Abstract
Natural killer (NK)–cell alloreactivity can be exploited in haploidentical hematopoietic stem cell transplantation (HSCT). NK cells from donors whose HLA type includes Bw4, a public epitope present on a subset of HLA-B alleles, can be alloreactive toward recipients whose cells lack Bw4. Serologically detectable epitopes related to Bw4 also exist on a subset of HLA-A alleles, but the interaction of these alleles with KIR3DL1 is controversial. We therefore undertook a systematic analysis of the ability of most common HLA-B alleles and HLA-A alleles with Bw4 serologic reactivity to protect target cells from lysis by KIR3DL1-dependent NK cells. All Bw4− HLA-B alleles failed to protect target cells from lysis. All Bw4+ HLA-B alleles with the exception of HLA-B*1301 and -B*1302 protected targets from lysis. HLA-A*2402 and HLA-A*3201 unequivocally protected target cells from lysis, whereas HLA-A*2501 and HLA-A*2301 provided only weak protection from lysis. KIR3DL1-dependent alloreactive NK clones were identified in donors with HLA-A*2402 but not in donors with HLA-B*1301 or -B*1302. These findings clarify the HLA types that donors and recipients need in haploidentical HSCT and other NK allotherapies in order to benefit from NK alloreactivity.
Introduction
Natural killer (NK)–cell alloreactivity can be exploited in haploidentical hematopoietic stem cell transplantation (HSCT) to improve graft survival, reduce graft-versus-host disease and decrease leukemic relapse.1 Infusion of alloreactive NK cells without stem cells has also been shown to result in hematologic remission in patients with acute myeloid leukemia (AML).2 NK cells lyse cells that have reduced expression of class I HLA molecules. In an allogeneic setting, donor NK cells are activated by the absence of donor (self) class I HLA molecules on recipient cells3,4 ; the absence of self-epitopes are detected by inhibitory killer immunoglobulin-like receptors (KIRs) on donor NK cells. HLA-C molecules with an asparagine at amino acid 80 provide the C1 epitope for the KIR2DL2 and KIR2DL3 inhibitory receptors and those with a lysine at amino acid 80 provide the self-epitope, C2, for the KIR2DL1 inhibitory receptor. The self-epitope of relevance to the KIR3DL1 receptor is the Bw4 epitope at amino acids 77 to 83 on some HLA-B alleles. All HLA-B alleles have either the Bw4 or the Bw6 epitope. NK cells from donors whose HLA-B alleles include the Bw4 epitope lyse cells lacking the Bw4 epitope because such target cells cannot supply the ligand for the NK inhibitory receptor KIR3DL1. However, it has been reported (reviewed in Ruggeri et al5 ) that only two-thirds of donors with Bw4+ HLA-B alleles make NK clones that lyse Bw4− targets. Therefore, selecting an NK or stem cell donor for a particular patient currently requires a lengthy, labor-intensive in vitro NK cloning procedure to confirm the donor has NK cells alloreactive toward the patient.1
It is not clear why some Bw4+ donors cannot make NK clones that lyse Bw4− targets. A likely explanation in some cases is that a common allele of KIR3DL1 (KIR3DL1*004) is not expressed at the cell surface.6 However, a systematic examination of the ability of HLA-B alleles to bind to KIR3DL1 has not been undertaken. Amino acid 80 in the HLA-B protein critically determines binding to KIR3DL1.7 Alleles with the Bw6 epitope have asparagine (N) at this position and do not bind to KIR3DL1, while alleles with Bw4 have either an isoleucine (I) or threonine (T) and are ligands for KIR3DL1. It has been reported that alleles with an isoleucine (80I) are stronger ligands than alleles with a threonine (80T),8,9 while other investigators have noted differences within the Bw4 80I family of alleles10 and peptide-dependent differences in the binding of Bw4 tetramers to different KIR3DL1 variants.11 Several HLA-A alleles are also known to react with anti-Bw4 antibodies, but their ability to behave as a ligand for KIR3DL1 is controversial.8,10,11 These studies used HLA-bare Epstein-Barr virus (EBV) cell lines transfected with individual HLA alleles or tetramers loaded with individual peptides to compare the effect of different alleles. A related question that has not been addressed is whether donors with the different forms of Bw4 epitope are all capable of producing NK clones that lyse cells lacking Bw4.
To improve the ability to select donors for haploidentical HSCT or NK-cell infusions based on HLA rather than NK cloning assays, we undertook a systematic analysis of the ability of common HLA-B alleles and Bw4+ HLA-A alleles to inhibit KIR3DL1-dependent NK clones. In addition, we tested the ability of individuals with Bw4+ HLA-A alleles to produce KIR3DL1-dependent clones. Because it is difficult to know whether results from HLA-bare cells transfected with single HLA alleles, or tetramers loaded with single peptides, accurately reflect the complex array of HLA ligands presented to NK cells (in vivo), we chose to use EBV cell lines that express a normal array of HLA antigens but which were homozygous for the HLA alleles of interest. Our results confirm that most, but not all, of the common Bw4+ HLA-B alleles are ligands for KIR3DL1. In addition, the status of the Bw4+ HLA-A alleles is clarified, and we demonstrate that individuals with a Bw4+ HLA-A allele are able to generate KIR3DL1-dependent clones. These results have implications for donor selection for haploidentical HSCT.
Methods
NK cells
Blood was obtained from laboratory volunteers and blood donors from The Australian Red Cross Blood Service, Western Australia, with informed consent obtained in accordance with the Declaration of Helsinki. Written approval to use blood samples was obtained from the Ethics Committee of Royal Perth Hospital. HLA typing was performed by DNA sequencing (Table 1). NK cells were purified by ficoll centrifugation with RosetteSep (StemCell Technologies, Vancouver, BC). Purified NK cells were plated at limiting dilutions into 96-well round-bottom plates in NK medium (RPMI-1640, 10% fetal calf serum [FCS], 0.1 mM modified Eagle medium [MEM], nonessential amino acids, and 1 mM sodium pyruvate; Invitrogen, Carlsbad, CA). Irradiated, allogeneic, ficoll-separated peripheral blood mononuclear cell (PBMC) feeder cells (pooled from 10 donors) were added to each well at a concentration of 105 cells/100 μL and cultured at 37°C in 5% CO2. A total of 100 μL of medium was replaced with fresh NK medium containing 400 IU/mL IL-2 (Chiron, Emeryville, CA) on day 1, with irradiated PBMC feeder cells added to each well at a concentration of 8 × 104 cells/100 μL on day 4, and with fresh NK medium containing 200 IU/mL IL-2 on day 11. After day 13, cell growth was monitored, cells were split if necessary, and cells were fed every 3 to 4 days with 105/mL irradiated RPMI-8866 cells.
HLA class I and KIR3DL1 allele typing of NK-cell donors
| Donor . | HLA-A . | HLA-B . | Bw4/6 . | HLA-C . | C1/2 . | KIR3DL1 typing . | KIR3DL1 expression . |
|---|---|---|---|---|---|---|---|
| 1 | 0201, 2902 | 4403, 5703 | 4 | 0701, 1601 | 1 | 01501/017, 008 | High |
| 2 | 0201, 2601 | 0702, 3801 | 4,6 | 0702, 1203 | 1 | 002, 00101 | High |
| 3 | 0101, 2402 | 0801, 5109 | 4,6 | 0102, 0701 | 1 | 00501-like, 01502 | High, low |
| 4 | 0201, 3303 | 4601, 5801 | 4,6 | 0102, 0302 | 1 | 00501, 01502 | High, low |
| 5 | 1101 | 1301, 1501 | 4,6 | 0304, 0401 | 1,2 | 007, 01502 | High, low |
| 6 | 1101 | 1301, 1501 | 4,6 | 0304, 0401 | 1,2 | 00501, 007 | Low |
| 7 | 0301, 3101 | 1302, 3501 | 4,6 | 0401, 0602 | 2 | 001/016, 002 | High |
| 8 | 0101, 0301 | 0801 | 6 | 0701 | 1 | NT | High |
| 9 | 0201, 1101 | 1501, 3501 | 6 | 0303, 0401 | 1,2 | NT | Low |
| 10 | 0101, 2402 | 0801, 5501 | 6 | 0303, 0701 | 1 | NT | High |
| Donor . | HLA-A . | HLA-B . | Bw4/6 . | HLA-C . | C1/2 . | KIR3DL1 typing . | KIR3DL1 expression . |
|---|---|---|---|---|---|---|---|
| 1 | 0201, 2902 | 4403, 5703 | 4 | 0701, 1601 | 1 | 01501/017, 008 | High |
| 2 | 0201, 2601 | 0702, 3801 | 4,6 | 0702, 1203 | 1 | 002, 00101 | High |
| 3 | 0101, 2402 | 0801, 5109 | 4,6 | 0102, 0701 | 1 | 00501-like, 01502 | High, low |
| 4 | 0201, 3303 | 4601, 5801 | 4,6 | 0102, 0302 | 1 | 00501, 01502 | High, low |
| 5 | 1101 | 1301, 1501 | 4,6 | 0304, 0401 | 1,2 | 007, 01502 | High, low |
| 6 | 1101 | 1301, 1501 | 4,6 | 0304, 0401 | 1,2 | 00501, 007 | Low |
| 7 | 0301, 3101 | 1302, 3501 | 4,6 | 0401, 0602 | 2 | 001/016, 002 | High |
| 8 | 0101, 0301 | 0801 | 6 | 0701 | 1 | NT | High |
| 9 | 0201, 1101 | 1501, 3501 | 6 | 0303, 0401 | 1,2 | NT | Low |
| 10 | 0101, 2402 | 0801, 5501 | 6 | 0303, 0701 | 1 | NT | High |
The KIR3DL1*005-like allele in donor 3 differs from KIR3DL1*005 by a valine-to-leucine substitution at amino acid 18 in the D0 domain. In all cases, HLA alleles were assigned on the basis of sequencing of exons 2 and 3. In some cases, the assignment of class I HLA alleles is based on the most common allele consistent with the sequence in exons 2 and 3, and alleles differing in other exons cannot be excluded. As the signal peptide of KIR3DL1 was not sequenced, KIR3DL1*01501 cannot be distinguished from KIR3DL1*017 in donor 1, and KIR*001 cannot be distinguished from KIR3DL1*016 in donor 7.
NT indicates not tested.
Purified polyclonal NK cells were expanded by culturing with irradiated allogeneic feeder cells at a 1:10 ratio for 12 days with 200 IU/mL IL-2, replacing the NK medium every 2 to 3 days. Before use in the CD107a assay, NK cells were cultured with irradiated RPMI-8866 at a 1:10 ratio for 7 days with 200 IU/mL IL-2, replacing medium every 2 to 3 days. Alternatively, when determining frequency of KIR3DL1-dependent clones, 12-day culture polyclonal NK cells were cultured at 106/mL for a further 48 hours with 400 IU/mL IL-2 before use in the CD107a assay.
EBV cell lines
EBV-transformed B lymphoblastoid cell lines (BLCLs) were either 10th International Histocompatibility Workshop cells, or generated in-house (Table 2). The 721.221 class I–negative cell line was a gift from J. McCluskey (University of Melbourne, Australia). The RPMI-8866 cell line was obtained from ATCC (Manassas, VA). All cells were cultured in RPMI-1640 (Invitrogen) with 10% heat-inactivated FCS (ThermoTrace, Melbourne, Australia).
HLA class I typing of BLCL target cell panel
| ID . | Short ID . | HLA-B . | AA 80 . | Bw4/6 . | HLA-A . | HLA-C . | C1/2 . |
|---|---|---|---|---|---|---|---|
| IHW 9084 | B13 | 1302 | T | 4 | 3001 | 0602 | 2 |
| IHW 9067 | B27 | 2705 | T | 4 | 0201 | 0102 | 1 |
| IHW 9009 | B37 | 3701 | T | 4 | 0101 | 0602 | 2 |
| IHW 9090 | B4402 | 4402 | T | 4 | 0201 | 0501 | 2 |
| IHW 9027 | B4403 | 4403 | T | 4 | 2902 | 1601 | 1 |
| IHW 9047 | B47 | 4701 | T | 4 | 0301 | 0602 | 2 |
| IHW 9062 | B38 | 3801 | I | 4 | 0201 | 1203 | 1 |
| IHW 9040 | B49 | 4901 | I | 4 | 0101 | 0701 | 1 |
| IHW 9016 | B51 | 5101 | I | 4 | 0204 | 1502 | 2 |
| IHW 9011 | B52 | 5201 | I | 4 | 0101 | 1202 | 1 |
| IHW 9010 | B53 | 5301 | I | 4 | 6802 | 0401 | 2 |
| IHW 9052 | B57 | 5701 | I | 4 | 0201 | 0602 | 2 |
| IHW 9157 | B58 | 5801 | I | 4 | 33 | 0302 | 1 |
| IHW 9029 | A23 | 1402 | I | 6 | 2301 | 0802 | 1 |
| IHW 9001 | A24 | 0702 | I | 6 | 2402 | 0702 | 1 |
| IHW 9008 | A25 | 1801 | I | 6 | 2501 | 1203 | 1 |
| Q94 0055016Y | A32 | 1501, 3901 | I | 6,6 | 3201, 0201 | 0303, 1203 | 1,1 |
| IHW 9065 | B07 | 0702 | N | 6 | 0301 | 0702 | 1 |
| IHW 9088 | B08 | 0801 | N | 6 | 0101 | 0701 | 1 |
| IHW 9029 | B14 | 1402 | N | 6 | 2301 | 0802 | 1 |
| IHW 9099 | B15 | 1501 | N | 6 | 0217 | 0303 | 1 |
| IHW 9019 | B18 | 1801 | N | 6 | 3002 | 0501 | 2 |
| IHW 9068 | B35 | 3501 | N | 6 | 0201 | 0401 | 2 |
| Q94 0052722Q | B39 | 0702, 3901 | N | 6,6 | 0201, 0301 | 0702, 1203 | 1,1 |
| IHW 9084 | B40 | 4002 | N | 6 | 0201 | 0202 | 2 |
| IHW 9043 | B41 | 4101 | N | 6 | 0101 | 1701 | 2 |
| IHW 9021 | B42 | 4201 | N | 6 | 3001, 6802 | 1701 | 2 |
| IHW 9058 | B45 | 4501 | N | 6 | 0201 | 1601 | 1 |
| IHW 9076 | B46 | 4601 | N | 6 | 0206, 0207 | 0102, 0801 | 1,1 |
| R98 0903165W | B50 | 5001, 0702 | N | 6,6 | 0301, 0201 | — | — |
| R04 0901000J | B55 | 5502, 0801 | N | 6,6 | 0101, 0206 | 0102, 0701 | 1,1 |
| R97 0330581F | B56 | 5601, 0801 | N | 6,6 | 01, 34 | — | — |
| ID . | Short ID . | HLA-B . | AA 80 . | Bw4/6 . | HLA-A . | HLA-C . | C1/2 . |
|---|---|---|---|---|---|---|---|
| IHW 9084 | B13 | 1302 | T | 4 | 3001 | 0602 | 2 |
| IHW 9067 | B27 | 2705 | T | 4 | 0201 | 0102 | 1 |
| IHW 9009 | B37 | 3701 | T | 4 | 0101 | 0602 | 2 |
| IHW 9090 | B4402 | 4402 | T | 4 | 0201 | 0501 | 2 |
| IHW 9027 | B4403 | 4403 | T | 4 | 2902 | 1601 | 1 |
| IHW 9047 | B47 | 4701 | T | 4 | 0301 | 0602 | 2 |
| IHW 9062 | B38 | 3801 | I | 4 | 0201 | 1203 | 1 |
| IHW 9040 | B49 | 4901 | I | 4 | 0101 | 0701 | 1 |
| IHW 9016 | B51 | 5101 | I | 4 | 0204 | 1502 | 2 |
| IHW 9011 | B52 | 5201 | I | 4 | 0101 | 1202 | 1 |
| IHW 9010 | B53 | 5301 | I | 4 | 6802 | 0401 | 2 |
| IHW 9052 | B57 | 5701 | I | 4 | 0201 | 0602 | 2 |
| IHW 9157 | B58 | 5801 | I | 4 | 33 | 0302 | 1 |
| IHW 9029 | A23 | 1402 | I | 6 | 2301 | 0802 | 1 |
| IHW 9001 | A24 | 0702 | I | 6 | 2402 | 0702 | 1 |
| IHW 9008 | A25 | 1801 | I | 6 | 2501 | 1203 | 1 |
| Q94 0055016Y | A32 | 1501, 3901 | I | 6,6 | 3201, 0201 | 0303, 1203 | 1,1 |
| IHW 9065 | B07 | 0702 | N | 6 | 0301 | 0702 | 1 |
| IHW 9088 | B08 | 0801 | N | 6 | 0101 | 0701 | 1 |
| IHW 9029 | B14 | 1402 | N | 6 | 2301 | 0802 | 1 |
| IHW 9099 | B15 | 1501 | N | 6 | 0217 | 0303 | 1 |
| IHW 9019 | B18 | 1801 | N | 6 | 3002 | 0501 | 2 |
| IHW 9068 | B35 | 3501 | N | 6 | 0201 | 0401 | 2 |
| Q94 0052722Q | B39 | 0702, 3901 | N | 6,6 | 0201, 0301 | 0702, 1203 | 1,1 |
| IHW 9084 | B40 | 4002 | N | 6 | 0201 | 0202 | 2 |
| IHW 9043 | B41 | 4101 | N | 6 | 0101 | 1701 | 2 |
| IHW 9021 | B42 | 4201 | N | 6 | 3001, 6802 | 1701 | 2 |
| IHW 9058 | B45 | 4501 | N | 6 | 0201 | 1601 | 1 |
| IHW 9076 | B46 | 4601 | N | 6 | 0206, 0207 | 0102, 0801 | 1,1 |
| R98 0903165W | B50 | 5001, 0702 | N | 6,6 | 0301, 0201 | — | — |
| R04 0901000J | B55 | 5502, 0801 | N | 6,6 | 0101, 0206 | 0102, 0701 | 1,1 |
| R97 0330581F | B56 | 5601, 0801 | N | 6,6 | 01, 34 | — | — |
HLA types of target cells used in flow cytometric and chromium release assays. In all cases, HLA alleles were assigned on the basis of sequencing of exons 2 and 3. In some cases, the assignment of class I HLA alleles is based on the most common allele consistent with the sequence in exons 2 and 3, and alleles differing in other exons cannot be excluded. Targets that were homozygous for HLA-B50, HLA-B55, and HLA-B56 were not available. Therefore, cells that were heterozygous for these antigens and had a common Bw6 antigen as the second antigen were selected. AA80 indicates the amino acid present at residue 80 of the HLA-B alleles.
— indicates HLA-C typing not performed.
Flow cytometry
KIR expression was identified on NK clones using PE-conjugated antibodies to NKG2A/B, CD158a, CD158b (Beckman Coulter, Fullerton, CA), and KIR3DL1 (BD Biosciences, Franklin Lakes, NJ). Flow cytometry was performed on a BD Biosciences FACSCanto instrument and analyzed using BD FACSDiva Software (BD Biosciences) and FlowJo Software (TreeStar, Ashland, OR). Bw4 or Bw6 expression on BLCL target cells was determined using a Bw4 or Bw6 monoclonal antibody (mAb; gifts from J. McCluskey). FITC-conjugated anti–human IgG (Chemicon, Temecula, CA) was used as the secondary antibody.
51Cr release cytotoxicity assay
NK cell–mediated killing was measured by the standard 4-hour 51Cr release assay12 at an effector-target ratio of 2:1. For blocking experiments, NK cells were preincubated with anti-KIR3DL1 antibody (DX9; BD Biosciences) or IgG1 isotype control (Biolegend, San Diego, CA) at concentrations of 2.5 μg/mL and 10 μg/mL, respectively, for 20 minutes at 37°C. Assays were performed in triplicate and standard errors of the mean were calculated.
CD107a cytotoxicity assay
NK-cell expression of CD107a was used to measure NK cytotoxicity by cultured polyclonal NK cells. These cells were added to BLCL target cells at a 1:1 ratio in a 96-well round-bottom plate. A total of 5 μL of anti-CD107a–FITC (BD Biosciences) was added to each well. After a 1-hour incubation at 37°C, 6 μg/mL monensin (BD GolgiStop; BD Biosciences) was added, and the cells were incubated a further 5 hours. NK cells were then stained with anti-CD56–PECy7, anti-KIR3DL1–PE (BD Biosciences), anti-CD158b–APC, and anti-CD158a–APC (Beckman Coulter) and analyzed by flow cytometry.
KIR3DL1 allele sequencing and KIR genotyping
Results
KIR3DL1 genotype of NK cells
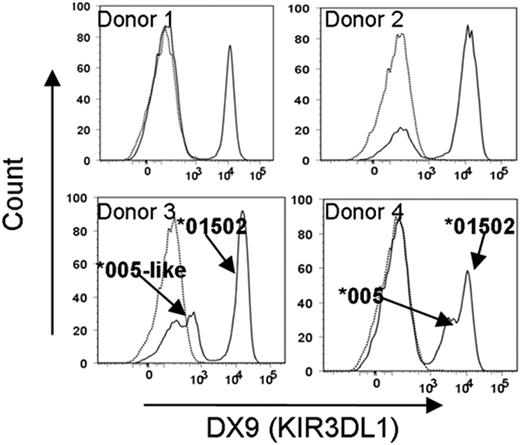
As different alleles of KIR3DL1 have high or low expression at the cell surface, we determined the KIR3DL1 alleles and receptor expression pattern on 10 NK donors (Table 1). To compare the behavior of high- and low-expressing alleles, 2 donors with only high-expression alleles and 2 donors heterozygous for high- and low-expression alleles (donors 1 and 2 and donors 3 and 4, respectively, in Table 1) were selected. In addition, because the high-expressing allele in both donors 3 and 4 was the same (KIR3DL1*01502), between-donor reproducibility could be assessed. All 4 donors had at least one Bw4+ HLA-B allele (Table 1). Interestingly, the low-expression receptors in donors 3 and 4 differed in their level of expression (Figure 1). This difference may be because the low-expression receptor in donor 3 differed from the low-expression receptor encoded by KIR3DL1*005 in donor 4 by a valine to leucine substitution at amino acid 18 in the D0 domain.
Low-level KIR3DL1 expression differs in 2 donors due to a nonsynonymous mutation at nucleotide position 115 in exon 3 encoding the D0 domain of the KIR3DL1*005 allele. NK cells from each donor were stained with anti-KIR3DL1 antibody (DX9; solid lines) or isotype control (IgG1; dotted lines). KIR3DL1 surface expression correlated with KIR3DL1 allele typing, with donors 1 and 2 having unimodal high-level expression and donors 3 and 4 having bimodal (high- and low-level) expression. The low-level receptor expression differed between donors 3 and 4. Comparison of both KIR3DL1 sequences from donors 3 and 4 revealed a single nonsynonymous nucleotide mutation at position 115 of the KIR3DL1*005 allele in donor 3.
Low-level KIR3DL1 expression differs in 2 donors due to a nonsynonymous mutation at nucleotide position 115 in exon 3 encoding the D0 domain of the KIR3DL1*005 allele. NK cells from each donor were stained with anti-KIR3DL1 antibody (DX9; solid lines) or isotype control (IgG1; dotted lines). KIR3DL1 surface expression correlated with KIR3DL1 allele typing, with donors 1 and 2 having unimodal high-level expression and donors 3 and 4 having bimodal (high- and low-level) expression. The low-level receptor expression differed between donors 3 and 4. Comparison of both KIR3DL1 sequences from donors 3 and 4 revealed a single nonsynonymous nucleotide mutation at position 115 of the KIR3DL1*005 allele in donor 3.
KIR3DL1-dependent NK cells lyse cells lacking the Bw4 epitope
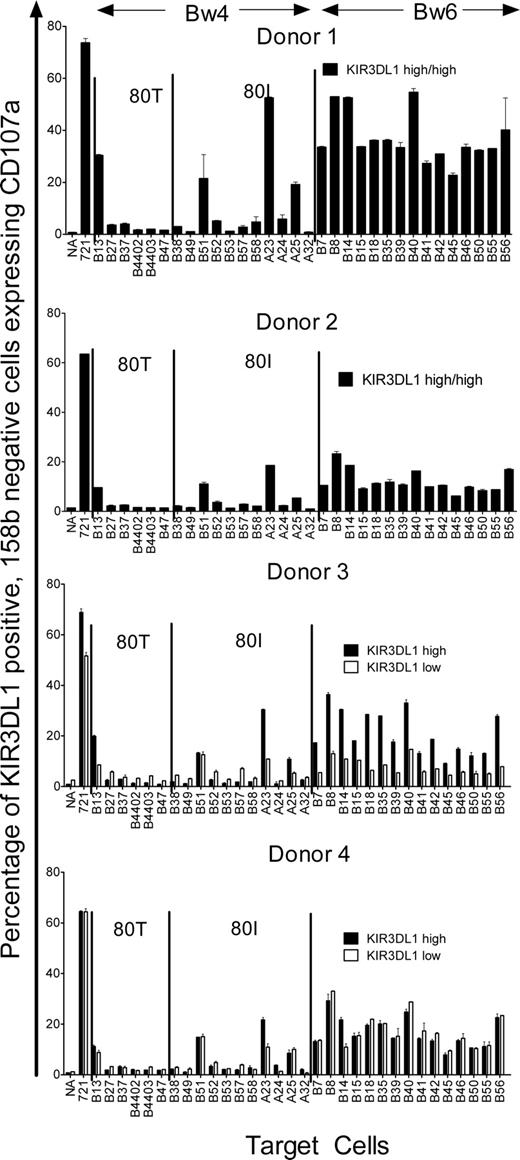
KIR expression on polyclonally expanded NK cells has been demonstrated not to alter the frequency of NK cells expressing particular KIR receptors.9 To detect NK-cell cytotoxicity toward Bw4− targets mediated by KIR3DL1-dependent clones, polyclonal NK cells from donors 1 to 4 were expanded by in vitro culture and then incubated with BLCL targets expressing common HLA-B alleles or the Bw4+ HLA-A alleles A*2301, A*2402, A*2501, and A*3201 (Table 2). Cytotoxicity was measured by CD107a expression on KIR3DL1+ (DX9+), CD158b− (KIR2DL2−, KIR2DL3−, KIR2DS2−) NK cells. Because all 4 NK donors were homozygous for C1-group HLA-C alleles and a significant proportion of KIR3DL1+ NK cells coexpressed CD158b, the inhibitory receptor for the C1-epitope, only KIR3DL1+, CD158b− cells were examined. KIR3DL1+, CD158b− NK cells up-regulated CD107a when incubated with either the class I HLA–negative target (721.221+ control) or targets homozygous for the Bw6 epitope (Figure 2). Bw4+ targets tended to inhibit CD107a expression. Overall, there was little difference between the 4 donors in terms of the target cells that showed greatest inhibition of CD107a expression. CD107a expression was inhibited by all Bw4-expressing targets with 4 exceptions: those expressing HLA-B*1302, HLA-B*5101, HLA-A*2301, and HLA-A*2501. There were some small differences in the ability of the other Bw4-expressing targets to inhibit CD107a expression, but these differences did not relate to the presence of 80I or 80T alleles.
CD107a expression by KIR3DL1+ polyclonal NK cells is inhibited by most Bw4+ targets. Polyclonally expanded NK cells from 4 donors (donors 1-4) were incubated alone (NA), with a positive control, the class I–negative BLCL 721.221 (721), and a range of Bw4+ and Bw6+ targets (Table 2) and CD107a expression were measured on KIR3DL1+, CD158b− NK cells. Donors 3 and 4 had NK cells that showed high or low expression of DX9, and for each, the percentage of CD107a+ cells among either the NK cells bearing the high level (■) or low level (□) of DX9 expression are shown. All donors lysed the 721.221 cell line and all Bw6-homozygous (Bw4−) targets. All donors were inhibited by most of the Bw4+ targets except for targets B13, B51, A23, and A25. For donor 3, the low-level expression allele was less cytotoxic against Bw6-expressing targets and not as well inhibited by most Bw4-expressing targets compared with the high-expression allele. By contrast for donor 4, the high- and low-level expression alleles behaved similarly. Error bars represent SEM.
CD107a expression by KIR3DL1+ polyclonal NK cells is inhibited by most Bw4+ targets. Polyclonally expanded NK cells from 4 donors (donors 1-4) were incubated alone (NA), with a positive control, the class I–negative BLCL 721.221 (721), and a range of Bw4+ and Bw6+ targets (Table 2) and CD107a expression were measured on KIR3DL1+, CD158b− NK cells. Donors 3 and 4 had NK cells that showed high or low expression of DX9, and for each, the percentage of CD107a+ cells among either the NK cells bearing the high level (■) or low level (□) of DX9 expression are shown. All donors lysed the 721.221 cell line and all Bw6-homozygous (Bw4−) targets. All donors were inhibited by most of the Bw4+ targets except for targets B13, B51, A23, and A25. For donor 3, the low-level expression allele was less cytotoxic against Bw6-expressing targets and not as well inhibited by most Bw4-expressing targets compared with the high-expression allele. By contrast for donor 4, the high- and low-level expression alleles behaved similarly. Error bars represent SEM.
Of the 2 donors with high KIR3DL1 expression, donor 1 had a higher percentage of NK cells expressing CD107a in response to Bw4− targets and therefore appeared to better discriminate between Bw4+ and Bw4− targets. However, this may simply reflect the fact that donor 1 had a higher proportion of NK cells dependent on KIR3DL1 for inhibition than did donor 2. The high-expression KIR3DL1 receptor in donors 3 and 4 and the low-expression allele (KIR3DL1*005) in donor 4 behaved in a similar way to NK cells in donor 1. However, in donor 3, NK cells with the low-expression KIR3DL1*005-like variant up-regulated CD107a relatively weakly in response to Bw6 targets and were less completely inhibited by Bw4 targets so that there was relatively poor discrimination between the Bw4 and Bw6 targets. Interestingly, in this donor, those NK cells expressing the KIR3DL1*005-like variant also exhibited less up-regulation of CD107a in response to the class I bare 721.221 cells than the NK cells expressing the KIR3DL1*01502 allele. These results suggest that the poor discrimination between Bw4 and Bw6 targets exhibited by the NK cells expressing the receptor encoded by the KIR3DL1*005-like variant may be due to these cells being relatively weakly armed rather than a smaller proportion being KIR3DL1-dependent for inhibition. Donor 3 was particularly informative in terms of the Bw4 alleles that are KIR3DL1 ligands. For almost all Bw4 targets, NK cells with the high-expression allele KIR3DL1*01502 were more strongly inhibited than NK cells with the weakly expressed KIR3DL1*005-like variant. For each Bw6 target, NK cells with the high-expression allele KIR3DL1*01502 were less inhibited than NK cells expressing the weakly expressed KIR3DL1*005-like variant. In this respect, HLA-B*1302, HLA-A*2301, and HLA-A*2501 all behaved like Bw6 alleles, reinforcing the conclusion that these alleles are not KIR3DL1 ligands. Interestingly, in donor 4, the only target that appeared to discriminate between NK cells expressing KIR3DL1*01502 and KIR3DL1*005 was the HLA-A*23 target. This suggests that some KIR3DL1 alleles may provide exceptions to the more general rules.
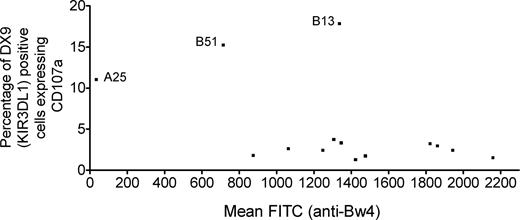
The ability of the different targets to inhibit CD107a expression on KIR3DL1-expressing effectors could be related not so much to the affinity of the ligand but rather to differences in the level of expression of the ligand on the various targets. Therefore, the level of Bw4 expression was checked by staining each target with an anti-Bw4 mAb. As shown in Figure 3, the average CD107a expression induced on KIR3DL1+ NK cells was not related to the level of Bw4 expression on the target. The A23, A24, and A32 targets also stained strongly, whereas the A25 target did not stain with the Bw4 mAb and did not inhibit NK-cell cytotoxicity despite being known to have the Bw4 motif. However, this mAb also did not react with 2 additional cell lines with HLA-A25, indicating that this mAb does not react with the HLA-A25–associated Bw4 epitope. The B51 target did have reduced HLA-B expression, which could explain the poor inhibition mediated by this target. However, the B13 target, which was also poorly inhibitory, had levels of Bw4 expression comparable with the other Bw4-expressing targets. Therefore, reduced HLA-B expression was not responsible for its lack of inhibition. There was no correlation between the level of Bw4 expression and ability to suppress CD107a expression when the HLA-A*2501 and B*5101 targets were excluded (r = −0.13; P = .68).
Level of Bw4 expression does not correlate with percentage of CD107a expression induced on KIR3DL1+ NK cells. Each Bw4+ target cell was stained with an anti-Bw4 mAb to detect Bw4 expression. The mean channel fluorescence (MCF) for each target was plotted against the percentage of CD107a+ NK cells induced by the target on NK cells with a high-expression KIR3DL1 receptor, averaged across the 4 NK-cell donors. Target A25 did not stain with the Bw4 mAb and does not inhibit NK-cell cytotoxicity. Target B51, which weakly inhibited NK-cell cytotoxicity, has lower expression of Bw4 than the other Bw4-expressing alleles. However, the B13 target, which also weakly inhibited NK-cell cytotoxicity, expressed levels of Bw4 comparable with most Bw4+ targets. After excluding the A25 and B51 targets, there was no significant correlation between percentage of KIR3DL1+ cells expressing CD107a and Bw4 expression on the target cell (r = −0.13; P = .68).
Level of Bw4 expression does not correlate with percentage of CD107a expression induced on KIR3DL1+ NK cells. Each Bw4+ target cell was stained with an anti-Bw4 mAb to detect Bw4 expression. The mean channel fluorescence (MCF) for each target was plotted against the percentage of CD107a+ NK cells induced by the target on NK cells with a high-expression KIR3DL1 receptor, averaged across the 4 NK-cell donors. Target A25 did not stain with the Bw4 mAb and does not inhibit NK-cell cytotoxicity. Target B51, which weakly inhibited NK-cell cytotoxicity, has lower expression of Bw4 than the other Bw4-expressing alleles. However, the B13 target, which also weakly inhibited NK-cell cytotoxicity, expressed levels of Bw4 comparable with most Bw4+ targets. After excluding the A25 and B51 targets, there was no significant correlation between percentage of KIR3DL1+ cells expressing CD107a and Bw4 expression on the target cell (r = −0.13; P = .68).
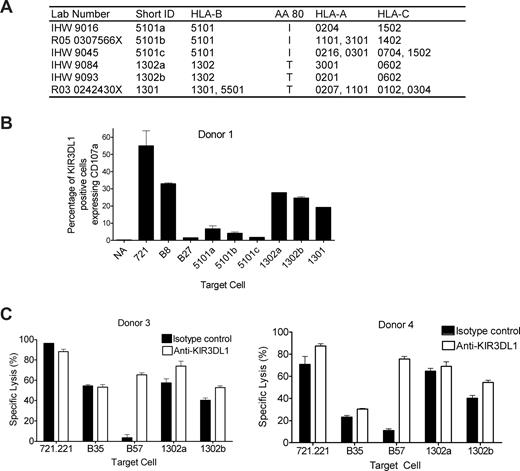
HLA-B*13 is not a KIR3DL1 ligand, but HLA-B*5101 is
To confirm the lack of inhibition seen with the targets bearing HLA-B*1302 and HLA-B*5101, additional examples of BLCL expressing B*1302 and B*5101, and one expressing B*1301 (Figure 4A), were tested in the CD107a assay against NK cells from donor 1. KIR3DL1+ NK cells up-regulated CD107a when incubated with either the positive control (721.221) or the Bw6 control (B8) as expected (Figure 4B). The Bw4 control (B27) inhibited CD107a expression as expected. Both examples of targets expressing HLA-B*1302 and the single example of a target expressing HLA-B*1301 failed to inhibit CD107a expression. In contrast, the original HLA-B*5101 target with reduced HLA-B expression (5101a) inhibited CD107a expression less strongly than the 2 additional examples of B*5101 targets. The additional B*13 and B*5101 targets had HLA-B expression comparable with the other Bw4+ targets as determined by staining with the Bw4 mAb (data not shown). These data suggest that the observation in relation to the original B51 target was due to reduced expression of HLA-B*5101 on that target, whereas HLA-B*13 appears to be a poor ligand for KIR3DL1. Lack of inhibition of cytotoxicity by HLA-B*1302 was confirmed using NK clones from donors 3 and 4 in a 4-hour 51Cr release assay (Figure 4C). A total of 2 KIR3DL1+ clones (clone C3 with low KIR3DL1 expression from donor 4 and clone D2 with high KIR3DL1 expression from donor 3) were tested. As shown in Figure 4C, cytotoxicity of clones C3 and D2 against the class I–negative cell line (721.221) and a Bw6 control (B35; Table 2) was not affected by blocking KIR3DL1 with DX9 antibody, whereas inhibition of cytotoxicity by a Bw4 control (B57; Table 2) was reversed by blocking with DX9 (Figure 4C). Cytotoxicity against the 2 HLA-B*1302 homozygous targets was relatively strong in the absence of blocking antibody and only weakly enhanced in the presence of DX9, confirming that HLA-B*1302 is a poor ligand for KIR3DL1.
HLA-B*1302 and HLA-B*1301 do not inhibit cytotoxicity of KIR3DL1-dependent NK cells. (A) Polyclonally expanded NK cells from donor 1 were incubated with the class I HLA–negative cell line, 721.221 (positive control), a Bw6 control (B8; Table 2), a Bw4 control (B27; Table 2), 2 HLA-B*1302 targets, 1 HLA-B*1301 target, and 3 HLA-B*5101 targets. (B) CD107a expression was measured on KIR3DL1+, CD158b− NK cells incubated with various target cells or alone (NA). CD107a expression was induced by the 721.221 cell line and the Bw6 control (B8), and not by the Bw4 control (B27) as expected. All targets expressing either HLA-B*1302 or HLA-B*1301 failed to inhibit CD107a expression. HLA-B*5101–expressing targets inhibited CD107a expression. (C) A total of 2 KIR3DL1+ NK clones (clone C3 from donor 4 expressing the low-expression KIR3DL1*005 allele and clone D2 from donor 3 expressing the high-expression KIR3DL1*01502 allele) were used in the 4-hour 51Cr release assay against the class I–negative cell line (721.221), a Bw6 control (B35; Table 2), a Bw4 control (B57; Table 2) and 2 HLA-B*1302 homozygous targets (panel A) to confirm lack of inhibition through KIR3DL1. KIR3DL1 was blocked with anti-DX9 (KIR3DL1; □) and isotype control (IgG1; ■). Both NK clones lysed 721.221 and the Bw6 control (B35). Both NK clones were inhibited by the Bw4 control (B57), and inhibition was reversed in the presence of anti-KIR3DL1 mAb. Neither clone was inhibited by either HLA-B*1302 target with very little or no reversal of inhibition in the presence of the anti-KIR3DL1 mAb. Error bars represent SEM.
HLA-B*1302 and HLA-B*1301 do not inhibit cytotoxicity of KIR3DL1-dependent NK cells. (A) Polyclonally expanded NK cells from donor 1 were incubated with the class I HLA–negative cell line, 721.221 (positive control), a Bw6 control (B8; Table 2), a Bw4 control (B27; Table 2), 2 HLA-B*1302 targets, 1 HLA-B*1301 target, and 3 HLA-B*5101 targets. (B) CD107a expression was measured on KIR3DL1+, CD158b− NK cells incubated with various target cells or alone (NA). CD107a expression was induced by the 721.221 cell line and the Bw6 control (B8), and not by the Bw4 control (B27) as expected. All targets expressing either HLA-B*1302 or HLA-B*1301 failed to inhibit CD107a expression. HLA-B*5101–expressing targets inhibited CD107a expression. (C) A total of 2 KIR3DL1+ NK clones (clone C3 from donor 4 expressing the low-expression KIR3DL1*005 allele and clone D2 from donor 3 expressing the high-expression KIR3DL1*01502 allele) were used in the 4-hour 51Cr release assay against the class I–negative cell line (721.221), a Bw6 control (B35; Table 2), a Bw4 control (B57; Table 2) and 2 HLA-B*1302 homozygous targets (panel A) to confirm lack of inhibition through KIR3DL1. KIR3DL1 was blocked with anti-DX9 (KIR3DL1; □) and isotype control (IgG1; ■). Both NK clones lysed 721.221 and the Bw6 control (B35). Both NK clones were inhibited by the Bw4 control (B57), and inhibition was reversed in the presence of anti-KIR3DL1 mAb. Neither clone was inhibited by either HLA-B*1302 target with very little or no reversal of inhibition in the presence of the anti-KIR3DL1 mAb. Error bars represent SEM.
HLA-A*2402 and HLA-A*3201 are ligands for KIR3DL1
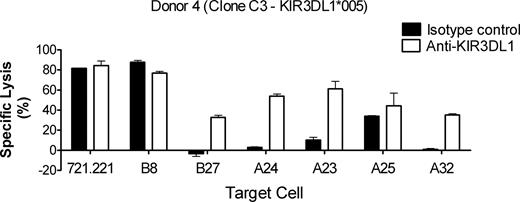
A total of 4 HLA-A alleles react with anti-Bw4 antibodies: HLA-A*23, HLA-A*24, HLA-A*25, and HLA-A*32. When assessed in terms of the ability of target cells expressing these alleles to inhibit CD107a expression of KIR3DL1+ NK cells (Figure 2), HLA-A*2402 and HLA-A*3201 were clearly KIR3DL1 ligands, while HLA-A*2501 appeared to have some weak inhibitory activity. HLA-A*2301 appeared devoid of inhibitory activity, particularly for NK cells expressing the high level allele (Figure 2 solid bars). Gumperz et al10 were unable to show reversibility of HLA-A*24–mediated inhibition of cytotoxicity using an anti-KIR3DL1 antibody. We therefore investigated the reversibility of inhibition by HLA-A alleles in a 51Cr release assay with an NK clone from donor 4 expressing the KIR3DL1*005 allele. As shown in Figure 5, the HLA bare (721.221) and Bw6− control target (B8) were lysed, and lysis was not enhanced by blocking KIR3DL1. The Bw4 control target (B27) completely inhibited lysis, and inhibition was reversed substantially by blocking with mAb. The A24 and A32 targets provided just as effective inhibition as the B27 control and again, inhibition was blocked by the mAb. In contrast, the A25 target inhibited lysis less effectively and showed only slightly enhanced lysis after blocking KIR3DL1, suggesting that HLA-A*2501 may have some weak binding to KIR3DL1. The A23 target provided intermediate inhibition, which was substantially reversed by blocking the receptor. Interestingly, in donor 4, A23 was the only target that showed a difference in CD107a expression between the high- and low-allele–expressing cells, suggesting a difference in the interaction of HLA-A*2301 with these 2 alleles.
Inhibition through Bw4-expressing HLA-A alleles can be reversed by addition of anti-KIR3DL1. Clone C3 from donor 4 expressing the low allele KIR3DL1*005 was tested in a 4-hour 51Cr release cytotoxicity assay against the class I–negative cell line (721.221), a Bw6 control (HLA-B*0801; Table 2), a Bw4 control (HLA-B*2705; Table 2), and targets expressing one of the 4 Bw4-expressing HLA-A alleles: A23, A24, A25, and A32 (Table 2). KIR3DL1 was blocked with either anti-KIR3DL1 antibody (DX9; □) or isotype control (IgG1; ■). Clone C3 lysed both 721.221 and the Bw6 control, and inhibition was not enhanced by anti-KIR3DL1 antibody. Clone C3 was inhibited by the Bw4 control and the A24 A23, and A32 targets, and this inhibition was reversed by addition of anti-KIR3DL1 antibody. Clone C3 lysed the A25 target and the anti-KIR3DL1 antibody only weakly enhanced specific lysis. Error bars represent SEM.
Inhibition through Bw4-expressing HLA-A alleles can be reversed by addition of anti-KIR3DL1. Clone C3 from donor 4 expressing the low allele KIR3DL1*005 was tested in a 4-hour 51Cr release cytotoxicity assay against the class I–negative cell line (721.221), a Bw6 control (HLA-B*0801; Table 2), a Bw4 control (HLA-B*2705; Table 2), and targets expressing one of the 4 Bw4-expressing HLA-A alleles: A23, A24, A25, and A32 (Table 2). KIR3DL1 was blocked with either anti-KIR3DL1 antibody (DX9; □) or isotype control (IgG1; ■). Clone C3 lysed both 721.221 and the Bw6 control, and inhibition was not enhanced by anti-KIR3DL1 antibody. Clone C3 was inhibited by the Bw4 control and the A24 A23, and A32 targets, and this inhibition was reversed by addition of anti-KIR3DL1 antibody. Clone C3 lysed the A25 target and the anti-KIR3DL1 antibody only weakly enhanced specific lysis. Error bars represent SEM.
Donors who have HLA-B*1301 or B*1302 can make KIR3DL1-dependent NK clones, but these NK clones are infrequent
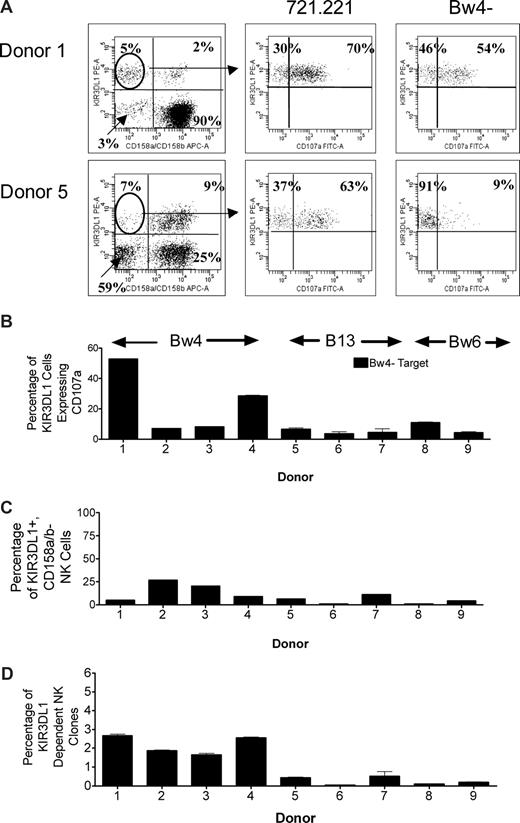
Because HLA-B*1302 and HLA-B*1301 are poor ligands for KIR3DL1, we asked whether donors who have HLA-B*13 as their only Bw4+ allele can generate KIR3DL1-dependent NK clones capable of lysing Bw4− targets. Using the CD107a assay, we tested the ability of 4 donors with Bw4+ alleles (donors 1-4; Table 1), 3 donors with B*1301 or B*1302 as their only Bw4+ allele (donors 5-7; Table 1) and 2 donors with only Bw6+ alleles (donors 8 and 9; Table 1) to generate KIR3DL1-dependent alloreactive NK clones. NK cells from each donor were incubated alone, with 721.221, with a target lacking only the Bw4 epitope (C1+, C2+, Bw4−), with a target lacking only the C1 epitope (C1−, C2+, Bw4+), and with a target lacking only the C2 epitope (C1+, C2−, Bw4+). KIR3DL1+, CD158a,b− cells were selected, and CD107a expression was measured against the range of target cells. Representative cytofluorograms of CD107a expression in the presence of 721.221 and the Bw4− target are shown in Figure 6A. As shown in Figure 6B, among the donors with Bw4 alleles other than B*13, donors 1 and 4 up-regulated CD107a expression on a large proportion of KIR3DL1+ NK cells in response to the Bw4− target (KIR3DL1-dependent), whereas for donors 2 and 3, a much lower percentage of KIR3DL1+ NK cells were KIR3DL1 dependent. In fact, donors 2 and 3 had similar proportions of KIR3DL1-dependent NK cells to the 3 HLA-B*13 and the 2 Bw6 homozygous donors. Thus, by this measure, HLA-B*13 donors were not distinguishable from some donors expressing other Bw4 alleles. However, as shown in Figure 6C, the proportion of KIR3DL1+, CD158a,b− NK cells also differed considerably between donors. When the proportion of KIR3DL1+, CD158a,b− NK cells that were KIR3DL1 dependent (Figure 6B) was multiplied by the proportion of all NK cells that were KIR3DL1+, CD158a,b− (Figure 6C) to determine the percentage of all NK cells from each donor that were KIR3DL1 dependent (Figure 6D), HLA-B*13+ donors could be distinguished from other Bw4+ donors and the Bw6 homozygous donors (donors 8 and 9). Donors with Bw4 alleles other than HLA-B*13 had 2% to 3% KIR3DL1-dependent NK clones, whereas donors with HLA-B*13 or homozygous for Bw6 had less than 0.5%. Donors with HLA-B*13 were, however, able to generate C1-dependent and C2-dependent clones (data not shown).
Donors who express HLA-B*1302 or HLA-B*1301 make very few KIR3DL1-dependent NK cells. NK cells from 9 donors (donors 1-9) were tested in the CD107a assay to determine their ability to make KIR3DL1-dependent NK clones. Each donor was incubated with the class I bare target 721.221, targets lacking either the C1 or C2 epitopes (data not shown), or targets lacking only the Bw4 epitope (HLA-A*0301, 1101, B*0702, 3501, C*0401, 0702). (A) Representative examples of flow cytometry showing identification of KIR3DL1+, CD158a,b− NK cells and their subsequent CD107a expression when incubated with 721.221 or the Bw4− target. Numbers on plots are percentages of total displayed cells. (B) After incubation with the Bw4− target, the proportion of KIR3DL1+, CD158a,b− NK cells that were CD107a+ did not distinguish donors with HLA-B*13 from donors with other Bw4+ alleles and donors homozygous for Bw6. (C) The proportion of KIR3DL1+, CD158a,b− NK cells does not distinguish HLA-B*13 donors from donors with other Bw4+ alleles and donors homozygous for Bw6. (D) The proportion of KIR3DL1-dependent NK clones of the total NK-cell population distinguishes HLA-B*13 donors from donors with other Bw4+ alleles. A total of 2% to 3% of NK cells from donors with other Bw4+ alleles are KIR3DL1 dependent, whereas this is true of less than 1% of NK cells from HLA-B*13 donors. Less than 0.5% of NK clones from donors homozygous for Bw6 are KIR3DL1 dependent, slightly less than donors with HLA-B13. Error bars represent SEM.
Donors who express HLA-B*1302 or HLA-B*1301 make very few KIR3DL1-dependent NK cells. NK cells from 9 donors (donors 1-9) were tested in the CD107a assay to determine their ability to make KIR3DL1-dependent NK clones. Each donor was incubated with the class I bare target 721.221, targets lacking either the C1 or C2 epitopes (data not shown), or targets lacking only the Bw4 epitope (HLA-A*0301, 1101, B*0702, 3501, C*0401, 0702). (A) Representative examples of flow cytometry showing identification of KIR3DL1+, CD158a,b− NK cells and their subsequent CD107a expression when incubated with 721.221 or the Bw4− target. Numbers on plots are percentages of total displayed cells. (B) After incubation with the Bw4− target, the proportion of KIR3DL1+, CD158a,b− NK cells that were CD107a+ did not distinguish donors with HLA-B*13 from donors with other Bw4+ alleles and donors homozygous for Bw6. (C) The proportion of KIR3DL1+, CD158a,b− NK cells does not distinguish HLA-B*13 donors from donors with other Bw4+ alleles and donors homozygous for Bw6. (D) The proportion of KIR3DL1-dependent NK clones of the total NK-cell population distinguishes HLA-B*13 donors from donors with other Bw4+ alleles. A total of 2% to 3% of NK cells from donors with other Bw4+ alleles are KIR3DL1 dependent, whereas this is true of less than 1% of NK cells from HLA-B*13 donors. Less than 0.5% of NK clones from donors homozygous for Bw6 are KIR3DL1 dependent, slightly less than donors with HLA-B13. Error bars represent SEM.
Donors who lack Bw4-expressing HLA-B alleles but express HLA-A*2402 can make KIR3DL1-dependent NK clones
Because HLA-A*2402 appears to be a ligand for KIR3DL1, we asked whether donors who lack Bw4-expressing alleles other than HLA-A*2402 are able to make KIR3DL1-dependent NK clones that could be exploited in haploidentical transplantation. A total of 129 NK clones were generated from a C1-homozygous, Bw6-homozygous, HLA-A*2402+ donor (donor 10; Table 1). Each NK clone was tested against 4 targets: 721.221 (positive control); a C1+, C2−, Bw4− target (IHW 9065); a C1−, C2+, Bw4− target (IHW 9019); and a C1+, C2−, Bw4+ target (IHW 9157) in the 4-hour 51Cr release assay. A total of 14% of all NK clones killed the targets in a pattern consistent with KIR2DL2/KIR2DL3-dependent NK clones and expressed CD158b by flow cytometry, indicating that this donor could make KIR2DL2/KIR2DL3-dependent clones. None of the clones killed targets in a KIR2DL1-dependent manner, as would be expected for an individual who does not have a C2+ HLA-C allele. However, a further 12.5% of all NK clones killed the targets in a pattern consistent with KIR3DL1-dependent NK clones (killed both Bw4− targets but not the Bw4+ target) and expressed KIR3DL1 by flow cytometry. Thus, donors who express HLA-A*2402 and lack Bw4+ HLA-B alleles can generate KIR3DL1-dependent NK clones.
Discussion
Our data confirm that KIR3DL1-dependent NK cells lyse targets that are homozygous for common Bw6 alleles but do not lyse targets with most of the common Bw4 alleles. These findings are consistent with the rule that the amino acid at position 80 of HLA-B molecules has the predominant influence on specificity for KIR3DL1.7,8 It has been reported that Bw4 alleles with an isoleucine at position 80 (80I) are better inhibitors of KIR3DL1-mediated lysis than those with a threonine at position 80 (80T).8 In particular, class I HLA-bare cells transfected with HLA-B*5801 (80I) have been reported to be stronger inhibitors of KIR3DL1-dependent clones than cells transfected with HLA-B*2705 and HLA-B*3701 (80T).15 We were unable to confirm these findings. The interpretation of the data from Cella and colleagues may have been complicated by the fact that most of the targets used in their study were not homozygous at the HLA-B locus. In addition, the difference between 80I and 80T alleles was only apparent when the Bw4-expressing HLA-A alleles were included. It is also possible that the effector cells used in their study had a KIR3DL1 allele that does interact differently with 80I or 80T and was not represented in our study. Nevertheless, our study included 5 high-expression and 2 low-expression alleles of KIR3DL1, including the common KIR3DL1*001, *015, and *005 alleles, and no evidence for an 80I/80T effect was observed. While the hierarchy of different Bw4+ HLA-B alleles reported in earlier publications may well exist if analyzed by the particular read-outs used in those studies, our data suggest that these subtleties may not be significant for target cells expressing a normal array of ligands and polyclonal NK cells.
Among Bw4+ HLA-B alleles, we found only HLA-B*1302 and B*1301 (which is more common in Asian populations) to be poor KIR3DL1 ligands. A total of 3 individuals in whom HLA-B*1302 or B*1301 was the only Bw4+ allele had a very low frequency (< 0.5%) of alloreactive NK cells dependent on KIR3DL1 compared with donors with other Bw4+ alleles (2%-3%). These flow cytometric estimates of clonal frequency are comparable with data from Ruggeri and colleagues obtained by direct NK cloning.5 HLA-B*1301 and HLA-B*1302 are unique among HLA-B and HLA-C alleles in that they have a leucine rather than arginine at position 145, a position that is important in salt bridge formation between HLA-C and KIRs.16 Mutation of asparagine 135 of KIR2DL2, which forms the salt bridge with arginine 145 of HLA-C, lowered HLA binding affinity by 20-fold relative to the wild-type receptor, suggesting a vital role for proper salt bridge formation in stabilizing KIR/HLA interaction. While the structure of the KIR3DL1/HLA-B complex has not been solved, it is thought that KIR3DL1 interaction with HLA-B may be similar to KIR2DL2 interaction with HLA-C, as many of the positions important in KIR recognition of HLA are conserved among HLA-B and HLA-C alleles.16 It is possible that the leucine at position 145, which prefers to be buried within the protein hydrophobic core and which also has a nonreactive side-chain, may not form an adequate salt bridge with KIR3DL1.
Conclusions from previous reports addressing the role of Bw4+ HLA-A alleles as ligands for KIR3DL1 have been inconsistent.8,10,11 In particular, Gumperz et al10 observed inhibition of a KIR3DL1+ NK clone by HLA-A*2501 and HLA-A*2403, but inhibition was not reversed by blocking with anti-KIR3DL1. Our data agree with the findings of Thananchai et al,11 which showed that HLA-A*2402 (the common subtype of HLA-A24) is an effective ligand. We show that inhibition by HLA-A24 is reversed by blocking with anti-KIR3DL1 and furthermore show that an individual whose only Bw4+ allele was HLA-A*2402 was able to generate KIR3DL1-dependent NK clones, indicating that, at least in this individual, A*2402 was an effective ligand and capable of “arming” NK cells for effector function. HLA-A*3201 is also an effective ligand. The status of HLA-A*2301 and HLA-A*2501 is less clear. HLA-A*2501–expressing targets exhibited, at best, a weak inhibitory effect in both the CD107a and 51Cr release assays. The interaction of HLA-A*2301 with KIR3DL1 is particularly interesting. The A23 target was virtually ineffective in the CD107a assay with NK cells expressing the KIR3DL1 high allele, but was the only target which clearly showed greater inhibition of NK cells expressing KIR3DL1*005 than those expressing KIR3DL1*01502 in donor 4. The inhibitory capacity of HLA-A*2301 for the KIR3DL1*005 allele was confirmed in the 51Cr release assay using an NK clone expressing KIR3DL1*005 from this same donor. These data suggest that the effectiveness of HLA-A*2301 as a KIR3DL1 ligand may be more dependent on the KIR3DL1 allele than other Bw4 alleles. However, studies using additional A23 targets and further examples of NK cells expressing KIR3DL1*005 and other alleles are required before definitive conclusions can be drawn.
Other groups have reported that different KIR3DL1 alleles interact with Bw4 to differing degrees.9,15,17,18 For alleles other than HLA-A*2301, we found little difference between the behavior of the high-expression allele KIR3DL1*01502 and the low-expression KIR3DL1*005 allele in donor 4. This result is consistent with the findings of Draghi et al,9 except they compared KIR3DL1*005 with KIR3DL1*002 rather than KIR3DL1*01502. (KIR3DL1*002 differs from KIR3DL1*01502 by a single amino acid in the D2 domain.) However, for donor 3, there was a clear difference between the NK cells expressing the high-expression and low-expression alleles. The KIR3DL1*005-like variant differs from KIR3DL1*005 by a single nonsynonymous mutation at nucleotide position 115, is weakly expressed, and the mutation appears to alter the ability of this receptor to interact with Bw4. Not only is it poorly inhibited by Bw4 alleles, but NK cells expressing this allele only weakly lyse cells lacking Bw4. Thus, KIR3DL1-dependent clones in donors whose only KIR3DL1 allele is the KIR3DL1*005-like variant may be weakly armed and may not be particularly effective alloreactive NK cells.
One-third of Bw4+ potential stem cell donors cannot make KIR3DL1-dependent clones. Many of these may have the KIR3DL1*004 allele, which is not expressed at the cell surface. However, our data suggest additional explanations. For example, our data suggest that individuals with HLA-B*1301 or HLA-B*1302 as their only Bw4+ allele will make relatively few KIR3DL1-dependent clones and may therefore be a poor choice of donor for a haploidentical transplantation. On the other hand, individuals who have HLA-A*2402 or HLA-A*3201 may well be suitable. The influence of KIR3DL1 polymorphism is also likely to be relevant. Our data suggesting KIR3DL1 polymorphism influences the arming of NK cells on the one hand and the effectiveness of HLA-A*2301 as a ligand on the other hand is consistent with reports of others demonstrating KIR3DL1 allele–dependent variation in strength of interaction with different Bw4 alleles.9,15,17,18
The exploitation of alloreactive NK cells in haploidentical transplantations requires both a patient amenable to such therapy and a suitable donor. In addition to Bw4, the HLA-C1 and HLA-C2 epitopes can also mediate NK allorecognition through their corresponding KIR.5 Only cells from patients who lack either Bw4 and/or the C1 or C2 ligand can be lysed by alloreactive NK cells. Only donors who have the corresponding epitope are capable of NK-mediated alloreactivity. The changed status of HLA-B*13, HLA-A*24, and HLA-A*32 shown in our study means that a number of individuals (patients and donors) will need reclassification. Individuals currently classified as Bw4− (Bw6 homozygous) would now be classified as Bw4+ if they have HLA-A24 or HLA-A32, while those whose only Bw4+ allele is HLA-B13 would now be classified as Bw4−. Although we do not have a database of HLA-C–typed, haploidentical pairs in which to directly analyze these effects, we can make some predictions from the general population. In a database of 200 white Western Australians typed at HLA-A, HLA-B, and HLA-C by sequencing based on the current definition of Bw4 positivity and the HLA-C alleles present, 28% of individuals would have all 3 NK-cell epitopes (Bw4, C1, C2) and thus not be amenable to NK allotherapy. However, HLA-A24, HLA-A32, and HLA-B13 have frequencies of 20%, 6%, and 6%, respectively, in this population, resulting in 15% of individuals (thus patients and donors) requiring reclassification. The net effect when examined empirically in this population is such that the proportion of individuals (and thus patients) who would now be considered to have all 3 NK-cell epitopes will increase from 28% to 32%. However, for Bw4− patients, an additional 11% of individuals (and thus donors) would now be expected to be Bw4+ and therefore suitable donors. Such effects will also be evident to various degrees in other ethnic groups depending upon the frequencies of the relevant alleles. HLA-B13, for instance, has a frequency of 25% in some Chinese populations.19
Until the rules governing the interaction of HLA alleles and KIR3DL1 alleles are better understood, methods to accurately estimate the frequency of alloreactive clones are required. Our data indicate that the simple flow cytometric assay we have used can do this allowing faster identification of donors suitable for haploidentical transplantations and other NK allotherapeutic applications. A formal evaluation of the CD107a assay for detecting C1-, C2-, and Bw4-dependent alloreactive clones is currently under way.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors gratefully acknowledge the support of the Ray and Bill Dobney Foundation of Royal Perth Hospital and the Child Health Research Foundation of Western Australia.
Authorship
Contribution: B.A.F. performed most of the bench work, including maintenance of cell lines, flow cytography, NK cloning, and chromium release assays, and helped draft the manuscript; D.D.S. helped develop the flow cytometric assays for CD107a, maintained cell lines, and performed NK cloning; E.V.B. performed the NK cloning of the HLA-A24 individual, chromium release assays, and flow cytography to characterize NK clones from this individual; L.J.L. assisted with assay development, laboratory management and design of experiments; F.T.C. designed and analyzed experiments and assisted with manuscript preparation; and C.S.W. designed and analyzed experiments, assisted with manuscript preparation, and had overall responsibility for the research program.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Campbell S. Witt, Department of Clinical Immunology and Immunogenetics, PathWest Royal Perth Hospital, Wellington St, Perth, Western Australia 6000, Australia; e-mail: campbell.witt@health.wa.gov.au.