Abstract
Formation of inhibitory antibodies is a common problem encountered in clinical treatment for hemophilia. Human factor VIII (hFVIII) plasmid gene therapy in hemophilia A mice also leads to strong humoral responses. We demonstrate that short-term therapy with an anti-ICOS monoclonal antibody to transiently block the inducible costimulator/inducible costimulator ligand (ICOS/ICOSL) signaling pathway led to sustained tolerance to hFVIII in hFVIII plasmid–treated hemophilia A mice and allowed persistent, high-level FVIII functional activity (100%-300% of normal). Anti-ICOS treatment resulted in depletion of ICOS+CD4+ T cells and activation of CD25+Foxp3+ Tregs in the peripheral blood, spleen, and lymph nodes. CD4+ T cells from anti-ICOS–treated mice did not proliferate in response to hFVIII stimulation and produced high levels of regulatory cytokines, including interleukin-10 and transforming growth factor-β. Moreover, CD4+CD25+ Tregs from tolerized mice adoptively transferred dominant tolerance in syngeneic hFVIII plasmid-treated hemophilia A mice and reduced the production of antibodies against FVIII. Anti-ICOS–treated mice tolerized to hFVIII generated normal primary and secondary antibody responses after immunization with the T-dependent antigen, bacteriophage Φx 174, indicating maintenance of immune competency. Our data indicate that transient anti-ICOS monoclonal antibody treatment represents a novel single-agent immunomodulatory strategy to overcome the immune responses against transgene product after gene therapy.
Introduction
The ultimate goal of gene therapy in the treatment of genetic disorders is to achieve persistent, therapeutic-levels of transgene expression. However, immune responses against exogenously introduced transgene products occur in many gene therapy model systems. Whereas transgene induced immune responses primarily comprise humoral responses,1-5 cytotoxic lymphocytes (CTLs) may also be induced in the presence of other strong signals, such as viral vector components.6-10 Transgene-specific antibodies and/or CTLs can significantly reduce or eliminate functional transgene products and/or transduced cells. Thus, in addition to development of efficient gene transfer vectors and delivery methods, novel approaches to establish transgene-specific tolerance are essential to the success of gene therapy
Hemophilia A is a congenital bleeding disorder caused by a deficiency of coagulation factor VIII (FVIII). Currently, hemophilia patients are treated with repeated infusions of FVIII protein concentrates. Gene therapy has been explored as a promising treatment in phase 1 clinical trials.11-13 However, to date, only transient, low-level FVIII protein expression has been achieved because of development of immune responses against FVIII and/or associated gene transfer vectors. In most preclinical experiments using immunocompetent hemophilia A murine and canine models, strong immune responses against FVIII after gene therapy have completely inhibited circulating FVIII activity and thus subverted the effect of gene therapy.2-5,8,9,14-16
Recent gene transfer studies1,5,9,17-20 indicate that the risk of transgene-specific immune responses depends on multiple factors, including the type and dose of the vector, the expression cassette and tissue specificity of the promoter, the type and level of transgene expression, route of administration, transduced cell type, and the age and the underlying mutation of the gene therapy model. Some of these factors have been extensively reviewed.21 Avoiding risk factors for the induction of antibody before gene therapy is highly desirable. However, some of these factors cannot be altered and some are not easy to overcome. Thus, safe and effective means to induce tolerance and prevent and/or modulate the transgene-specific immune responses after gene therapy need to be developed.22
Limited success has been achieved to induce tolerance against transgene product on prolonged exposure to antigens, including mucosal administration of FVIII-C2 domain,23 B-cell gene therapy,24 or hepatic gene transfer.25 However, in most cases tolerance was established in only a fraction of the treated animals. Common immunosuppressive drugs nonspecifically targeting T-cell activation, clonal expansion or differentiation into effector T cells have also been used to prevent transgene-specific responses. A recent study of combining 2 drugs, mycophenolate mofetil (MMF) and rapamycin (RPA), demonstrated that antibody responses against factor IX (FIX) was prevented after adeno-associated virus (AAV)–mediated gene transfer into the livers of nonhuman primates.26 However, administration of either a single agent, or 2-agent combinations of MMF, cyclosporine A (CSA), and RPA were shown to have limited effects in a hemophilia A mouse model by only delaying immune responses after nonviral gene transfer.27 Inhibitory antibodies appeared shortly after withdrawal of the drug(s). This difference in the immune responses may depend on the transgene product (eg, FVIII protein) is more immunogenic than FIX. Other strategies to induce peripheral tolerance to transgene products have included elimination of activated/effector T cells by depleting antibodies, generation of T-cell apoptosis, or antigen-specific nonresponsiveness (anergy) by costimulation blockade, and active suppression by regulatory T cells (Tregs). We have previously shown that human factor VIII (hFVIII) transgene expression in mice was prolonged after treatment with a combined immunomodulation regimen using murine CTLA4-Ig and an antimurine CD40L antibody (MR1) to block T-cell costimulation via CD28/CTLA4:B7 and CD40L/CD40 pathways.27 Unfortunately, antihuman CD40L is currently not available for clinical use. Therefore, the identification of other effective and less toxic single agent(s) would be beneficial for eventual clinical applications.
Inducible costimulator (ICOS) is the third member of the CD28/CTLA4 costimulatory family.28-30 ICOS binds specifically to its ligand (ICOS-L, B7-related protein-1[B7RP-1, B7h]), which is constitutively expressed by B cells.31 The interaction of ICOS with ICOS-L permits terminal differentiation of B cells to antibody-secreting plasma cells. ICOS expression, although readily detectable on resting T cells, rises to levels comparable with those of CD28 after activation of T cells.32 In the absence of ICOS (eg, in ICOS knockout mice), T-cell activation and proliferation are defective and antibody responses to T-dependent antigens are reduced.33,34 Anti-ICOS monoclonal antibody (mAb) alone or in combination with other agents, such as soluble CD40-Ig or anti-CD40L, has been shown to inhibit allograft rejection in transplantation animal models35,36 and to induce dominant tolerance to islet cell allografts in the NOD mouse.37 These models suggest the involvement of antigen-specific regulatory T cells in tolerance induction after anti-ICOS treatment. In this study, we demonstrate that immune modulation with anti-ICOS mAb alone down-regulates FVIII-specific immune responses in hemophilia A mice after nonviral gene therapy. In addition, we have used this model to explore the mechanisms responsible for immune tolerance induction by anti-ICOS mAb.
Methods
Mice
All mice were kept in accordance with National Institutes of Health (NIH) guidelines for animal care and the guideline of Seattle Children's Hospital Research Institute and maintained at a specific pathogen-free facility. Hemophilia A mice in a 129/SV × C57BL/6 mixed genetic background were generated by targeted disruption of exon 16 of the FVIII gene38 and bred in our animal facility.
Gene transfer of hFVIII into hemophilia A mice and immunomodulation with anti-ICOS
Hemophilia A mice were injected with hFVIII plasmid (pBS-HCRHP-FVIIIA; see the third paragraph in this section) and treated with different immunomodulatory agents. The regimens included intraperitoneal administration of anti-ICOS mAb (hybridoma 7E.17G9,39 rat IgG2b; produced at National Cell Culture, Minneapolis, MN) at a dose of 10 mg/kg given on days 1 to 5, 8, 11, and 14 (∼ 220 μg per injection); a combination of anti-ICOS mAb (same dosing schedule) and soluble CTLA4-Ig27 (BD Biosciences PharMingen, San Diego, CA) at a dose of 5 mg/kg given on days 0 and 2; and a combination of anti-ICOS (same dosing schedule) and anti-CD40L27 (clone MR1; Bio Express, West Lebanon, NH) at a dose of 10 mg/kg given on days −1, 0, 2, 7, and 14.
Hemophilia A mice designated for extended immunomodulation received 16 intraperitoneal injections of anti-ICOS (10 mg/kg/treatment)36 between days −1 and 30; the injections were given daily during the first week, then 2 or 3 times each week for the next 3 weeks.
The methods of plasmid DNA preparation and infusion have been described previously.5,40 Briefly, 50 μg of hFVIII plasmids in 2 mL of phosphate-buffered saline (PBS) were injected into the tail vein of 20- to 24-g hemophilia A mice over 6 to 8 seconds. Scheduled blood samples were taken from the retro-orbital plexus.
Antibodies
Antimurine Foxp3 (FJK-16s)-fluorescein isothiocyanate, antimurine CTLA-4 (UC10-4B9)-phycoerythrin (PE), anti-CD62L (MEL-14)-PE-Cy5, anti-CD45RB (C363.16A)-antigen-presenting cells (APCs), and antimurine ICOS (C398.4A)-fluorescein isothiocyanate were purchased from eBioscience (San Diego, CA). Anti-GITR (DTA-1)-PE-Cy7, anti-CD25 (PC61)-allophycocyanin-Cy7, and anti-CD4 (L3T4)-Alexa Fluor 700 were purchased from BD Biosciences PharMingen.
T-cell staining and flow cytometric analysis
Single-cell suspensions were prepared by mechanical disruption of superficial cervical lymph nodes (LNs) and spleens of tolerized, anti-ICOS alone treated, hFVIII plasmid only treated, or untreated control mice, respectively. Peripheral blood mononuclear cells (PBMCs) were isolated from heparinized blood after lysis of red cells. Cells were first stained for surface markers CD4, CD25, CD62L, CD45RB, GITR, and ICOS. Subsequently cells were fixed, permeabilized, and stained intracellularly for CTLA-4 and Foxp3. Flow cytometric analysis was performed using a FACS LSRII flow cytometer (BD Biosciences, San Jose, CA) and analyzed using FlowJo software (TreeStar, Ashland, OR)
Assays for measuring hFVIII activity and anti-hFVIII antibodies
hFVIII activity was measured by a chromogenic assay measuring factor Xa generation (Chromogenix Coatest SP Factor VIII kit, Diapharma, West Chester, OH), and a modified clotting assay using reagents to measure activated partial thromboplastin time and FVIII-deficient plasma. hFVIII activity was calculated from a standard curve generated by serially diluted normal human pooled plasma.
Inhibitory antibodies against hFVIII were measured by Bethesda assay as previously described.41
Proliferation assays
CD4+ T cells were isolated by negative selection from spleens of tolerized or anti-ICOS alone treated, or hFVIII plasmids only treated, or untreated mice by using the mouse CD4+ T-cell isolation kit form Miltenyi Biotec (Auburn, CA). CD4+ T cells (105 cells in 200 μL per well) were stimulated in vitro with hFVIII concentrate at 10 U/mL (1 U = 100 ng of hFVIII protein) or with phytohemagglutinin (PHA; Sigma-Aldrich, St Louis, MO) at 5 μg/mL or cultured without stimulant as control for 72 hours in the presence of T cell–depleted, irradiated APCs (105 per well) in a 96-well round-bottom plate, and pulsed with 1 μCi [3H]-thymidine per well for the final 18 hours. All data are shown as mean plus or minus SD counts per minute for [3H]-thymidine incorporation (mean ± SD of triplicate cultures).
Adoptive transfer of CD4+CD25+ Tregs into syngeneic hemophilia A mice
Single-cell suspensions were prepared and pooled from the spleens of tolerized mice, hFVIII plasmids only treated mice, or untreated control mice. The pooled cells were purified to obtain CD4+ T cells using a CD4+ isolation kit (Miltenyi Biotec). The CD4+CD25− and CD4+CD25+ populations were isolated from the bound and unbound fractions, respectively, using the CD25+ Treg isolation kit (Miltenyi Biotec). A total of 5 × 106 CD4+, or 106 CD4+CD25−, or CD4+CD25+ cells suspended in 300 μL PBS was injected into syngeneic mice via the tail vein. Mice were challenged by hydrodynamic delivery of hFVIII plasmids the next day. hFVIII expression levels and inhibitory antibody titers were followed over time.
Measurements of cytokine secretion
CD4+ T cells were prepared from the spleens of tolerized mice, hFVIII plasmids only treated mice, or untreated control mice as described in “Proliferation assays.” Cells were cultured alone (control) or in the presence of 10 U/mL of hFVIII, or 5 μg/mL of PHA, for 144 hours together with T cell–depleted, irradiated APCs. Cytokine concentrations of interleukin-10 (IL-10) and transforming growth factor-β1 (TGF-β1) in the supernatants were determined by enzyme-linked immunosorbent assay (BD Biosciences) according to the manufacturer's recommendation and the data interpolated against the linear range on the standard curves.
Immunization of mice with bacteriophage Φx 174
Bacteriophage Φx 174 was prepared as previously described.42 The stock solution of 1011 plaque-forming units (PFUs) per milliliter was diluted and injected intraperitoneally into tolerized and untolerized hemophilia A mice 6 months after plasmid injection at a dose of 1010 PFU/kg (2 × 108 PFU/mouse). A secondary immunization was carried out 4 weeks after the primary immunization.
Approximately 200 μL of peripheral blood was collected before immunization and at 1, 2, and 4 weeks after each immunization. Aliquots of sera were stored at −80°C and subsequently analyzed for phage-neutralizing antibody activity and expressed as the rate of phage inactivation (K value [Kv]) using the standard formula.27,42
Statistics
Data were expressed as mean plus or minus SD. The statistical significance of the difference between means was determined using the 2-tailed Student t test. Differences were considered significant at P less than .05.
Results
Short-term anti-ICOS treatment does not prevent formation of anti-hFVIII antibodies
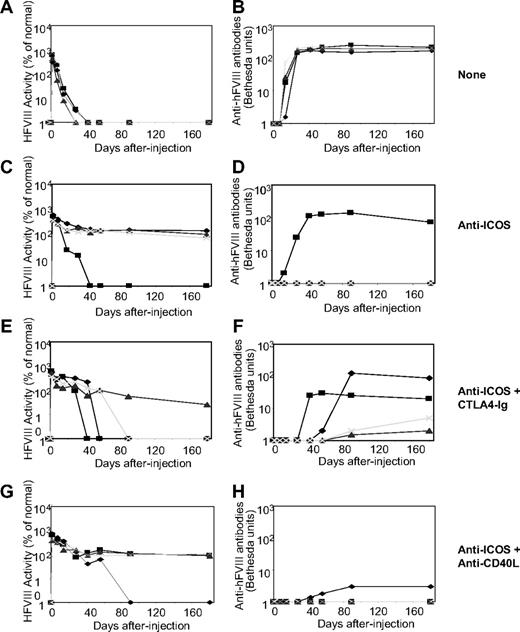
As shown in our previous study,5 administration of hFVIII plasmids with a liver specific promoter into hemophilia A mice using hydrodynamic injection can produce high levels of transgene expression. However, expression levels rapidly diminished because of the development of inhibitor antibodies (Figure 1A,B). To modulate the immune responses against hFVIII, we tested agents that block the ICOS/inducible costimulator/inducible costimulator ligand (ICOSL) pathway with or without additional agents disrupting alternative costimulatory pathways. Three groups of hFVIII plasmid-treated mice were transiently immunosuppressed after gene transfer by (1) administration of anti-ICOS mAb alone (given as 8 intraperitoneal treatments over a 2-week period), (2) combination of anti-ICOS (same dosing protocol) and 2 doses of soluble CTLA4-Ig (given at days 0 and 2), and (3) combination of anti-ICOS (same dosing protocol) and anti-CD40L mAb (given as 5 treatments between days −1 and 14). One of 4 mice from the anti-ICOS only group (Figure 1C,D), 3 of 4 mice from the combination anti-ICOS and CTLA4-Ig group (Figure 1E,F), and 1 of 4 mice from the combination anti-ICOS and anti-CD40L mAb group (Figure 1G,H) developed inhibitory antibodies by 4 to 8 weeks after gene transfer. These results imply that 8 treatments of anti-ICOS alone over a 2-week period prevented anti-hFVIII antibody responses in 75% of the animals and that addition of either CTLA4-Ig or anti-CD40L mAb does not further enhance the immunomodulatory effect of anti-ICOS.
Naked plasmid transfer of hFVIII plasmids into hemophilia A mice with short-term anti-ICOS treatment either alone or in combination with CTLA4-Ig or antimurine CD40L antibody (MR1). A total of 50 μg of the plasmid in 2 mL of saline solution was injected into the tail vein of hemophilia A mice (n = 4) in 5 to 8 seconds. Immunosuppressive agents were administered intraperitoneally. Anti-ICOS mAb was administrated 8 times in a 2-week period, CTLA4-Ig was injected 2 times at day 0 and 2, and anti-CD40L mAb was administered 5 times over a 2-week period. Circulating hFVIII activity was evaluated in plasma at regular intervals by a modified clotting assay (A,C,E,G) and confirmed by a chromogenic COATEST assay. Inhibitory antibody titers were evaluated by Bethesda assay and expressed as Bethesda units/mL (B,D,F,H). (A,B) No immunosuppression. (C,D) Anti-ICOS mAb alone. (E,F) Anti-ICOS + CTLA4-Ig. (G,H) Anti-ICOS + anti-CD40L mAb. The respective set of figures (A,B, C,D, E,F, and G,H) show data from the same experiment, and an individual mouse was represented by the same symbol in a set.
Naked plasmid transfer of hFVIII plasmids into hemophilia A mice with short-term anti-ICOS treatment either alone or in combination with CTLA4-Ig or antimurine CD40L antibody (MR1). A total of 50 μg of the plasmid in 2 mL of saline solution was injected into the tail vein of hemophilia A mice (n = 4) in 5 to 8 seconds. Immunosuppressive agents were administered intraperitoneally. Anti-ICOS mAb was administrated 8 times in a 2-week period, CTLA4-Ig was injected 2 times at day 0 and 2, and anti-CD40L mAb was administered 5 times over a 2-week period. Circulating hFVIII activity was evaluated in plasma at regular intervals by a modified clotting assay (A,C,E,G) and confirmed by a chromogenic COATEST assay. Inhibitory antibody titers were evaluated by Bethesda assay and expressed as Bethesda units/mL (B,D,F,H). (A,B) No immunosuppression. (C,D) Anti-ICOS mAb alone. (E,F) Anti-ICOS + CTLA4-Ig. (G,H) Anti-ICOS + anti-CD40L mAb. The respective set of figures (A,B, C,D, E,F, and G,H) show data from the same experiment, and an individual mouse was represented by the same symbol in a set.
Extended anti-ICOS treatment prevents inhibitory antibody production
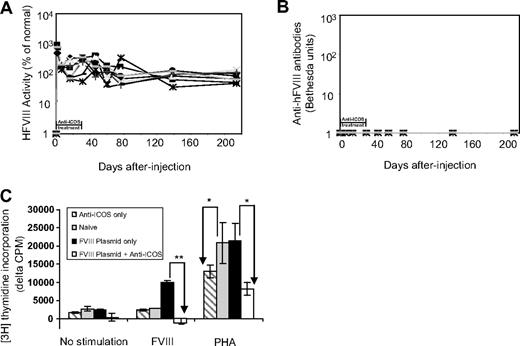
Because of the incomplete suppression of anti-FVIII antibody production by short-term anti-ICOS treatment, we next evaluated an extended anti-ICOS treatment regimen (16 treatments in a 4-week period). Strikingly, we observed persistent and high-level transgene expression in all treated mice (n = 9) for at least 6 months (Figure 2A). In line with extended transgene expression, no inhibitory antibodies developed in these mice throughout the study period (Figure 2B).
Effects of extended anti-ICOS treatment in mice after hFVIII plasmid transfer.hFVIII plasmid-treated mice (n = 9) were injected intraperitoneally with anti-ICOS 16 times during a 4-week period. (A) Persistent high levels of FVIII were expressed in mice as shown by hFVIII activity. (B) No inhibitors developed in mice as evaluated by Bethesda assay. (C) Absence of in vitro proliferation of CD4+ T cells isolated from tolerized mice in response to stimulation with hFVIII. Eight weeks after hFVIII plasmid transfer and anti-ICOS treatment, spleen CD4+ T cells were isolated and stimulated with hFVIII or PHA or cultured without stimulation for 72 hours in the presence of irradiated APCs. A total of 1 μCi of 3H-thymidine per well was added for the last 18 hours of culture. Spleen CD4+ T cells from mice treated with anti-ICOS alone, hFVIII plasmid alone, and untreated naive mice were used as controls. Data are presented as the mean counts per minute plus or minus SD by subtracting data obtained from T cells only in the absence of APCs and are representative of 2 separate experiments. *P < .05, **P < .01, compared with groups indicated by arrows.
Effects of extended anti-ICOS treatment in mice after hFVIII plasmid transfer.hFVIII plasmid-treated mice (n = 9) were injected intraperitoneally with anti-ICOS 16 times during a 4-week period. (A) Persistent high levels of FVIII were expressed in mice as shown by hFVIII activity. (B) No inhibitors developed in mice as evaluated by Bethesda assay. (C) Absence of in vitro proliferation of CD4+ T cells isolated from tolerized mice in response to stimulation with hFVIII. Eight weeks after hFVIII plasmid transfer and anti-ICOS treatment, spleen CD4+ T cells were isolated and stimulated with hFVIII or PHA or cultured without stimulation for 72 hours in the presence of irradiated APCs. A total of 1 μCi of 3H-thymidine per well was added for the last 18 hours of culture. Spleen CD4+ T cells from mice treated with anti-ICOS alone, hFVIII plasmid alone, and untreated naive mice were used as controls. Data are presented as the mean counts per minute plus or minus SD by subtracting data obtained from T cells only in the absence of APCs and are representative of 2 separate experiments. *P < .05, **P < .01, compared with groups indicated by arrows.
CD4+ T cell responses to hFVIII stimulation are reduced after anti-ICOS treatment
We next investigated whether anti-ICOS treatment induced T-cell tolerance to hFVIII in hemophilia A mice. We evaluated the proliferative responses of CD4+ T cells isolated from spleens of mice treated with hFVIII plasmids with or without anti-ICOS immunomodulation using a [3H]-thymidine incorporation assay (Figure 2C). As expected, CD4+ T cells derived from untreated mice did not proliferate in the presence of APCs and hFVIII. CD4+ T cells from hFVIII plasmid-only treated mice proliferated robustly in the presence of APCs and hFVIII. In contrast, the proliferative response of CD4+ T cells derived from hFVIII plasmid plus anti-ICOS-treated mice was completely abrogated compared with that of hFVIII plasmid-only treated mice (P < .05). CD4+ T cells from each of the 3 groups of animals responded to PHA stimulation in the presence of APCs. CD4+ T cells from hFVIII plasmid plus anti-ICOS-treated mice and anti-ICOS alone treated mice had 40% to 60% reduction (P < .05) in the PHA-induced response under these conditions, implying minor nonspecific (bystander) suppression. As expected, treatment with anti-ICOS alone had no effect on FVIII-induced proliferation (Figure 2C). These results indicate that CD4+ T cells derived from hFVIII plasmid plus anti-ICOS–treated mice have significantly impaired proliferation in response to hFVIII stimulation, suggesting the induction of hyporesponsiveness specific to hFVIII in hemophilia A mice after gene transfer.
Depletion of CD4+ICOS+ T cells and activation of Tregs after extended anti-ICOS treatment
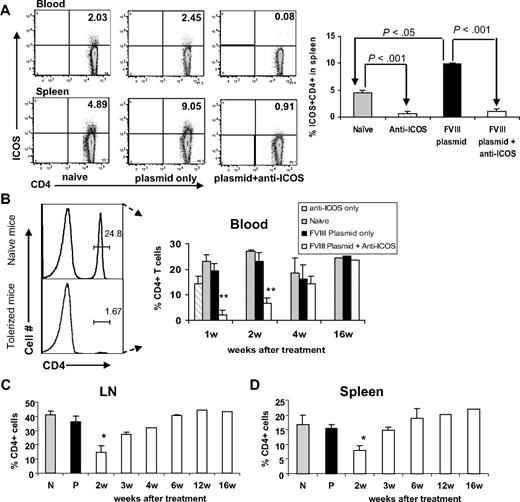
We also explored the possible mechanism leading to tolerance induction after extended anti-ICOS treatment. CD4+ T cells were isolated from peripheral blood, spleen, and lymph nodes of (1) anti-ICOS alone treated mice, (2) untreated naive mice, (3) hFVIII plasmid-only treated mice, or (4) hFVIII plasmid plus anti-ICOS–treated mice. We first analyzed ICOS expression on blood and splenic CD4+ T cells, which is thought to be up-regulated on antigen challenge.33 We observed that hFVIII plasmid transfer resulted in an approximate 2-fold increase in the percentage of CD4+ICOS+ T cells within the spleen (Figure 3A). This change also correlated with increased expression of the early activation marker CD25 at 48 hours after plasmid treatment compared with that of naive mice (data not shown). In hFVIII plasmid plus anti-ICOS–treated and anti-ICOS alone treated hemophilia A mice, CD4+ICOS+ T cells were virtually undetectable in either the blood or spleen (Figure 3A), and the remaining T cells also failed to exhibit any increase in CD25 expression (data not shown).
Depletion of CD4+ICOS+ T cells in mice with extended 4-week anti-ICOS treatment. Lymphocytes isolated from secondary lymphoid organs of 4 groups of mice, namely, untreated control, anti-ICOS alone, hFVIII plasmid only, and hFVIII plasmid plus anti-ICOS–treated mice, were stained for ICOS and CD4 markers. (A) Left panel, representative dot plots of flow cytometry of blood and spleen samples at day 2 after indicated treatment. Right panel, statistical analysis of percentage of ICOS+CD4+ cells in 4 groups of mice (n = 3). (B-D) Time course of percentage of CD4+ T cells in peripheral blood (B), LNs (C), and spleen (D) against isolated total mononuclear cells. *P < .05, **P < .01, compared with groups of naive, anti-ICOS only, and plasmid-only treated mice. N and P indicate untreated naive mice and mice treated with hFVIII plasmid only, respectively.
Depletion of CD4+ICOS+ T cells in mice with extended 4-week anti-ICOS treatment. Lymphocytes isolated from secondary lymphoid organs of 4 groups of mice, namely, untreated control, anti-ICOS alone, hFVIII plasmid only, and hFVIII plasmid plus anti-ICOS–treated mice, were stained for ICOS and CD4 markers. (A) Left panel, representative dot plots of flow cytometry of blood and spleen samples at day 2 after indicated treatment. Right panel, statistical analysis of percentage of ICOS+CD4+ cells in 4 groups of mice (n = 3). (B-D) Time course of percentage of CD4+ T cells in peripheral blood (B), LNs (C), and spleen (D) against isolated total mononuclear cells. *P < .05, **P < .01, compared with groups of naive, anti-ICOS only, and plasmid-only treated mice. N and P indicate untreated naive mice and mice treated with hFVIII plasmid only, respectively.
Notably, repeated injections of anti-ICOS into hFVIII plasmid-treated hemophilia A mice led to a significant reduction in the percentage of CD4+ T cells within peripheral blood (Figure 3B), spleen (Figure 3C), and lymph nodes (Figure 3D) in the first and second weeks after gene transfer. The depletion of CD4+ T cells during the first 2 weeks of anti-ICOS treatment was more substantial probably because of more frequent injections of anti-ICOS mAb (10 total at the first 2 weeks vs 6 injections total at the last 2 weeks of treatment). However, we cannot rule out the possibility that the animals developed antirat antibody during the treatment period. The percentage of CD4+ T cells gradually returned to normal in tolerized mice within 4 to 6 weeks after plasmid treatment (Figure 3B-D). No change in the percentage of CD4+ T cells was observed in hFVIII plasmid-only treated mice or untreated control mice. In anti-ICOS alone treated group (independent on exposure to hFVIII plasmid), only minor reduction in the percentage of CD4+ T cells was observed (Figure 3B right panel), indicating that significant CD4+ T-cell reduction in the hFVIII plasmid plus anti-ICOS–treated group was induced by persistent exposure to hFVIII antigen, which caused activation and increased ICOS expression of CD4+ T cells.
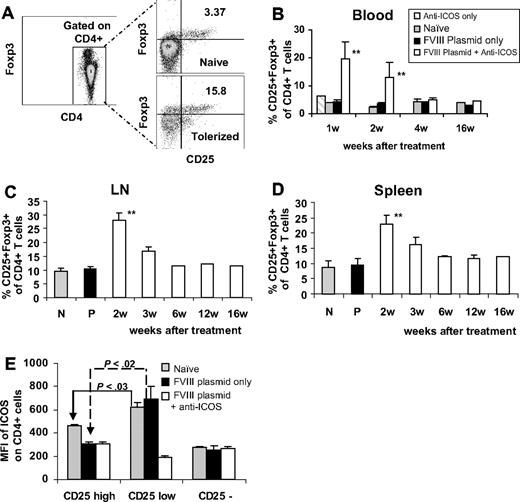
We also analyzed the CD4+ population for the expression of CD25 and Foxp3 (Figure 4A). At 1 to 2 weeks after gene transfer, the relative proportion of CD25+Foxp3+ regulatory T cells present in peripheral blood of tolerized mice was significantly higher than that observed in anti-ICOS alone, hFVIII plasmid-only treated, or naive mice. This difference disappeared within 4 weeks after gene transfer (Figure 4B). Because of the depletion of total CD4+ T cells, the absolute number of circulating CD25+Foxp3+ cells in tolerized mice during the first week of anti-ICOS treatment were comparable with or slightly less than those in untreated mice. Similar results were obtained in the analysis of Tregs in spleen and lymph nodes.43 However, at late time points, the proportions as well as absolute numbers of Tregs were slightly higher than those observed in naive and in plasmid-treated mice (Figure 4C,D). None of the control groups of either anti-ICOS only or isotype control treated mice has significant elevation of CD4+CD25+Foxp3+ T cells (Figure 4B), indicating these effects are predominantly associated with gene transfer/antigen presentation. When analyzed 2 to 4 days after plasmid transfer, ICOS expression in different subpopulations of CD4 cells was significantly higher in CD4+CD25low effector T cells than in CD4+CD25high regulatory T cells in the spleens of both naive and plasmid-only treated mice (Figure 4E). The same trend was also observed in LNs and blood. We conclude that CD4+CD25+ Tregs with lower ICOS expression were more resistant to depletion when treated with anti-ICOS mAb.
Immunomodulation of gene therapy with extended anti-ICOS treatment results in increased percentage of CD4+ CD25+ Foxp3+ cells. Lymphocytes of secondary lymphoid organs from 3 groups of mice treated with different regimens were stained for CD4, CD25, and intracellularly for Foxp3. (A) Representative dot plots of flow cytometry of blood samples at day 14. Time course of percentage of CD25+Foxp3+ was evaluated in CD4+ T cells isolated from (B) peripheral blood, (C) LNs, and (D) spleen. **P < .01, compared with groups of naive, anti-ICOS alone, and plasmid only treated mice. (E) ICOS expression on CD4+CD25high, CD4+CD25low, and CD4+CD25− cell populations isolated from spleen of 3 groups of mice at day 2 to 4 after plasmid transfer. Results were shown as MFI of ICOS.
Immunomodulation of gene therapy with extended anti-ICOS treatment results in increased percentage of CD4+ CD25+ Foxp3+ cells. Lymphocytes of secondary lymphoid organs from 3 groups of mice treated with different regimens were stained for CD4, CD25, and intracellularly for Foxp3. (A) Representative dot plots of flow cytometry of blood samples at day 14. Time course of percentage of CD25+Foxp3+ was evaluated in CD4+ T cells isolated from (B) peripheral blood, (C) LNs, and (D) spleen. **P < .01, compared with groups of naive, anti-ICOS alone, and plasmid only treated mice. (E) ICOS expression on CD4+CD25high, CD4+CD25low, and CD4+CD25− cell populations isolated from spleen of 3 groups of mice at day 2 to 4 after plasmid transfer. Results were shown as MFI of ICOS.
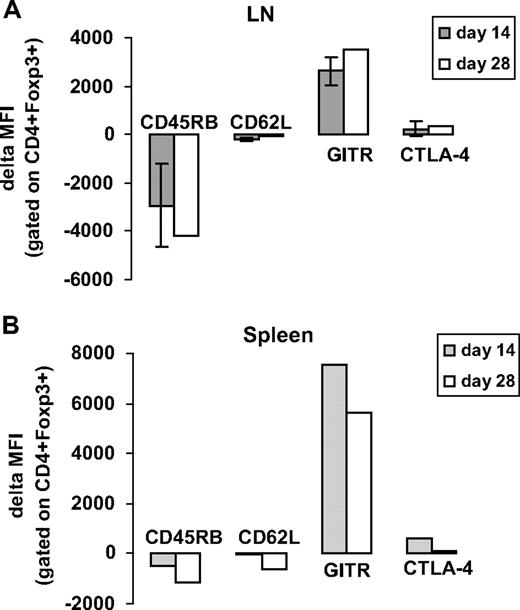
To characterize the activation status of CD4+CD25+Foxp3+ Tregs in hFVIII plasmid plus anti-ICOS–treated animals, we evaluated the expression of several surface markers known to be expressed on regulatory T cells including CD45RB, CD62L, GITR, and CTLA-4. Based on median fluorescence intensity (MFI), anti-ICOS immunomodulation led to a reduction in the expression of CD45RB and CD62L and an increase in the relative expression of GITR and CTLA-4 both in spleen- and LN-derived CD4+FoxP3+ cells (Figure 5). These findings suggested that Tregs in hFVIII plasmid plus anti-ICOS–treated animals were highly activated. The change was particularly prominent during the first 2 weeks after anti-ICOS treatment.
Immunomodulation of gene therapy with extended anti-ICOS treatment-induced activation of CD4+Foxp3+ Tregs. Lymphocytes isolated from secondary lymphoid organs of hFVIII plasmid plus anti-ICOS–treated mice were stained for various regulatory surface and intracellular markers, including CD45RB, CD62L, GITR, and CTLA4 at day 14 and 28 after plasmid transfer. For analysis, cells were gated on CD4+Foxp3+ population. (A) Expression of regulatory markers in LNs. (B) Expression of regulatory markers in spleen. The data represent delta MFI obtained by subtracting the background MFI observed in untreated naive mice.
Immunomodulation of gene therapy with extended anti-ICOS treatment-induced activation of CD4+Foxp3+ Tregs. Lymphocytes isolated from secondary lymphoid organs of hFVIII plasmid plus anti-ICOS–treated mice were stained for various regulatory surface and intracellular markers, including CD45RB, CD62L, GITR, and CTLA4 at day 14 and 28 after plasmid transfer. For analysis, cells were gated on CD4+Foxp3+ population. (A) Expression of regulatory markers in LNs. (B) Expression of regulatory markers in spleen. The data represent delta MFI obtained by subtracting the background MFI observed in untreated naive mice.
Increased secretion of regulatory cytokines in response to anti-ICOS treatment
To further test the role of Tregs in tolerance induction after anti-ICOS immunomodulation, we analyzed the capacity of T cells from tolerized, hFVIII plasmid-only treated, and control naive mice to produce regulatory cytokines after hFVIII-specific in vitro stimulation. Splenic CD4+ T cells were stimulated with an optimal concentration of hFVIII (10 U/mL) and the cytokine content in culture supernatants analyzed. The CD4+ T cells from tolerized mice 8 weeks after plasmid treatment produced significantly higher concentrations of the “regulatory” cytokines IL-10 and TGF-β compared with T cells derived from mice treated with hFVIII plasmid only or from untreated control mice (Table 1). In addition, T cells from tolerized mice exhibited an increase in the basal level of IL-10 secretion.
In vitro cytokine secretion by splenic CD4+ T cells from naive mice, FVIII plasmid-only treated mice, and FVIII plasmid plus anti-ICOS–treated mice
| In vitro stimulation . | TGF-β1, pg/mL . | IL-10, pg/mL . |
|---|---|---|
| No stimulation | ||
| Naive control | 112 ± 6 | 70 ± 2 |
| FVIII plasmid only | 116 ± 12 | 73 ± 3 |
| FVIII plasmid + anti-ICOS | 103 ± 87 | 498 ± 7§ |
| FVIII stimulation | ||
| Naive control | 221 ± 3 | 106 ± 33 |
| FVIII plasmid only | 521 ± 4*† | 380 ± 40*† |
| FVIII plasmid + anti-ICOS | 706 ± 55†‡ | 1432 ± 40†§ |
| In vitro stimulation . | TGF-β1, pg/mL . | IL-10, pg/mL . |
|---|---|---|
| No stimulation | ||
| Naive control | 112 ± 6 | 70 ± 2 |
| FVIII plasmid only | 116 ± 12 | 73 ± 3 |
| FVIII plasmid + anti-ICOS | 103 ± 87 | 498 ± 7§ |
| FVIII stimulation | ||
| Naive control | 221 ± 3 | 106 ± 33 |
| FVIII plasmid only | 521 ± 4*† | 380 ± 40*† |
| FVIII plasmid + anti-ICOS | 706 ± 55†‡ | 1432 ± 40†§ |
*P < .05 versus naive control under FVIII stimulation.
P < .01 versus group with same treatment but under different stimulation regimens.
P < .05 versus naive control and FVIII plasmid-only treated mice within same stimulation regimen.
P < .01 versus naive control and FVIII plasmid-only treated mice within same stimulation regimen.
Adoptive transfer of Tregs from tolerized mice can transfer dominant tolerance
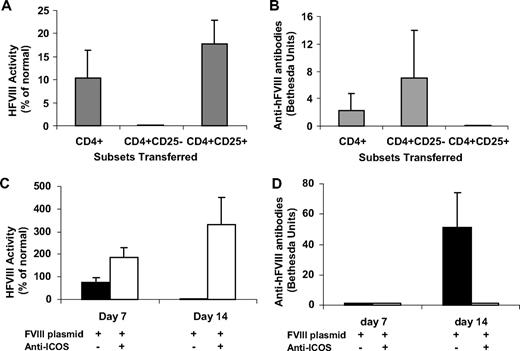
Adoptive transfer experiments were performed to determine whether Tregs are actively involved in modulating immune responses in anti-ICOS–treated mice. Distinct T-cell populations (total CD4+, CD4+CD25+, CD4+CD25− cells) were isolated from hFVIII tolerized mice 4 weeks after plasmid treatment and one day after ending anti-ICOS mAb treatment and transferred into sygeneic naive hemophilia A mice. The recipient mice were injected with hFVIII plasmids one day after adoptive transfer and their immune responses monitored. At day 14 after transfer and challenge, mice receiving total CD4+ T cells (5 × 106 cells/mouse) had modest serum levels of hFVIII and developed inhibitory antibodies. Mice that received CD4+CD25+ T cells (106 cells/mouse) had the highest levels of FVIII activities among the 3 groups without generation of inhibitory antibodies (Figure 6A,B). Conversely, mice that received CD4+CD25− T cells (106 cells/mouse) had undetectable FVIII activity and the highest levels of inhibitor antibodies (Figure 6A,B). The transfer of nonspecific CD4+CD25+ Tregs from naive mice had a less suppressive effect on antibody responses (data not shown). These results indicate that tolerance induction in anti-ICOS–treated hemophilia A mice was associated with generation of antigen-specific CD25+Foxp3+ Tregs. We have evaluated the recipient mice at later time points and found that the adoptive tolerance did not persist beyond 4 to 8 weeks after gene transfer.
CD4+ and CD4+CD25+ T lymphocytes from hFVIII plasmid plus extended anti-ICOS–treated mice exert dominant tolerance after adoptive transfer. (A,B) A total of 5 × 106 CD4+ cells, 1 × 106 CD4+CD25− cells, or 1 × 106 CD4+CD25+ cells from tolerized mice 4 weeks after gene transfer were adoptively transferred into naive hemophilia A mice. The recipient mice were subsequently challenged with hFVIII plasmid 1 day after adoptive transfer. (A) hFVIII activity and (B) anti-hFVIII antibodies were examined in recipient mice at day 14 after adoptive transfer of indicated cell populations isolated from tolerized mice. (C,D) 5 × 106 CD4+ cells were obtained from donor mice 8 weeks after gene transfer and adoptively transferred into naive hemophilia A mice. The recipient mice were then challenged with hFVIII plasmid 1 day after adoptive transfer. (C) hFVIII activity and (D) anti-hFVIII antibodies were measured in recipient mice at day 7 and day 14 after adoptive transfer of total CD4+ T cells from mice treated as indicated.
CD4+ and CD4+CD25+ T lymphocytes from hFVIII plasmid plus extended anti-ICOS–treated mice exert dominant tolerance after adoptive transfer. (A,B) A total of 5 × 106 CD4+ cells, 1 × 106 CD4+CD25− cells, or 1 × 106 CD4+CD25+ cells from tolerized mice 4 weeks after gene transfer were adoptively transferred into naive hemophilia A mice. The recipient mice were subsequently challenged with hFVIII plasmid 1 day after adoptive transfer. (A) hFVIII activity and (B) anti-hFVIII antibodies were examined in recipient mice at day 14 after adoptive transfer of indicated cell populations isolated from tolerized mice. (C,D) 5 × 106 CD4+ cells were obtained from donor mice 8 weeks after gene transfer and adoptively transferred into naive hemophilia A mice. The recipient mice were then challenged with hFVIII plasmid 1 day after adoptive transfer. (C) hFVIII activity and (D) anti-hFVIII antibodies were measured in recipient mice at day 7 and day 14 after adoptive transfer of total CD4+ T cells from mice treated as indicated.
To test whether the dominant tolerance mediated by transferred Treg cells can persist over the long term, we adoptively transferred total CD4+ T cells isolated from tolerized mice or from plasmid only treated mice 2 months after initial gene transfer and 1 month after ending immunomodulation to recipient hemophilia A mice. Recipient mice were challenged with hFVIII plasmid 1 day after adoptive transfer of T cells. At day 7 after transfer and challenge, mice receiving total CD4+ T cells from tolerized mice had FVIII activity (average, 200% of normal), whereas mice receiving total CD4+ T cells from hFVIII plasmid-only treated mice had much lower levels of FVIII activity (average, 80% of normal) (Figure 6C). At day 14 after transfer and challenge, mice receiving T cells from tolerized mice maintained high-level FVIII activity, whereas mice receiving total CD4+ T cells from hFVIII plasmid-treated animals no longer exhibited FVIII activity (Figure 6C). In agreement with these results, mice receiving total CD4+ T cells from hFVIII plasmid-only treated mice developed high-titer inhibitors at day 14 after transfer and challenge (Figure 6D). The group of mice receiving CD4+ T cells from tolerized mice eventually developed low-titer inhibitory antibodies at approximately week 4 after plasmid challenge (data not shown). The rapid onset and high magnitude of inhibitor formation in mice receiving total CD4+ T cells from hFVIII plasmid-only treated mice indicated the transfer of antigen-specific memory effector T cells. In contrast, the slow onset and low magnitude of inhibitory antibody formation in mice receiving total CD4+ T cells from tolerized mice signified the absence of memory T-cell transfer and protection from high-titer antibody production by Tregs.
T-cell tolerance induced by anti-ICOS treatment is FVIII-specific
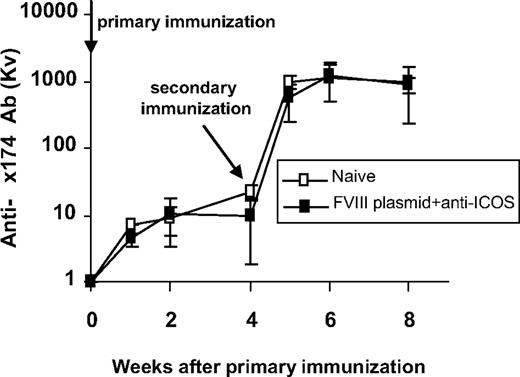
To demonstrate that anti-ICOS treatment did not result in general immune deficiency, tolerized mice were challenged 6 months after plasmid injection with the T-dependent neoantigen, bacteriophage Φx 174.42,44 Two groups of mice, FVIII-tolerized mice and untreated control mice (n = 4 per group), were immunized twice, 4 weeks apart. As shown in Figure 7, mice tolerized to hFVIII displayed normal primary and secondary responses to bacteriophage Φx 174 immunization with a strong amplification of antibody titers and isotype switching after secondary immunization, which were identical to the antibody responses observed in untreated control mice. ICOS-deficient mice immunized with Φx 174 have impaired primary and secondary antibody responses with lack of memory (amplification) and failure to isotype switch, suggesting that anti-ICOS treatment when given during exposure to Φx 174 will interfere with antibody responses to Φx 174 (B.P., H.D.O., and C.H.M., unpublished results, January 2008). These results strongly suggest that transient immunomodulation with anti-ICOS in hFVIII plasmid-treated hemophilia A mice can promote long-term hFVIII-specific immune tolerance without altering subsequent immune responses to other T cell–dependent antigens.
Tolerance induced by anti-ICOS immunomodulation is specific to FVIII. Six months after anti-ICOS mAb treatment, tolerized hemophilia A mice (n = 4) were challenged twice 4 weeks apart with the neoantigen bacteriophage Φx 174 (2 × 108 PFU/each challenge). Untreated hemophilia A mice (n = 4) were used as control. Phage-neutralizing antibody activity was expressed as the rate of phage inactivation (Kv) using a standard formula. Mice receiving no bacteriophage did not produce neutralizing antibody (data not shown). All mice showed adequate antibody titers, amplification, and isotype switching (100% IgG) after secondary immunization.
Tolerance induced by anti-ICOS immunomodulation is specific to FVIII. Six months after anti-ICOS mAb treatment, tolerized hemophilia A mice (n = 4) were challenged twice 4 weeks apart with the neoantigen bacteriophage Φx 174 (2 × 108 PFU/each challenge). Untreated hemophilia A mice (n = 4) were used as control. Phage-neutralizing antibody activity was expressed as the rate of phage inactivation (Kv) using a standard formula. Mice receiving no bacteriophage did not produce neutralizing antibody (data not shown). All mice showed adequate antibody titers, amplification, and isotype switching (100% IgG) after secondary immunization.
Discussion
To test the hypothesis that blockade of selected costimulatory pathways can modulate transgene-specific immune responses in a gene therapy setting, we injected hFVIII plasmid-treated hemophilia A mice with an anti-ICOS mAb. The outcome of these experiments demonstrated that transient anti-ICOS treatment successfully ablated antibody formation after nonviral gene transfer of hFVIII.
Multiple T-cell costimulatory pathways, including CD28 and B7 molecules (CD80, CD86), ICOS and ICOSL, CD-40L and CD40, OX40 (CD134), and OX40-L, provide a functional redundancy to ensure robust T-cell activation to mount immune responses against foreign antigens. Previously, we have shown that the combination of CTLA4-Ig and anti-CD40L could effectively prevent the transgene-specific immune responses in this model. It has also been shown that combined blockade of CD28 and CD40L signaling45,46 or CD40L and ICOS signaling47 resulted in improved solid organ graft survival. However, no single agent (eg, CTLA4-Ig or anti-CD40L alone) was effective.27 Based on these observations, we anticipated that transient blockade of only a single pathway (eg, the interaction of ICOS and ICOSL by anti-ICOS mAb) would not prevent the immune responses against hFVIII in hemophilia A mice treated with hFVIII plasmid. Our data, however, show clearly that administration of anti-ICOS mAb over an extended period effectively promotes tolerance to the transgene. This is different from the results reported in a hemophilia mouse model reported by Hausl et al,48 suggesting that the interference with ICOS-ICOSL interaction by anti-ICOSL monoclonal antibody does not inhibit restimulation and differentiation of FVIII-specific memory B cell. This difference could be because the model used was one of an established disease and/or that the blocking antibodies anti-ICOS and anti-ICOSL act differently. On conjugation, anti-ICOSL may indeed initiate B-cell activation bypassing the ICOS-ICOSL costimulatory pathway, similar to the effect of anti-CD40 antibody,49 which achieves B-cell activation by directly crosslinking CD40. Alternatively, anti-ICOSL antibody may be less effective because memory B-cell responses are less T-dependent.
Established animal models of tolerance have demonstrated that the presence of antigen is required to maintain the tolerant state.50,51 The minimum blood concentration of FVIII needed to maintain hyporesponsiveness was estimated to be in the range of 10−11 to 10−10 M, a level readily achieved by the sustained hFVIII gene expression (10−9 to 10−8 M) in our gene transfer model. In addition to persistent high-level hFVIII gene expression, the tolerance induced in anti-ICOS–treated hemophilia A mice after gene transfer is characterized by lack of inhibitory antibody formation in vivo and failure of CD4+ T cells to proliferate in response to hFVIII stimulation in vitro. These events correlated with increased secretion of regulatory cytokines, including IL-10 and TGF-β by cultured CD4+ T cells from tolerized mice. At least 2 mechanisms may have contributed to the unresponsiveness of CD4+ T cells to subsequent in vitro hFVIII stimulation: (1) deletion of or anergy induction in antigen-specific effector T cells or (2) generation of antigen-specific CD4+CD25+Foxp3+ Tregs.
We observed that hFVIII plasmid transfer triggered increased ICOS expression on a subset of CD4+ T cells, which we consider hFVIII specific effector T cells, detectable as early as 24 hours after gene transfer. On day 7 after treatment with anti-ICOS mAb, almost 90% of this pool of CD4+ICOS+ T cells within the peripheral lymphoid compartment were depleted. Concomitantly, the percentage, but not the absolute numbers, of CD4+CD25+Foxp3+ Tregs within the CD4+ cell population increased significantly in anti-ICOS–treated mice compared with plasmid-only treated mice and naive mice. Finally, Tregs isolated from anti-ICOS–treated mice exhibited evidence of cell activation as indicated by the up-regulation of CTLA-4 and GITR and down-regulation of CD45RB and CD62L. The mechanisms leading to the activation of Tregs in anti-ICOS mAb-treated mice are unknown and need further investigation. One possible reason for the relative increase in the percentage of Tregs within the CD4+ T-cell compartment is the fact that Tregs express less ICOS than effector T cells and therefore are more resistant to anti-ICOS treatment. As shown in Figure 4E, ICOS expression was significantly higher in CD4+CD25low effector T cells than that in CD4+CD25high Tregs in the spleen of both naive and plasmid-only treated mice. Therefore, in contrast to the general immune suppression achieved by a 2-drug combination (MMF and RPA),26 anti-ICOS antibody aimed preferentially at ICOShigh effector T cells induced by antigen activation after gene transfer/antigen presentation. This dynamic process is dependent on continuous exposure to antigen after gene transfer and leads to significant depletion of antigen-specific CD4+ effector T cells. CD4+CD25+ Tregs with lower ICOS expression are more resistant to anti-ICOS depletion. Therefore, hFVIII plasmid plus anti-ICOS treatment induced an active state where Treg numbers were proportionally higher than those in the steady state, thus leading to antigen-specific tolerance. Thus, the ratio of regulatory/effector T cells appears to be the major determining factor in inducing tolerance in our animal model.
Several recent studies have shown that ICOS signaling is important for the induction of IL-10–producing Tregs,52,53 and/or for the regulatory functions of CD4+ Tregs.54,55 In a murine model of type 1 diabetes, significantly higher levels of IL-10 were secreted by and ICOS expressed on Tregs isolated from pancreatic tissues than from LNs.56,57 On the other hand, the combination of CD40-Ig and anti-ICOS induced the generation of Tregs in a rat cardiac transplant model.58 In a cardiac allograft mouse model, ICOS blockade induced a novel antigen-specific CD8+PD1+ regulatory T-cell population.59 Collectively, these results suggest that the ICOS-ICOSL costimulatory pathway is complex and its blockade leads to different effects depending on the immunologic challenge, timing of the blockade, and/or disease model.60
In our model, anti-ICOS mAb treatment during the early phase of gene therapy effectively eliminated FVIII specific effector T cells in the spleen, LNs, and PBMCs of hFVIII plasmid-treated mice. In addition, we observed that total CD4+ T cells derived from tolerized mice 8 weeks after plasmid treatment produced significantly higher concentrations of the “regulatory” cytokines IL-10 and TGF-β. Maynard et al61 reported recently that, in peripheral lymphoid tissue, only a small subset of Treg, mostly Foxp3+, secret IL-10; however, after activation, IL-10 secretion is greatly increased. This observation is consistent with our finding that anti-ICOS treatment resulted in significantly higher proportions of activated Foxp3+ Tregs, which are presumably responsible for secretion of high concentrations of IL-10.
Adoptive transfer experiments demonstrated that dominant tolerance can be established by the transfer of CD4+CD25+ Tregs, but not by CD4+CD25− effector T cells. These findings suggest that CD4+CD25+ Tregs are actively involved in inducing/maintaining hFVIII-specific tolerance and are in agreement with previous reports demonstrating that activation of CD4+CD25+ Tregs and IL-10/TGF-β–secreting cells are directly responsible for the induction of mucosal tolerance to FIX62 and that Tregs play an active role in tolerance induction to FIX after AAV-mediated hepatic gene transfer into mice63 and nonhuman primates.26 However, the capacity of CD4+CD25+ Tregs to transfer tolerance was only partially effective as suggested by the gradual development of anti-hFVIII antibodies in the recipient mice, possibly because of transfer of insufficient numbers, or disappearance and insufficient regeneration, of CD4+CD25+ Tregs.
Finally, adoptive transfer of CD4+ T cells from tolerized mice and hFVIII plasmid-only treated mice was performed at 2 months after initiation of treatment, when effector T cells are expected to have matured into memory T cells.64 Interestingly, mice receiving CD4+ T cells from plasmid only treated donor mice developed FVIII neutralizing antibodies much faster than did mice receiving cells from tolerized donor mice. The rapid onset and high magnitude of inhibitor formation in the former experiment suggest transfer of memory T cells,65 whereas the slower onset and low magnitude of inhibitor formation in the latter group indicate the absence of memory effector T cells, a direct result of the dominant suppression by Tregs transferred from tolerized mice. It has been reported that ICOS is involved in modulation of secondary immune responses66 and is expressed at low concentration on resting memory T cells28,52 This is consistent with our data that memory effector T cells were depleted/absent in anti-ICOS–treated mice.
The interaction of ICOS with ICOSL provides critical signals that mediate T help to B cells.29,33,34,67,68 Studies using pathway antagonists and transgenic or knockout mice have revealed the important role of ICOS in B-cell differentiation, CD40-mediated immunoglobulin class switching, germinal center formation, and memory B-cell development.69 The majority of anti-hFVIII antibody observed in hFVIII plasmid-treated hemophilia A mice is of the IgG1 isotype, but IgG2a, IgG2b, and IgG3 antibodies are also present,27 suggesting a predominantly Th2 mechanism. ICOS was found to play a key role in Th2 responses,70 as ICOS−/− mice showed marked defects in Th2 cytokine production and T-dependent antibody responses.33 Therefore, ICOS blockade may have effectively inhibited Th2 effector function in our model. However, ICOS−/− mice are resistant to induction of high-dose tolerance to encephalitogenic myelin oligodendrocyte glycoprotein 35-55 peptide,71 suggesting that absence of the CD4+ICOS+ cell population alone is probably insufficient to induce tolerance. On the other hand, CD4+CD25+ Tregs have been reported to be able to inhibit the maturation but not the initiation of autoantibody responses in an Ig transgenic model.72 Thus, we hypothesize that the tolerance induced by anti-ICOS immunomodulation in our study is the result of the combined effect of depleting CD4+ICOS+ effector T cells and activating CD4+CD25+Foxp3+ Tregs. In the first 1 to 2 weeks after transgene expression, depletion of antigen-specific CD4+ICOS+ T cells and activation of Tregs appear to be crucial in inducing tolerance to hFVIII. Thereafter, long-term tolerance appears to be maintained through the persistent absence of antigen-specific memory effector T cells and active suppression mediated by the expanded population of Tregs, without causing general immune suppression.
In conclusion, 4-week treatment of anti-ICOS mAb results in transient depletion of CD4+ T cells during the initial treatment period and induces short-term elimination of effector T cells and long-term loss of antigen-specific memory T cells. Simultaneously, anti-ICOS treatment promotes Treg activation and, possibly, long-term Treg survival or homing. Based on the results of our adoptive transfer experiments, we suggest that antigen-specific regulatory T cells play a prominent role in the induction and maintenance of tolerance after anti-ICOS treatment. As a consequence, transgene expression in tolerized mice is maintained life-long at high levels without affecting normal responses to other T cell–dependent neoantigens. Thus, transient administration of anti-ICOS mAb represents a highly effective regimen for modulating transgene-specific humoral immune responses and supports the use of similar approaches in other preclinical gene therapy models.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
C.H.M. was supported by a Career Development Grant from the National Hemophilia Foundation and NIH grants R01 HL069049 and HL82600. G.J.F. and B.R.B. were supported by NIH grant P01 A156299.
National Institutes of Health
Authorship
Contribution: B.P. performed research, analyzed data, and wrote the paper; P.Y. performed research; B.R.B. and G.J.F. contributed anti-ICOS and helped revise the paper; D.J.R. revised the paper; H.D.O. provided helpful ideas and revised the paper; and C.H.M. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Carol H. Miao, Department of Pediatrics, University of Washington and Seattle Children's Hospital Research Institute, 1900 Ninth Avenue, Seattle, WA 98101; e-mail: miao@u.washington.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal