C.W., K.K., L.F., and C.-C.C. helped with experiments and writing the paper; N.P.R. supervised the research design and the writing of the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Pawel Muranski or Nicholas P. Restifo, National Cancer Institute, National Institutes of Health, Bethesda, MD 20892; e-mail: muranskp@mail.nih.gov or restifo@nih.gov.
References
Author notes
*P.M., A.B., and P.A.A. contributed equally to this work.

![Figure 3. In vitro–polarized effector CD4+ T-cell subsets acquire distinct phenotypes and gene expression profiles. (A) In vitro polarizing conditions alter the phenotype of TRP-1 cells. Th0, Th1, and Th17 TRP-1 T cell were analyzed using flow cytometry for the expression of selected activation markers and adhesion molecules: CD62L, CD45RB, SCA1 (Ly6a), CD38, and CD49d (integrin α4, VLA-4). Percentage of positive cells is calculated based on the comparison with an isotype control antibody. (B) Relative alterations in mRNA quantities of selected genes are observed using microarray analysis. Th1 versus Th17 TRP-1 cells were compared, using Th0 mRNA as a reference. mRNAs are grouped by their function (integrins and adhesion molecules, matrix metalloproteinases [MMPs] and related molecules, cytokines and their receptors, chemokines, and chemokine receptors). A cutoff for significant difference in the level of mRNA message expression was set at 2-fold.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/112/2/10.1182_blood-2007-11-120998/4/m_zh80110819970003.jpeg?Expires=1769079611&Signature=kwoe2cbsEhbdY9tNKdQRWvqdupY7ytz0G731IRNL3LricVhP4X~d3kQuLYY~jbudQhdnVdT~TSa81p-eOplOMP-lw~D9vZTPOCQ3AqjgOF9NsO5vNtr7C8LOdYkgPS5EkQQ646iBOlm4ejbbRQ145m4nRvYZByvLWVuXcD8abLR967YcgLGyJk4b1BGUM6tMvRxjZPCPQu8o4XEvflkcnEFxLI8ZgjJc2rP1f9d7lpnPF4A7KvWAppxMvjSaMqUJVxYR~h3Y2sMRePM2Uen5mZDUpWKC3ukyiaPr1XqX2L01tMU080maRSgxee~xTjIZ7MlUXF2Xg49QRCyi~JWxKw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)