In this issue of Blood, Devine and colleagues give evidence that AMD3100 (Plerixafor), the direct antagonist of CXCR4/SDF-1, can induce mobilization of CD34+ hematopoietic stem cells with rapidity and in quantities sufficient for an allogeneic transplant. Twenty patients with hematologic malignancies showed prompt engraftment after myeloablative conditioning and infusion of AMD3100-mobilized donor cells, with no unexpected adverse events. A single-day stem-cell collection procedure has become reality.
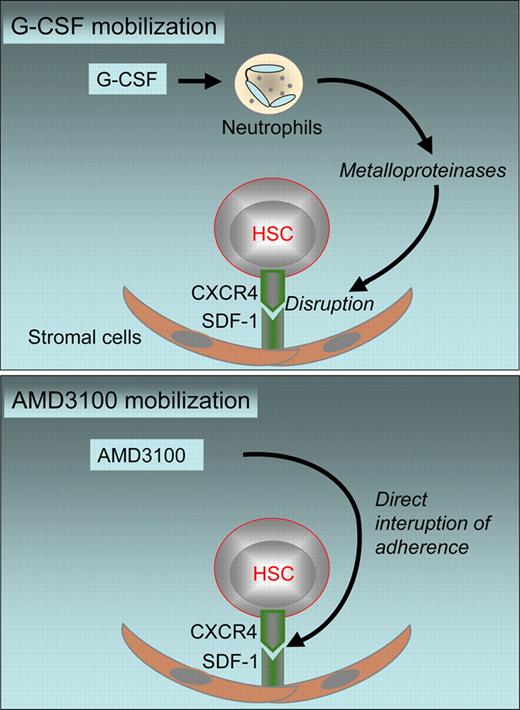
Peripheral blood stem cells (PBSCs) have become the stem-cell source of choice for autologous hematopoietic stem cell transplants (HSCTs) and for two-thirds of allogeneic HSCTs.1 Their more rapid engraftment and ease of collection, compared with cumbersome bone marrow (BM) harvest procedures, have been the key elements for this change. Still, the collection process is complex and time-consuming, and requires logistical adjustments. Stem cells need to be mobilized from their hematopoietic niche. Currently, mobilization is induced by chemotherapy (in the autologous setting) alone or in combination with granulocyte colony–stimulating factor (G-CSF) or by G-CSF alone (in the allogeneic setting). The mobilization process is indirect. G-CSF induces a release of metalloproteinases, which disrupt the CXCR4/SDF-1 axis and thus provoke the exit of stem cells (see top panel of figure).2 Donors have to inject the drug over a 4- to 6-day period. Timing and coordination between many departments, clinicians, and other participants is required.
Mechanisms of HSC mobilization with G-CSF (top panel) and AMD3100 (bottom panel).
Mechanisms of HSC mobilization with G-CSF (top panel) and AMD3100 (bottom panel).
A direct antagonist of the CXCR4/SDF-1 axis (see bottom panel of figure) has been developed as an alternative mobilization option.2 Animal models and preliminary data have shown promise. Plerixafor (AMD3100) alone has induced rapid mobilization of CD34+ cells within a few hours. Numbers of circulating CD34+ cells in G-CSF–primed patients increased significantly when AMD3100 was added on the last day of mobilization. Transplants with these cells showed rapid engraftment after autologous HSC transplantation.3
In a convincing study, Devine and coauthors show that this novel approach can be used successfully in allogeneic HSCTs as well. For allogeneic HSCTs, 25 donors were treated with a single dose of 240 μg/kg AMD3100 and underwent apheresis as soon as 4 hours later, with collection of enough cells for a transplant (a sufficient cell dose was defined as > 2 × 106 CD34+ cells/kg) in two-thirds of the donors with a single dose and collection. Twenty stem-cell products were transplanted into patients with hematologic malignancies. All had received myeloablative conditioning. Engraftment was rapid and complete. Acute graft-versus-host disease (GVHD) occurred in 35% of patients. No unexpected adverse events were observed. Pain at the injection site was noted. Lightheadedness, nausea, bloating, flatulence, perioral paresthesias, loose stools, diapheresis, and headache were seen during mobilization. All toxicities were mild and resolved within a few hours without intervention.
These data are convincing. Will Plerixafor replace mobilization with G-CSF from now on? The ease of the collection procedure is an argument toward the affirmative. Harvesting teams will be excited. Donors can come in early in the morning, receive mobilization under supervision, and be free to go home by evening.
There are some caveats; it is unlikely that everything will change. Graft products show significant differences. CD34+ cell content is lower, and CD3+ cell content significantly higher, after AMD3100 than after G-CSF mobilization. More recipients of AMD3100-mobilized cells need to be followed for a longer period of time to ascertain safety. Even with G-CSF, too little information is yet available to compare BM and PBSC grafts in allogeneic recipients. There is an advantage to the use of PBSC transplants early on, what with more rapid engraftment and lower mortality in high-risk patients. On the other hand, there is a higher incidence of GVHD and higher transplant-related mortality during follow-up. For patients with aplastic anemia and for patients with low-risk disease, such as chronic myeloid leukemia in first chronic phase, survival is significantly worse with PBSC than with BM transplants.4 The higher T-cell count after AMD3100 collection might increase the risk of GVHD even further.
Lastly, little is known about the impact of AMD3100 on the donors at median or long-term follow-up. No information is yet available on toxicities with higher donor numbers. In a recent report, 1 donor death was reported to occur per roughly 10 000; severe adverse events, in 1 of about 1000 donors.5 The potential risk of hematological malignancies after G-CSF mobilization still remains a matter of debate. Experience with AMD3100 is far too limited to exclude potential toxicity. The supplier of AMD3100 and the transplant community will both face challenges in collecting appropriate long-term data.
Despite these reservations, there is proof of principle now that stem-cell collection in sufficient numbers can become feasible within 1 day. A great relief for donors and harvest centers is in sight.
Conflict-of-interest disclosure: A.G. is a consultant to Polyphor (Allschwil, Switzerland) and has received research support from Amgen (Zug, Switzerland) and Roche (Basel, Switzerland). A.W.-F. declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal