Abstract
Over the years, methods of cytogenetic analysis evolved and became part of routine laboratory testing, providing valuable diagnostic and prognostic information in hematologic disorders. Karyotypic aberrations contribute to the understanding of the molecular pathogenesis of disease and thereby to rational application of therapeutic modalities. Most of the progress in this field stems from the application of metaphase cytogenetics (MC), but recently, novel molecular technologies have been introduced that complement MC and overcome many of the limitations of traditional cytogenetics, including a need for cell culture. Whole genome scanning using comparative genomic hybridization and single nucleotide polymorphism arrays (CGH-A; SNP-A) can be used for analysis of somatic or clonal unbalanced chromosomal defects. In SNP-A, the combination of copy number detection and genotyping enables diagnosis of copy-neutral loss of heterozygosity, a lesion that cannot be detected using MC but may have important pathogenetic implications. Overall, whole genome scanning arrays, despite the drawback of an inability to detect balanced translocations, allow for discovery of chromosomal defects in a higher proportion of patients with hematologic malignancies. Newly detected chromosomal aberrations, including somatic uniparental disomy, may lead to more precise prognostic schemes in many diseases.
Routine cytogenetics diagnostics and its limitations
Cytogenetic analysis has provided fundamental insights into the molecular pathogenesis of a variety of hematologic disorders. The discovery of the Philadelphia chromosome and other chromosomal aberrations paved the way for the characterization of molecular lesions that lead to phenotypic characteristics of a number of hematologic malignancies. Metaphase cytogenetics (MC) has proven an extremely valuable diagnostic tool, providing definite diagnoses, for example, in the case of balanced pathognomonic translocations in chronic myeloid leukemia (CML), acute promyelocytic leukemia (APL), or core binding factor acute myelogenous leukemias (AMLs).1,2 Chromosomal aberrations support the cytomorphologic diagnosis and provide prognostic information, for example, in myelodysplastic syndromes (MDSs),3-5 AML with unbalanced translocations,6-10 multiple myeloma (MM),11,12 and chronic lymphocytic leukemia (CLL).13,14 Irrespective of their location, size, and corresponding phenotype, chromosomal defects also represent suitable markers of clonality suggestive of clonal dominance by the malignant clone or, in rare circumstances, of oligoclonality due to a profound depletion of stem cell reserves15 (Figure 1). Such a scenario explains the occasional occurrence of chromosomal abnormalities in otherwise typical aplastic anemia (AA; M. Wlodarski, L. Gondek, C. L. O'Keefe, R. Tiu, A. Haddad, A. Risitano, J.P.M., manuscript submitted May 2008).
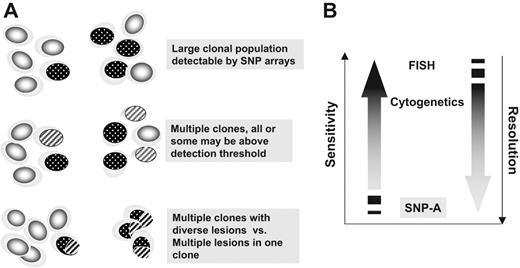
Clonality in hematopoietic diseases. Chromosomal abnormalities or somatic mutations can be used as markers of clonality. Clonal defects (a pathogenic lesion or a marker indicative only of clonality) can be detected by, for example, SNP-A–based karyotyping (sensitivity problem) only if present in a significant proportion of cells. True malignant expansion of the dominant clone has to be contrasted with the clonality due to contraction of the cell compartment resulting in a recruitment of only one or a few stem cells at any given time.
Clonality in hematopoietic diseases. Chromosomal abnormalities or somatic mutations can be used as markers of clonality. Clonal defects (a pathogenic lesion or a marker indicative only of clonality) can be detected by, for example, SNP-A–based karyotyping (sensitivity problem) only if present in a significant proportion of cells. True malignant expansion of the dominant clone has to be contrasted with the clonality due to contraction of the cell compartment resulting in a recruitment of only one or a few stem cells at any given time.
Technical advantages and limitations of MC
Routine MC allows for detection of large clonal populations (> 10% of dividing cells), indicating the presence of significantly expanded and dominant clones.16-19 Moreover, multiple clones may be detected and can be distinguished from complex karyotypic lesions occurring within a single clone.
Many invariant chromosomal lesions affect the behavior of malignant clones, their progression, response to therapy, and patient survival. This information has been incorporated in predictive schemes in various hematologic malignancies. For example, the International Prognostic Scoring System (IPSS) for MDS provides an algorithm that is predominantly based on the types of cytogenetic lesions.20
Chromosomal abnormalities are present only in a proportion of cases including, for example, 50% of MDSs or AMLs and to a lesser extent in other myeloid malignancies. This inability to detect lesions may be due to the relatively low resolution of MC, estimated to be 1 to 10 Mb.16-19 Although a normal MC result by itself tends to indicate a more favorable prognosis, there is great clinical diversity within patients with normal MC, and this large group cannot be further stratified using cytogenetics as a parameter. In addition, reliance on cell division leaves a significant proportion of cases unresolved due to lack of growth or specimen hypocellularity. Theoretically, cryptic clonal lesions are present in many, if not most, cases of AML, MM, and MDS, but cannot be detected due to their size or due to technical shortcomings of MC. Such additional “hidden” lesions may also exist in patients with known abnormalities, further contributing to clinical heterogeneity and modifying the prognosis assigned to known invariant aberrations. Moreover, smaller chromosomal changes may allow for better mapping of causative genes in commonly deleted regions (CDRs).
New cytogenetic methods
Increased sensitivity and resolution are likely to improve the diagnostic value of cytogenetic diagnostics. Various karyotypic methods have been introduced, including spectral karyotyping (SKY) and fluorescent in situ hybridization (FISH). To date, only FISH is routinely applied for targeted detection of specific lesions with established clinical relevance.21 However, as in the case of unbalanced translocations, the increased sensitivity of FISH for unbalanced chromosomal defects is difficult to translate into prognostic information. For example, the presence of a small proportion of abnormal cells may simply indicate that hematopoiesis is not entirely clonal. Moreover, if the difference between the “background” intrinsic to the technique seen in otherwise normal bone marrow and the size of the abnormal clone in a patient is small, its relevance may not be clear. However, due to the speed and ease of application and lack of reliance on cell growth, FISH is widely applied as a reliable diagnostic tool in the search for specific balanced translocations.21 Clinical FISH is performed on interphase cells, including more mature nondividing progeny, whereas MC requires hematopoietic progenitor cells that have been stimulated to divide (Table 1). Even though FISH is very sensitive, it can be applied only in a targeted fashion. Hence, a comprehensive screening for chromosomal aberrations cannot be carried out using this technique.
Comparison of MC with novel methods of karyotyping using array-based whole genome scanning
| Feature . | Technique . | |||||
|---|---|---|---|---|---|---|
| MC . | SNP-A . | SNP/Copy no. oligo arrays . | Oligo/BAC CGH-A . | FISH . | SKY . | |
| Resolution | + | ++++ | ++++ | ++++/++ | ++ | +++ |
| Sensitivity | + | + | + | + | +++ | + |
| Copy neutral LOH | − | + | + | − | − | − |
| Need for cell division | + | − | − | − | − | + |
| Detection of balanced lesions | + | − | − | − | + | − |
| Distinction of multiple clones | + | − | − | − | + | − |
| Screening for unknown defects | + | + | + | + | − | + |
| Feature . | Technique . | |||||
|---|---|---|---|---|---|---|
| MC . | SNP-A . | SNP/Copy no. oligo arrays . | Oligo/BAC CGH-A . | FISH . | SKY . | |
| Resolution | + | ++++ | ++++ | ++++/++ | ++ | +++ |
| Sensitivity | + | + | + | + | +++ | + |
| Copy neutral LOH | − | + | + | − | − | − |
| Need for cell division | + | − | − | − | − | + |
| Detection of balanced lesions | + | − | − | − | + | − |
| Distinction of multiple clones | + | − | − | − | + | − |
| Screening for unknown defects | + | + | + | + | − | + |
Plus and minus signs indicate increases and decreases in the quality of individual methods.
LOH indicates loss of heterozygosity.
DNA whole genome scanning technology using DNA arrays
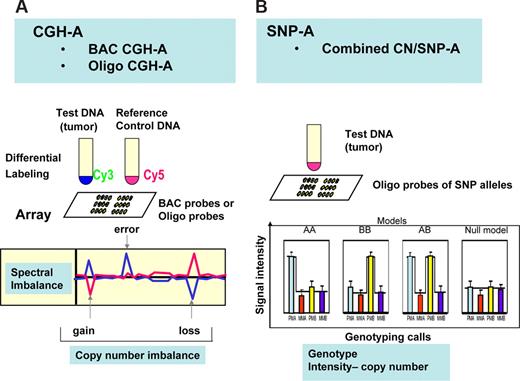
Array-based technologies applied for karyotyping of inherited and somatic chromosomal aberrations include single nucleotide polymorphism arrays (SNP-As), copy number polymorphism oligonucleotide arrays (oligo-As), and comparative genome hybridization arrays (CGH-As) with oligonucleotide and bacterial artificial chromosome (BAC) probes (Figure 2).
General principles of CGH-A and SNP-A arrays. (A) In CGH-A, control DNA is used as reference for the test DNA obtained from putative tumor DNA. Analysis of spectra generated through hybridization of differentially labeled DNA to oligo or BAC probes on array is shown below. Decreased copy number in the tumor DNA results in decreased intensity of the signal for the test and increased signal for reference DNA. (B) In SNP-A, hybridization of amplified and labeled DNA to probes corresponding to alleles for each locus results in a genotyping pattern allowing for determination of the heterozygosity or homozygosity for each allele. At the same time, intensity of the hybridization signals allows for determination of copy number changes.
General principles of CGH-A and SNP-A arrays. (A) In CGH-A, control DNA is used as reference for the test DNA obtained from putative tumor DNA. Analysis of spectra generated through hybridization of differentially labeled DNA to oligo or BAC probes on array is shown below. Decreased copy number in the tumor DNA results in decreased intensity of the signal for the test and increased signal for reference DNA. (B) In SNP-A, hybridization of amplified and labeled DNA to probes corresponding to alleles for each locus results in a genotyping pattern allowing for determination of the heterozygosity or homozygosity for each allele. At the same time, intensity of the hybridization signals allows for determination of copy number changes.
Single-nucleotide polymorphism arrays
SNP-As were developed to genotype germ line–encoded polymorphisms and use chips with oligonucleotide probes homologous to areas located throughout human genome. DNA probes are designed to distinguish various genotypes, and their distribution is dictated by the location of the SNPs.22,23 Consequently, the resolution of SNP-A depends upon the density of the probes and the distribution of SNPs throughout the genome.24,25 Areas known to contain more SNPs can be better covered, whereas, as expected, the resolution within “SNP deserts” is poor.
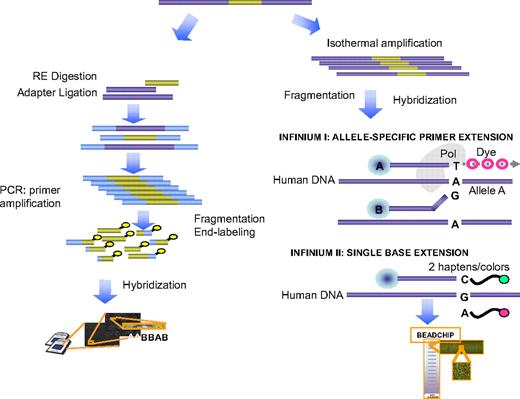
Chip- and bead-based platforms of SNP-A are available. In the technology used by Affymetrix (Santa Clara, CA), genomic DNA is digested by restriction endonucleases, amplified, and labeled. After hybridization to the arrays, a readout can be generated that includes genotyping calls. Furthermore, the strength of the hybridization signal corresponds to the copy number of the genomic region (Figures 2,3). In bead-based platforms, the whole genome amplification and fragmentation steps are followed by hybridization to an oligonucleotide bead array. One of 2 bead types correspond to each allele in the SNP locus and allelic specificity is conferred by enzymatic (allele-specific primer or single base) extension and fluorescent staining. Both technologies provide independent measurements of loss of heterozygosity (LOH) by genotyping (frequency of heterozygous calls) and determination of hybridization intensity, a feature that allows for reduction of the variability of results24,26 (Figure 4). Analysis of these 2 parameters increases the precision of copy number determination.24,26 The data output can be generated using several biostatistical and genetic software packages based on various bioanalytical principles; one excellent example of such an algorithm is CNAG,23,27 which combines copy number analysis and LOH (Figures 4,5).
Principles of basic methods of SNP-A and its application for karyotyping. The generalized principle behind the technology used in Affymetrix SNP-A is shown in the left portion of the figure, whereas the bead array platform used by Illumina (San Diego, CA) is depicted in the right with both 1- and 2-color variants used in Infinium I and II arrays (Illumina). Below, the principle of genotyping based on the hybridization pattern for allelic probes is shown. For a detailed description, see “Single-nucleotide polymorphism arrays.”
Principles of basic methods of SNP-A and its application for karyotyping. The generalized principle behind the technology used in Affymetrix SNP-A is shown in the left portion of the figure, whereas the bead array platform used by Illumina (San Diego, CA) is depicted in the right with both 1- and 2-color variants used in Infinium I and II arrays (Illumina). Below, the principle of genotyping based on the hybridization pattern for allelic probes is shown. For a detailed description, see “Single-nucleotide polymorphism arrays.”
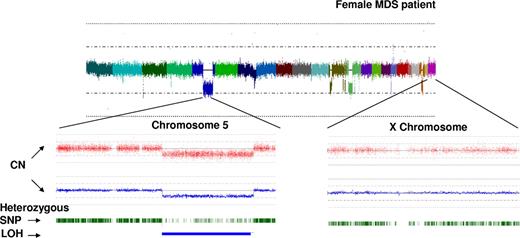
Principle of bioinformatic analysis of array output. Analysis of hybridization signal intensity for each SNP probe allows for construction of karyograms. In this example, CNAG v.2 software (www.genome.umin.jp/CNAGtop2.html) was used for analysis: colors result from merging of individual signals grouped based on the topographic distribution throughout the genome and correspond to each of the autosomes and the X-chromosome. The hybridization signal intensity plot results in trace colors oscillating around the diploid signal intensity value. In the example shown, multiple areas of hypoploid signal intensity can be distinguished corresponding to several genomic losses. Zooming in on 2 exemplary chromosomes (5 and X, below the whole genome view) demonstrates the copy number determination plot (red dots symbolize hybridization signals of single SNP probes; blue line represents average copy number) as well as heterozygosity tracing depicted using individual green ticks (which merge when the density of heterozygosity calls is high). Areas of deletion are recognizable by the decrease in the hybridization signal intensity (below the diploid line) and corresponding decrease in the expected density of heterozygosity calls. Of note is that residual heterozygosity calls (here in an example of del5q31) correspond to signals derived from nonclonal cells contaminating the sample.
Principle of bioinformatic analysis of array output. Analysis of hybridization signal intensity for each SNP probe allows for construction of karyograms. In this example, CNAG v.2 software (www.genome.umin.jp/CNAGtop2.html) was used for analysis: colors result from merging of individual signals grouped based on the topographic distribution throughout the genome and correspond to each of the autosomes and the X-chromosome. The hybridization signal intensity plot results in trace colors oscillating around the diploid signal intensity value. In the example shown, multiple areas of hypoploid signal intensity can be distinguished corresponding to several genomic losses. Zooming in on 2 exemplary chromosomes (5 and X, below the whole genome view) demonstrates the copy number determination plot (red dots symbolize hybridization signals of single SNP probes; blue line represents average copy number) as well as heterozygosity tracing depicted using individual green ticks (which merge when the density of heterozygosity calls is high). Areas of deletion are recognizable by the decrease in the hybridization signal intensity (below the diploid line) and corresponding decrease in the expected density of heterozygosity calls. Of note is that residual heterozygosity calls (here in an example of del5q31) correspond to signals derived from nonclonal cells contaminating the sample.
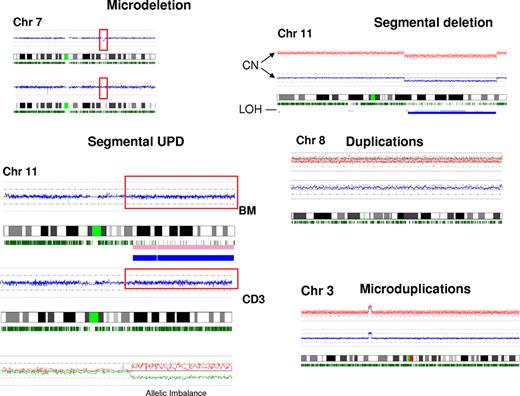
Examples of types of defects detected by SNP-A. Various types of chromosomal lesions detectable by SNP-A are presented, including microdeletions and microgains, large segmental or numeric gains, losses, and segmental acquired UPD. For demonstration purposes, the somatic nature of an exemplary microdeletion detected in bone marrow is demonstrated by comparison with sorted, nonclonal CD3 cells from the same patient. The karyogram of CD3 cells shows 2 normal chromosomes 7. Below, a similar demonstration is provided for an exemplary UPD with a disparity in the density of heterozygosity calls between clonal bone marrow sample and nonclonal sorted CD3 T cells.
Examples of types of defects detected by SNP-A. Various types of chromosomal lesions detectable by SNP-A are presented, including microdeletions and microgains, large segmental or numeric gains, losses, and segmental acquired UPD. For demonstration purposes, the somatic nature of an exemplary microdeletion detected in bone marrow is demonstrated by comparison with sorted, nonclonal CD3 cells from the same patient. The karyogram of CD3 cells shows 2 normal chromosomes 7. Below, a similar demonstration is provided for an exemplary UPD with a disparity in the density of heterozygosity calls between clonal bone marrow sample and nonclonal sorted CD3 T cells.
Higher density arrays provide a greater resolution that can be further increased by inclusion of sequential oligonucleotide probes that do not depend upon the presence of SNPs. SNP-As allow for assessment of the gene copy number-neutral LOH, such as autozygosity or uniparental disomy (UPD).25 To increase resolution, arrays containing both SNP and genomic probes are now available; various combinations of tagging, nonsynonymous, and copy number variants (CNV) probes can be included in such mixed SNP/oligonucleotide copy number probe arrays, which combine the advantages of SNP-A and some of the advantages of CGH-A.
Comparative genomic hybridization arrays
In contrast to SNP-A and oligo A, CGH-A relies on the difference in the copy number between test and reference DNA samples and is based on dual-color labeling of test and reference control DNA (Figure 2). The analytic principle involves competition between differentially labeled fragmented test (eg, tumor) and control diploid DNA; imbalances due to copy number changes in tester DNA result in a shift in fluorescence spectra. Both BAC-based as well as oligonucleotide-based CGH-A (oligo CGH-A) are currently available. The ability to compare the hybridization signals of test and control DNA affords a high level of precision and exclusion of artifacts, a clear advantage of CGH-A over SNP-A. High-density and very precise oligo CGH-As are now available from Agilent (Palo Alto, CA)28 or NimbleGen (Madison, WI),29-31 which differ mostly in the technology used to generate arrays. In contrast, to SNP-A, CGH-A allows for even or targeted distribution of probes or for targeting probes to areas of known CNVs25 but does not allow for detection of UPD. The major drawback of BAC CGH-A is the need for large amounts of BAC DNA and the high number of BACs required to achieve good resolution.25
Sensitivity and resolution of SNP-A and CGH-A
The detection principle relies on the presence of sufficient numbers of cells sharing a clonal abnormality (Figure 6). This detection threshold indicates the minimal clonal size. If multiple clones with distinct lesions are contained in the cellular specimen, individual defects may not be detectable. Paradoxically, this relative insensitivity may be a strength of array-based karyotyping, as only large clonal expansions are detectable, whereas smaller irrelevant clones are omitted. Depending on the biostatistical detection algorithm, in dilution studies, the presence of approximately 25% of abnormal cells with uniform unbalanced lesions may be detectable by 250 K SNP-A.32
The concepts of sensitivity and resolution in the context of SNP-A–based karyotyping, metaphase cytogenetics, and FISH. (A) Numerically large clones characterized by a chromosomal defect are easily detectable by SNP-A (top portion). The presence of clonal mosaicism may be detected if individual clones reach the detection threshold. Compound defects (bottom portion) cannot be distinguished by SNP-A from clonal mosaicism (middle portion). (B) Comparison of sensitivity (size of the clone) and resolution (size of the lesion) between SNP-A, metaphase cytogenetics, and FISH.
The concepts of sensitivity and resolution in the context of SNP-A–based karyotyping, metaphase cytogenetics, and FISH. (A) Numerically large clones characterized by a chromosomal defect are easily detectable by SNP-A (top portion). The presence of clonal mosaicism may be detected if individual clones reach the detection threshold. Compound defects (bottom portion) cannot be distinguished by SNP-A from clonal mosaicism (middle portion). (B) Comparison of sensitivity (size of the clone) and resolution (size of the lesion) between SNP-A, metaphase cytogenetics, and FISH.
In contrast to sensitivity (minimal proportion of cells allowing for detection by SNP-A), resolution refers to the minimal size of lesions that can be detected by SNP-A (Figure 6). Resolution depends on the density of the arrays, distribution of probes, and biostatistical algorithms used to detect copy number changes. Theoretically, the high-density arrays (eg, 500 K probes) with an average 10 probes per call allow for a resolution of around 50 K, but arrays with even higher numbers of probes can decrease the minimal detectable size of a deletion to around 25 Kb.25 However, it appears that increasing the density of the arrays from 250 to 500 K does not necessarily result in a higher detection rate for either microdeletions or UPD.33 Examples of types of chromosomal lesions detectable by SNP-A are shown in Figure 5. In oligo-CGH-A, because probe spacing does not depend upon the location of SNPs, probes can be evenly distributed, allowing for median probe spacing (eg, in 385 K arrays is every 6 Kb). In addition, CGH-As targeting individual chromosomes allow for an even higher resolution that enables mapping of break points within 5-Kb intervals.
In SNP-A, there is an expected variability in the minimal size of the area of LOH that can be detected, with regions of more than 200 Kb having a high chance of detection, whereas less than 50 Kb LOH segments are less reliably identified.24 The limitations of LOH detection by SNP genotyping are likely due to the frequency of heterozygous loci, distribution of SNPs to be tested throughout the chromosome, and the size of the affected region. In addition, contamination with normal diploid cells can further complicate the interpretation of the results. A further drawback of SNP- or CGH-A as a karyotyping platform is its inability to distinguish mosaicism from complex lesions occurring in one clone (Figure 6; Table 1).
A variety of algorithms have been developed that allow for a reduction in experimental variation of regions with a similar copy number.26,27,34,35 Although very large invariant lesions detected by SNP-A or CGH-A are unlikely to be inherited, comparison with a DNA sample containing a germ-line configuration is imperative to exclude normal CNV and to distinguish them from truly somatic defects. This is of utmost importance for microdeletions and duplications that have to be distinguished in their somatic forms from normal CNVs that can span up to 12% of human genome34 and may involve many known genes.36 In particular for LOH, such an allelic imbalance study between the somatic (tumor) and normal (germ line) tissues can definitively resolve the issue as to whether the observed change is of somatic/pathologic nature (Figure 5). The initial identification of normal variants is particularly easy in the case of well-characterized CNVs, but novel lesions for which a populational frequency has not been described or rare changes should all be investigated by comparison of germ-line and tumor samples. As a general rule, CNVs are smaller (in our analysis of 120 controls ≤ 1 Mb) and may have characteristic locations, but the validity of this assumption remains to be elucidated in a large, formal series. The process of evaluation of individual lesions should also require cross-references with CNVs described in public databases and internal controls performed by individual laboratories. In addition, different array platforms produce considerable variation among the numbers and types of CNVs.24
Uniparental disomy
A major advantage of SNP-A over MC and CGH-A is the ability to detect stretches of homozygosity present throughout the genome due to 3 mechanisms: (1) miotic and (2) somatic uniparental disomy (UPD) and (3) autozygosity. Whereas autozygosity is inherited from both parents, UPD can result from errors at meiosis leading to both copies of a chromosome or chromosomal region being derived from one parent. Finally, of importance for SNP-A karyotyping is acquired UPD (Figure 5) or copy-neutral LOH after segmental deletions and subsequent replacement of the lost fragment by a copy of the remaining allele, or mitotic recombination (Figure 7). Similar mechanisms, but without chromosomal loss, may result in duplication leading to trisomy and/or even uniparental trisomy. For diagnostic applications, the distinction of somatic UPD and an inherited form of homozygosity is of utmost importance and can be best accomplished through comparison of DNA from the malignant tissue with remission or constitutional DNA, although to a degree, it can perhaps be inferred from the size of the affected region. Somatic UPD was first identified using microsatellites and has been found in several malignancies and many distinct chromosomal locations. The first detection of acquired UPD in hematologic disorders was in polycythemia vera (PV),37 a discovery that paved the way for subsequent discovery of the V617F JAK2 mutation.38 However, systematic studies with precise mapping of regions affected by UPD were not performed until SNP-A became available. The utility of DNA arrays to conveniently detect UPD in AML was initially reported in a study of 60 AML patients using 10 K arrays.39 Regions of homozygosity likely representing somatic UPD were identified in 12% of cases. SNP-A also allowed for very efficient identification of UPD9p.27,40 By analogy to this lesion, UPD in other areas of the genome can indicate the presence of activating mutations in important genes, for example, homozygous mutation in CEBPA (19q)41 or UPD13q and Flt-3 mutations.39 Similarly, an inactivating NF-1 mutation in a homozygous constellation was found in patients with juvenile myelomonocytic leukemia (JMML) with UPD17q42 (Table 2). The presence of biallelic mutations such as in cases of AML with Flt-3 ITD may be associated with worse prognostic features compared with heterozygous genotypes.43
LOH and copy-neutral LOH and their consequences for the pathogenesis of malignant myeloid disorders. SNP-As facilitate detection of LOH. Two types of LOH are depicted using chromosome 7 as an example. SNP karyograms demonstrate monosomy-7 on the right and UPD7q on the left. In the bottom portion, theoretic pathogenetic pathways resulting in LOH due to deletion or UPD are shown. UPD can result in duplication of a somatic activating mutation, acquired homozygosity of a germ line–encoded polymorphism occurring normally in heterozygous form, or duplication of maternal or paternal methylation pattern with either activation or total inactivation of the duplicated allele. In deletion, the remaining allele may harbor a somatic inactivating mutation, leading to hemizygosity of a germ-line polymorphism that carries functional consequences or haploinsufficiency.
LOH and copy-neutral LOH and their consequences for the pathogenesis of malignant myeloid disorders. SNP-As facilitate detection of LOH. Two types of LOH are depicted using chromosome 7 as an example. SNP karyograms demonstrate monosomy-7 on the right and UPD7q on the left. In the bottom portion, theoretic pathogenetic pathways resulting in LOH due to deletion or UPD are shown. UPD can result in duplication of a somatic activating mutation, acquired homozygosity of a germ line–encoded polymorphism occurring normally in heterozygous form, or duplication of maternal or paternal methylation pattern with either activation or total inactivation of the duplicated allele. In deletion, the remaining allele may harbor a somatic inactivating mutation, leading to hemizygosity of a germ-line polymorphism that carries functional consequences or haploinsufficiency.
Examples of copy neutral LOH and corresponding gene mutations in homozygous form
| Area of UPD . | Gene . | Disease . | Reference . |
|---|---|---|---|
| UPD9q | Jak2 | MPD | Kralovics et al37,38 |
| UPD13q | FTL-ITD | AML | Raghavan et al39 |
| UPD17q | NF-1 | JMML | Flotho42 |
| UPD21q | AML-1/Runx1 | AML | Raghavan et al39 |
| UPD19q | CEBPA | AML | Raghavan et al39 ; Fitzgibbon et al41 |
| UPD4q | c-Kit | AML | Unpublished results* |
| UPD1p | c-Mpl | MDS/MPD, MPD | Unpublished results† |
| UPD11p | WT1 | AML | Raghavan et al39 |
| Area of UPD . | Gene . | Disease . | Reference . |
|---|---|---|---|
| UPD9q | Jak2 | MPD | Kralovics et al37,38 |
| UPD13q | FTL-ITD | AML | Raghavan et al39 |
| UPD17q | NF-1 | JMML | Flotho42 |
| UPD21q | AML-1/Runx1 | AML | Raghavan et al39 |
| UPD19q | CEBPA | AML | Raghavan et al39 ; Fitzgibbon et al41 |
| UPD4q | c-Kit | AML | Unpublished results* |
| UPD1p | c-Mpl | MDS/MPD, MPD | Unpublished results† |
| UPD11p | WT1 | AML | Raghavan et al39 |
A. Dunbar, L.P. Gondek, C.H. O'Keefe, M.A. McDevitt, J.P.M. (manuscript submitted March 2008).
H. Szpurka, February 2008.
The pathophysiology of UPD may include various mechanisms, several shared with deletions. Somatic UPD may result in duplication of an activating somatic mutation or homozygosity for a disease-prone minor allele present in the germ line. UPD can also lead to increased or decreased gene expression due to duplication of a methylation pattern. A similar mechanism may operate in deletion with loss of the inactivated or unmethylated allele. In addition, deletion may result in haploinsufficiency or loss of the intact allele with the remaining allele deficient due to somatic polymorphism or an inactivating somatic mutation.
The presence of somatically acquired UPD as a clonal marker has to be evaluated in the context of corresponding normal tissues. Smaller areas of UPD involving limited numbers of SNPs are more frequent and may not be a result of germ-line mitotic recombination or autozygosity but due to the variability of the normal distribution of heterozygous calls.24 In some individuals, larger regions can be affected by UPD; in the Hapmap project, 15% of control individuals showed areas of homozygosity, all smaller than 2 Mb.33 These regions are unlikely to correspond to somatic UPD and are germ line encoded. In controls used for the SNP-A study in MDS, 72% of samples contained areas of homozygosity, but most of the changes were small, and nonclonal UPD regions more than 2 MB were found only in 12% of cases.32,33 Similarly, areas of shared homozygosity were found both in blood samples and isolated myeloma cells in 2 of 12 cases, implying that this UPD may be nonclonal and can involve even whole arms of chromosomes in the germ-line configuration.44 Although it is likely that some smaller areas resembling UPD are a technical artifact or autozygosity, the large numbers of consecutive SNPs in homozygous configuration found in controls suggest true germ-line UPD.
The detection rate depends on the distance between the SNPs, size of the region, and the numbers of SNPs. As a consequence, the minimum detectable length of copy-neutral LOH decreases with a higher density of SNP-A.45 Although we observed germ line– encoded UPD as large as 18 Mb, as a general rule, larger regions of UPD are less likely to be of germ-line origin and point toward a clonal event.32 In conclusion, comparison of tumor and paired constitutional samples is necessary to identify a truly somatic event in the cases of any new copy-neutral LOH that does not affect the invariant areas of genome known to be pathologic. Of note is that the sensitivity of SNP-A for deletions and UPD may differ and depends both on the computational algorithms as well as the density of the arrays. In one study, as few as 20% of clonal cells in the sample allowed for a clear identification of somatic UPD.46
In addition to its diagnostic value, whole genome scanning-based karyotyping constitutes an excellent mapping tool, thereby allowing for delineation of boundaries of commonly deleted/duplicated regions or, in the case of SNP-A, areas of segmental UPD overlap (Figure 8). Moreover, genotyping arrays can be used for genotypic analysis within areas of UPD. Changes in the heterozygous genotype in the germ-line configuration to hemizygosity (deletion) or homozygosity (UPD) may point toward causative genes, in the case of minor alleles with an exceedingly low homozygous frequency in the general population. Further improvement of mapping is possible using multiple sample analysis algorithms assigning either an average intensity (copy number) or a combined LOH genotyping score over a region of interest across multiple samples.35
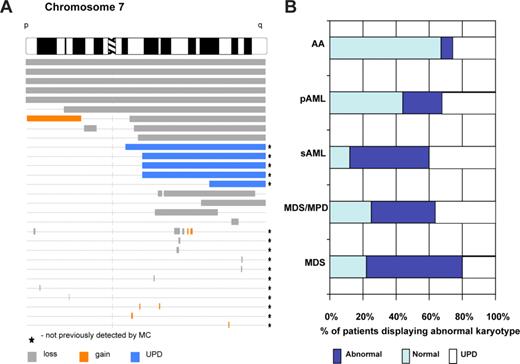
SNP-A karyotyping allows both mapping of invariant lesions and improvement of detection rate of cytogenetic abnormalities. (A) Mapping of chromosomal aberrations in myeloid malignancies. Karyograms generated by SNP-A allow for mapping of the location of chromosomal aberrations and delineation of minimal commonly deleted regions (as an example, topography of lesions on chromosome 7 is shown based on the analysis of a cohort of patients with AML and MDS). (B) Summary of the SNP-A karyotyping results. In representative studies of patients, including 78% of MDS, 75% of MPD/MDS, 87% of sAML,32 30% of AA (M. Wlodarski, L. Gondek, C. L. O'Keefe, R. Tiu, A. Haddad, A. Risitano, J.P.M., manuscript submitted May 2008), and 56% of primary AML (R. Tiu, C. L. O'Keefe, M. Sekeres, M. A. McDevitt, J. Karp, J.P.M., manuscript submitted June 2008) patients were analyzed using SNP-A, and the rates of detection of chromosomal abnormalities, including UPD, were calculated.
SNP-A karyotyping allows both mapping of invariant lesions and improvement of detection rate of cytogenetic abnormalities. (A) Mapping of chromosomal aberrations in myeloid malignancies. Karyograms generated by SNP-A allow for mapping of the location of chromosomal aberrations and delineation of minimal commonly deleted regions (as an example, topography of lesions on chromosome 7 is shown based on the analysis of a cohort of patients with AML and MDS). (B) Summary of the SNP-A karyotyping results. In representative studies of patients, including 78% of MDS, 75% of MPD/MDS, 87% of sAML,32 30% of AA (M. Wlodarski, L. Gondek, C. L. O'Keefe, R. Tiu, A. Haddad, A. Risitano, J.P.M., manuscript submitted May 2008), and 56% of primary AML (R. Tiu, C. L. O'Keefe, M. Sekeres, M. A. McDevitt, J. Karp, J.P.M., manuscript submitted June 2008) patients were analyzed using SNP-A, and the rates of detection of chromosomal abnormalities, including UPD, were calculated.
Comparison of whole genome scanning technologies as a karyotyping tool with other methods
Overall, SNP-A allows for detection of unbalanced cytogenetic defects and UPD in nondividing cells and assesses distribution of cytogenetic defects among all cells, not only in dividing progenitors. Its sensitivity is comparable with that of metaphase cytogenetics. In contrast, SNP-A arrays demonstrate excellent resolution and also allow, unlike CGH-A, the detection of UPD. Comparison between SNP-A, high-density BAC CGH-A, and oligo-CGH-A showed comparable results but clearly bioinformatic tools influence the data output. It appears that oligo-CGH can achieve the best resolution and precision in identifying copy number changes, including CNVs47,48 (Table 1).
Implications of array-based karyotyping in hematologic diseases
Myelodysplastic syndromes.
Early studies used BAC CGH for the investigation of genomic changes in various solid tumors and hematopoietic malignancies, including MDS.49-53 CGH-A allowed for a wider application of this technology than very labor-intense chromosomal CGH.54 The quality of CGH-A–based analysis is limited by the platform on which it is performed and depends on the density of BAC probes, varying from thousands to hundreds of thousands.25 By comparison, metaphase CGH can only detect lesions of 2 to 10 Mb when present in more than 50% of the cells analyzed.55,56
In a series of 65 MDS patients, using a commercial 2.4 BAC array, a variety of chromosomal defects were found patients,57 but their relatively low density precluded precise mapping of the lesions and some of the BAC probes included areas of CNV, often giving inconsistent results. Nevertheless, CGH demonstrated the presence of cryptic abnormalities in addition to those that were detected by MC, for example, in MDS patients with trisomy-853 or patients with CML.58 In accelerated phase and blast crisis of CML, by analogy to the higher rate of chromosomal aberration found during evolution to AML, discrete copy number changes were detected frequently by CGH-A.58 Investigations using SNP-A initially included 10 K arrays but, with the evolution of the technology, studies with 50, 250, and 500 K arrays have been conducted.32,33,40,59 In a series of 64 patients with MDS, 50 K SNP-As reveal more lesions than traditional MC; a majority of unbalanced defects seen by MC were confirmed and new lesions were found in both patients with normal MC and those with previously known abnormalities.32 A surprising finding was a high prevalence of segmental UPD present in patients with MDS and MDS/MPD, including chronic myelomonocytic leukemia (CMML). UPD of various isolated chromosomes, including UPD1 and UPD9, was previously detected using microsatellites (for example, in earlier studies of chromosome 1 in MDS37,60 ), and this and subsequent studies revealed how surprisingly common UPD occurs in MDS.32
In the next published series, 250 and 500 K arrays were applied to larger cohorts of patients.33 When 119 patients with low-risk MDS were analyzed, UPD was found in 46%; deletions, in 10%; and amplifications, in 8% of cases. Univariate analysis showed that deletions (P = .04) and IPSS (IPSS; P < .001) scores correlated with overall survival; however, on multivariate analysis, only IPSS scores retained prognostic significance (P < .001). Similarly, in a study of 175 patients with MDS, MDS/MPD, and AML that evolved from MDS (sAML), lesions were found in up to 80% of patients.32 In addition, in 50% of patients with unsuccessful MC, chromosomal aberrations were found. UPD was detected in 20% to 30% of patients including sAML, but was particularly frequent in patients with CMML. Allelic imbalances for somatic defects were demonstrated through combined analysis of marrow samples and DNA derived from nonclonal CD3-sorted lymphocytes. Inclusion of SNP-A–detected defects allowed for substratification of patients with normal MC or of those with established aberrations. No impact on survival of patients with low-risk MDS was found, but this may have been related to the relatively short observation period in comparison with the median survival expected for this group.32
In general, investigations performed with 250 K and 50 K arrays suggested that SNP-A–detected lesions, including UPD, may have clinical relevance and can effectively “upgrade” the IPSS score to more advanced disease. For example, in a recent study of MPD and MDS/MPD patients, UPD9 was easily identified using SNP arrays but UPD6 and UPD11 was also frequently detected. In particular, UPD11q was frequently encountered in patients with CMML. Previously, in JMML, a large region of UPD17q was found in cases with NF-1 mutation.42 Similarly, invariant UPD on 4q was identified in 25% of refractory anemia with ringed sideroblasts (RARS), 12% of refractory cytopenia with multilineage dysplasia (RCMD) with normal cytogenetics, 17% of refractory anemia with excess blasts (RAEB), and 6% of 5q− syndrome cases.33 In addition, more complex defects appear to be more frequent and include both UPDs of various chromosomes and deletions. Another conclusion that can be derived from SNP-A–based analysis of AA is that submicroscopic cytogenetic defects can be used as markers of clonality and in some cases provide a distinction between hypocellular MDS (abnormal SNP-A cytogenetics) and typical AA (M. Wlodarski, L. Gondek, C. L. O'Keefe, R. Tiu, A. Haddad, A. Risitano, J.P.M., manuscript submitted May 15, 2008).
AML and ALL.
Both SNP-A and oligo CGH-A used as a karyotyping platform revealed cryptic chromosomal lesions in cytotogenetically normal AML.41,50,53,61,62 Similar to MDS, CGH-A demonstrated the presence of smaller copy number changes in a high proportion of patients with AML.54 An early application of 10-K SNP-A in AML demonstrated the importance of this tool for the detection of UPD.39,41 Similar to the principle governing occurrence of a homozygous Jak2 mutation through acquisition of UPD9,37,38 homozygous mutations of CEBPA, WT1, Flt-3, and AML1 were found in patients with UPD19q, 11p, 13q, or 21q, respectively39,41 (Table 2). Thus, invariant areas of UPD may point toward genes harboring mutations as duplication of the mutated allele may provide selection advantage to the affected clone. Later studies showed that SNP-A can provide insights into the origin of AML transformation in MPD. Upon transformation to AML, serially studied patients showed evolution of AML either from Jak2V617F+ (with UPD9 present in the blast population and in granulocytes before and after transformation) or Jak2V617F− hematopoietic clone (UPD9 present in granulocytes but not in AML blasts) with appearance of new additional lesions, for example, UPD7, UPD6, or del7.40 This result is in agreement with other studies of MPD-derived AML showing that AML may evolve from a Jak2V617F− precursor and only less frequently from the original Jak2 mutation-containing clone63 and is consistent with low incidence of Jak2 mutations in AML.64-66 In a study of patients with MDS-derived secondary AML, UPD of various chromosomes was found in 23% and additional lesions conveyed shorter survival.32 Overall, the results of MC with regard to the detection of chromosomal abnormalities in AML were improved by the application of SNP-A, allowing for the identification of clonal defects in 87% of patients (R. Tiu, C. L. O'Keefe, M. Sekeres, M. A. McDevitt, J. Karp, J.P.M., manuscript submitted June 2008). The newly detected clonal lesions, including UPD, seemed to impact on the survival of cases with previously normal MC as well as in those in whom additional lesions were found. These findings are consistent with the recognized association of poor prognosis and complex chromosomal abnormalities.32 In the most recent study of 140 cases of AML, abnormalities were found in 56% of cases and allowed for more precise prognosis with regard to overall survival or event-free survival independent of the status of Flt-3 and NPM-1 mutation (R. Tiu, C. O'Keefe, A. Sekeres, M. A. McDevitt, J. Karp, J.P.M., manuscript submitted June 2008). In general, in myeloid disorders tested, including MDS, MPD/MDS, MPD, primary AML, SML and AA, SNP-A showed a higher detection rate of chromosomal abnormalities than MC (Figure 8).
Similar to AML, CGH-A–based karyotyping allows for improving the detection rate of chromosomal abnormalities over MC in ALL (eg, copy number changes in 73% of cases).67,68 When oligo-CGH-A was applied to study ALL, various previously undetected lesions were found. Among them, deletions of various sizes involving chromosome 9p were detected in 46% of patients with the CDR involving the CDKN2A gene.69 Similar deletions of 9p21 were observed in another study using high-resolution BAC CGH-A that also allowed for detection of gains in 1q23-qter in low-risk and 2p11.2 in high-risk disease.70 In 2 other studies, intrachromosomal 21q amplifications involving RUNX1 were found.71,72 Of particular importance is that CGH-A allowed for detection of cytogenetic abnormalities in patients with normal or failed MC.73 A large series of patients with ALL were investigated by SNP-A.46,74 In a study of 399 pediatric patients, SNP-A provided very informative results showing presence of various deletions of duplications not previously seen by MC. The most common genomic abnormalities included CDRs on 9p and 12p involving p16INK4A and ETV6, respectively. As in other hematologic malignancies, regions of segmental UPD were frequently encountered 95 of 399 patients with the most predominant UPD involving chromosome 9.46 The most complex and illustrative analysis combining various molecular methods, including SNP-A, was performed on a large cohort of patients with ALL.74 In this study, SNP-A allowed for detection of previously cryptic lesions in areas of the genome that were characterized by diploid copy number by MC. Various structural abnormalities, including deletions and amplifications, were detected in 40% of patients with precursor B-cell ALL. SNP-A pointed toward deletions involving the PAX5 gene on 9p that was found to be an invariant target of somatic mutations in approximately 30% of patients. Various other consequential deletions have been identified using this strategy including, for example, EBF1 on 5q33. A similar approach of combined high-resolution CGH-A and expression arrays was taken in ETV6/RUNX1-positive ALL; deletions involving 9p and 12p were detected and several potential target genes were identified for further investigation.75
CLL and MM.
Both B-CLL as well as MM have, over the years, posed specific problems for successful MC because of the difficulties of inducing analyzable metaphases in the neoplastic cells, making these diseases ideal for SNP-A studies. High-resolution CGH-A was applied to study CLL and identified previously known losses of 13q,11q and gains of 12q and allowed for more a precise definition of commonly affected regions.68,76 Similarly, high-resolution 10 K and 50 K Affymetrix SNP-As were evaluated as a diagnostic tool for CLL and revealed chromosomal imbalances in 66% of 70 consecutive cases.44 Among the prognostically important aberrations, del13q14 was present in 36 (51.4%); trisomy 12, in 9 (12.8%); del11q22, in 9 (12.8%); and del17p13, in 4 cases (5.7%). A prominent clustering of breakpoints on both sides of the MIRN15A/MIRN16-1 genes indicated the presence of recombination hot spots in the 13q14 region. Patients with a monoallelic del13q14 had slower lymphocyte growth kinetics than patients with biallelic deletions.44 UPD regions with loss of heterozygosity were identified in 14 cases and, similar to the findings in MDS with UPD7q, 6q, or 11q, corresponded to areas frequently affected by deletions. Of note is that some of the controls also contained copy-neutral UPD, and comparison with germ-line DNA suggested that some of the presumed UPDs may not be somatic events.44 In another study, cytogenetic changes were investigated in 56 CLL patients using 50 K SNP-A, which confirmed nearly all changes detected by FISH. Moreover, new abnormalities were found, including UPD in 4 of 46 patients.77
In MM, a number of prognostically important chromosomal abnormalities can be detected using SNP-A. In the initial study using 50 K SNP-A involving 30 MM patients and purified plasma cells, a large number of deletions and gains were reported.78 MC showed cytogenetic abnormalities in 44% of cases studied, whereas using SNP-A chromosomal defects were found in most of the patients. UPD was identified at a large frequency in most of the patients, showing up to 19 regions of UPD per patient. However, most of these lesions were small and, in view of the fact that the confirmation through comparison with germ-line samples was not given, not all of these lesions represented true somatic events.
When 500 K SNP-As were applied to study the karyotypes of 55 MM cases,79 many of the known lesions, for example, involving chromosome 16, were found and, by analogy to the findings in MDS, also included UPD, such as UPD16, present in 75% of patients. SNP-A analysis allowed for precise mapping of the lesions and delineation of commonly deleted region. Expression analysis of genes contained in the CDR was used to identify 2 genes with a significantly decreased expression in CLL, including WWOX and CYLD. Haploinsuffciency of these genes provides molecular insights into the poor prognosis of patients with LOH16q. This study exemplifies how SNP-A can contribute to the clarification of pathogenic molecular lesions.
Conclusions
Whole genome scanning technology has ushered in a new era in the detection of genomic defects in malignant disease and has led to a markedly increased resolution of karyotyping. It is likely that this technology will be introduced into the clinical practice to complement MC. Its independence from the presence of dividing cells and ability to detect copy-neutral LOH are clear advantages. Initial clinical analyses of cryptic lesions detected by SNP-karyotyping suggest that some of them, including UPD detected by SNP-A, provide important information about the prognosis. As an investigative tool, array-based karyotyping, through recognition of novel and cryptic microdeletions, can define various common deleted regions, allowing for a better delineation of causative genes. Karyotypic analysis using CGH- and SNP-A has to follow stringent criteria of exclusion of somatic defects and a definition of a minimal size of lesion that can be reliably identified through various algorithms that include assessment of the number of probes involved, consistency of LOH calls and copy number changes, and physical size of the lesions and their locations.
Acknowledgments
The authors thank Prof S. Ogawa for his contribution to the field and provision of invaluable bioanalytic tools that facilitated progress in this field.
This work was supported by National Institutes of Health (NIH, Bethesda, MD) R01 HL082983 (J.P.M.), U54 RR019391 (J.P.M.), K24 HL077522 (J.P.M.), and a charitable donation from the Robert Duggan Cancer Research Fund (Cleveland, OH).
National Institutes of Health
Authorship
Contribution: J.P.M. conceived the idea; and J.P.M. and G.J.M. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jaroslaw P. Maciejewski, Experimental Hematology and Hematopoiesis Section, Taussig Cancer Center R-40, Cleveland Clinic, 9500 Euclid Ave, Cleveland, OH; e-mail: maciejj@ccf.org.