Abstract
Venous thromboembolism (VTE) is increasingly diagnosed among individuals with hematologic malignancies. However, the risk of VTE among patients undergoing hematopoietic stem cell transplantation (HSCT) is unclear. We examined the incidence and risk factors for VTE and bleeding among 1514 patients undergoing in-patient HSCT. No protocolized VTE prophylaxis was used. By HSCT day 180, 75 symptomatic VTE occurred in 70 patients (4.6%; 95% confidence interval [CI], 3.6%-5.8%). Fifty-five (3.6%) were catheter-associated, 11 (0.7%) were non–catheter-associated deep venous thromboses, and 9 (0.6%) were pulmonary emboli. Thirty-four percent of VTE occurred at a platelet count less than 50 ×109/L; 13% occurred at a platelet count less than 20 ×109/L. In multivariate analysis, VTE was associated with prior VTE (odds ratio [OR], 2.9; 95% CI, 1.3-6.6) and with graft-versus-host disease (GVHD; OR, 2.4; 95% CI, 1.4-4.0). Clinically significant bleeding occurred in 230 patients (15.2%; 95% CI, 13.4%-17.1%); 55 patients (3.6%; 95% CI, 2.7%-4.7%) had fatal bleeding. Bleeding was associated with anticoagulation (OR, 3.1; 95% CI, 1.8-5.5), GVHD (OR, 2.4; 95% CI, 1.8-3.3), and veno-occlusive disease (OR, 2.2; 95% CI, 1.4-3.6). In HSCT patients, VTE is primarily catheter-related and 3-fold less common than clinically significant bleeding. These findings warrant consideration when selecting VTE prophylaxis in HSCT patients.
Introduction
Venous thromboembolism (VTE) is a common complication among individuals with cancer.1,2 Although it has been most closely associated with solid tumor malignancies, VTE is increasingly recognized as a complication among patients with hematologic malignancies.3,4 VTE rates of 6% to 12% have been reported for lymphoma5-7 and acute leukemia.8,9 With thalidomide-based therapy, up to 35% of multiple myeloma patients may develop VTE.10
However, relatively little information is available regarding the risk of VTE and the optimal approach to VTE prevention among individuals undergoing hematopoietic stem cell transplantation (HSCT).11 Patients undergoing HSCT have a number of identifiable risk factors for VTE, including prolonged hospitalization, indwelling vascular catheters, exposure to cytotoxic chemotherapy and immune modulators,12-16 infections,17 and graft-versus-host disease (GVHD).18,19 However, perhaps even more so than other patients with hematologic malignancies, patients undergoing HSCT also face an increased risk of bleeding. This risk is multifactorial, arising from prolonged thrombocytopenia, acquired hepatic dysfunction, and specific end-organ toxicities such as hemorrhagic cystitis, diffuse alveolar hemorrhage, and GVHD-associated gastrointestinal bleeding.11,20,21
Despite its proven efficacy,22 VTE prophylaxis remains underused in medical and surgical patients.23 In response to this apparent quality deficit, hospital accreditation and patient safety organizations, networks of academic medical centers, and professional societies have developed guidelines recommending universal VTE prophylaxis for hospitalized patients.24-26 However, large randomized clinical trials demonstrating the efficacy and safety of VTE prophylaxis have not included patients undergoing HSCT.24-26 Consequently, clinicians lack sufficient data to make informed decisions on the risks and benefits of VTE prophylaxis in this population. In this study, we sought to determine the incidence of and risk factors for VTE and bleeding among patients undergoing HSCT, thereby providing data with which to weigh the competing risks of thrombosis and bleeding in this medically complex patient population. We hypothesized that VTE would occur less frequently than clinically significant bleeding, findings that might influence the selection of VTE prophylaxis for these patients.
Methods
Database and medical record abstraction
This study was approved by the Institutional Review Board of the Johns Hopkins Medical Institutions. We conducted a retrospective cohort study of patients who had undergone HSCT. In a hospital discharge database, we used International Classification of Disease 9 (ICD-9),27 procedure code 41.0 to identify patients who had undergone an inpatient HSCT between July 1, 1993, and June 30, 2005. Patients undergoing outpatient HSCT during this time period were excluded.
From the discharge database, we obtained the following patient information: age, sex, race, cancer diagnosis, date and type of HSCT, duration of hospitalization, and overall survival. We extracted data from the patients' electronic medical records, including HSCT conditioning regimen, HSCT complications (GVHD, veno-occlusive disease [VOD], cytomegalovirus infection [CMV]), use of immune modulators (cyclosporine, corticosteroids, etc), VTE events, VTE treatment, and clinically significant bleeding events, as defined in the 6 subsections that follow. In reviewing individual patient data, we evaluated all HSCT discharge summaries, subsequent outpatient clinic notes and hospital discharge summaries, radiology studies, and, when available, postmortem analyses.
Management of indwelling central venous catheters
All patients had indwelling central venous catheters, which were placed on or before the date of admission. Patients undergoing autologous peripheral blood stem cell transplantation had double-lumen hemapheresis catheters placed for stem cell collection up to 14 days before admission. When not in continuous use, hemapheresis catheter lumens were flushed twice daily with 10 mL normal saline followed by 2 mL unfractionated heparin (1000 units/mL). In the absence of complications, these catheters were used throughout the entire course of HSCT. Patients undergoing other forms of transplantation (allogeneic or autologous bone marrow) had double-lumen Hickman catheters placed. Hickman catheters were flushed at least once daily with 10 mL normal saline followed by 6 mL unfractionated heparin (10 units/mL) per lumen during the inpatient stay and during outpatient follow up when not in continuous use. Catheters were removed once patients no longer required routine transfusions or intravenous antimicrobials.
VTE prophylaxis
Throughout hospitalization, VTE prophylaxis consisted of encouraged ambulation only. Patients did not receive mechanical or pharmacologic prophylaxis. Pharmacologic VOD prophylaxis was not used. Asymptomatic patients did not undergo routine VTE screening.
HSCT conditioning regimens and immune modulators
HSCT conditioning regimens included primarily busulfan-cyclophosphamide or cyclophosphamide–total body irradiation for hematologic malignancies, cyclophosphamide-thiotepa for breast cancer, cyclophosphamide alone for aplastic anemia, and carboplatin-based regimens for ovarian cancer and other solid tumors. Depending on the transplant protocol, patients undergoing allogeneic HSCT received cyclosporine or tacrolimus as GVHD prophylaxis, as did certain patients undergoing autologous transplantation protocols featuring GVHD induction. First-line treatment of GVHD usually consisted of systemic corticosteroids.
Platelet transfusions
Once patients developed myelosuppression, platelets were transfused to maintain a platelet count 20 ×109/L or more until stem cell engraftment (defined as white blood cell [WBC] count ≥ 0.5 ×109/L). Thereafter, a minimum platelet count 10 ×109/L or more was maintained. Patients with fever or pulmonary infection were transfused to achieve a platelet count of 20 ×109/L or more. Individuals with active bleeding or requiring anticoagulation were transfused to maintain a platelet count of 50 ×109/L or more.
Patient follow-up
Patients were usually admitted to the transplantation unit to begin their conditioning regimens 7 to 10 days before the date of HSCT. The day of stem cell infusion was designated HSCT day 0. In the absence of other complications, patients remained hospitalized until evidence of stem cell engraftment. After hospital discharge, patients undergoing autologous HSCT had daily follow-up in the transplantation center through at least transplantation day 30; patients undergoing allogeneic HSCT had daily follow-up through day 60. Thereafter, patients were typically evaluated every 3 months for 1 year, and then transitioned to less frequent and more variable follow-up in the outpatient clinic.
Definition of variables
We defined VTE based on objective radiographic and clinical data. Symptomatic VTE events occurring between HSCT admission and HSCT day 180 were considered HSCT-associated events. Deep vein thrombosis (DVT)–associated symptoms/signs included extremity pain and swelling, and catheter malfunction. Pulmonary embolism (PE)–associated symptoms/signs included dyspnea, chest pain, tachypnea, and hypoxemia. Diagnostic criteria for DVT required objective documentation of an intravascular thrombus as demonstrated by Duplex ultrasound imaging or an intravascular filling defect on contrast computed tomography (CT) venography or contrast venography. Catheter-associated thrombosis was defined as thrombosis of the vein(s) in which the catheter was placed or a contiguous vein. Fibrin sheaths on or intraluminal clots within central venous catheter tips, without evidence of endovascular thrombus, were not recorded as VTE events. A pulmonary embolus was diagnosed if pulmonary angiography or contrast CT demonstrated a pulmonary arterial filling defect, or if a patient with compatible symptoms had a high- or intermediate-probability ventilation-perfusion scan.
We defined clinically significant bleeding as a bleeding event between HSCT admission and HSCT day 180 that led to a specific clinical intervention. These interventions included but were not limited to hospitalization, transfusion, endoscopy, bronchoscopy, intubation, or continuous bladder irrigation. Bleeding episodes not requiring intervention (eg, most mucosal and soft tissue bleeding, microscopic hematuria) were not recorded. Thrombotic and bleeding events were recorded based on the consensus of 2 reviewers (D.E.G. and M.B.S.).
We recorded GVHD that was documented histopathologically and required systemic treatment (generally grades II-IV skin GVHD and all grades of liver and gut GVHD). VOD was defined by the Jones criteria (bilirubin level ≥ 34.2 μmol/L [2 mg/dL] and 2 of 3 of the following: hepatomegaly, ascites, and ≥ 5% weight gain) and was supported in most cases by findings on ultrasonography and transjugular liver biopsy.28 CMV infection was defined by microbiologic data (either antigenemia or end-organ histopathology).
Statistical analysis
We used the Kaplan-Meier method to calculate the incidence of VTE during the 180 days of interest. Patients were censored at the time of death or at the time of loss to follow-up, such as when the patient's referring institution resumed care. We also calculated the proportion of patients who had VTE and the proportion that had bleeding among all who underwent HSCT. We calculated 95% confidence intervals around the proportions assuming a binomial distribution. We tested the significance of differences in proportions using Fisher exact test, and tested continuous outcomes with the Wilcoxon signed-rank test. Odds ratios in the bivariate analyses were generated with logistic regression. We used multivariate logistic regression to assess the independent odds of thrombosis or bleeding associated with the clinical factors. Variables associated with VTE in the bivariate analyses, as well as those hypothesized to be associated with thrombosis were put into multivariable models. Likelihood ratio tests were used to test for improved model fit with the addition of covariates. In addition, stepwise forward and backward selection techniques were used to confirm the choice of model.
Results
Characteristics of the study cohort
We identified 1596 patients with eligible ICD-9 procedure codes. Of these patients, 26 were excluded because they had been coded incorrectly and had undergone outpatient HSCT (n = 19) or had inadequate data in the electronic medical record (n = 7). Of the remaining 1570 patients, we excluded 56 who were receiving therapeutic anticoagulation at the time of HSCT admission and were therefore at low risk of VTE. Thus, 1514 patients were included in our analysis.
Median follow-up was 642 days (mean, 960 days), and total follow up was 3978 person-years. The median survival for patients who died during follow-up was 269 days. One hundred nine (7.2%) were lost to follow-up in the first 180 days (generally, care was assumed by the referring institution) and 306 patients (20.2%) died in that interval. One hundred eight of the 306 patients (35.3%) who died during the first 180 days after HSCT underwent a postmortem examination. The more common causes of death identified at autopsy included infections (53 patients, 37.1%), bleeding (24 patients, 16.8%), VOD (19 patients, 13.3%), GVHD (14 patients, 9.8%), and diffuse alveolar damage (12 patients, 8.4%). In a number of instances, death was attributed to multiple causes. Only one patient had pulmonary embolism identified at autopsy, but it was not believed to be the cause of death.
Baseline demographic and clinical characteristics are shown in Table 1. Patients with hematologic malignancies accounted for 85% of the study cohort. Hematologic malignancies classified among “other” diagnoses included paroxysmal nocturnal hemoglobinuria (n = 1) and Waldenström macroglobulinemia (n = 1). Among solid tumor patients, diagnoses classified as “other” included sarcoma (n = 4), germ cell tumors (n = 3), and neuroblastoma (n = 1). One patient received a transplant for adrenoleukodystrophy.
Baseline patient characteristics
| Characteristic . | No. (%) . |
|---|---|
| Total patients in analysis | 1514 |
| Age, y | |
| Mean | 45 |
| Standard deviation | 12 |
| Sex | |
| Male | 769 (51) |
| Female | 745 (49) |
| Race | |
| White | 1311 (87) |
| Black | 155 (10) |
| Other | 48 (3) |
| Disease category | |
| Hematologic malignancy | 1291 (85) |
| Solid tumor | 223 (15) |
| Diagnosis | |
| Hematologic malignancies | |
| Non-Hodgkin lymphoma | 508 (34) |
| Acute myeloid leukemia | 219 (14) |
| Multiple myeloma | 147 (10) |
| Hodgkin disease | 138 (9) |
| Chronic myeloid leukemia | 114 (8) |
| Acute lymphocytic leukemia | 59 (4) |
| Myelodysplastic syndrome | 47 (3) |
| Chronic lymphocytic leukemia | 37 (2) |
| Aplastic anemia | 20 (1) |
| Other | 2 (<1) |
| Solid tumors | |
| Breast | 191 (13) |
| Ovarian | 23 (2) |
| Other | 9 (<1) |
| Type of transplantation | |
| Autologous | 928 (61) |
| Allogeneic | 586 (39) |
| Conditioning regimen | |
| Busulfan-cyclophosphamide | 830 (55) |
| Cyclophosphamide-total body irradiation | 397 (26) |
| Cyclophosphamide-thiotepa | 191 (13) |
| Carboplatin-based | 32 (2) |
| Cyclophosphamide | 16 (1) |
| Melphalan | 9 (1) |
| Other | 5 (<1) |
| Unknown | 34 (2) |
| Duration of hospitalization, d | |
| Median | 26 |
| IQR 25 to 75 | 21-36 |
| Vital status at discharge | |
| Alive | 1384 (91) |
| Dead | 130 (9) |
| Overall survival after transplantation, d | |
| Median | 269 |
| IQR | 86-826 |
| Complications of transplantation | |
| Graft-versus-host disease | 300 (20) |
| Veno-occlusive disease | 99 (7) |
| Cytomegalovirus | 86 (6) |
| Immune modulators | |
| Cyclosporine | 673 (44) |
| Corticosteroids | 293 (19) |
| Characteristic . | No. (%) . |
|---|---|
| Total patients in analysis | 1514 |
| Age, y | |
| Mean | 45 |
| Standard deviation | 12 |
| Sex | |
| Male | 769 (51) |
| Female | 745 (49) |
| Race | |
| White | 1311 (87) |
| Black | 155 (10) |
| Other | 48 (3) |
| Disease category | |
| Hematologic malignancy | 1291 (85) |
| Solid tumor | 223 (15) |
| Diagnosis | |
| Hematologic malignancies | |
| Non-Hodgkin lymphoma | 508 (34) |
| Acute myeloid leukemia | 219 (14) |
| Multiple myeloma | 147 (10) |
| Hodgkin disease | 138 (9) |
| Chronic myeloid leukemia | 114 (8) |
| Acute lymphocytic leukemia | 59 (4) |
| Myelodysplastic syndrome | 47 (3) |
| Chronic lymphocytic leukemia | 37 (2) |
| Aplastic anemia | 20 (1) |
| Other | 2 (<1) |
| Solid tumors | |
| Breast | 191 (13) |
| Ovarian | 23 (2) |
| Other | 9 (<1) |
| Type of transplantation | |
| Autologous | 928 (61) |
| Allogeneic | 586 (39) |
| Conditioning regimen | |
| Busulfan-cyclophosphamide | 830 (55) |
| Cyclophosphamide-total body irradiation | 397 (26) |
| Cyclophosphamide-thiotepa | 191 (13) |
| Carboplatin-based | 32 (2) |
| Cyclophosphamide | 16 (1) |
| Melphalan | 9 (1) |
| Other | 5 (<1) |
| Unknown | 34 (2) |
| Duration of hospitalization, d | |
| Median | 26 |
| IQR 25 to 75 | 21-36 |
| Vital status at discharge | |
| Alive | 1384 (91) |
| Dead | 130 (9) |
| Overall survival after transplantation, d | |
| Median | 269 |
| IQR | 86-826 |
| Complications of transplantation | |
| Graft-versus-host disease | 300 (20) |
| Veno-occlusive disease | 99 (7) |
| Cytomegalovirus | 86 (6) |
| Immune modulators | |
| Cyclosporine | 673 (44) |
| Corticosteroids | 293 (19) |
IQR indicates interquartile range.
VTE incidence and clinical associations
Between HSCT admission and day 180, 75 symptomatic VTE events occurred in 70 patients (4.6%; 95% CI, 3.6%-5.8%). Our estimates using survival analysis were comparable with a VTE incidence of 4.9% (95% CI, 3.9% to 6.2%). Thrombotic events included 55 catheter-associated upper-extremity DVT (3.6%; 95% CI, 2.7%-4.7%), 11 non–catheter-associated lower-extremity DVT (0.7%; 95% CI, 0.4%-1.3%), and 9 pulmonary emboli (0.6%; 95% CI, 0.2%-1.0%). No deaths were directly attributable to VTE. Prior VTE, development of GVHD, corticosteroid use, and hospital length of stay were significantly associated with the development of VTE in bivariate analyses (Table 2). In multivariate analyses, prior VTE (OR, 2.9; 95% CI, 1.3-6.6) and GVHD (OR, 2.4; 95% CI, 1.4-4.0) remained significantly associated with VTE.
Clinical characteristics associated with VTE in bivariate analyses
| Characteristic . | Odds ratio . | 95% CI . | P . |
|---|---|---|---|
| Age, for each 1-y increase | 0.99 | 0.97-1.01 | .32 |
| Race, nonwhite relative to white | 0.99 | 0.70-1.41 | .97 |
| Sex, female compared with male | 1.57 | 0.96-2.56 | .07 |
| Disease category, heme vs solid | 0.96 | 0.48-1.92 | .92 |
| Year of transplantation, for every 1-y increase | 0.97 | 0.91-1.04 | .46 |
| Duration of hospitalization, for every additional day | 1.01 | 1.00-1.03 | .05* |
| Overall survival, for every additional day | 1.00 | 1.00-1.00 | .22 |
| Prior VTE | 8.36 | 2.62-21.0 | <.001* |
| Type of transplantation, autologous vs allogeneic | 0.89 | 0.58-1.54 | .82 |
| Complications of transplantation | |||
| GVHD | 2.05 | 1.22-3.45 | .006* |
| VOD | 0.86 | 0.31-2.41 | .77 |
| CMV | 1.00 | 0.36-2.82 | .99 |
| Immune modulators | |||
| Cyclosporine | 1.26 | 0.78-2.04 | .34 |
| Corticosteroids | 1.87 | 1.09-3.14 | .02* |
| Characteristic . | Odds ratio . | 95% CI . | P . |
|---|---|---|---|
| Age, for each 1-y increase | 0.99 | 0.97-1.01 | .32 |
| Race, nonwhite relative to white | 0.99 | 0.70-1.41 | .97 |
| Sex, female compared with male | 1.57 | 0.96-2.56 | .07 |
| Disease category, heme vs solid | 0.96 | 0.48-1.92 | .92 |
| Year of transplantation, for every 1-y increase | 0.97 | 0.91-1.04 | .46 |
| Duration of hospitalization, for every additional day | 1.01 | 1.00-1.03 | .05* |
| Overall survival, for every additional day | 1.00 | 1.00-1.00 | .22 |
| Prior VTE | 8.36 | 2.62-21.0 | <.001* |
| Type of transplantation, autologous vs allogeneic | 0.89 | 0.58-1.54 | .82 |
| Complications of transplantation | |||
| GVHD | 2.05 | 1.22-3.45 | .006* |
| VOD | 0.86 | 0.31-2.41 | .77 |
| CMV | 1.00 | 0.36-2.82 | .99 |
| Immune modulators | |||
| Cyclosporine | 1.26 | 0.78-2.04 | .34 |
| Corticosteroids | 1.87 | 1.09-3.14 | .02* |
indicates statistically significant.
VTE timing and platelet counts
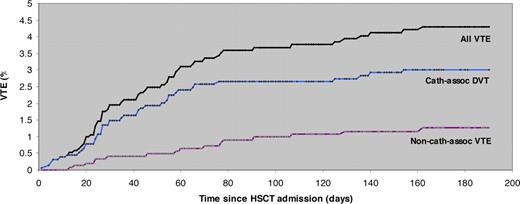
The median time to development of VTE was 35 days (interquartile range [IQR], 16-64 days) after HSCT admission. (Figure 1) For catheter-associated upper-extremity DVT (n = 55), the median time to the event was 33 days (IQR, 12-53 days) after HSCT admission and 40 days (IQR, 27-75 days) after catheter placement. The median time after HSCT admission for non–catheter-associated lower-extremity DVT was 63 days (IQR, 28-116 days). Pulmonary embolism occurred a median of 66 days (IQR, 37-78 days) after HSCT admission.
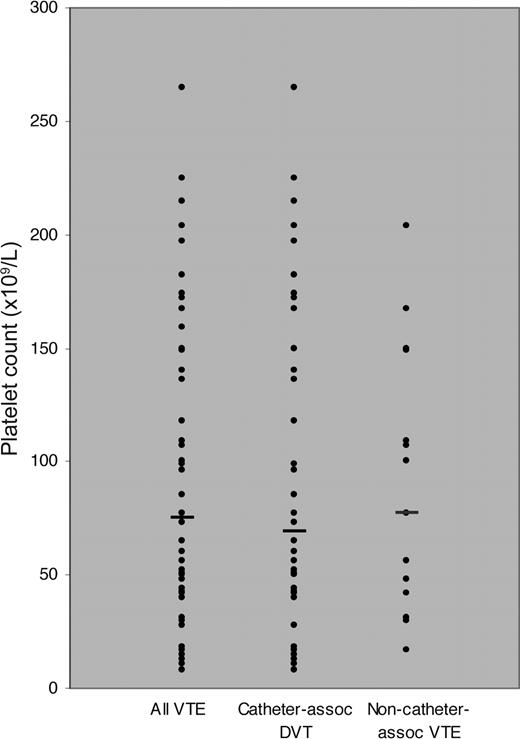
Platelet counts at the time of VTE were available for 52 VTE events (70%; Figure 2). The median platelet count at the time of VTE events overall was 75 ×109/L (IQR, 42-150 ×109/L). For catheter-associated DVT and non–catheter-associated VTE (lower extremity DVT and PE), median platelet counts were 69 ×109/L (IQR, 42-167 ×109/L) and 77 ×109/L (IQR, 31-149 ×109/L). Among all VTE events, 31 (60%) occurred at a platelet count less than 100 ×109/L, 17 (34%) occurred at a platelet count less than 50 ×109/L, and 7 (13%) occurred at a platelet count less than 20 ×109/L.
Platelet counts at time of VTE events, according to VTE event type. Horizontal bars indicate median counts.
Platelet counts at time of VTE events, according to VTE event type. Horizontal bars indicate median counts.
Bleeding events
Two hundred thirty patients (15.2%; 95% CI, 13.4%-17.1%) experienced clinically significant bleeding. Among these patients, 277 bleeding events occurred: 110 gastrointestinal (39%), 63 genitourinary (23%), 47 pulmonary (17%), 27 central nervous system (10%), 12 ophthalmologic (4%), 6 gynecologic (2%), 6 mucosal (2%), 5 cardiovascular (2%), and 4 at other locations (1%). Bleeding was fatal in 55 patients (3.6%; 95% CI, 2.7%-4.7%) for a case fatality rate of 24% (95% CI, 18.5%-30.0%): 28 pulmonary (50%), 13 gastrointestinal (23%), 13 central nervous system (23%), and 2 cardiovascular (4%).
After excluding the 62 patients who initiated anticoagulation during days 1 to 180 (all of whom did so for newly diagnosed VTE) to better understand the baseline risk of bleeding for these patients, we found that 207 of 1452 patients (14.3%; 95% CI, 12.5%-16.1%) had clinically significant bleeding, and 52 patients (3.6%; 95% CI, 2.7%-4.7%) had fatal bleeding for a case-fatality rate of 25% (95% CI, 19.1%-31.6%).
The initiation of anticoagulation during HSCT days 1 to 180 was the strongest predictor of bleeding with an OR of 3.1 (95% CI, 1.8-5.5). Other independent predictors of bleeding were nonwhite race (OR, 1.3; 95% CI, 1.1-1.6), VOD (OR, 2.2; 95% CI, 1.4-3.6), and GVHD (OR, 2.4; 95% CI 1.8-3.3). Autologous rather than allogeneic transplantation was protective (OR, 0.46; 95% CI, 0.33-0.64) and bleeding decreased in each subsequent calendar year (OR, 0.94; 95% CI, 0.90-0.98).
Discussion
This cohort study of 1514 subjects represents the largest analysis of the incidence of and risk factors associated with VTE and bleeding among patients undergoing HSCT. In the first 180 days after HSCT, 70 patients (4.6%) suffered a symptomatic thromboembolic event: 3.6% of patients had a catheter-associated upper-extremity DVT, and 1.3% had a lower-extremity DVT or pulmonary embolism. In comparison, 230 patients (15.2%) developed clinically significant bleeding, and 55 patients (3.6%) suffered fatal bleeding during their initial transplantation course. Among the 62 patients who were initiated on anticoagulation for newly diagnosed VTE during the first 180 days after transplantation, 23 (37%) experienced a bleeding event, and 3 (5%) had fatal bleeding. If we exclude these patients, 207 (14.3%) of 1452 patients developed clinically significant bleeding and 52 patients (3.6%) suffered fatal bleeding. Therefore, the incidence of clinically significant bleeding was 3-fold higher than the incidence of VTE and resulted in 55 deaths compared with no confirmed fatalities due to VTE. With increasing focus on expanding the use of VTE prophylaxis, this study provides meaningful data to guide policy makers and practitioners in the management of VTE in patients who underwent transplantation.
The findings from our HCST cohort complement previous investigations. In their retrospective study of 447 patients who underwent bone marrow transplantation, Pihusch et al noted catheter thrombosis in 3.6% of patients and DVT and/or pulmonaryembolism in 2.2% of patients during the first 100 days after transplantation.11 Importantly, patients in that series received pharmacologic VOD prophylaxis (unfractionated heparin 100 units per kilogram intravenously daily). This measure—which is not routinely used at most transplantation centers—may have influenced the incidence of both VTE and hemorrhagic complications. Of note, 168 patients (37.6%) suffered severe bleeding and 13 patients (2.2%) succumbed to fatal bleeding in their study. In an earlier study from our institution (18% of patients from this study are included in our patient cohort that underwent HSCT), moderate bleeding occurred in 11.3% of patients, severe bleeding in 12% of patients, and fatal bleeding in 5% of patients.20 Although the definitions of bleeding differ slightly among these studies, the frequency of clinically significant bleeding identified in each of these series is at least 10-fold higher than has been previously noted in the general medical oncology population undergoing treatment (0.9%).29-31 Therefore, an approach to VTE prophylaxis that is appropriate for most medical oncology patients may not be tolerated by hematopoietic stem cell transplant recipients or other profoundly thrombocytopenic patient populations.
One potential strategy to optimize the benefits of VTE prophylaxis is to target subgroups of patients at the greatest risk. We found that a previous history of VTE and GVHD were associated with an increased risk of VTE during HSCT. Previous VTE is a well-recognized risk factor for VTE that may help target prophylaxis in patients undergoing HSCT.32,33 However, GVHD is less likely to serve as a useful marker for targeting pharmacologic prophylaxis, as GVHD was also a risk factor for bleeding in our patient cohort. The association between GVHD and thrombosis has been reported previously11 and has been attributed to endothelial damage and release of tumor necrosis factor alpha.11,18,19 Likewise, clinically significant bleeding is a well-established complication of GVHD.11,21
Profound thrombocytopenia is a common feature of HSCT that further complicates management of VTE in these patients. In our series of patients who underwent transplantation, we found that thrombocytopenia was only partially protective against the development of VTE. Sixty percent of thrombotic events occurred when the platelet count was less than 100 ×109/L, 34% of events occurred with a platelet count less than 50 ×109/L, and 13% occurred with a platelet count less than 20 ×109/L. This distribution is likely to complicate decisions regarding VTE prevention, as past clinical trials have either excluded thrombocytopenic patients or withheld pharmacologic prophylaxis during periods of thrombocytopenia.22,34 Mechanical prophylaxis has been proposed as an alternative means to prevent VTE in thrombocytopenic cancer patients.25 However, the benefits of sequential/pneumatic compression devices in HSCT patients remain unknown and may be limited because of the theoretic risk of soft-tissue bleeding in profoundly thrombocytopenic patients and the presumed lesser efficacy of the devices in prevention of catheter-associated DVT. For catheter-associated DVT, the predominant VTE event in this study, even pharmacologic prophylaxis is of unclear benefit. Although 3 recent randomized, controlled trials demonstrated LMWH and low-dose warfarin to be well tolerated in cancer patients with indwelling catheters, neither approach led to a decrease in catheter-associated thrombosis.35-37
As a retrospective analysis, this study has a number of limitations. First, VTE prophylaxis, in the form of encouraged ambulation, was not standardized or quantified in the medical record. Therefore, we cannot determine whether ambulation is protective against venous thromboembolism or what is the minimal amount of activity required to prevent lower-extremity DVT. In contrast, a standardized policy of central venous catheter management was used during the study period, facilitating interinstitutional comparisons of catheter care and event rates. Second, the diagnosis of VTE depended upon clinical suspicion, as there was no active radiographic surveillance for thromboembolism. Therefore, the number of thrombotic events recorded during our study is likely an underestimate. Nevertheless, these results reflect usual practice and, therefore, have relevance to other physicians involved in the care of patients undergoing HSCT. Furthermore, the clinical significance of asymptomatic catheter-associated thrombi remains unknown.38 Third, although the precise timing of VTE diagnosis was available (using the diagnostic imaging study), less detail was available for the timing of bleeding events, the identification of which relied upon daily progress notes, discharge summaries, and clinic notes in the electronic medical record. Nonetheless, it is unlikely that a substantial number of clinically significant bleeding events were missed after reviewing all of these clinical data sources.
We did not capture data on VTE risk factors that have been documented in other patient populations, such as intercurrent infection (apart from CMV), medical comorbidities, and obesity. However, these traditional risk factors may be less applicable to a population undergoing HSCT. Infections are exceedingly common in these patients and often are not documented microbiologically (eg, fungal pulmonary infections). Conversely, patients who qualify for HSCT have undergone rigorous pretransplantation cardiac and pulmonary evaluations and thus are generally medically fit apart from their cancer diagnosis. Although obesity has been associated with an increased risk of VTE in some studies,33 others have been unable to confirm this association.32,39 Furthermore, obesity has not been identified as a risk factor for VTE in cancer patients.40 One might question the inclusion of patients with solid tumors (15% of all patients, primarily breast cancer), in the cohort, as breast cancer is no longer considered an indication for HSCT. Importantly, the rate of VTE among these patients did not differ from that among patients with hematologic malignancies (P = .999). Thus, the findings reported in this study are applicable to contemporary populations undergoing HSCT.
A limitation of our data analysis is that the censored patients may not have been truly uninformative, an assumption required for using techniques of survival analysis. The 2 groups of censored patients were those who died (n = 306) and patients who transferred back to the referring institution (n = 109). These populations may represent the opposite poles of thrombosis risk; patients who died would have been at increased risk of thrombosis had they lived, whereas the transferred patients (enriched with more clinically stable patients less likely to have transplantation complications such as severe GVHD) would have been at a decreased risk of thrombosis had they been observed through 180 days. However, available autopsy data from 108 of the 306 patients who died before day 180 suggest that even among this population VTE occurred rarely. Only one patient (0.9%) had evidence of VTE (a PE deemed not to be the cause of death). In contrast, 24 patients had bleeding as a cause of death.
Despite the retrospective nature of this study, data were generally quite complete; patients undergoing HSCT have standardized follow-up, are treated according to strict protocols, and are often enrolled in clinical trials. By evaluating individual patient records, rather than relying exclusively on administrative data, we were able to provide information on VTE timing and associated platelet counts, relevant details that are rarely reported in studies of this size. In addition, we selected clinically significant study end points. Specifically, we limited the study period to the 6 months after HSCT. In contrast, in their retrospective study of hemostatic complications among 447 patients who underwent HSCT, Pihusch et al reported a higher overall rate of non–catheter-associated DVT and PE (5.8% among patients who underwent allogeneic HSCT), but these events occurred at a median time of 180 days after HSCT (range: HSCT day −4 to day 998).11 Given the complex disease and treatment courses of patients with hematologic malignancies, it seems likely that some of these late events may be attributable to conditions or procedures other than HSCT. Similarly, it seems unlikely that VTE prophylaxis used during HSCT admission would impact VTE events occurring more than 2 years later.
In conclusion, we have demonstrated that VTE occurs infrequently among patients undergoing HSCT and is predominantly catheter associated. In this population, the efficacy and safety of thromboprophylaxis remain unknown. Until safe and effective prevention strategies are identified, if prophylaxis is considered for these patients, the following points should be noted: (1) VTE occurs even during periods of severe thrombocytopenia—approximately one-third of events occurred when the platelet count was less than 50 × 109/L; (2) the subset of patients at highest risk for VTE (ie, those with GVHD) are also at highest risk of bleeding; and (3) clinically significant bleeding occurs in a substantial proportion of patients after HSCT. Physicians should therefore carefully consider the complex hemostatic milieu of patients undergoing transplantation when contemplating VTE prophylaxis.
Presented in part at the 49th annual meeting of the American Society of Hematology, Atlanta, GA, December 8, 2007.41
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: D.E.G. and M.B.S. designed the study, performed research, analyzed data, and wrote the paper; J.B.S. designed the study, analyzed the data, and wrote the paper; M.Y.L. and J.K. performed research; and R.J.J. analyzed data and wrote the paper.
Conflict-of-interest disclosure: M.B.S. is a consultant for Sanofi-Aventis, GlaxoSmithKline, and Eisai, Inc, and has received honoraria from Sanofi-Aventis, GlaxoSmithKline, and Eisai, Inc. The remaining authors declare no competing financial interests.
Correspondence: Michael B. Streiff, Associate Professor of Medicine, Division of Hematology, Department of Medicine, Johns Hopkins Medical Institutions, 1830 E Monument St, Suite 7300, Baltimore, MD 21205; e-mail: mstreif@jhmi.edu.