Abstract
While the extravasation cascade of lymphocytes is well characterized, data on their intraepithelial positioning and morphology are scant. However, the latter process is presumably crucial for many immune functions. Integrin αE(CD103)β7 has previously been implicated in epithelial retention of some T cells through binding to E-cadherin. Our current data suggest that αE(CD103)β7 also determines shape and motility of some lymphocytes. Time-lapse microscopy showed that wild-type αE(CD103)β7 conferred the ability to form cell protrusions/filopodia and to move in an amoeboid fashion on E-cadherin, an activity that was abrogated by αE(CD103)β7-directed antibodies or cytochalasin D. The αE-dependent motility was further increased (P < .001) when point-mutated αE(CD103) locked in a constitutively active conformation was expressed. Moreover, different yellow fluorescent protein–coupled αE(CD103) species demonstrated that the number and length of filopodia extended toward purified E-cadherin, cocultured keratinocytes, cryostat-cut skin sections, or epidermal sheets depended on functional αE(CD103). The in vivo relevance of these findings was demonstrated by wild-type dendritic epidermal T cells (DETCs), which showed significantly more dendrites and spanned larger epidermal areas as compared with DETCs of αE(CD103)-deficient mice (P < .001). Thus, integrin αE(CD103)β7 is not only involved in epithelial retention, but also in shaping and proper intraepithelial morphogenesis of some leukocytes.
Introduction
Positioning and locomotion of leukocytes within tissues provide the basis for the molecular crosstalk with other cells and are prerequisites for a functional immune system. To date, the recruitment cascade of initial endothelial adhesion, activation, firm adhesion, transmigration, and subsequent localization into the connective tissue is one of the best established concepts in leukocyte biology.1,2 However, many effector functions of immunocytes are exerted within parenchymatous organs, mostly epithelia. It is therefore somewhat surprising that we know relatively little about locomotion and function-determining morphogenesis of lymphocytes within epithelial tissues, such as the epidermis of the skin.3
An adhesion receptor that is thought to mediate retention of lymphocytes within epithelial tissues is integrin αE(CD103)β7.4 First described 2 decades ago as a selective marker for intestinal intraepithelial lymphocytes,5 αE(CD103)β7 has been implicated in epithelial T-cell retention through binding to E-cadherin.6,7 Indeed, αE(CD103)-deficient mice exhibited a reduced number of mucosal intraepithelial T cells.8 However, αE(CD103)β7 has later been found to be also expressed by some lymphocytes within other epithelia, such as the epidermis of the skin,9 where it presumably contributes to recruitment of T cells in inflamed human skin10 as well as dendritic epidermal T cells (DETCs) in murine skin.11 Thymic DETC precursor cells express integrin αE(CD103)β7 before their migration into the periphery, suggesting that αE(CD103)β7 is involved in guiding tissue-specific epidermal localization of DETCs.12 Expression of αE(CD103)β7 has been described on some CD4+CD25+13,14 and CD8+15,16 regulatory T cells (Treg). In several experimental models αE(CD103)β7 is involved in guiding tissue localization of lymphocyte subsets in inflammatory conditions and/or allograft rejection.14,17,18 Although αE(CD103)β7 is highly expressed by some lymphocytes and CD11high/MHC-IIhigh dendritic cells at mucosal and other epithelial sites, its role in immune regulation remains largely elusive. Expression of αE(CD103)β7 appears to be associated with cytotoxic activity of CD8+ T cells in graft-versus-host disease and allogeneic transplantation.19,20 Along this line, interactions of αE(CD103)β7 with E-cadherin are crucial for lysis of E-cadherin+ tumor cells by cytotoxic T cells in some cases.21 Moreover, expression of αE(CD103)β7 is associated with several important cellular activities such as antigen presentation22 or stimulation of Treg23 and some mucosal CD8+ T cells24,25 by αE(CD103)β7+ dendritic cells. It is, however, currently unclear whether and to what extent αE(CD103)β7 itself contributes directly to such functions.
Overall, a robust body of evidence has accumulated indicating that αE(CD103)β7 is involved in tissue-specific retention and/or effector functions of some immune cells. Yet, it is still unclear whether αE(CD103)β7 is involved in other cellular functions such as fine-tuning of shape and motility, similar to what has been shown for some other integrins,26 which are presumably crucial for the exertion of effector functions within epithelial compartments. We present here the first experimental data showing that integrin αE(CD103)β7 is indeed involved in sculpting the shape as well as stimulating the motility of cells on ligand contact. Thus, integrin αE(CD103)β7 also determines the ligand-directed shape and locomotion of cells, a novel function that extends beyond mere adhesion and retention on the substrate.
Methods
Plasmids
Yellow fluorescent protein was fused C-terminally to 3 different integrin αE-constructs. The integrin αE stop codon was eliminated by site-directed mutagenesis (primer: sense 5′-GTCTGCTCCAAGATCAGCCCCCTGCTTC; antisense 5′-GAAGCAGGGGGCTGATCTTGGAGCAGAC) using the QuikChange II XL Site-Directed Mutagenesis Kit (Stratagene, Amsterdam, The Netherlands). The YFP-gene was amplified by polymerase chain reaction (PCR) using YFP-primers linked with a NotI restriction site (sense 5′-AGGCTGTGAGGCGGCCGCTAATGGTGAGCAAGGGCGAG, antisense 5′-CTCTAGCGTTGCGGCCGCCTACTTGTACAGCTCGTC) and Platinum Pfx DNA Polymerase (Invitrogen, Karlsruhe, Germany). The PCR product as well as the 3-point mutated plasmids were digested with NotI (NEB, Frankfurt/Main, Germany) and ligated with T4 DNA Ligase (Invitrogen). The following PCR conditions were used: mutagenesis 95°C 1 minute, followed by 18 cycles 95°C 50 seconds, 60°C 50 seconds, 68°C 9 minutes; YFP 94°C 2 minutes, followed by 30 cycle, 94°C 15 seconds, 58°C 30 seconds, 68°C 1 minute. All plasmids were sequenced (SEQLAB, Göttingen, Germany).
Cell culture and transfections
K562 cells were cultured in RPMI and PAM212 cells were cultured in DMEM (PAA, Pasching, Germany). The Nucleofector Kit V (Amaxa, Cologne, Germany) was used for transient transfections. The transfection efficiency was determined by flow cytometry. For coating, slides were overlayered with 1 μg/mL recombinant murine E-cadherin (R&D Systems, Wiesbaden, Germany) in 2 mM CaCl2/PBS over night at 4°C. Kollagenreagens Horm (NYCOMED, Linz, Austria) was diluted 1:1 in PBS and coated on cover slips overnight at 37°C. For antibody or cytochalasin D treatment the transfected K562 were incubated for 60 minutes with the 2E7 mAb (20 μg/mL) or cytochalasin D (0.5 μM), respectively. As determined by both trypan blue exclusion and flow cytometry assessing annexin V/propidium iodide staining, cytochalasin D treatment did not affect cell viability under the conditions used in our study (> 95% viable cells).
Confocal microscopy
Cells were cultured for 90 minutes on E-cadherin–coated cover slips. Imaging was performed by serial 1.0 μm Z-steps using a TCS-4D microscope and SCANware software (Leica, Wetzlar, Germany). For coculture experiments, 1.5 × 105 K562 and 4 × 105 PAM212 were coincubated in RPMI. In other experiments, cryostat-cut sections or epidermal sheets were overlayered with transfected cells. Confocal microscopy was performed after 24 hours by serial 0.2 to 0.5 μm Z-steps using a DMI6000 microscope equipped with a TCS Sp5 scanner and LAS AF software (Leica Microsystems, Mannheim, Germany). Morphometric analyses were performed using ImageJ software (version 1.38; National Institutes of Health, Bethesda, MD).
Time-lapse microscopy
Cells were allowed to attach to E-cadherin for 30 minutes, and were then monitored for 60 to 90 minutes using an Axiovert 200M microscope equipped with a LD Achroplan 20×/0.4 lens (Zeiss, Göttingen, Germany). Images were taken every 150 seconds using a CoolSnap ES camera (Photometrics, Tucson, AZ). Morphometric analyses were performed using MetaMorph Software (version 6.3r2; Visitron Systems, Puchheim, Germany). The following numbers of independent experiments were performed: mock-transfected cells, 7 experiments; wild-type αE(CD103)/β7, 3 experiments; αE-open/β7, 6 experiments; and αE-closed/β7, 4 experiments.
Mice
The isolation of animal material was approved by governmental authorities (Regierung von Unterfranken [District Government of Lower Franconia], Germany) and was performed according to institutional guidelines. Mice (C57/BL6 background) were maintained under specific pathogen-free conditions.
Epidermal cell suspensions and flow cytometry
Mouse ears were incubated with dispase (Boehringer, Mannheim, Germany) at 6 mg/mL for 1 hour at 37°C. The epidermis was then peeled off and digested again for 5 minutes at 37°C under gentle stirring in dispase (6 mg/mL) and DNase (0.1%). The resulting single-cell suspension was subjected to 2-color flow cytometry using monoclonal antibodies directed against CD3ϵ (PE-conjugated), CD11a, CD18, CD25, CD29, CD44, CD49b, CD49d, CD49e, CD49f, and CD103 (FITC-conjugated; BD Pharmingen, Heidelberg, Germany). Dead cells were excluded by propidium-iodide staining. Cells were analyzed in a FACScan using CellQuest software (Becton Dickinson, Heidelberg, Germany).
Isolation of epidermal sheets and immunohistochemistry
Ears were removed and depilated. The split skin was floated for 20 minutes on 0.5 M NH4SCN and the epidermal sheet was peeled off. After fixation in acetone for 20 minutes at −20°C and washing in phosphate-buffered saline (PBS), the epidermal sheets were soaked for 90 minutes with antimurine CD3ϵ (Becton Dickinson) followed by anti-hamster fluorescein isothiocyanate FITC (Vector Laboratories, Burlingame, CA) for 60 minutes. The sheets were analyzed using an Axioscop 2 microscope equipped with a Plan-Neofluar 40×/0.75 lens (Zeiss), and images were recorded using an AxioCam HRc (Zeiss). Morphometric analysis was performed using AxioVision AC software (Zeiss).
Statistical analysis
Data are displayed as mean (± SD or ± SEM as indicated), P values were determined using the 2-tailed t test, and P values less than .05 (confidence interval [CI] of 95%) were considered statistically significant. All statistical analyses were 2-sided.
Results
Integrin αE(CD103)β7 increases cell motility on E-cadherin
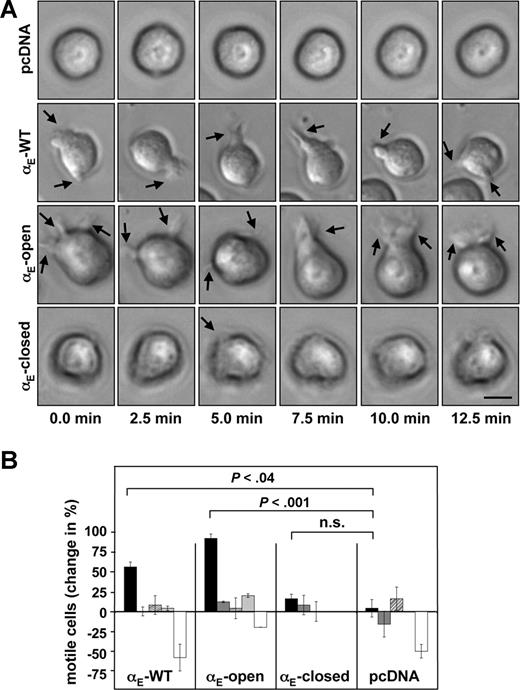
Given that little is known about the role of αE(CD103)β7 for the motility of leukocytes, we addressed this issue by transfecting cells with the murine receptor, in which the αE(CD103) chain was locked in different functional conformations by point mutations.27 Unfortunately, transfection of several populations of primary murine lymphocytes under various conditions did not result in the expression of functional heterodimers, whereas control proteins were readily expressed (data not shown). In addition, Jurkat cells showed constitutive expression of the human β7 integrin subunit, thus precluding functional studies of the murine heterodimer (data not shown). However, when the erythroleukemia cell line K562 was double-transfected with the wild-type αE(CD103) and β7 integrin subunits, surface expression and binding to E-cadherin were readily detected. The overall migration (determined by single-cell-tracking) on immobilized murine E-cadherin was similar in all transfectant lines expressing αE(CD103)β7 (mean95 μm/h, ± 18), while mock-transfected cells migrated significantly longer distances (average 159 μm/h ± 37, P < .001 compared with either of the αE(CD103)β7 transfectants). However, some remarkable differences became apparent regarding the motility of cells transfected with the different αE(CD103) species at their site of attachment: Time-lapse microscopy demonstrated that expression of wild-type (αE-WT) or a point-mutated αE(CD103) species locked in a constitutively active conformation (αE-open) resulted in a significantly increased ability of the cells to move in an amoeboid fashion on E-cadherin–coated surfaces and to extend cellular protrusions and filopodia in a highly dynamic manner. Integrin αE-open/β7 and αE-WT/β7 transfected K562 cells showed markedly more pronounced changes in morphology as compared with a constitutively inactive species (αE-closed/β7)or mock (pcDNA3.1) transfected cells (Figure 1A; Videos S1,S2, available on the Blood website; see the Supplemental Materials link at the top of the online article). Quantitative analyses demonstrated that the number of cells showing amoeboid motility was significantly increased by 50% (± 19) in cultures transfected with integrin αE-WT/β7 (Figure 1B) as compared with mock transfectants (P < .04; Figure 1B). When the cells were transfected with integrin αE-open/β7, the number of motile cells within the cultures was even increased by 85% (± 27, P < .001 compared with controls; Figure 1B). In addition, the magnitude of cellular movement and the size of the protrusions formed by the cells appeared to be markedly greater in transfectants expressing functionally active αE(CD103)β7 species as compared with the controls (visualized in Videos S1,S2). Transfection of integrin αE-closed/β7 did not result in enhanced motility of the transfectants (P = .5 compared with mock-transfected controls; Figure 1B). Culture of the cells on uncoated plastic surfaces, on collagen-coated surfaces, in the presence of αE(CD103)-directed antibodies, or cytochalasin D completely abrogated the increases in amoeboid movement (Figure 1B). Thus, the observed gains of motility were strictly dependent on interactions of functional αE(CD103)β7 with E-cadherin and appeared to be relayed through the actin-based cytoskeleton.
Expression of integrin αE(CD103)β7 capacitates K562 cells to move in an amoeboid fashion on E-cadherin. (A) K562 cells were double-transfected with the murine β7 integrin subunit and either wild-type αE(CD103) or αE(CD103) locked in a constitutively active (αE-open) or inactive (αE-closed) conformation. Control cells were transfected with the empty vector (pcDNA). Transfectant cells were seeded on recombinant murine E-cadherin and images were taken every 2.5 minutes. Shape changes of a representative cell for each condition are shown.  indicates examples of filopodia and/or cell protrusions. This panel corresponds to Videos S1,S2. Scale bar = 10 μm. (B) K562 cells were transfected with integrin αE-WT/β7, integrin αE-open/β7, integrin αE-closed/β7, or the empty vector (pcDNA) as detailed in “Cell culture and transfections.” The transfectants were monitored for 60 minutes on immobilized E-cadherin (
indicates examples of filopodia and/or cell protrusions. This panel corresponds to Videos S1,S2. Scale bar = 10 μm. (B) K562 cells were transfected with integrin αE-WT/β7, integrin αE-open/β7, integrin αE-closed/β7, or the empty vector (pcDNA) as detailed in “Cell culture and transfections.” The transfectants were monitored for 60 minutes on immobilized E-cadherin ( ), on uncoated plastic surfaces (
), on uncoated plastic surfaces ( ), or on collagen (▨). In addition, transfectants plated on E-cadherin were treated with a function-blocking antibody directed against αE(CD103) (
), or on collagen (▨). In addition, transfectants plated on E-cadherin were treated with a function-blocking antibody directed against αE(CD103) ( ) or were cultured in the presence of cytochalasin D (□). The number of cells moving in an amoeboid fashion and producing protrusions/filopodia is depicted relative to the control (wild-type αE(CD103)β7-transfected cells adhering to fetal calf serum–coated plastic surfaces). Values shown represent the means (± SEM).
) or were cultured in the presence of cytochalasin D (□). The number of cells moving in an amoeboid fashion and producing protrusions/filopodia is depicted relative to the control (wild-type αE(CD103)β7-transfected cells adhering to fetal calf serum–coated plastic surfaces). Values shown represent the means (± SEM).
Expression of integrin αE(CD103)β7 capacitates K562 cells to move in an amoeboid fashion on E-cadherin. (A) K562 cells were double-transfected with the murine β7 integrin subunit and either wild-type αE(CD103) or αE(CD103) locked in a constitutively active (αE-open) or inactive (αE-closed) conformation. Control cells were transfected with the empty vector (pcDNA). Transfectant cells were seeded on recombinant murine E-cadherin and images were taken every 2.5 minutes. Shape changes of a representative cell for each condition are shown.  indicates examples of filopodia and/or cell protrusions. This panel corresponds to Videos S1,S2. Scale bar = 10 μm. (B) K562 cells were transfected with integrin αE-WT/β7, integrin αE-open/β7, integrin αE-closed/β7, or the empty vector (pcDNA) as detailed in “Cell culture and transfections.” The transfectants were monitored for 60 minutes on immobilized E-cadherin (
indicates examples of filopodia and/or cell protrusions. This panel corresponds to Videos S1,S2. Scale bar = 10 μm. (B) K562 cells were transfected with integrin αE-WT/β7, integrin αE-open/β7, integrin αE-closed/β7, or the empty vector (pcDNA) as detailed in “Cell culture and transfections.” The transfectants were monitored for 60 minutes on immobilized E-cadherin ( ), on uncoated plastic surfaces (
), on uncoated plastic surfaces ( ), or on collagen (▨). In addition, transfectants plated on E-cadherin were treated with a function-blocking antibody directed against αE(CD103) (
), or on collagen (▨). In addition, transfectants plated on E-cadherin were treated with a function-blocking antibody directed against αE(CD103) ( ) or were cultured in the presence of cytochalasin D (□). The number of cells moving in an amoeboid fashion and producing protrusions/filopodia is depicted relative to the control (wild-type αE(CD103)β7-transfected cells adhering to fetal calf serum–coated plastic surfaces). Values shown represent the means (± SEM).
) or were cultured in the presence of cytochalasin D (□). The number of cells moving in an amoeboid fashion and producing protrusions/filopodia is depicted relative to the control (wild-type αE(CD103)β7-transfected cells adhering to fetal calf serum–coated plastic surfaces). Values shown represent the means (± SEM).
Cellular morphology in vitro determined by integrin αE(CD103)β7 on contact with E-cadherin
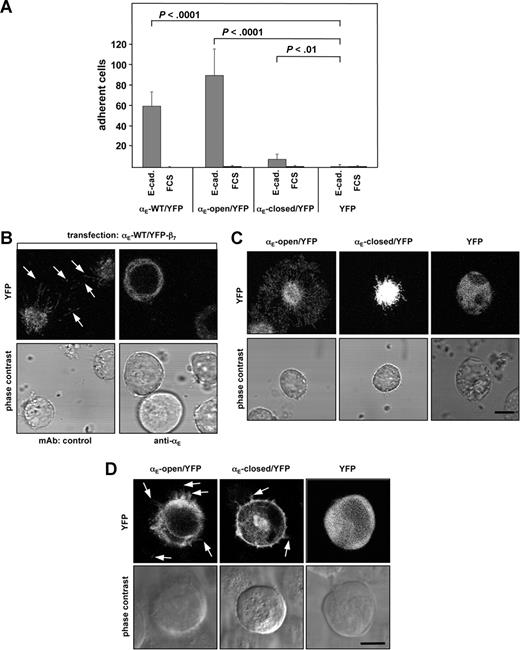
To visualize the formation of cellular protrusions and filopodia in relation to αE(CD103)β7 expression, αE-WT, constitutively active (αE-open) and constitutively inactive (αE-closed) species, respectively, were fused to YFP and transfected into K562 cells. The cells were cotransfected with the unlabeled β7 subunit. As readily detectable by fluorescence microscopy and flow cytometry, all of the transfectants showed robust expression of the integrin αE-YFP/β7 fusion proteins (data not shown). The expected functional properties were readily exhibited by the transfectants: Using wild-type (αE-WT/YFP/β7) or the point-mutated species locked in a constitutively active conformation (αE-open/YFP) resulted in significantly increased cell adhesion to E-cadherin (P < .001 in both cases as compared with mock-transfected cells), while expression of a constitutively inactive species (αE-closed/YFP) led to significantly weaker binding (P < .01 compared with the control; Figure 2A), indicating the suitability of the YFP fusion proteins for further functional experiments. To further ascertain that fusion of YFP did not alter the function of αE(CD103)β7, additional control experiments were performed in which αE(CD103) was fused to YFP using either 5 amino acid or 21 amino acid spacer sequences, both yielding essentially the same results in functional adhesion experiments (data not shown).
Expression of functional integrin αE(CD103)β7 influences the cellular shape and induces filopodia formation in a ligand-dependent fashion in vitro. (A) Wild-type or mutant αE(CD103) subunits were fused to YFP as outlined in “Plasmids.” K562 cells were cotransfected with the wild-type β7 subunit and either wild-type αE(CD103)/YFP, αE-open/YFP, αE-closed/YFP, or YFP alone as indicated. Adhesion experiments (determined as bound cells/20× field, n = 3 experiments) performed on E-cadherin– or FCS-coated surfaces demonstrate the functionality of the integrin αE-YFP-fusion-proteins. (B) K562 cells cotransfected with wild-type αE(CD103)/YFP and β7 subunits were plated on E-cadherin in the presence of a control mAb or a mAb directed against αE(CD103). Images were taken by confocal microscopy in z-stacks (top panels) and phase contrast microscopy (bottom panels). Arrows indicate examples of protrusions/filipodia in the E-cadherin contact zone. (C) The indicated transfectants were seeded on E-cadherin, and the formation of filopodia was monitored by confocal microscopy. The E-cadherin contact zone of a representative cell for each condition is shown. Scale bar = 10 μm. (D) The indicated transfectants were mixed and cocultured with PAM212 keratinocytes. Analysis was performed by confocal microscopy in z-steps of 0.2 to 0.5 μm. One section within the contact zone between the K562 and PAM212 is depicted, in the top row the fluorescence and in the bottom row the phase contrast image of the cells. Arrows indicate examples of protrusions/filopodia extended toward the keratinocytes. Scale bar = 10 μm.
Expression of functional integrin αE(CD103)β7 influences the cellular shape and induces filopodia formation in a ligand-dependent fashion in vitro. (A) Wild-type or mutant αE(CD103) subunits were fused to YFP as outlined in “Plasmids.” K562 cells were cotransfected with the wild-type β7 subunit and either wild-type αE(CD103)/YFP, αE-open/YFP, αE-closed/YFP, or YFP alone as indicated. Adhesion experiments (determined as bound cells/20× field, n = 3 experiments) performed on E-cadherin– or FCS-coated surfaces demonstrate the functionality of the integrin αE-YFP-fusion-proteins. (B) K562 cells cotransfected with wild-type αE(CD103)/YFP and β7 subunits were plated on E-cadherin in the presence of a control mAb or a mAb directed against αE(CD103). Images were taken by confocal microscopy in z-stacks (top panels) and phase contrast microscopy (bottom panels). Arrows indicate examples of protrusions/filipodia in the E-cadherin contact zone. (C) The indicated transfectants were seeded on E-cadherin, and the formation of filopodia was monitored by confocal microscopy. The E-cadherin contact zone of a representative cell for each condition is shown. Scale bar = 10 μm. (D) The indicated transfectants were mixed and cocultured with PAM212 keratinocytes. Analysis was performed by confocal microscopy in z-steps of 0.2 to 0.5 μm. One section within the contact zone between the K562 and PAM212 is depicted, in the top row the fluorescence and in the bottom row the phase contrast image of the cells. Arrows indicate examples of protrusions/filopodia extended toward the keratinocytes. Scale bar = 10 μm.
Serial stacks of the K562 cells analyzed by confocal microscopy demonstrated that numerous filopodia were extended by the cells in the contact zone to E-cadherin–coated surfaces. As demonstrated by αE(CD103) specific antibody-mediated abrogation of filopodia formation, this function was strictly dependent on αE(CD103)β7 (Figure 2B). Strikingly, the number and length of the filopodia clearly depended on the conformational state of the αE-YFP fusion protein transfected. Transfection of αE-open/YFP resulted in formation of the largest filopodia, whereas transfection of αE-WT/YFP led to an intermediate size, and cells expressing integrin αE-closed/YFP exhibited the shortest filopodia (Figure 2C). Statistical analyses verified the significant difference (P < .02 comparing the ratios cell body/filopodia between αE-open/YFP/β7- and αE-closed/YFP/β7-transfected K562 cells). Control experiments using YFP-transfected cells on E-cadherin (Figure 2C) or integrin αE(CD103)/YFP/β7-transfected cells on collagen (not shown) did not provoke the formation of filopodia, further indicating that the filopodia of the integrin αE(CD103)β7-transfected cells were dependent on the specific interaction between αE(CD103)β7 and its ligand, E-cadherin.
Confirming the functional properties in an experimental system approaching the natural situation within the skin, the formation of filopodia induced by functionally active αE(CD103)β7 was also observed when K562 transfectants were cocultured with PAM212, an E-cadherin-expressing murine keratinocyte line.28 In contrast, K562 cells expressing αE-closed/YFP/β7 showed only few and short filopodia, and K562 cells transfected with YFP alone did not show formation of filopodia when cocultured with PAM212 keratinocytes (Figure 2D).
Generation of an artificial dendritic cell-like phenotype of leukocytes on natural epidermis through expression of αE(CD103)β7
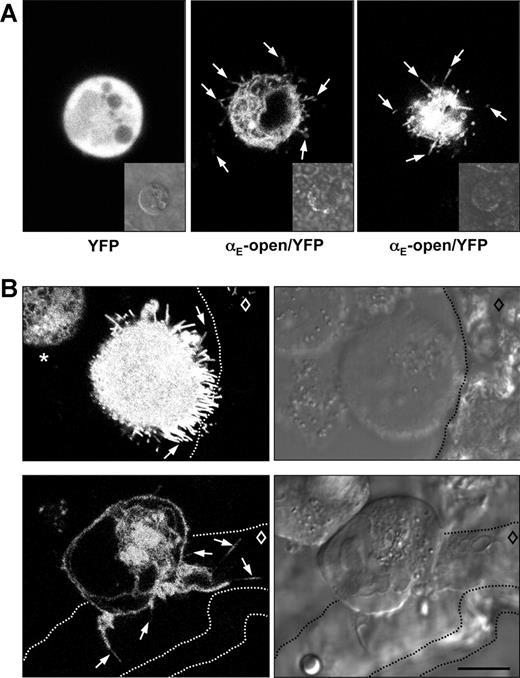
To more closely mimic the in vivo situation where immune cells interact with epidermal cells organized in a polarized and stratified epithelium, we performed 2 complementary series of experiments: First, K562 cells expressing αE(CD103)/YFP constructs and the wild-type β7 subunit were seeded on cryostat-cut sections of murine skin. In the second series of experiments, epidermal sheets were prepared from mouse ears, and the sheets were placed upside-down into plastic dishes. The αE(CD103)β7 transfectants were then plated onto the exposed undersurface of the epidermal sheets. Indeed, in both types of experiments some of the transfectant cells expressing active αE(CD103)β7, but not the controls, formed conspicuous filopodia and protrusions extending clearly toward the epidermal keratinocytes, thus generating a striking dendritic cell-like morphology on intact epidermis (examples depicted in Figure 3A,B).
Dendritic morphology of αE(CD103)β7-expressing cells contacting natural murine epidermis in 2 complementary settings. (A) Epidermal sheets were separated from the dermis and prepared as outlined in “Isolation of epidermal sheets and immunohistochemistry.” K562 cells transfected with YFP alone (left panel) or YFP-fused integrin αE-constructs and wild-type β7 (αE-open/YFP and β7 shown here in middle and right panels) were seeded on the exposed undersurface of the epidermis and the formation of dendrites was visualized by confocal microscopy. Examples of filopodia extending across or between epidermal keratinocytes are indicated by arrows. The middle and the right panels show 2 different cells at a higher (middle) or lower level (right). The inserts depict the respective phase contrast images. (B) K562 cells transfected with integrin αE-open/YFP and β7 were seeded onto cryostat-cut sections of murine skin. The top row shows a situation where the epidermis is separated from the dermis and 2 transfectant cells are located in the resulting cleft, one cell with contact to the epidermis and one cell without. Arrows point to examples of dendrites directed toward the epidermis. The asterisk in the top left panel identifies a transfectant cell that has no contact to the epidermis and shows no formation of filopodia. In the bottom row, a transfectant cell is located across the dermo-epidermal junction. Arrows identify some dendrites extending across the epidermis. The dashed lines indicate the borders of the epidermis (including stratum corneum in the 2 bottom panels), and the diamond indicates the location of the viable epidermal cell layers. Scale bar = 10 μm. These images correspond to Videos S3 to S6.
Dendritic morphology of αE(CD103)β7-expressing cells contacting natural murine epidermis in 2 complementary settings. (A) Epidermal sheets were separated from the dermis and prepared as outlined in “Isolation of epidermal sheets and immunohistochemistry.” K562 cells transfected with YFP alone (left panel) or YFP-fused integrin αE-constructs and wild-type β7 (αE-open/YFP and β7 shown here in middle and right panels) were seeded on the exposed undersurface of the epidermis and the formation of dendrites was visualized by confocal microscopy. Examples of filopodia extending across or between epidermal keratinocytes are indicated by arrows. The middle and the right panels show 2 different cells at a higher (middle) or lower level (right). The inserts depict the respective phase contrast images. (B) K562 cells transfected with integrin αE-open/YFP and β7 were seeded onto cryostat-cut sections of murine skin. The top row shows a situation where the epidermis is separated from the dermis and 2 transfectant cells are located in the resulting cleft, one cell with contact to the epidermis and one cell without. Arrows point to examples of dendrites directed toward the epidermis. The asterisk in the top left panel identifies a transfectant cell that has no contact to the epidermis and shows no formation of filopodia. In the bottom row, a transfectant cell is located across the dermo-epidermal junction. Arrows identify some dendrites extending across the epidermis. The dashed lines indicate the borders of the epidermis (including stratum corneum in the 2 bottom panels), and the diamond indicates the location of the viable epidermal cell layers. Scale bar = 10 μm. These images correspond to Videos S3 to S6.
Integrin αE(CD103)β7 affects the in vivo morphology of dendritic epidermal T cells (DETC) in mice
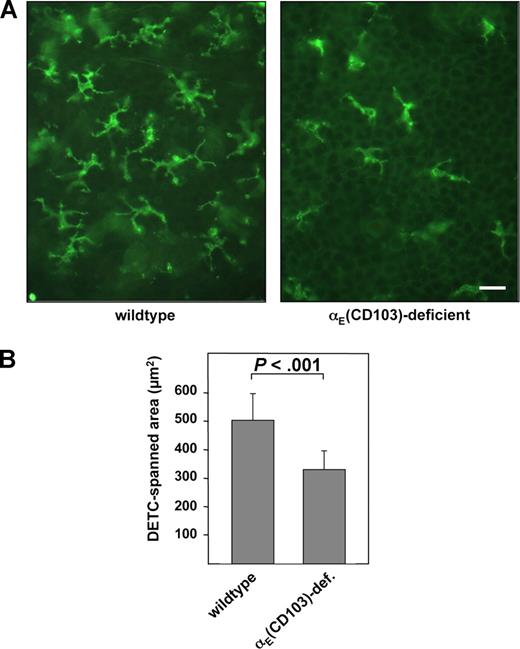
The relevance of our findings for properties of naturally occurring lymphocytes in vivo can be deduced from our further experiments in which we analyzed wild-type and αE(CD103)-deficient mice.8 To address the question whether αE(CD103)β7 affected shape and morphology of natural cells in vivo, we investigated murine DETCs, a CD3+ cell population of the γδ T-cell lineage that constitutively expresses αE(CD103)β7 under normal conditions.12 As expected,11 the number of DETCs was reduced in αE(CD103)-deficient mice as compared with the wild-type controls (littermates, C57/BL6 genetic background). However, there were conspicuous morphologic differences between DETCs within the epidermis of wild-type mice and their counterparts in the skin of αE(CD103)-deficient mice.
When whole mounts of epidermal sheets were stained by a CD3-directed monoclonal antibody, it became apparent that both number and size of dendrites per cell in αE(CD103)-deficient mice were significantly reduced as compared with wild-type animals (Figure 4A). Extensive quantitative morphometric analyses revealed that the dendrites of DETCs from integrin αE-deficient mice spanned an average area of 325 μm2 (± 64.6), as compared with 495 μm2 (± 91.9) in wild-type littermates, which was larger by 52.3% (P < .001; Figure 3B). In addition, the average number of dendrites per DETC was reduced significantly in αE(CD103)-deficient mice (P < .001). DETCs isolated from the epidermis of wild-type mice showed a significantly higher proportion of cells forming protrusions/filopodia when plated on E-cadherin as compared with DETCs isolated from αE(CD103)-deficient mice (P < .003). Again, the formation of such protrusions was abrogated by antibodies directed against αE(CD103), β7, or E-cadherin (Figure S1). Given that flow cytometric analysis of single-cell suspensions of murine epidermis showed no compensatory alterations in other adhesion molecules including the integrin chains αL(CD11a), β2(CD18), β1(CD29), α2(CD49b), α4(CD49d), α5(CD49e) and α6(CD49f) on DETCs of αE(CD103)-deficient mice (data not shown), the morphologic differences between DETCs of αE(CD103)β7-deficient mice and DETCs of wild-type mice can most likely be attributed to the expression status of αE(CD103)β7.
Dendritic morphology of dendritic epidermal T cells is influenced by integrin αE(CD103) in vivo. (A) Epidermal sheets of wildtype and integrin αE(CD103)-deficient mice on the C57BL/6 genetic background (littermates) were prepared and DETCs were visualized using an mAb directed against CD3 as outlined in “Isolation of epidermal sheets and immunohistochemistry.” The scale bar indicates 20 μm. (B) The surface area spanned by DETC dendrites in wild-type (left bar) and integrin αE-deficient littermates (right bar) was analyzed using ImageJ software. The values shown represent data from 50 DETCs that were analyzed from 3 mice of each genotype.
Dendritic morphology of dendritic epidermal T cells is influenced by integrin αE(CD103) in vivo. (A) Epidermal sheets of wildtype and integrin αE(CD103)-deficient mice on the C57BL/6 genetic background (littermates) were prepared and DETCs were visualized using an mAb directed against CD3 as outlined in “Isolation of epidermal sheets and immunohistochemistry.” The scale bar indicates 20 μm. (B) The surface area spanned by DETC dendrites in wild-type (left bar) and integrin αE-deficient littermates (right bar) was analyzed using ImageJ software. The values shown represent data from 50 DETCs that were analyzed from 3 mice of each genotype.
Discussion
Our results indicate that expression of functional αE(CD103)β7 integrin is important for some cells to adapt and sculpt their shape on encountering E-cadherin, a ligand that is constitutively expressed within many epithelial tissues, in a fashion that allows close contact with ligand-bearing structures. This is the first example of such a function of a lymphocyte adhesion receptor that acts primarily within parenchymatous tissues, where many target cells for immune reactions are located and where effector functions of immigrating immune cells are exerted. In contrast, the cascade of lymphocyte extravasation and locomotion within the connective tissue is well established.1,2 It is therefore conceivable that this novel functional property of αE(CD103)β7 plays a role for the intraepithelial functions of αE(CD103)β7-bearing cells in vivo.
First described as a marker for intestinal intraepithelial lymphocytes,4-7,29 integrin αE(CD103)β7 has been an enigmatic and tantalizing heterodimeric receptor. Although αE(CD103)β7 is expressed at high levels by CD8+ mucosal and epidermal T cells, it is also found on small subsets of T lymphocytes elsewhere. Approximately 30% of splenic CD4+CD25+ regulatory T cells express αE(CD103)β7, apparently independent of T-cell activation.14,30,31 The receptor has been implicated in guiding tissue localization of lymphocyte subsets under inflammatory conditions10,14,18 and/or allograft rejection.17,19,32,33 Expression of αE(CD103)β7 appears to be associated with cytotoxic activity of CD8+ T cells.19,20,34,35 Along this line, interactions of αE(CD103)β7 with E-cadherin appear to be crucial for lysis of autologous E-cadherin+ tumor cells by cytotoxic T cells in some cases.21 Expression of αE(CD103)β7 is associated with several important cellular activities of mucosal dendritic antigen presenting cells, such as antigen presentation13,22,36 or stimulation of Treg23 and some mucosal CD8+ T cells24,25 by αE(CD103)β7+ dendritic cells. Overall, a robust body of circumstantial evidence suggests an association of αE(CD103)β7 expression with important immune functions. It is nevertheless currently unclear whether and to what extent αE(CD103)β7 itself contributes directly to such cellular functions, so the precise role of αE(CD103)β7 in immune regulation still remains largely elusive. However, it appears reasonable to assume that the proper exertion of all of the above-mentioned immunologic functions attributed to αE(CD103)β7-expressing cells requires that the interacting immune cells adapt their shape according to preformed tissue structures that need to come in close contact to each other. Based on our results it is now conceivable that αE(CD103)β7 contributes to this process in some cell types.
One example of a naturally occurring cell type of the T-cell lineage, DETCs, has been investigated in this study using αE(CD103)β7-deficient mice and their wild-type counterparts. Supporting the hypothesis that αE(CD103)β7 facilitates the formation of cellular protrusions and filopodia “squeezing” between epidermal keratinocytes, DETCs of αE(CD103)-deficient mice showed significantly fewer and shorter dendrites as compared with wild-type DETCs. Forming dendrites and taking an appropriate shape are presumably important prerequisites for proper contact formation of DETCs with a maximum number of neighboring keratinocytes and other epidermal cells and, therefore, may play a role for at least some of the functions attributed to DETCs including maintenance of epidermal integrity, epithelial defense, wound healing, regression of skin tumors, or suppression of cutaneous graft-versus-host disease.37-40 It is therefore conceivable that αE(CD103)β7 is indirectly involved in such functions through facilitating the proper shape of DETCs within the epidermis. The hypothetical concern that the observed functional differences between wild-type and αE(CD103)β7-deficient DETCs might result from compensatory changes in other adhesion molecules appears unlikely, because no such changes could be detected in several candidate molecules. New transfection strategies, such as RNA electroporation into primary lymphocyte populations,41 might help to further investigate such issues in the future.
How the αE(CD103)β7 integrin influences the cellular shape and formation of filopodia is not entirely clear yet, but likely involves signaling interactions with components of the cytoskeleton, as evidenced by the abrogation in the presence of cytochalasin D. Such signaling events have been demonstrated for some other integrins including αIIbβ3 or α1β1.26,42 Likewise, it is possible that adaptor molecules contribute to the intracellular transmission of “outside-in” signals generated through the αE(CD103)β7 integrin, as has also been demonstrated for other integrins such as α4β1 or αLβ2.43-45 In any case, the morphologic differences in a defined population of naturally occurring T cells strongly suggest that αE(CD103)β7 influences the cellular shape not just in transfection-based artificial systems, but also in vivo, where the situation might be more complex and might involve a plethora of other adhesive interactions and/or mediators.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by a Rudolf Virchow Award and a research grant from the Deutsche Forschungsgemeinschaft (M.P.S.). G.H., M.J.L., and M.P.S. are members of the Rudolf Virchow Center.
Authorship
Contribution: S.S. generated and validated YFP-coupled constructs, performed transfections, video microscopy, animal experiments, and most of the functional experiments, and drafted the manuscript; H.S. generated YFP-coupled constructs and performed transfections; M.F., G.H., and M.J.L. critically contributed to the confocal microscopy, set up experimental conditions, contributed to the images containing photomicrographs, and made significant intellectual input to the manuscript; P.K. generated point-mutated αE-constructs and provided significant intellectual input to the manuscript; M.P.S. conceived and planned the study, contributed to the morphometric analyses, coculture studies, and preparation of epidermal sheets, and wrote the manuscript. All authors critically read and approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Michael P. Schön, Department of Dermatology and Venereology, Georg August University Göttingen, von Siebold Str 3, 37075 Göttingen, Germany, e-mail: michael.schoen@med.uni-goettingen.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal