Abstract
Early results of the fludarabine, cyclophosphamide, and rituximab (FCR) regimen in 224 patients showed that it was highly active as initial therapy of chronic lymphocytic leukemia. In this report, we present the final results of all 300 study patients at a median follow up of 6 years. The overall response rate was 95%, with complete remission in 72%, nodular partial remission in 10%, partial remission due to cytopenia in 7%, and partial remission due to residual disease in 6%. Two patients (< 1%) died within 3 months of starting therapy. Six-year overall and failure-free survival were 77% and 51%, respectively. Median time to progression was 80 months. Pretreatment characteristics independently associated with inferior response were age 70 years or older, β2-microglobulin twice the upper limit of normal (2N) or more, white cell count 150 × 109/L or more, abnormal chromosome 17, and lactate dehydrogenase 2N or more. No pretreatment characteristic was independently associated with decreased complete remission duration. The risk of late infection was 10% and 4% for the first and second years of remission, respectively, and less than 1.5% per year for the third year onward. In a multivariate analysis of patients receiving fludarabine-based therapy at our center, FCR therapy emerged as the strongest independent determinant of survival.
Introduction
Recent advances in the treatment of chronic lymphocytic leukemia (CLL) have seen the development of highly effective regimens capable of producing true complete remissions (CRs), as confirmed by the absence of disease on bone marrow biopsy. The ability to achieve such remissions on a consistent basis is a prerequisite for the eventual cure of the disease. Fludarabine and cyclophosphamide (FC), the most active chemotherapy regimen developed to date, achieves CR in only 24% to 39% of patients.1-4 There is substantial preclinical evidence to suggest that the addition of the anti-CD20 antibody rituximab to chemotherapeutic agents may further increase cytotoxicity.5,6 We have previously shown that the combination of FC and rituximab (FCR) was highly effective, achieving CR in 70% of chemotherapy-naive patients.7 However, at a median 2 years of follow up, the durability of remissions and the impact of treatment on overall survival were not defined.7 The purpose of the current report is to present the long-term outcome of the FCR regimen at a median follow up of 6 years. These results establish FCR as the most effective regimen for the initial therapy of CLL. Furthermore, analysis of pretreatment factors led to the development of a predictive model for patients receiving chemoimmunotherapy, thus providing the rationale for a risk-adapted CLL induction strategy.
Methods
Approval was obtained from the M. D. Anderson Cancer Center (MDACC) institutional review board for these studies. Informed consent was obtained in accordance with the Declaration of Helsinki.
Synopsis of study design and treatment plan
Between July 1999 and November 2003, 300 patients aged 18 years or older with previously untreated CLL and symptomatic or progressive disease as defined by the National Cancer Institute sponsored Working Group (NCI-WG) 1996 criteria8 were enrolled in an open-label, phase 2 evaluation of FCR as initial therapy. Details of the study, treatment plan, and associated supportive care measures were previously reported.7 Briefly, patients received rituximab (375-500 mg/m2) on day 1 and fludarabine (25-30 mg/m2 daily) and cyclophosphamide (250-300 mg/m2 daily) on days 1 to 3 of each course (days 2 to 4 for the first course only). Treatment was repeated every 4 weeks for a planned total of 6 courses.
Patients were restaged after 3 and 6 courses. Responses were graded according to the NCI-WG 1996 criteria. In addition to standard definitions for complete response (CR) and nodular partial response (PR-nod), patients in partial response were further subdivided into those with residual disease in blood, nodes, spleen, marrow, or other sites (PR-d) and those who met all criteria for CR except for incomplete recovery of blood counts (PR-i; hemoglobin level < 110 g/L, absolute neutrophil count < 1.5 × 109/L, and/or platelet count < 100 × 109/L). Computerized tomography was not routinely used in the assessment of disease response. Following completion of therapy, patients were followed every 3 to 6 months for disease progression and survival.
Bone marrow specimens were evaluated for minimal residual disease (MRD) at the end of treatment by flow cytometry (FL-C) and by a polymerase chain reaction (PCR)–based ligase assay for patient-specific clonal IgVH. FL-C negativity was defined as less than 1% cells coexpressing CD5 and CD19 in the marrow lymphocyte gate with a normal kappa-lambda ratio. The technique for PCR evaluation had been previously published and has a sensitivity of at least 1 in 104 mononuclear cells.9 PCR results were normalized to the RAS oncogene, with ratios between 0.001 to 0.10 being considered low positive and higher ratios being considered positive.
Statistical consideration
Overall survival (OS), failure-free survival (FFS), and time to progression (TTP) were calculated from the first day of FCR therapy. Failure was defined as primary refractory disease, CLL progression (including development of Richter transformation), therapy-related myelodysplasia, or death. TTP was censored for therapy-related myelodysplasia and death in remission. Continuous variables were evaluated using the Mann-Whitney test, and categoric variables were evaluated using the Fisher exact or chi-square tests, as appropriate. Actuarial survival was calculated using the method of Kaplan and Meier, and comparisons were made using the log-rank test. Multivariate analyses were performed using logistic binary regression (for response) and Cox regression (for survival) as appropriate. Regression tree analysis was used to construct a prognostic model based on significant pretreatment factors. All P values were 2 sided.
Comparison group
Patients enrolled in previous MDACC studies of fludarabine-based treatment as initial therapy of CLL were included as a historical comparison group. These included 190 patients who received fludarabine alone (n = 77) or with prednisone (n = 113; F),10 and 140 patients who received FC4 (n = 107) or fludarabine and mitoxantrone11 (n = 33; FC/M). Previous nonrandomized comparisons had shown similar outcomes for individual regimens within each category of therapy.4,10,11 Cox multivariate analysis was used to adjust for differences in pretreatment characteristics, to define the independent impact of therapy on survival.
Results
Patient characteristics
Pretreatment characteristics of the 300 patients are listed in Table 1. Median age was 57 years, with 14% 70 years or older. The majority of patients had Rai stage I to II (61%) or III to IV (36%) disease. Patients with Rai stage 0 disease were treated because of marked constitutional symptoms and rapid doubling time. Beta-2-microglobulin (β2m) was equal to or elevated above twice the upper limit of laboratory reference (≥ 2N) in 43% of patients. Bone marrow flow cytometry for CD38 was available for 253 patients, of whom 52 (21%) had 30% or more CD19/38 coexpression. Cytogenetic status was determined by conventional karyotyping in marrow aspirates in 222 patients; of these patients, 66 (30%) had clonal abnormalities and 8 (4%) had abnormalities involving chromosome 17. This study preceded the clinical availability of IgVH mutation determination,12,13 ZAP-70 phenotyping,14,15 and fluorescent in situ hybridization (FISH) testing for occult cytogenetic aberrations16 ; the impact of these factors on treatment outcome was therefore not evaluable.
Pretreatment characteristics
| Pretreatment characteristic . | Value . | Complete response (%) . |
|---|---|---|
| Median age, y (range) | 57 (17-86) | — |
| Younger than 60 y | 186 (62) | 140 (75) |
| 60 to 69 y | 73 (24) | 56 (77) |
| 70 y or older | 41 (14) | 21 (51) |
| Male | 211 (70) | 154 (73) |
| Female | 89 (30) | 63 (71) |
| Median time from diagnosis, mo (range) | 24 (0-155) | — |
| Rai stage 0 | 11 (4) | 10 (91) |
| Rai stages I to II | 182 (61) | 136 (75) |
| Rai stages III to IV | 107 (36) | 71 (66) |
| Zubrod performance status 0 | 119 (40) | 96 (81) |
| Zubrod performance status 1 | 171 (57) | 116 (68) |
| Zubrod performance status 2 | 10 (3) | 5 (50) |
| Median white cell count ×109/L (range) | 76 (2-620) | — |
| Less than 50 ×109/L | 110 (37) | 82 (75) |
| 50 to 149 ×109/L | 139 (46) | 107 (77) |
| 150 ×109/L or more | 51 (17) | 28 (55) |
| Median serum β2-microglobulin, mg/L (range) | 3.7 (1.6-16.4) | — |
| Less than 2× upper limit normal | 168 (57) | 141 (84) |
| 2× or more upper limit normal | 127 (43) | 73 (58) |
| Median serum lactate dehydrogenase, IU/L (range) | 548 (103-1828) | — |
| Less than 2× upper limit normal (%) | 293 (98) | 72 (71) |
| 2× or more upper limit normal (%) | 6 (2) | 2 (33) |
| Diploid cytogenetics | 156 (70) | 118 (76) |
| Abnormal cytogenetics, not chromosome 17 | 58 (26) | 42 (72) |
| Abnormality of chromosome 17 | 8 (4) | 2 (25) |
| Bone marrow lymphocyte CD19/38 less than 30% | 201 (79) | 150 (75) |
| Bone marrow lymphocyte CD19/38 30% or more | 52 (21) | 34 (65) |
| Pretreatment characteristic . | Value . | Complete response (%) . |
|---|---|---|
| Median age, y (range) | 57 (17-86) | — |
| Younger than 60 y | 186 (62) | 140 (75) |
| 60 to 69 y | 73 (24) | 56 (77) |
| 70 y or older | 41 (14) | 21 (51) |
| Male | 211 (70) | 154 (73) |
| Female | 89 (30) | 63 (71) |
| Median time from diagnosis, mo (range) | 24 (0-155) | — |
| Rai stage 0 | 11 (4) | 10 (91) |
| Rai stages I to II | 182 (61) | 136 (75) |
| Rai stages III to IV | 107 (36) | 71 (66) |
| Zubrod performance status 0 | 119 (40) | 96 (81) |
| Zubrod performance status 1 | 171 (57) | 116 (68) |
| Zubrod performance status 2 | 10 (3) | 5 (50) |
| Median white cell count ×109/L (range) | 76 (2-620) | — |
| Less than 50 ×109/L | 110 (37) | 82 (75) |
| 50 to 149 ×109/L | 139 (46) | 107 (77) |
| 150 ×109/L or more | 51 (17) | 28 (55) |
| Median serum β2-microglobulin, mg/L (range) | 3.7 (1.6-16.4) | — |
| Less than 2× upper limit normal | 168 (57) | 141 (84) |
| 2× or more upper limit normal | 127 (43) | 73 (58) |
| Median serum lactate dehydrogenase, IU/L (range) | 548 (103-1828) | — |
| Less than 2× upper limit normal (%) | 293 (98) | 72 (71) |
| 2× or more upper limit normal (%) | 6 (2) | 2 (33) |
| Diploid cytogenetics | 156 (70) | 118 (76) |
| Abnormal cytogenetics, not chromosome 17 | 58 (26) | 42 (72) |
| Abnormality of chromosome 17 | 8 (4) | 2 (25) |
| Bone marrow lymphocyte CD19/38 less than 30% | 201 (79) | 150 (75) |
| Bone marrow lymphocyte CD19/38 30% or more | 52 (21) | 34 (65) |
Data are numbers (%) except where specified.
Treatment outcome
The response rate was 95%, with CR in 72%, PR-nod in 10%, PR-i 7%, and PR-d in 5% of patients (Table 2). Fifteen patients (5%) failed therapy due to disease resistance (n = 13) or early death (n = 2). Posttreatment bone marrow was negative for MRD by FL-C and PCR, respectively, in 82% and 42% of patients in CR, 39% and 36% of patients in PR-nod, and 55% and 31% of patients in PR-I.
Results of the fludarabine, cyclophosphamide, and rituximab (FCR) regimen
| Response category . | No. (%) . | Marrow FL-C negative, n/N (%) . | Marrow PCR negative, n/N (%) . | Time to progression . | Overall survival . | ||
|---|---|---|---|---|---|---|---|
| Median, mo . | 6-y prog free, % . | Median, mo . | 6-y surv, % . | ||||
| Complete response | 217 (72) | 173/210 (82) | 73/173 (42) | 85 | 67 | Not reached | 88 |
| Nodular partial response | 31 (10) | 10/26 (39) | 8/22 (36) | 71 | 49 | Not reached | 77 |
| Partial response due to cytopenia (PR-i) | 21 (7) | 11/20 (55) | 4/13 (31) | 50 | 31 | 66 | 42 |
| Partial response due to residual disease (PR-d) | 16 (5) | 1/10 (10) | 0/4 (0) | 19 | - | 34 | 24* |
| Failure | |||||||
| Resistant disease | 13 (4) | 0/10 (0) | - | - | - | 20 | 15* |
| Death within 3 mo | 2 (<1) | - | - | - | - | 1 | - |
| Response category . | No. (%) . | Marrow FL-C negative, n/N (%) . | Marrow PCR negative, n/N (%) . | Time to progression . | Overall survival . | ||
|---|---|---|---|---|---|---|---|
| Median, mo . | 6-y prog free, % . | Median, mo . | 6-y surv, % . | ||||
| Complete response | 217 (72) | 173/210 (82) | 73/173 (42) | 85 | 67 | Not reached | 88 |
| Nodular partial response | 31 (10) | 10/26 (39) | 8/22 (36) | 71 | 49 | Not reached | 77 |
| Partial response due to cytopenia (PR-i) | 21 (7) | 11/20 (55) | 4/13 (31) | 50 | 31 | 66 | 42 |
| Partial response due to residual disease (PR-d) | 16 (5) | 1/10 (10) | 0/4 (0) | 19 | - | 34 | 24* |
| Failure | |||||||
| Resistant disease | 13 (4) | 0/10 (0) | - | - | - | 20 | 15* |
| Death within 3 mo | 2 (<1) | - | - | - | - | 1 | - |
Response categories were that of the National Cancer Institute (NCI) 1996 guidelines with the exception of partial response due to residual disease, which included patients meeting all clinical and laboratory parameters for complete response but with incomplete marrow recovery (hemoglobin level < 110 g/L, neutrophil count < 1.5 × 109/L, and/or platelet count < 100 × 109/L). Total numbers (N) in columns 3 and 4 are smaller than column 2 because not all patients had FL-C on PCR testing.
Results at 5 years.
At a median survivor follow-up of 72 months (range: 32-100 months), actuarial 6-year OS and FFS for the entire cohort (n = 300) were 77% and 51%, respectively. Among patients with a partial response or better (n = 285), median TTP was 80 months, with a projected 60% progression free at 6 years.
The impact of response category on remission duration and survival
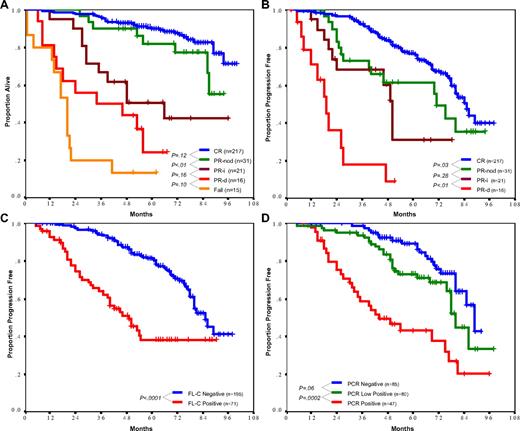
Patients in CR had the most favorable TTP (median: 85 months) and survival (88% at 6 years; Figure 1A,B), followed by patients in PR-nod who had a shorter TTP (median: 71 months, P = .03) but similar survival (77% at 6 years, P = .12). Compared with PR-nod, patients in PR-i had similar TTP (median: 50 months, P = .28), but experienced shorter survival (42% at 5 years, P = .01). The reason for inferior survival despite similar remission duration was because of differences in salvage therapy after relapse, which was limited by persistent myelosuppression in 7 of 10 PR-i patients (vs 2 of 15 PR-nod patients, P = .006), resulting in second remission in only 1 of 8 PR-i patients (vs 6 of 10 PR-nod patients, P = .05). Five patients in PR-i died during ongoing remission: 2 patients from complications of treatment-related myelodysplasia, 1 patient from neutropenic sepsis, 1 patient from carcinoma of the lung, and 1 patient from undetermined cause. Patients with residual disease (PR-d) after FCR therapy had a TTP of only 19 months and a short survival that was not significantly superior to that of nonresponders (Figure 1A, P = .10).
Overall survival (OS) and time to progression (TTP). (A) OS by treatment response. Patients in complete remission (CR) and nodular partial response (PR-nod) had similarly favorable survival (6-year OS 88% and 77% respectively, P = .12). Survival for other categories was as follows: partial response due to incomplete recovery (PR-i), 6-year OS 42%; partial remission due to residual disease (PR-d), 5-year OS 24%; and resistant disease, 5-year OS 15%. (B) TTP by treatment response. Median TTP was longest in CR patients (85 months), followed by PR-nod and PR-i (71 months and 50 months, respectively), and was only 19 months for patients in PR-d. (C) Impact of flow cytometry (FL-C) status on TTP. Patients with less than 1% CD5/19 coexpressing cells in the bone marrow at the end of therapy had a significantly longer TTP (median 85 vs 49 months for FL-C negative vs positive, P < .001). (D) Impact of PCR status on TTP. PCR-negative patients had longer TTP than low positive patients (median 89 vs 80 months, respectively, P = .06), who in turn had longer TTP than PCR-positive patients (median 40 months, P = .001 compared with low positive patients).
Overall survival (OS) and time to progression (TTP). (A) OS by treatment response. Patients in complete remission (CR) and nodular partial response (PR-nod) had similarly favorable survival (6-year OS 88% and 77% respectively, P = .12). Survival for other categories was as follows: partial response due to incomplete recovery (PR-i), 6-year OS 42%; partial remission due to residual disease (PR-d), 5-year OS 24%; and resistant disease, 5-year OS 15%. (B) TTP by treatment response. Median TTP was longest in CR patients (85 months), followed by PR-nod and PR-i (71 months and 50 months, respectively), and was only 19 months for patients in PR-d. (C) Impact of flow cytometry (FL-C) status on TTP. Patients with less than 1% CD5/19 coexpressing cells in the bone marrow at the end of therapy had a significantly longer TTP (median 85 vs 49 months for FL-C negative vs positive, P < .001). (D) Impact of PCR status on TTP. PCR-negative patients had longer TTP than low positive patients (median 89 vs 80 months, respectively, P = .06), who in turn had longer TTP than PCR-positive patients (median 40 months, P = .001 compared with low positive patients).
Bone marrow FL-C negativity was associated with superior TTP (median: 85 months, vs 49 months for FL-C positive, P < .001; Figure 1C) and survival (84% at 6 years, vs 65% for FL-C positive, P = .001). PCR status was also predictive of outcome, with median TTP for positive, low positive, and negative PCR being 40, 80, and 89 months, respectively (Figure 1D, P < .001). However, when PCR was applied to patients already known to be FL-C negative there was little additional impact, with a difference of only 12 months in median remission between PCR-positive and -negative patients (median TTP 77 vs 89 months, respectively, P = .07).
Pretreatment characteristics associated with CR rate, CR duration, and survival
The following pretreatment factors were examined for association with treatment outcomes: age, sex, time from diagnosis, performance status, creatinine clearance, Rai stage, white cell count, β2m, LDH, albumin, cytogenetics, and CD38 status. Factors independently associated with CR rate were age younger than 70 years (P = .02, odds ratio [OR] 2.8), β2m less than 2N (P = .002, OR 3.0), white cell count less than 150 × 109/L (P = .02, OR 2.8), and absence of chromosome 17 abnormalities (P = .01, OR 9.2). Survival was independently associated with age younger than 70 years (P = .001, hazard ratio [HR]: 0.35), β2m less than 2N (P = .003, HR: 0.38), white cell count less than 150 × 109/L (P = .02, HR: 0.46), LDH less than 2N (P = .001, HR: 0.12), and absence of chromosome 17 abnormalities (P = .002, HR: 0.20). Karyotypic abnormalities other than those involving chromosome 17 had no impact on CR or survival. No factors were independently associated with TTP for the patients who achieved CR.
Regression tree analysis was used to construct a model that stratified patients into risk groups by pretreatment characteristics. This analysis was performed for both CR rate and survival, with identical outcomes. The final model stratified patients by β2m, with patients having β2m 2N or more further subdivided by age (Table 3). Favorable risk (β2m < 2N) patients had a CR rate of 84% and a 6-year survival of 85% that was not significantly different between patients aged younger than 70 years or 70 years or older (6-year survival 84% vs 90%, respectively, P = .73). Intermediate risk patients (β2m ≥ 2N, age < 70 years) had a CR rate of 60% and a 6-year survival of 72%. Unfavorable risk patients (β2m ≥ 2N, age ≥ 70 years) had a CR rate of 48% and a 6-year survival of 48%. As a group, patients 70 years or older were significantly less likely to complete 6 cycles of therapy (46%, vs 79% for patients < 70 years, P < .001), with early cessation due to prolonged cytopenia (50%), disease progression/resistance (18%), patient choice (18%), early death (5%), severe infection (5%), or hemolytic anemia (5%).
Prognostic model based on regression tree analysis
| Category/age, y . | No. (%) . | Complete response, no. (%) . | CR duration . | Overall survival . | ||
|---|---|---|---|---|---|---|
| Median, mo . | 6-y prog free, % . | Median, mo . | 6-y surv, % . | |||
| β2-microglobulin less than 2N, any age | 168 (57) | 141 (84) | 86 | 72 | Not reached | 85 |
| β2-microglobulin 2N or more | ||||||
| Less than 70 y | 96 (33) | 58 (60) | 81 | 66 | Not reached | 72 |
| 70 y and older | 31 (11) | 15 (48) | 85 | 72 | 48 | 48 |
| Category/age, y . | No. (%) . | Complete response, no. (%) . | CR duration . | Overall survival . | ||
|---|---|---|---|---|---|---|
| Median, mo . | 6-y prog free, % . | Median, mo . | 6-y surv, % . | |||
| β2-microglobulin less than 2N, any age | 168 (57) | 141 (84) | 86 | 72 | Not reached | 85 |
| β2-microglobulin 2N or more | ||||||
| Less than 70 y | 96 (33) | 58 (60) | 81 | 66 | Not reached | 72 |
| 70 y and older | 31 (11) | 15 (48) | 85 | 72 | 48 | 48 |
Patients with β2-microglobulin (β2m) less than twice upper limit of reference (< 2N) had a favorable 6-year survival of 85%, with no significant difference and between younger (< 70 years, n = 158) and older (≥ 70 years, n = 10) patients (6-year survival 84% vs 90%, respectively, P = .73). Patients with β2m 2N or more and age younger than 70 years had intermediate survival (6-year survival 72%, P = .003 compared with low β2m patients), and those with β2m 2N or more and age 70 years or older had the most unfavorable survival (6-year survival 48%, P = .007 compared with intermediate risk patients). For those patients who achieved complete remission, remission duration was similar regardless of pretreatment risk category.
Patients with abnormal chromosome 17 (n = 7), LDH 2N or more (n = 5), or both (n = 1) constituted high risk, low frequency populations with CR rates of 33% or less (Table 1). None of the patients with LDH 2N or more had known large cell transformation or active hemolysis to explain the LDH elevation. Interestingly, even among the 13 high-risk patients, β2m remained strongly prognostic with low β2m patients (< 2N, n = 5) experiencing significantly superior CR rate and survival than high β2m patients (≥ 2N, n = 8; CR rate 80% vs 0% and OS 80% [at 6 years] vs 13% [at 4 years], respectively; P < .01 for both comparisons).
Late events after completion of therapy
Early hematologic and infectious toxicity during FCR therapy had been previously reported.7 The extended follow up of patients permitted evaluation of late toxicity during remission. Following completion of therapy, 19% patients had persistent cytopenia (neutrophil count < 109/L and/or platelet count < 50 × 109/L) lasting more than 3 months. Following recovery of blood counts, recurrent late cytopenia episodes occurred in 69 (28%) of 245 patients; these occurred predominantly during the first year of remission, with actuarial 1- and 6-year incidences of 18% and 23%, respectively. No active treatment other than discretionary use of growth factor support was required for late cytopenia, and 8% of episodes were associated with infections. Advanced Rai stage was associated with an increased risk of persistent cytopenia longer than 3 months (27% vs 15% for stages III-IV and 0-II, respectively, P = .03), but not with the risk of late recurrent episodes. Age, performance status, and β2m were not significantly associated with persistent or recurrent cytopenia.
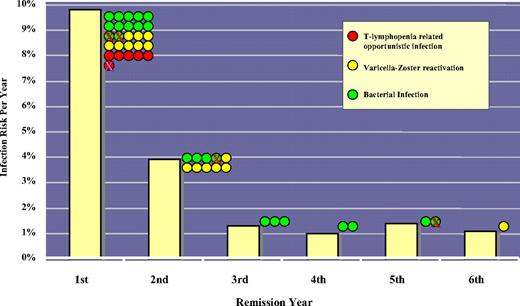
The risk of serious (grade ≥ 3) or opportunistic infection was 10% and 4% during the first and second years of remission, respectively. T-lymphopenia–associated opportunistic infections (2 Pneumocystis jiroveci pneumonia, 1 Legionella pneumonia, 1 pulmonary aspergillosis, 1 endemic fungus infection, and 1 disseminated listeriosis) were restricted entirely to the first year of remission, and 14 of the 15 episodes of varicella-zoster reactivation occurred within the first 2 years of remission. From the third year of remission onward, serious infections were uncommon (< 1.5% per year) and predominantly bacterial (Figure 2).
The risk of late infections during remission. Bars represent the risk of serious (grade ≥ 3, viral, or opportunistic) infections for each year of ongoing remission. The infection risk was highest in the first year and rapidly declined to a baseline risk of less than 1.5% per year from the third year onward. The colored dots represent individual episodes per year; crosses, infection episodes that were fatal. The occurrence of opportunistic infections was limited to the first year, and varicella-zoster reactivation was limited predominantly to the first 2 years.
The risk of late infections during remission. Bars represent the risk of serious (grade ≥ 3, viral, or opportunistic) infections for each year of ongoing remission. The infection risk was highest in the first year and rapidly declined to a baseline risk of less than 1.5% per year from the third year onward. The colored dots represent individual episodes per year; crosses, infection episodes that were fatal. The occurrence of opportunistic infections was limited to the first year, and varicella-zoster reactivation was limited predominantly to the first 2 years.
Richter syndrome (RS; diffuse large cell lymphoma) occurred in 6 patients: RS was diagnosed at disease progression from CR or PR in 5 patients, and beyond 6 months after CLL relapse in 1 patient. The actuarial risk of RS was 2.5% at 6 years. Myelodysplasia occurred in 8 patients, 2 of whom were in PR-i (10% crude rate for PR-i, vs 2% for other response categories P = .10). Five patients had refractory cytopenia with multilineage dysplasia, and 3 patients had refractory anemia with excess blasts-2 (RAEB-2). All 8 patients developed myelodysplasia during ongoing CLL remission, and no patient had been exposed to genotoxic agents other than FCR therapy. The actuarial risk of myelodysplasia was 2.8% at 6 years.
Historical comparison with patients receiving F or FC/M
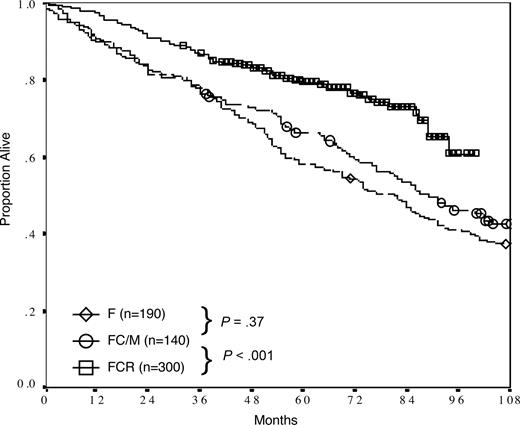
In a comparison of FCR with previous generations of frontline fludarabine-based regimens at our institution, treatment with FCR was associated with a significantly superior overall survival (P < .001, Figure 3). After adjusting for differences in pretreatment variables using Cox regression multivariate analysis, FCR therapy emerged as the strongest independent predictor of survival (P < .001, HR: 0.48), with age 70 years or older (P < .001, HR: 2.1) and β2m 2N or more (P < .001, HR: 1.8) remaining as significant covariates.
Survival of patients receiving fludarabine (F), fludarabine and cyclophosphamide or mitoxantrone (FC/M), and FCR as initial therapy of CLL at the M. D. Anderson Cancer Center. Six-year overall survivals were 54%, 59%, and 77%, respectively.
Survival of patients receiving fludarabine (F), fludarabine and cyclophosphamide or mitoxantrone (FC/M), and FCR as initial therapy of CLL at the M. D. Anderson Cancer Center. Six-year overall survivals were 54%, 59%, and 77%, respectively.
Discussion
The addition of rituximab to fludarabine and cyclophosphamide (FC) doubled the complete remission rate and remission duration of FC to 72% and 80 months, respectively.1-4 In a historical comparison with a group of patients receiving previous generations of CLL therapy, FCR therapy emerged as the most important determinant of long-term survival. The safety profile of FCR was favorable, with early deaths occurring in less than 1% of patients. One-fifth of patients had delayed marrow recovery after completion of therapy, and one-quarter of patients developed self-limiting late cytopenic episodes. The risk of late infection was highest in the first year of remission, and decreased rapidly to a baseline level of less than 1.5% per year by the third year. Consistent with previously established kinetics of immune recovery following fludarabine-based therapy,10 the occurrence of severe opportunistic infections was limited entirely to the first year of remission.
Complete remission was the most important determinant of long-term survival. Inferior survival and decreased CR rate were associated with the same constellation of adverse pretreatment factors, and patients with adverse factors who achieved CR enjoyed durable remissions similar to their nonadverse counterparts. The importance of CR achievement as an “equalizer” of risk was highlighted by the TTP analysis showing that no pretreatment factor was independently associated with decreased CR duration. This observation underscores the ability of modern therapy to alter the natural history of CLL, with traditional prognostic factors losing their significance in the face of treatment advances. Patients in PR due to persistent cytopenia (PR-i) experienced longer time to disease progression than patients in PR due to persistent disease (PR-d). The separation of patients in partial response by disease status is not a validated end point under the current consensus guidelines,8,17 and requires further prospective evaluation prior to its incorporation into routine clinical practice. The simple flow cytometric gating strategy used in this study was prognostic for remission duration and survival. An international standardized approach for high-sensitivity flow cytometric assessment was recently published and will further evaluate the impact of MRD in future therapeutic trials.18
Specific subgroups with disproportionately low CR rates were patients with chromosome 17 abnormalities and patients with LDH 2N or more. Deletion of the p53 locus on chromosome 17 had been associated with poor survival and chemoresistance.16,19 Although 7 of 8 patients with chromosome 17 abnormalities in the current study had changes that would have resulted in the loss of at least one p53 locus, FISH screening was not performed and it is not known how many other patients harbored occult deletions. The results of the current study were therefore not generalizable to patients with p53 deletion by FISH, but with apparently normal chromosome 17 by conventional karyotyping. It is known that the cytogenetic yield of CLL cells is poor under routine culture conditions,20 and FISH screening as well as determination of IgVH mutation and ZAP-70 status should be incorporated into clinical trials to prospectively assess their impact on treatment outcome. The reason for the association between elevated LDH and resistant disease was incompletely defined and may be related to its association with proliferative disease.
Beta-2-microglobulin, the invariant light chain of HLA class I molecules, was selected by the regression tree analysis to be the most important determinant of CR and survival in the total patient population. Elevated β2m levels had been associated with inferior survival in several hematologic malignancies, including acute myelogenous leukemia,21 mantle cell lymphoma,22 and multiple myeloma,23 and is a consistent marker of poor-risk CLL in our institutional experience.24,25 The biologic reason for its prognostic significance is unclear, and may be related to high tumor burden. In the current study, β2m was prognostic even among patients with high-risk disease features (chromosome 17 abnormalities and/or LDH ≥ 2N), with a CR rate of 80% and a 6-year survival of 80% among high-risk patients with β2m less than 2N. A simple risk model based on β2m and age effectively stratified patients into risk groups for future risk-adapted studies (Table 3). High β2m was significantly associated with decreased CR probability and inferior survival, and identified those patients most in need of targeted interventions. Younger (< 70 years) patients may benefit from therapy intensification with addition of other active agents such as alemtuzumab,26 as their excess risk of death from uncontrolled leukemia currently outweighs the risk of therapy-related death. Older (≥ 70 years) patients were underrepresented in this and other27-29 chemoimmunotherapy studies, largely due to a tendency for community physicians to preferentially refer their younger patients to academic centers for investigational therapy. In our experience, older patients were less likely to complete 6 cycles of FCR therapy and may therefore benefit from alternative, marrow-sparing treatment approaches.
The FCR regimen has the highest CR rate, longest remission duration, and most favorable survival of frontline regimens for the treatment of CLL reported to date. It is an appropriate therapeutic backbone for future studies in the pursuit of a cure for CLL.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors acknowledge the contribution of physicians within the University of Texas M. D. Anderson Cancer Center and in the community who assisted with the clinical care of patients on this trial.
Authorship
Contribution: M.J.K. designed, performed, and analyzed the trial and coauthored the paper; C.S.T. analyzed results and wrote the paper; S.O., W.W., H.K., D.A.T., and J.C. provided clinical care to patients, assisted in the analysis of data and development of critical themes, and coauthored the paper; S.W. and K.-A.D. analyzed data and coauthored paper; and S.L. collected and verified patient information, analyzed data, and coauthored the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Michael J. Keating, Leukemia Department, Unit 428, The University of Texas M. D. Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030; e-mail mkeating@mdanderson.org.