Abstract
Cutaneous mastocytosis (CM) in children is a usually benign skin disorder caused by mast cell proliferation. Progressive disease leading to systemic involvement and fatal outcomes has been described. C-kit receptor mutations have been identified as causative for CM, some of which potentially respond to imatinib treatment as described for patients with systemic mastocytosis. We report successful therapy of progressive CM with imatinib in a 23-month-old boy. KIT gene analysis revealed not only a somatic deletion of codon 419 in exon 8 (c.1255_1257delGAC) which responds to imatinib therapy, but also a novel germ line p. Ser840Asn substitution encoded by exon 18 in the c-kit kinase domain. Family history suggests this exchange does not affect receptor function or cause disease. Imatinib therapy was well tolerated, stopped symptoms and disease progression, and appeared to shorten the course of the disease. Imatinib could possibly represent a novel therapeutic option in patients with progressive CM.
Introduction
Cutaneous mastocytosis (CM) is a usually benign disease caused by neoplastic mast cell proliferation.1 The disease leads to various degrees of hyperpigmented skin lesions, usually accompanied by pruritus.1,2 Extracutaneous involvement has to be ruled out to distinguish CM from systemic mastocytosis (SM), which requires aggressive treatment.1,3–5 In most children, CM usually resolves by adolescence, in contrast to adults.6–9 However, progressive disease leading to systemic involvement and fatal outcome has been described.10
c-Kit receptor mutations play a causative role in mast cell proliferation observed in both CM and SM.1,11 Imatinib, a tyrosine kinase inhibitor that effectively targets the bcr-abl kinase in chronic myeloid leukemia,12 also specifically inhibits the c-Kit receptor.13,14 Consequently, imatinib has been successfully used for the treatment of SM; however, clinical response has been observed to depend on the underlying KIT mutation.3,15,16 The role of imatinib in CM therapy, in contrast, is not clear. A recent study in children with CM revealed KIT mutations comparable with patients with SM.11 It can therefore be assumed that aberrant c-Kit signaling in CM can also be targeted by imatinib. However, most probably because of the usually benign course of CM, to date there is no information regarding the role of imatinib in CM treatment. We report successful and uneventful therapy of progressive CM with imatinib in a 23-month-old boy.
Methods
Genomic DNA from skin biopsy was isolated using the QIAamp DNA Mini kit (Qiagen, Hilden, Germany), followed by polymerase chain reaction amplification of KIT exons 8, 9, 10, 11, 17, 18, and 19. Sequencing was performed with the ABI PRISM Big Dye Terminator, version 3.1 Ready Reaction Cycle Sequencing kit (Applied Biosystems, Foster City, CA). Reaction products were separated on an ABI 3130 Genetic Analyzer (Applied Biosystems).
Case report, results, and discussion
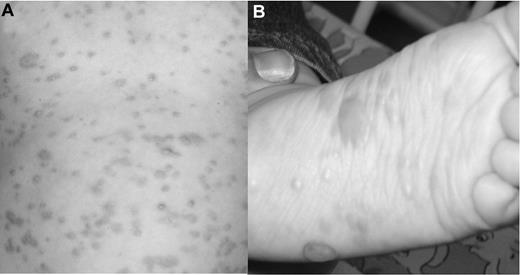
An 8-month-old boy presented with a maculo-papular-bullous exanthema affecting head, trunk, and extremities (Figure 1). The patient had pruritus, and Darier sign was positive. The rash had started at 5 months and showed constant progression. Internal and neurologic examination was unremarkable. The tentative diagnosis of CM was confirmed histologically. Serum tryptase, and serum and urine histamine levels were normal. Histologic bone marrow examination showed no pathologic mast cell infiltration, and 99m-TC-MDP scintigraphy was negative.
Skin changes before imatinib therapy. (A) Generalized macules and papules accompanied by severe pruritus on trunk and extremities. (B) The patient showed increasing numbers of bullous lesions on the extremities, especially on both feet.
Skin changes before imatinib therapy. (A) Generalized macules and papules accompanied by severe pruritus on trunk and extremities. (B) The patient showed increasing numbers of bullous lesions on the extremities, especially on both feet.
Despite conservative treatment, macular and bullous lesions gradually increased in size and number, and pruritus continuously worsened. At 23 months, serum tryptase levels started to rise but remained within normal levels (< 20 μg/L). Sequence analysis of genomic DNA from a skin biopsy demonstrated a somatic deletion of the GAC nucleotide triplet encoding amino acid 419, aspartic acid, in exon 8 of the KIT gene (c.1255_1257delGAC; p.Asp419del), a known mutation responding to imatinib therapy. Furthermore, a novel germ line mutation resulting in the replacement of serine (AGC), residue 840, by asparagine (AAC; p.Ser840Asn or S840N) was found in exon 18. Family screening identified the patient's father as the carrier of the new mutation. None of the father's family members was ever diagnosed with mastocytosis, a gastrointestinal stromal tumor, abdominal tumors, leukemia, germ line tumors, or skin pigment changes.
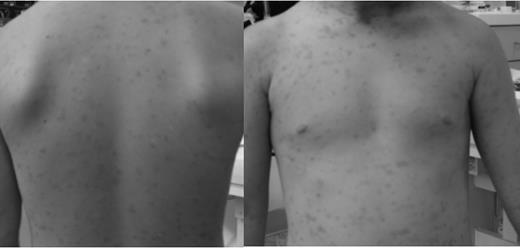
Treatment with 100 mg of imatinib daily was begun, after the patient's parents had given their written, informed consent in accordance with the Declaration of Helsinki. Within 2 months, the pruritus completely resolved, the bullous lesions disappeared, and the maculo-papular cutaneous lesions decreased to a faint macular rash (Figure 2). After 13 months, imatinib treatment was discontinued for the first time. Within a week, the patient again showed a marked increase of lesion size and pruritus, which prompted the parents to restart therapy after only 5 days. After another 13 months, dose tapering (100 mg every other day) was successful without recurrence. Imatinib therapy was discontinued after a total of 32 months. At the age of 6, the patient shows only faint hyperpigmented macules, no pruritus, normal tryptase levels, and no recurrence.
Skin status after imatinib therapy. The patient shows faint macular lesions without any accompanying symptoms.
Skin status after imatinib therapy. The patient shows faint macular lesions without any accompanying symptoms.
The patient tolerated imatinib treatment well without clinical side effects. White blood count and serum parameters remained within normal limits. Growth (parallel to the 25th percentile), weight gain (parallel to the 50th percentile), and neurologic and cognitive development were unimpaired.
Imatinib has been successfully used to treat patients with SM.12 Aberrant c-Kit signaling caused by receptor mutations is assumed to be the cause of most cases of mastocytosis.1 Mutations in the intracellular kinase domain, the juxtamembranous domain, the transmembrane domain, and the extracellular domain of the c-Kit receptor have been described in patients with mastocytosis.1,5,11,17 p.Asp816Val is the most common mutation but is insensitive to imatinib treatment.1 Only few c-Kit mutations have been reported to clinically respond to imatinib therapy (eg, p.Asp816Thr, p.Lys509Ile, p.Phe522Cys, p.Val560Gly, p.Asp419del).18–22 KIT mutation analysis in our patient yielded not only a somatic p.Asp419del mutation but also a novel germ line p.Ser840Asn substitution in the intracellular kinase domain of the receptor. The boy had inherited the mutation from his father; his siblings were not affected. A mutation of the adjacent amino acid (p.Asp839Lys) paradoxically inactivates the c-Kit receptor and causes mastocytosis in children.23 Surprisingly, however, the father and his family were not affected by diseases linked to c-Kit receptor mutations. We therefore assume that this novel c-Kit mutation neither affects receptor function nor causes disease.
Probably because of the usually benign course, there is no information about the use of imatinib in CM. Current guidelines recommend imatinib treatment only for patients with SM.1 Yanagihori et al showed KIT mutation patterns in pediatric CM patients comparable with patients with SM.11 Our patient showed disease progression despite conservative treatment and carried a somatic p.Asp419del mutation, which responds to imatinib and was originally described as a germ line mutation causing familial mastocytosis and gastrointestinal stromal tumor.20 Murphy et al reported cases of progressive CM with fatal outcome in children.10 Taken together, this information prompted us to initiate imatinib treatment.
Our patient tolerated therapy without clinical or serologic side effects. Furthermore, our patient showed normal growth and development throughout treatment and follow-up. Imatinib is safe in adults and is currently used to treat malignancies in children, including chronic myeloid leukemia, gastrointestinal stromal tumors, brain tumors, osteosarcoma, Ewing sarcoma, neuroblastoma, synovial sarcomas, and dermatofibrosarcoma protuberans.13,24–27 Series in children with solid tumors, acute lymphoblastic leukemia, and chronic myeloid leukemia reported dose-dependent and usually transient grade 1 to 4 side effects but no apparent negative influence on child growth and development.12,24–26 These studies indicate that imatinib treatment is safe in children, which would support its use in less life-threatening illnesses.
Similar to patients with SM, our patient showed a relapse of CM when treatment first was stopped, which resolved after reintroducing the drug.15 However, after dose tapering, imatinib treatment could be stopped in our patient. Pediatric series with long-term follow-up showed that CM takes a prolonged course and resolves in adolescence. Disease duration is usually not affected by conservative treatment, and symptoms improve only in a minority of patients.6–8 Our case suggests that imatinib treatment not only alleviates symptoms and stops disease progression but could possibly also shorten the natural course of CM in children.
In conclusion, we successfully treated progressive CM in a 23-month-old boy with imatinib. This was tolerated very well, stopped progression, led to the resolution of symptoms, and appeared to shorten disease duration compared with previous reports. Imatinib could therefore be an option to treat progressive CM in children in the future.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: K.M.H. wrote the manuscript; A.M. cowrote the paper and treated the patient; P.L. performed the KIT mutational analysis; B.B. performed the skin biopsy and histologic studies; A.W., P.S., M.B., W.S., and H.L. were actively involved in the decision process to treat with imatinib and cowrote the paper; and C.U. cowrote and supervised the paper and treated the patient.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Karl M. Hoffmann, Department of Pediatrics and Adolescent Medicine, Medical University Graz, Auenbruggerplatz 30, A-8036 Graz, Austria; e-mail: hoffmaka@mac.com.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal