Abstract
Dissociating graft-versus-tumor (GVT) effect from acute graft-versus-host disease (GVHD) still remains a great challenge in allogeneic bone marrow transplantation (allo-BMT). Bortezomib, a proteasome inhibitor, has shown impressive efficacy as a single agent in patients with hematologic malignancies but can result in toxicity when administered late after allogeneic transplantation in murine models of GVHD. In the current study, the effects of T-cell subsets and their associated cytokines on the efficacy of bortezomib in murine allogeneic BMT were investigated. Increased levels of serum tumor necrosis factor-α (TNFα) and interferon-γ (IFNγ) were observed after allo-BMT and continuous bortezomib administration. Bortezomib-induced GVHD-dependent mortality was preventable by depletion of CD4+ but not CD8+ T cells from the donor graft. The improved survival correlated with markedly reduced serum TNFα but not IFNγ levels. Transfer of Tnf−/− T cells also protected recipients from bortezomib-induced GVHD-dependent toxicity. Importantly, prolonged administration of bortezomib after transplantation of purified CD8+ T cells resulted in enhanced GVT response, which was dependent on donor CD8+ T cell–derived IFNγ. These results indicate that decreased toxicity and increased efficacy of bortezomib in murine allo-BMT can be achieved by removal of CD4+ T cells from the graft or by inhibiting TNFα.
Introduction
Allogeneic hematopoietic stem cell transplantation (HSCT) is an effective treatment for many hematologic malignancies due, in part, to the graft-versus-tumor (GVT) responses mediated by donor T cells in the graft. However, significant obstacles such as tumor relapse and graft-versus-host disease (GVHD) still limit the efficacy of this procedure.1-5 Even though GVHD and GVT are closely linked, recent clinical and experimental data indicate that separation of effective GVT from acute GVHD can be achieved.6
Although both CD4+ and CD8+ T cells can independently induce GVHD target organ damage, there are significant differences in their mechanisms of action.7,8 CD4+ T cells rely predominantly on the Fas/FasL pathway, whereas CD8+ T cells mediate pathology via the perforin/granzyme pathway.9 Cytokine-mediated cytotoxicity also has a central role in CD4+ T cell–mediated acute GVHD.10 Donor CD4+ T cells are the predominant T-cell population to produce interleukin (IL)–2 in the first several days after GVHD induction, which increases both the severity and mortality of GVHD.7,8 In vitro, the bulk of cytokine production occurs in human CD4+ T-cell populations after alloantigen stimulation.11 In addition, donor CD4+ T cell–derived tumor necrosis factor-α (TNFα) significantly contributes to the early proinflammatory events of GVHD.12
Proteasome inhibition using bortezomib has emerged as an effective anticancer therapy and may have far-reaching potential in allogeneic bone marrow transplantation (allo-BMT) due to its antitumor and immunomodulatory effects.13 We and others have independently demonstrated that bortezomib administered immediately after allogeneic HSCT resulted in marked inhibition of acute GVHD while still preserving GVT responses.14-16 However, prolonged or delayed administration of bortezomib in murine allogeneic BMT resulted in exacerbated GVHD-dependent mortality due to severe gut pathology. This finding was correlated with significant increases of proinflammatory cytokines in the serum and TNF receptor expression in the gut. Therefore, further studies are necessary to develop strategies to improve the therapeutic efficacy of bortezomib and for the separation of GVT from GVHD.
The current results demonstrate that removal of CD4+ donor T cells from the graft significantly decreased the degree of acute GVHD and bortezomib-induced lethal toxicity in 2 murine major histocompatibility complex (MHC)–mismatched allogeneic bone marrow transplantation (BMT) models. Notably, the removal of CD4+ donor T cells from the graft resulted in unique posttransplantation serum cytokine patterns with decreased levels of TNFα but not interferon-γ (IFNγ) in the serum. Furthermore, transplantation of donor CD4+ T cell–replete grafts from Tnf−/− donors in combination with prolonged bortezomib administration resulted in amelioration of acute GVHD lethality. Transplantation of allogeneic bone marrow cells with donor splenocytes depleted of CD4+ T cells or donor bone marrow and purified CD8+ T cells followed by prolonged administration of bortezomib promoted GVT. This increased antitumor effect was dependent on CD8+ T cell–derived IFNγ. These results suggest that modification of donor T-cell composition leading not only to increased IFNγ but also to decreased TNFα levels correlates with increased therapeutic efficacy of bortezomib and also allows for separation of GVT from GVHD.
Methods
Animals
All animal protocols were approved by the University of Nevada at Reno, Animal Care and Use Committee. Female BALB/cAnN (BALB/c, H2d) and C57BL/6N (B6, H2b) mice were purchased from the Animal Production Area of the National Cancer Institute (Frederick, MD). FVB/NJ (FVB, H2q) and B6.129S7-Ifn-γtm1Ts (Ifnγ−/−, H2b) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). C57BL/6 (H2b) Tnf−/− mice were provided by Dr Sergei Nedospasov (Engelhardt Institute of Molecular Biology, Moscow, Russia).17 Animals were kept in specific pathogen–free conditions. All animal protocols were approved and in vivo studies were performed at University of Nevada at Reno. Mice were between 8 and 13 weeks of age at the start of the experiments.
Reagents
Bortezomib was kindly provided by Millennium Pharmaceuticals (Cambridge, MA). Stock bortezomib solution (1 mg/mL) was prepared in Dulbecco phosphate-buffered saline solution (PBS) and stored at −70°C for up to 2 months before use. Bortezomib solutions were always protected from light. The stock solutions were thawed and diluted in PBS immediately before use. Monoclonal antibodies to mouse CD4 (clone GK1.5) or CD8 (clone 19-178) were produced in ascites fluid. Recipient C57BL/6 or BALB/c mice received PBS or bortezomib in PBS at a dose of 15 to 20 μg (C57BL/6) or 7.5 to 10 μg (BALB/c) intraperitoneally at the indicated time point after allo-BMT. T-cell subsets were depleted with either anti-CD8 or anti-CD4 (300 μg/dose) administered intraperitoneally resulting in less than 2% CD8+ T cells of total spleen cells and less than 1% CD4+ T cells, respectively, although after administration of anti-CD4 a proportion of CD4−CD8−CD3+ T cells appears in the spleen suggesting 55% to 90% of the CD4+ T cells were depleted and up to 45% of the remaining cells down-regulated CD4 expression. Controls were given rIgG (300 μg/dose; Jackson ImmunoResearch Laboratories, West Grove, PA). CD4+ or CD8+ T cells were purified (> 93% enrichment) by negative selection with a magnetic cell separation system (Miltenyi Biotec, Auburn, CA).
Cell preparation
Bone marrow cell (BMC) suspensions were prepared by gently releasing cells from the backbones, femurs, and tibiae into PBS with a mortar and pestle, filtering the cells through a mesh filter to remove particulates, and washing the cell suspensions twice. Spleen-cell preparations were prepared by gently crushing the tissues to release the cells. Preparations were filtered to remove debris and washed twice in PBS for injection. Cell counts were performed on a Coulter Z1 cell counter (Coulter Electronics, Hialeah, FL).
In vivo studies
Induction of GVHD studies was performed at University of Nevada according to the guidelines of the Animal Care and Use Committees. C57BL/6 (B6, H2b) or BALB/c (H2d) mice were used as recipients in the GVHD/GVT model systems. Recipient mice received total body irradiation (950 cGy to B6 and 650 cGy to BALB/c) from a 137Cs source. Irradiation was followed by the infusion of 10 to 15 × 106 allogeneic donor BMCs intravenously with or without splenocytes (SCs; 2-50 × 106 cells intravenously) as a source of allogeneic T cells. Recipient mice then received PBS, or bortezomib in PBS at a dose of 15 to 20 μg (C57BL/6) or 7.5 to 10 μg (BALB/c) intraperitoneally at the indicated time phase after cell infusion. Mice were monitored and weighed weekly. All moribund mice were humanely killed. All experiments were performed at least 3 times with 5 to 10 mice per group. For tumor studies, BALB/c (H2d) mice received 3 × 105 A20 tumor cells 6 days before irradiation and infusion of B6 (H2b) allogeneic donor cells. A20 is a spontaneously derived BALB/c B-cell lymphoma cell line.18
Serum cytokine analysis
Serum was collected on day 6 after BMT. Serum cytokine levels were determined by multiplex analysis on a Luminex instrument (Austin, TX), using mouse cytokine-specific bead sets and standards according to the manufacturer's instructions (R&D Systems, Minneapolis, MN). Serum samples (n = 3-5 per group) were analyzed from each of 3 independent experiments.
Intracellular cytokine analysis
Mesenteric lymph nodes were collected from mice on day +9 after BMT and cell suspensions were restimulated with 40 ng/mL phorbol 12-myristate 13-acetate (PMA), 800 ng/mL ionomycin, and GolgiStop (BD Pharmingen, San Diego, CA) for 5 hours. Cells were surface labeled with FITC anti-H2b and phycoerythrin, -cyanine 5 (PEC5), anti-CD4 (clone GK1.5), or anti-CD8 (clone 53-6.7) followed by fixation and permeabilization with IntraPrep (Beckman Coulter, Hialeah, FL). Cells were stained intracellularly with PE-TNFα (clone MP6-XT22), PE-IFNγ (clone XMG1.2), or isotype PE-labeled rat IgG1 (BD Pharmingen). Labeled cells were measured on a 3-color FACScan flow cytometer using Cell Quest software (Becton Dickinson, Fullerton, CA). All data sets were analyzed using FlowJo software (TreeStar, Ashland, OR).
Antibodies and flow cytometry
Cell suspensions from mesenteric lymph nodes were incubated with antibodies labeled with FITC, PE, and/or PE-cyanine 5 (PC5), and all samples were resuspended in 1% formaldehyde (Sigma-Aldrich, St Louis, MO) in 1 × phosphate-buffered saline solution. Mouse anti-CD3 (clone 17A2), anti-CD4 (clone H129.19), and anti-CD8 (clone 53-6.7; BD Pharmingen) were used to assess in vivo cell depletion. Listmode data files were collected on a 3-color FACScan flow cytometer using CellQuest software (Becton-Dickinson). All data sets were analyzed using FlowJo software.
Real-time quantitative polymerase chain reaction analysis
A section of the ileum was collected from mice on day 7 after BMT and stored in RNAlater (Qiagen, Valencia, CA) until processing. mRNA was extracted from tissues using RNA STAT-60 (Tel-Test, Friendswood, TX) according to the manufacturer's instructions followed by RNA cleanup using Qiagen RNAeasy columns (Valencia, CA). First-strand cDNA will be generated using Bio-Rad iScript cDNA Synthesis Kit (Hercules, CA). TNFR1 and ACTB primers were purchased from SuperArray Bioscience (Frederick, MD). Gene expression was analyzed by the real-time quantitative polymerase chain reaction (qRT-PCR) procedure and SYBR green detection method using an ABI Prism 7000 sequence detection system (Applied Biosystems, Foster City, CA) and normalized to ACTB expression. Gene expression is reported using the ΔΔCT method in which the average value measured in the control group served as the reference value.
Statistics
Survival data were plotted by the Kaplan-Meier method and analyzed by the log-rank test. Serum data were analyzed by one-way analysis of variance with Tukey posttest comparison. Flow cytometric data were analyzed by Student t test or Mann-Whitney test as appropriate for distribution of data. P values less than .05 were considered significant.
Results
Differential cytokine profiles of donor T-cell subsets during acute GVHD murine model
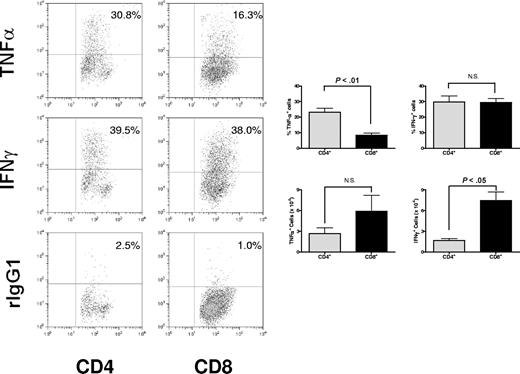
Although both CD4+ and CD8+ T cells can independently induce GVHD-associated organ damage, there are significant differences in their mechanisms of action that may be determined, at least in part, by the cytokines they produce. Therefore, we assessed whether there were differential cytokine polarization profiles in donor CD4+ and CD8+ T-cell subsets during the development of acute GVHD in a full MHC- and minor histocompatibility antigen–mismatched murine model. Lymphocytes were isolated from the mesenteric lymph nodes on day 9 after BMT and stimulated ex vivo with PMA and ionomycin to assess cytokine expression and analyzed for intracellular cytokine production in CD4+ and CD8+ T cells (Figure 1). The results demonstrate that significantly more (P < .01) CD4+ T cells than CD8+ T cells can produce TNFα, although in the mesenteric lymph nodes the ratio of CD4+ and CD8+ T cells favors the latter resulting in no significant difference in TNFα+CD4+ and TNFα+ CD8+ T cells. Conversely, there was no significant difference in frequency of intracellular cytokine expression of IFNγ T cells. However, there were significantly greater numbers of IFNγ+CD8+ T cells than IFNγ+CD4+ T cells in the mesenteric lymph nodes. These results are consistent with the previously reported findings that CD4+ T cells are a primary source of TNFα during the early phases of GVHD.12
Differential cytokine profiles in CD4+ and CD8+ T cells during acute murine GVHD. Mesenteric lymph nodes were collected on day +9 after BMT from irradiated BALB/c (H2d) recipients of 15 million B6 (H2b) bone marrow cells (BMCs) and 5 million splenocytes (SCs). Cells were assessed for intracellular TNFα or IFNγ expression in CD4+ cells and CD8+ cells. Significantly higher frequency of mesenteric lymph node (MLN) CD4+ T cells than CD8+ T cells (P < .01) expresses TNFα after nonspecific activation in vitro. The absolute number of TNFα+CD4+ and TNFα+CD8+ cells were not significantly different in the MLNs. Representative data from 1 of 3 independent experiments are presented as means plus or minus SEM (error bars).
Differential cytokine profiles in CD4+ and CD8+ T cells during acute murine GVHD. Mesenteric lymph nodes were collected on day +9 after BMT from irradiated BALB/c (H2d) recipients of 15 million B6 (H2b) bone marrow cells (BMCs) and 5 million splenocytes (SCs). Cells were assessed for intracellular TNFα or IFNγ expression in CD4+ cells and CD8+ cells. Significantly higher frequency of mesenteric lymph node (MLN) CD4+ T cells than CD8+ T cells (P < .01) expresses TNFα after nonspecific activation in vitro. The absolute number of TNFα+CD4+ and TNFα+CD8+ cells were not significantly different in the MLNs. Representative data from 1 of 3 independent experiments are presented as means plus or minus SEM (error bars).
Delayed bortezomib administration results in GVHD-dependent mortality in 2 different GVHD models of disease
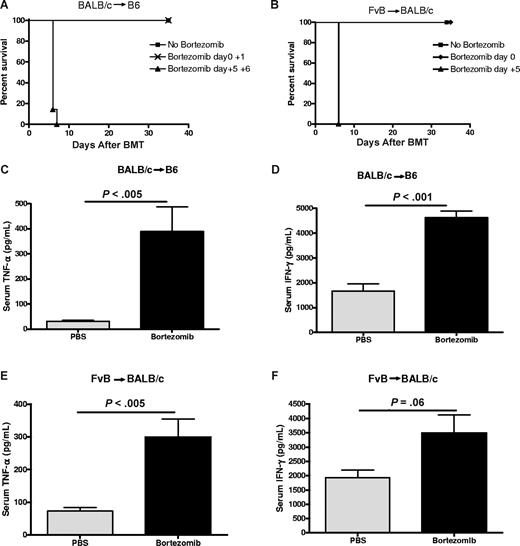
We and others have observed that although administration of the proteasome inhibitor, bortezomib, early after BMT can prevent GVHD, prolonged administration results in exacerbated acute GVHD–dependent mortality due to severe gut pathology.15,16 We had also shown that the pathology was associated with significant increases in inflammatory cytokines in the serum, including TNFα, and with increased expression of TNFR1 in the intestine. Moreover, this pathology was completely dependent on GVHD and potentially limits the use of continuous bortezomib administration as a cancer therapy after BMT. Based on the link between CD4+ T cells and TNFα production during GVHD, we next assessed whether there were differential effects of CD4+ and CD8+ T cells on this GVHD-dependent pathology. Bone marrow cells and a nonlethal dose of spleen cells from BALB/c (H2d) donors were transplanted into C57BL/6 (H2b) recipients after a myeloablative dose of total body irradiation (TBI). Delayed administration of bortezomib after BMT (days +5 and +6) resulted in rapid GVHD-dependent mortality, in contrast to nontreated controls (Figure 2A). This strain combination results in GVHD that is dependent on CD4+ T cells. Using another strain combination, we show that in an acute GVHD model in which both CD4+ and CD8+ donor T cells can independently contribute to acute GVHD induction (FvB donor cells [H2q] into BALB/c [H2d] recipients), severe bortezomib-induced lethality also occurred after administration on day +5 but not after administration immediately after BMT (day 0; Figure 2B). Marked increases in both TNFα and IFNγ levels were observed in the serum after delayed bortezomib treatment in both the BALB/c into B6 strain combination (Figure 2C,D) and the FvB into BALB/c strain combination (Figure 2E,F). Taken together, these results demonstrate that delayed administration of bortezomib results in GVHD-dependent lethality in 2 different strain combinations and is associated with significantly increased induction of inflammatory cytokines.
Delayed administration of bortezomib results in lethal toxicity in murine models of GVHD. (A) B6 (H2b) recipients of 15 million BALB/c (H2d) BMCs and 12 million spleen cells (SCs) were treated with or without 15 μg bortezomib per dose on days 0 to 1 or 5 to 6 after BMT (n = 6-7 mice/group). Representative data from 1 of 2 independent experiments are shown. (B) BALB/c (H2d) recipients of 10 million FvB (H2q) BMCs and 2 million SCs were treated with or without 7.5 μg bortezomib per dose on days 0 or 5 after BMT. Representative data from 1 of 2 independent experiments are shown. (C,D) B6 (H2b) recipients underwent transplantation and were treated with bortezomib on day + 5 after BMT as described in panel A. Serum was collected 12 hours after bortezomib administration and analyzed for TNFα and IFNγ levels (n = 3-8 mice/group). Representative data from 1 of 2 independent experiments are shown. Results are shown as means plus or minus SEM. (E,F) BALB/c (H2d) recipients of 10 million FvB (H2q) BMCs and 2 million SCs were treated with or without 7.5 μg bortezomib on day 5 after BMT. Serum was collected 12 hours after bortezomib administration and analyzed for TNFα and IFNγ levels (n = 5 mice/group). Results are shown as means plus or minus SEM. Representative data from 1 of 2 independent experiments.
Delayed administration of bortezomib results in lethal toxicity in murine models of GVHD. (A) B6 (H2b) recipients of 15 million BALB/c (H2d) BMCs and 12 million spleen cells (SCs) were treated with or without 15 μg bortezomib per dose on days 0 to 1 or 5 to 6 after BMT (n = 6-7 mice/group). Representative data from 1 of 2 independent experiments are shown. (B) BALB/c (H2d) recipients of 10 million FvB (H2q) BMCs and 2 million SCs were treated with or without 7.5 μg bortezomib per dose on days 0 or 5 after BMT. Representative data from 1 of 2 independent experiments are shown. (C,D) B6 (H2b) recipients underwent transplantation and were treated with bortezomib on day + 5 after BMT as described in panel A. Serum was collected 12 hours after bortezomib administration and analyzed for TNFα and IFNγ levels (n = 3-8 mice/group). Representative data from 1 of 2 independent experiments are shown. Results are shown as means plus or minus SEM. (E,F) BALB/c (H2d) recipients of 10 million FvB (H2q) BMCs and 2 million SCs were treated with or without 7.5 μg bortezomib on day 5 after BMT. Serum was collected 12 hours after bortezomib administration and analyzed for TNFα and IFNγ levels (n = 5 mice/group). Results are shown as means plus or minus SEM. Representative data from 1 of 2 independent experiments.
Donor CD4+ T cells are required for GVHD lethality associated with delayed bortezomib administration after allogeneic BMT
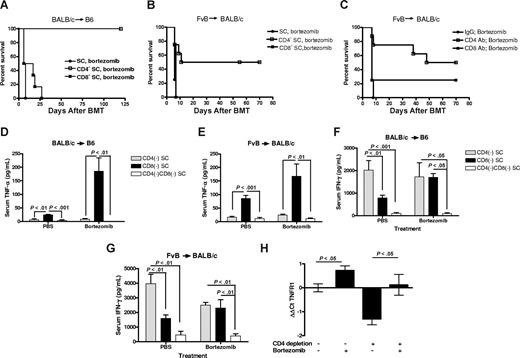
Cytokine-mediated cytotoxicity has been shown to be more actively involved in the early phase of GVHD induction and to have a central role in CD4-dependent GVHD.19,20 TNFα, particularly that derived from CD4+ T cells,12 has been associated with the gut damage and weight loss of GVHD.21,22 We therefore investigated whether selective removal of donor CD4+ cells would decrease GVHD target organ injury and reduce bortezomib-induced lethality. Donor mice were depleted of either CD4+ or CD8+ T cells using depleting antibodies in vivo. Allogeneic BMT was then performed using CD4- or CD8-depleted splenocytes. Significant (P < .01) increases in survival were observed in recipients of donor CD4+ T cell–depleted splenocytes compared with recipients of donor CD8+ T cell–depleted splenocytes or whole splenocytes in both the BALB/c into B6 and FvB into BALB/c GVHD models after delayed bortezomib administration (Figure 3A,B). Thus, these results demonstrate that modulating the donor graft through removal of CD4+ T cells can protect mice from the acute GVHD–induced gut damage.
Removal of donor CD4+ T cells decreases the risk of delayed bortezomib-related lethal toxicity. (A) B6 (H2b) recipients of 15 million BALB/c (H2d) bone marrow and 12 million SCs from donors depleted of CD4+ or CD8+ T cells in vivo, before tissue collection, were treated with 15 μg bortezomib per dose on day +5 and day +16 (n = 6 mice/group) and monitored for survival. Representative data from 1 of 2 independent experiments are shown. (B) BALB/c (H2d) recipients of 10 million FvB (H2q) bone marrow and 2 million SCs from donors depleted of CD4+ or CD8+ T cells in vivo, before tissue collection, were treated with 7.5 μg bortezomib on day +6 (n = 6 mice/group) and monitored for survival. (C) BALB/c (H2d) recipients of 10 million FvB (H2q) bone marrow and 2 million SCs were treated with indicated depleting mAb on day +4 and with 7.5 μg bortezomib per dose on day +6 after BMT (n = 5 mice/group) and monitored for survival. (D,F) B6 recipients of BALB/c cells underwent transplantation as described for panel A. Serum was collected on day +6 after BMT (n = 3-7 mice/group) and analyzed for TNFα and IFNγ. Representative data from 1 of 2 independent experiments are shown as means plus or minus SEM. (E,G) BALB/c recipients of 10 million FvB BMCs and 3 million SCs from donors that received CD4 and/or CD8 depleting antibody before tissue collection and treated as described in panel B. Serum was collected on day +6 after BMT (n = 5 mice/group) and analyzed for TNFα and IFNγ. Results are shown as means plus or minus SEM; representative data from 1 of 2 independent experiments are shown. (H) BALB/c recipients of FvB T cell–depleted bone marrow and 3 million SCs from untreated donors or in vivo CD4+ cell–depleted donors were treated with 10 μg bortezomib or PBS on day +6 and the ileum was collected for RT-qPCR analysis on day +7 (n = 3-6 mice/group). Data are expressed by the ΔΔCt method in which TNFR1 gene expression is normalized to ACTB in individual samples and then relative expression is compared with the mean value from the group receiving unfractionated spleen cells and PBS. Significant increases in TNFR1 gene expression are shown as means (± SEM; P < .05; Student t test).
Removal of donor CD4+ T cells decreases the risk of delayed bortezomib-related lethal toxicity. (A) B6 (H2b) recipients of 15 million BALB/c (H2d) bone marrow and 12 million SCs from donors depleted of CD4+ or CD8+ T cells in vivo, before tissue collection, were treated with 15 μg bortezomib per dose on day +5 and day +16 (n = 6 mice/group) and monitored for survival. Representative data from 1 of 2 independent experiments are shown. (B) BALB/c (H2d) recipients of 10 million FvB (H2q) bone marrow and 2 million SCs from donors depleted of CD4+ or CD8+ T cells in vivo, before tissue collection, were treated with 7.5 μg bortezomib on day +6 (n = 6 mice/group) and monitored for survival. (C) BALB/c (H2d) recipients of 10 million FvB (H2q) bone marrow and 2 million SCs were treated with indicated depleting mAb on day +4 and with 7.5 μg bortezomib per dose on day +6 after BMT (n = 5 mice/group) and monitored for survival. (D,F) B6 recipients of BALB/c cells underwent transplantation as described for panel A. Serum was collected on day +6 after BMT (n = 3-7 mice/group) and analyzed for TNFα and IFNγ. Representative data from 1 of 2 independent experiments are shown as means plus or minus SEM. (E,G) BALB/c recipients of 10 million FvB BMCs and 3 million SCs from donors that received CD4 and/or CD8 depleting antibody before tissue collection and treated as described in panel B. Serum was collected on day +6 after BMT (n = 5 mice/group) and analyzed for TNFα and IFNγ. Results are shown as means plus or minus SEM; representative data from 1 of 2 independent experiments are shown. (H) BALB/c recipients of FvB T cell–depleted bone marrow and 3 million SCs from untreated donors or in vivo CD4+ cell–depleted donors were treated with 10 μg bortezomib or PBS on day +6 and the ileum was collected for RT-qPCR analysis on day +7 (n = 3-6 mice/group). Data are expressed by the ΔΔCt method in which TNFR1 gene expression is normalized to ACTB in individual samples and then relative expression is compared with the mean value from the group receiving unfractionated spleen cells and PBS. Significant increases in TNFR1 gene expression are shown as means (± SEM; P < .05; Student t test).
To confirm that selective removal of donor CD4+ cells can significantly decrease bortezomib-induced GVHD-dependent lethal toxicity, we performed selective in vivo CD4+ or CD8+ T-cell depletion in the recipients 4 days after allogeneic BMT followed by administration of bortezomib on day 6. This protocol would allow us to determine whether later intervention, by T-cell subset removal, is protective. In agreement with the data in which the CD4+ T cells were removed before the BMT, in vivo depletion of CD4+ T cells after BMT abrogated the GVHD-dependent mortality resulting from delayed bortezomib treatment (Figure 3C), whereas in vivo depletion of CD8+ T cells showed no significant improvement on survival compared with mice receiving control antibody treatment. These results demonstrate that removal of the CD4+ T cells in the recipients, even if performed after allogeneic BMT, can result in protection from this augmented GVHD lethality.
Removal of CD4+ donor T cells from the graft significantly alters posttransplantation serum cytokine patterns in acute GVHD
We then ascertained the effect of CD4+ or CD8+ T-cell depletion on serum TNFα and IFNγ levels in both GVHD models after delayed bortezomib administration. One day after administration of bortezomib, TNFα levels were significantly decreased by the depletion of CD4+ cells compared with the depletion of CD8+ cells from the donor grafts in both strain combinations (Figure 3D,E), which correlated with the effects on survival. IFNγ levels were still observed after CD4+ T-cell depletion (Figure 3F,G) although reduced compared with whole spleen cell transfer (Figure 2C). In sharp contrast, depletion of CD8+ cells from the donor graft resulted in significant decreases in IFNγ compared with depletion of CD4+ cells in both GVHD models (Figure 3F,G). These data indicate that an increased level of TNFα in the serum is a characteristic predictor correlating with the degree of acute GVHD and is predominantly produced by CD4+ T cells.
We had previously reported that elevated gene expression of TNFR1 in the intestine of B6 recipients of unfractionated BALB/c grafts mice was also associated with the toxicity observed after delayed bortezomib administration.15 We therefore examined the effect of delayed bortezomib administration on intestinal TNFR1 gene expression in BALB/c recipients of either unfractionated or CD4-depleted spleen cell grafts. As shown in Figure 3H, delayed administration of bortezomib significantly increased intestinal TNFR1 gene expression compared with mice that received the same type of donor graft. However, there was a marked reduction in TNFR1 gene expression in the mice that received CD4-depleted spleen grafts compared with mice that received unfractionated spleen grafts. These data suggest that donor CD4+ T cells sensitize the intestine to bortezomib-mediated toxicity, at least in part by up-regulating TNFR1 expression.
Use of Tnf−/− donor CD4+ T cells protects bortezomib-treated mice from GVHD-dependent lethality
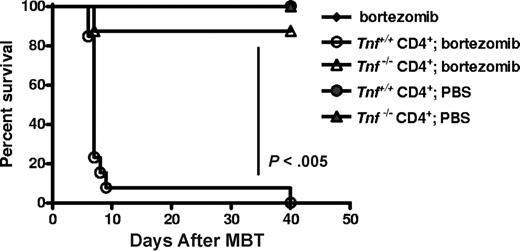
Donor CD4+ T cells have been reported to be the major contributor to increased serum TNFα associated with GVHD and T cells from Tnf−/− mice have been reported to exhibit less GVHD.12,23 We assessed the effect of donor CD4+ T cell–derived TNFα on bortezomib-induced GVHD lethality using T cells from Tnf−/− mice. Transplantation of Tnf−/− CD4+ T cells with Tnf+/+ BMCs followed by delayed bortezomib administration (day 5 after BMT) resulted in significant protection and survival (P < .005; Figure 4) compared with mice receiving CD4+ T cells from Tnf+/+ donors. These data demonstrate that donor CD4+ T cell–derived TNFα plays a critical role in the early acute GVHD associated with bortezomib-induced lethal toxicity and are further supported by the cytokine data in which CD4+ T-cell removal resulted in lower TNFα levels.
Recipients of Tnf−/− donor CD4+ T cells display decreased bortezomib-induced GVHD-dependent lethal toxicity. BALB/c (H2d) recipients of 15 million B6 (H2b) Tnf+/+ BM with or without either Tnf+/+ or Tnf−/− CD4+ T cells (5 × 105) were treated with 10 μg/dose bortezomib on day +5 after BMT (n = 7-14 mice/group). Significant increases in survival (P < .005; log rank test). Combined data from 2 independent experiments are shown.
Recipients of Tnf−/− donor CD4+ T cells display decreased bortezomib-induced GVHD-dependent lethal toxicity. BALB/c (H2d) recipients of 15 million B6 (H2b) Tnf+/+ BM with or without either Tnf+/+ or Tnf−/− CD4+ T cells (5 × 105) were treated with 10 μg/dose bortezomib on day +5 after BMT (n = 7-14 mice/group). Significant increases in survival (P < .005; log rank test). Combined data from 2 independent experiments are shown.
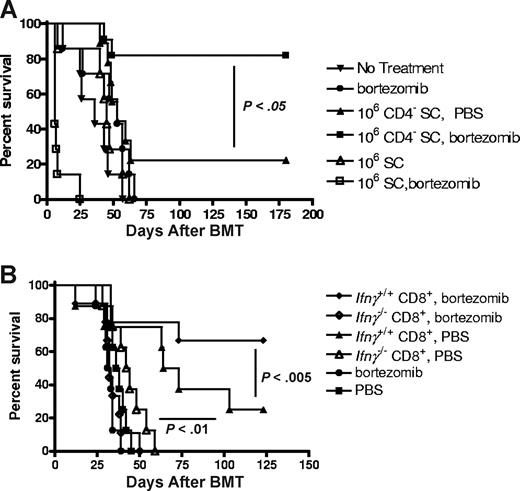
Posttransplantation bortezomib therapy with CD4+ T cell–depleted grafts results in enhanced GVT in advanced tumor-bearing mice
Removal of CD4+ donor T cells from the graft significantly decreased the severity of acute GVHD–associated bortezomib-induced toxicity. This finding suggests that this approach would allow for continuous bortezomib administration to be applied in tumor-bearing recipients, perhaps resulting in greater antitumor activity. Because removal of CD4+ donor T cells from the graft lowered TNFα levels yet preserved IFNγ production, we evaluated whether the GVT response can be effectively enhanced by posttransplantation bortezomib therapy. The spontaneously derived BALB/c B-cell lymphoma/leukemia, A20,18 was intravenously transplanted into BALB/c mice 6 days before allogeneic BMT with FvB donor cells. No significant difference was observed between recipients of allogeneic BM cells and untreated animals (Figure 5A). Administration of prolonged bortezomib therapy (days 0, +6, +11, and +16) after transplantation without donor T cells also did not improve survival, demonstrating the importance of GVT responses in this model. When the tumor-bearing mice received an allogeneic bone marrow transplant of donor splenocytes that were depleted of CD4+ T cells, significant increases in survival were observed compared with BMT treatment alone (P < .005; Figure 5A), indicating that significant GVT had been obtained. Importantly, a significantly greater increase in survival was observed in tumor-bearing mice that received an allogeneic bone marrow transplant of donor CD4+ T cell–depleted splenocytes and prolonged posttransplantation bortezomib therapy, compared with mice that received an allogeneic bone marrow transplant and either treatment alone, indicating additional antitumor responses had been obtained with the combination drug and cellular therapies (P < .05; Figure 5A). In agreement with the GVHD data, mice receiving the same numbers of whole splenocytes and prolonged posttransplantation bortezomib therapy quickly succumbed to GVHD-dependent lethality from delayed bortezomib (P < .05; Figure 5A), indicating that removal of CD4+ donor T cells in the graft allows for posttransplantation bortezomib administration and augmentation of GVT.
Posttransplantation bortezomib therapy enhances GVT in the absence of donor CD4+ T cells and is dependent on IFNγ production from donor CD8+ T cells. (A) BALB/c (H2d) mice received 3 × 105 A20 cells on day 6. On day 0, BALB/c (H2d) mice received 10 million FvB (H2q) bone marrow with or without 1 million unfractionated splenocytes or CD4 in vivo–depleted splenocytes. Mice received PBS or 7.5 μg/dose bortezomib on days 0, +6, +11, and +16 after BMT (n = 7-11 mice/group) and were monitored for survival. Representative data from 1 of 2 independent experiments are presented. (B) BALB/c (H2d) mice received 3 × 105 A20 cells on day 6. On day 0, BALB/c (H2d) mice received 10 million B6 (H2b) bone marrow with/without 4 × 105Ifnγ+/+ or Ifnγ−/− CD8+ T cells followed by 7.5 μg/dose bortezomib on days 0, +6, +11, and +16 after BMT (n = 8-9 mice/group). Representative data from 1 of 3 independent experiments are presented. Prolonged bortezomib therapy after allogeneic BMT result in significant decreases in survival in the mice that received Ifnγ−/− CD8+ T cells compared with mice that received Ifnγ+/+ CD8+ T cells (P < .005; log rank test).
Posttransplantation bortezomib therapy enhances GVT in the absence of donor CD4+ T cells and is dependent on IFNγ production from donor CD8+ T cells. (A) BALB/c (H2d) mice received 3 × 105 A20 cells on day 6. On day 0, BALB/c (H2d) mice received 10 million FvB (H2q) bone marrow with or without 1 million unfractionated splenocytes or CD4 in vivo–depleted splenocytes. Mice received PBS or 7.5 μg/dose bortezomib on days 0, +6, +11, and +16 after BMT (n = 7-11 mice/group) and were monitored for survival. Representative data from 1 of 2 independent experiments are presented. (B) BALB/c (H2d) mice received 3 × 105 A20 cells on day 6. On day 0, BALB/c (H2d) mice received 10 million B6 (H2b) bone marrow with/without 4 × 105Ifnγ+/+ or Ifnγ−/− CD8+ T cells followed by 7.5 μg/dose bortezomib on days 0, +6, +11, and +16 after BMT (n = 8-9 mice/group). Representative data from 1 of 3 independent experiments are presented. Prolonged bortezomib therapy after allogeneic BMT result in significant decreases in survival in the mice that received Ifnγ−/− CD8+ T cells compared with mice that received Ifnγ+/+ CD8+ T cells (P < .005; log rank test).
Donor IFNγ+ CD8 T cells are the primary contributors to bortezomib-enhanced GVT responses
Because elevated IFNγ levels were observed in the sera of recipients of donor CD4+ T cell–depleted splenocytes, and because posttransplantation bortezomib therapy enhanced GVT of CD8+ T cells, it is possible that IFNγ may represent an important pathway for CD8+ T cell–mediated GVT. We next investigated the mechanism of bortezomib-enhanced GVT response in the recipients of donor CD8+ T cells from Ifnγ−/− or Ifnγ+/+ B6 mice together with B6 Ifnγ+/+ bone marrow cells. A20 tumor–bearing BALB/c mice underwent an allogeneic BMT 6 days after getting the tumor. As expected, significant increases in survival were observed in recipients of CD8+ T cells from WT donors compared with recipients of BM alone (P < .01; Figure 5B), indicating that the GVT response resulted from donor CD8+ T cells. Similarly, the CD8+ T cell–mediated GVT response could be further enhanced by prolonged bortezomib administration after allogeneic BMT (P < .005). However, protection was significantly impaired in recipients of Ifnγ−/− donor CD8+ T cells (P < .01; Figure 5B). A dose of donor CD8+ T cells was used in which negligible effects on GVHD were obtained despite the absence of IFNγ. Necropsy of the recipients indicated that the majority of the recipients of Ifnγ−/− CD8+ T cells succumbed to tumor. These data indicate that donor CD8+ T cell–derived IFNγ is critical for bortezomib-enhanced GVT responses after allogeneic BMT in tumor-bearing recipients and protects from GVHD lethality.
Discussion
During allogeneic BMT, donor T-cell composition of the allograft is an important factor affecting the incidence and severity of acute GVHD.3-6 Because total reduction of donor T cells by pan-T depletion from the allograft also significantly attenuates GVT, selective removal of donor T-cell subsets represents one of the optimal strategies to preserve allogeneic T-cell responses with reduced GVHD.6,24,25 One of the novel findings in our study was that removal of CD4+ T cells results in marked protection from bortezomib-mediated GVHD even in a murine model in which CD4+ T cells and CD8+ T cells can independently induce disease, and yet still allowed GVT to occur. We also found that TNFα production by CD4+ T cells was a major mediator of bortezomib-mediated GVHD, whereas IFNγ production by CD8+ T cells was crucial for GVT and that continuous bortezomib treatment significantly enhanced the antitumor response. We had previously shown not only that could bortezomib be given safely immediately after allo-BMT but also that it resulted in reduction in acute murine GVHD.14 However, we and others had also shown that delaying administration resulted in severe, lethal exacerbation of GVHD.15,16 GVT was retained with peritransplantation administration of bortezomib, but the limited and early time course of treatment limited the ability of this drug to sensitize tumor cells to immune-mediated killing.26 Thus, these observations allowed for the use of delayed, continuous bortezomib administration to promote antitumor effects after allo-BMT. Our studies suggest that bortezomib-enhanced GVT activity and bortezomib-induced GVHD-dependent gut toxicity can be at least partially separated based on their different cytokines, and this may represent mechanisms by which these cytokines mediate their effector function.
It has been demonstrated that allogeneic donor T cells use different cytotoxicity pathways to mediate tissue-specific destruction of GVHD target organs.27,28 The gut, a major GVHD target organ, has been demonstrated to be highly sensitive to injury by TNFα.23 Previous studies have demonstrated the importance of donor T cell–derived TNFα in murine models of GVHD,23 particularly in CD4+ T cell–mediated disease.12,29 Indeed, in clinical studies, elevated serum TNFα and soluble TNF receptor levels have been shown to be predictive of GVHD.2 Consistent with these observations, we have found that bortezomib-mediated GVHD-dependent toxicity is most severe during the early phase of GVHD induction, and that recipients of Tnf−/− donor CD4+ T cells were protected. Although our data would implicate CD4+ T cells in bortezomib-mediated GVHD pathology, it is not absolute. As others have shown that TNFα can potentiate CD8+ T-cell GVHD but to a lesser effect than in CD4+ T cell–mediated disease,29 we also found that transplantation of much greater numbers of purified allogeneic CD8+ T cells correlated with increasing TNFα levels, and can also result in significant GVHD-dependent mortality after delayed bortezomib administration (data not shown), suggesting that the safety window of opportunity for posttransplantation bortezomib therapy is not limitless when alloreactive T cells are given, regardless of subset or cytokine profile. In addition, although protection was observed using Tnf−/− T cells, other proinflammatory cytokines (ie, IL-6) likely also play a role.
In contrast to the effects of TNFα on GVHD, there is accumulating experimental data that suggest IFNγ may also mediate protection against acute GVHD.30-32 However, increased IFNγ production in serum does correlate with the increased severity of acute GVHD, which may be contingent on timing of assessment.7 We and others have previously demonstrated that the absence of donor T cell–derived IFNγ can be deleterious in murine acute GVHD models.30,32 It has been shown that at least one mechanism underlying this increased GVHD was through an impaired ability of the donor T cells to undergo activation-induced cell death via fas.33
Others have shown that IFNγ is necessary for the optimal induction of allogeneic CD8+ T cell–mediated GVT responses,33 by directly suppressing tumor cell proliferation and promoting donor T cell–mediated immune response.34-36 Therefore, donor T cell–derived IFNγ would be a critical factor for the separation of GVT from acute GVHD.33 In our current study, removal of CD4+ donor T cells from the graft resulted in decreased levels of serum TNFα but not IFNγ. We also found that although IFNγ was required for CD8+ T cell–mediated GVT, the enhanced GVT response was did not correlate with increased serum IFNγ levels. Taken together, these observations suggest that IFNγ is a critical requirement for CD8+ T cell–mediated GVT but not direct cytokine-mediated cytotoxicity. However, we cannot rule out that CD4+ T-cell production of IFNγ may also contribute to CD8+ T cell–mediated GVT. Our findings are consistent with the recent study demonstrating the requirement for cognate interaction between T cells and tumor in either CD4+ or CD8+ T cell–mediated GVT.37 It should be noted that A20 tumor cells are sensitive to both CD4+ and CD8+ T cell–mediated allogeneic antitumor responses. A20 expresses both MHC I and MHC II,38 and enriched FvB CD4+ T cells do mediate GVT in BALB/c recipients (data not shown). However, the association of bortezomib-mediated GHVD toxicity with donor CD4+ T cells prevents the evaluation of bortezomib effects on CD4+ T cell–mediated GVT. Thus, our data demonstrate that donor-derived IFNγ is required for bortezomib-enhanced CD8+ T cell–mediated GVT responses, as the GVT response was lost in recipients of allogeneic Ifnγ−/− CD8+ T cells, either with or without posttransplantation bortezomib therapy.
In summary, the data presented here demonstrate that TNFα produced by CD4+ T cells is a major contributor to bortezomib-mediated GVHD toxicity and that selective CD4+ T-cell depletion or the absence of donor-derived TNFα can result in protection and prolonged administration of bortezomib after transplantation. Importantly, IFNγ produced by CD8+ T cells is required for GVT responses, and continuous treatment with bortezomib after transplantation results in significantly enhanced antitumor responses than either modality alone.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Drs Ruth Gault, William H. D. Hallett, and Kory Alderson for assisting in the preparation of the paper and helpful discussions, and Millennium Pharmaceuticals for providing bortezomib. We thank Weihong Ma and Megan Whitaker for their technical assistance with animal studies; Danice E. C. Wilkins, Angela Panoskaltsis-Mortari, Melinda Berthold, and Ryan Fremming for their technical assistance with the cytokine analysis; and Dane Bay for technical assistance with the qPCR assay.
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Health Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US government.
This work was supported in part by National Institutes of Health (NIH, Bethesda, MD) grant R01 CA102282 and with federal funds from the National Cancer Institute of the National Institutes of Health under contract no. N01-CO-12400, Intramural Research Program of the Center for Cancer Research.
National Institutes of Health
Authorship
Contribution: K.S. designed and performed research, analyzed data, and wrote the paper; M.L. designed and performed research and analyzed data; T.J.S. designed research; L.A.W. designed research, analyzed data, and participated in writing the paper; and W.J.M. designed research and participated in writing the paper.
Conflict-of-interest disclosure: W.J.M. has received honoraria from Millennium Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: William J. Murphy, Professor and Chair, Department of Microbiology & Immunology, University of Nevada School of Medicine, Mail Stop 199, Reno, NV 89557; e-mail: wmurphy@medicine.nevada.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal