Abstract
The importance of the lymphangiogenic factor VEGF-D and its receptor VEGFR-3 in early lymphatic development remains largely unresolved. We therefore investigated their role in Xenopus laevis tadpoles, a small animal model allowing chemicogenetic dissection of developmental lymphangiogenesis. Single morpholino antisense oligo knockdown of xVEGF-D did not affect lymphatic commitment, but transiently impaired lymphatic endothelial cell (LEC) migration. Notably, combined knockdown of xVEGF-D with xVEGF-C at suboptimal morpholino concentrations resulted in more severe migration defects and lymphedema formation than the corresponding single knockdowns. Knockdown of VEGFR-3 or treatment with the VEGFR-3 inhibitor MAZ51 similarly impaired lymph vessel formation and function and caused pronounced edema. VEGFR-3 silencing by morpholino knockdown, MAZ51 treatment, or xVEGF-C/D double knockdown also resulted in dilation and dysfunction of the lymph heart. These findings document a critical role of VEGFR-3 in embryonic lymphatic development and function, and reveal a previously unrecognized modifier role of VEGF-D in the regulation of embryonic lymphangiogenesis in frog embryos.
Introduction
The lymphatic vascular system is critical for reabsorption of extravasated fluid and dietary fat into the circulation. By transporting immune cells to lymphoid organs, it also plays a critical role in inflammation, infection, immunity, and numerous other diseases.1-4 Excessive formation of new lymphatics (lymphangiogenesis) in tumors facilitates malignant cells to escape via lymph vessels and to metastasize to distant organs.4-7 When lymph vessels fail to develop or function properly because of genetic, traumatic, infectious, or iatrogenic reasons, lymphedema develops. Although administration of the lymphangiogenic VEGF-C gene or protein reduces lymphedema in preclinical models,8,9 no treatment has been approved yet. Thus, strategies to stimulate or inhibit lymphangiogenesis could benefit treatment of lymphedema and cancer, respectively. This mandates better understanding of the molecular basis of this process. Compared with angiogenesis, the molecular mechanisms underlying lymphatic development are less extensively characterized. Gene inactivation studies in mice identified the critical involvement of transcription factor Prox1 and of VEGF-C in early lymphatic development,10,11 and the importance of other factors in lymph vessel formation, patterning, and function is gradually being revealed (reviewed in Alitalo et al4 ).
We recently reported that Xenopus tadpoles develop a functional lymphatic vasculature within the first 4 days of development.12 The usefulness of the tadpole as a genetic model to study lymphangiogenesis was evidenced by the similarities in lymphatic phenotypes between knockdown (KD) tadpoles and knockout mice, lacking Prox1 or VEGF-C.12 The existence of a lymphatic system also was recently demonstrated in zebrafish, and genetic studies showed that its development depended on Prox1 and on VEGF-C signaling,13,14 indicating at least partial conservation of the molecular players between zebrafish, Xenopus, and mammals.
VEGF-C and VEGF-D are the 2 known VEGF homologues, which bind VEGF receptor-3 (VEGFR-3) on lymphatic endothelial cells (LECs).4,15,16 Loss of VEGF-C in mouse embryos impairs LEC sprouting,11 whereas VEGF-D appears to have a negligible role in development in mice.17 Here, we evaluated possible interactions of VEGF-D with other lymphangiogenic factors, using single and combined gene silencing in Xenopus tadpoles by morpholino knockdown. The defective lymphatic phenotypes observed in combined knockdown morphants suggest a previously undocumented role of VEGF-D as a modifier of lymphangiogenesis. In addition, using morpholino knockdown and chemical inhibition, we show that VEGFR-3 blockage severely impairs early lymphatic development in the tadpole.
Methods
Morpholino injections
Fertilized Xenopus eggs from frogs purchased from Nasco Biology (Fort Atkinson, WI) were injected with 15 to 100 ng morpholino (Gene Tools, Philomath, OR) into the one-cell stage as described.12,18 The ATG-targeted antisense morpholinos were designed based on published GenBank19 Xenopus laevis sequences of xVEGF-C (no. CA973641),12 xProx1 (no. AB008773),12 and xVEGF-D (no. TC250621); when unavailable, the upstream sequence up to the ATG start codon was obtained by 5′ rapid amplification of cDNA ends (RACE; performed for xVEGFR-3 based on Xenopus tropicalis gene sequence; Ensembl Gene ID ENSXETT0000000839920 ). For morpholino sequences, see Document S1, Table 1 (available on the Blood website; see the Supplemental Materials link at the top of the online article). Injected embryos were staged according to Nieuwkoop and Faber21 and grown at 18°C until gastrulation was completed and subsequently at 22°C. Live screening of general development, heart beating, blood flow, lymph heart (LH) beating, and the presence or absence of edema was performed at stage 45 using a Zeiss SV11 stereomicroscope (Carl Zeiss, Zaventum, Belgium). This study was approved by the ethical committee for animal experimentation of the Katholieke Universiteit Leuven, Leuven, Belgium.
MAZ51 treatment of Xenopus laevis tadpoles
At stage 32/33, tadpoles were placed in 6-well dishes (15-20 tadpoles/well), and MAZ51 (3-(4-dimethylamino-naphthelen-1-ylmethylene)-1,3-dihydroindol-2-one in DMSO; Calbiochem–Merck Biosciences, Nottingham, United Kingdom)22 was added to the tadpole growth media at 5- to 50-μM concentrations. Control tadpoles were treated with the corresponding amount of DMSO. Compound/DMSO and growth medium were refreshed every day. At stage 35/36 (2.1-2.5 dpf), tadpoles were fixed for xProx1 in situ hybridization and at stage 45 (5 dpf), angiographies and lymphangiographies were performed to visualize the blood or lymphatic vasculature12 as detailed in “Angiography and lymphangiography of Xenopus tadpoles.”
In situ hybridization, histology, and immunostaining
Fixation of tadpoles and whole-mount in situ hybridization using probes for lymphatic marker xProx1 and blood vessel marker xMsr were performed as described.12 For xVEGFR-3, a probe spanning nucleotides 673 to 2685, corresponding to the sequence encoding immunoglobulin-like domain 3 to 7, the transmembrane domain, and approximately 200 nucleotides of the intracellular domain of VEGFR-3, was used. This probe was amplified on cDNA of X laevis based on sequence information from X tropicalis (Ensembl Gene ID ENSXETT0000000839920 ). The probe for blood vessel marker xFli23 was a kind gift of Dr A. Ciau-Uitz. Sections of whole-mount stained embryos were counterstained with nuclear fast red. Detection of erythrocytes was performed by whole-mount staining of unfixed embryos in o-dianisidine solution (40% ethanol with 0.01 M sodium acetate, 0.65% H2O2, and 0.6 mg/mL o-dianisidine [Sigma-Aldrich, Bornem, Belgium]) for 15 minutes, followed by rinsing with water and MEMFA (MOPS/EGTA/magnesium sulfate/formaldehyde) buffer fixation (adapted from Huber et al24 ). For viewing, embryos were bleached as described.12 For immunostaining, MEMFA-fixed embryos were paraffin-embedded and sectioned as described.12 Staining of myogenic cells using the antimyofibril monoclonal antibody 12/101 (obtained from the Developmental Studies Hybridoma Bank, University of Iowa, Iowa City) was as described.12,25
Morphometric analysis
Images of whole-mount tadpoles (tail region) or of tissue sections were acquired with the Zeiss AxioVision 4.6 software on an inverted Zeiss AxioVert 200M equipped with a Zeiss AxioCam MrC5 digital camera and afterward analyzed with the Zeiss KS300 morphometric analysis software with in-house–developed macros. Analysis of xProx1-stained areas in the tadpole tail was performed as follows12 : in the anterio-posterior axis, each region was defined from the location of the rectal diverticle to the tip of the tail; in the ventro-dorsal axis, area 1 was defined from the ventral border of the trunk to the dorsal margin of the endoderm, and area 2, from the dorsal margin of the endoderm to the dorsal border of the trunk. When specified, area 2 was further subdivided into area 2a and 2b, delineated by the dorsal margin of the endoderm and floor plate of the neural tube, and by the floor plate and dorsal roof, respectively (as denoted by the yellow line in Figure 1C). All analyses were performed by investigators blinded for the test condition, on tadpoles, matched for their stage and tail length. Morphometric analysis of LH sizes was performed on a series of consecutive cross-sections of stage-45 tadpoles. The value of the largest cross-sectional area per LH was used to calculate average maximal cross-sectional areas per condition.
Angiography and lymphangiography of Xenopus tadpoles
Stage-45 tadpoles were anesthetized in 0.02% 3-aminobenzoic acid ethyl ester, and placed on agarose gel. Tetramethylrhodamine-dextran (TRITC-dextran, Mr 2000 kDa) and fluorescein isothiocyanate-dextran (FITC-dextran, Mr 2000 kDa) dyes were injected with glass capillaries using a micromanipulator, to label functional blood and lymph vessels, respectively. Images were acquired with the Zeiss AxioVision 4.6 software on a Zeiss Lumar V.12 fluorescence stereomicroscope equipped with a Zeiss AxioCam MrC5 digital camera. (For all photomicrographs, magnification bars were calculated using mm- or μm-scale rulers photographed at the same magnification as the images.)
Statistical analysis
Absolute values were used to calculate means and SEMs. Within each experiment, gene-specific morpholino (or compound dose) was compared with control morpholino (or vehicle). Significance levels were calculated with the general linear model multivariate and univariate statistical model (full factorial), considering area 1 and area 2 (or 2a and 2b) as dependent variables, and morpholino dose and type and tadpole stage as fixed factors. Pairwise comparisons were performed between the different doses, after Bonferroni correction for multiple testing. Asterisks on figures represent genotypic or treatment difference at a significance level of P less than .05. Penetrance of a phenotype was determined by counting the number of tadpoles exhibiting the phenotype. Chi-square analysis was used to establish fraction differences between control and treated groups. The percentage of tadpoles with morphant phenotypes and its corresponding P value are presented below each bar graph in the figures. SPSS 13 was used to perform statistical analysis (Chicago, IL).
Results
Impaired lymphatic sprouting and migration in VEGF-DKD tadpoles
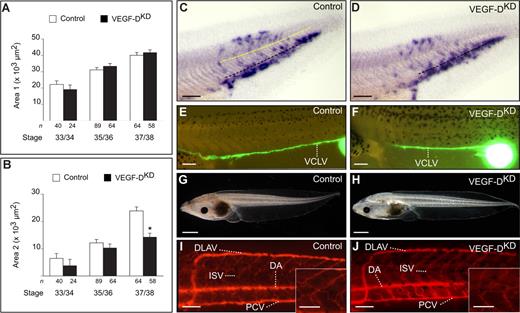
The role of VEGF-D in lymphangiogenesis was investigated using morpholino knockdown of mRNA expression in developing Xenopus laevis tadpoles, focusing on the rostral lymph sac in the head, the lymph hearts (LHs) in the anterior trunk, and the caudal lymph vessels in the posterior trunk. To facilitate the readability of the paper, the key properties of this tadpole model12 are described in Document S1 Note 1 and Figure S1. Cross-sections of xVEGF-D knockdown (VEGF-DKD) tadpoles (75 ng morpholino; Document S1 Note 2; stage 30-37/38) were analyzed after staining by whole-mount in situ hybridization for xMsr, a marker of (progenitor) cells of the blood vascular endothelial cell (BEC) lineage, or for the LEC marker xProx1. This analysis revealed that lymphatic commitment was normal in the rostral lymph sac area, as well as in the anterior and the posterior trunk where the LHs and the caudal lymph vessels form, respectively (Figure 1A,C,D; Document S1Figures 1, 2, and Note 3 for more details). We next analyzed whether VEGF-D regulated LEC sprouting and migration in the posterior trunk.12 Histologic analysis of cross-sections revealed that several xProx1+ LECs were capable of sprouting and migrating dorsally in VEGF-DKD tadpoles. However, compared with control tadpoles, fewer xProx1+ LECs migrated dorsally in VEGF-DKD tadpoles, especially at stage 37/38, as revealed by morphometric quantification of xProx1+“area 2,” which reflects sprouting and migration of LECs (Figure 1B-D, Document S1 Note 1 and Figure S1G,H).
Lymph vessel development in the posterior trunk in xVEGF-D knockdown tadpoles is impaired moderately. (A,B) Morphometric measurement of area 1 (LEC commitment; A) and area 2 (migration; B) in xProx1-stained tadpoles at the indicated stage after morpholino knockdown of xVEGF-D (75 ng morpholino). *P < .05 versus control. The number of tadpoles (n) is indicated. Error bars represent SEM. (C,D) Whole-mount xProx1 in situ hybridization of stage-37/38 tadpoles, revealing the presence of abundant xProx1+ LECs in area 1 but fewer cells in area 2 in the posterior trunk in VEGF-DKD (D) than in control (C) tadpoles. Black dashed line indicates dorsal margin of the endoderm; yellow dashed line, floor plate of the neural tube. (E,F) Lymphangiography in stage-45 control (E) or VEGF-DKD tadpoles (F), revealing normal filling of the VCLV. (G,H) Normal appearance of VEGF-DKD tadpoles at stage 45 (H), comparable with control (G). (I,J) Angiography in stage-45 control (I) and VEGF-DKD tadpoles (J), revealing normal blood vessel development. The insets in panels I and J represent larger magnifications, showing that the ISVs in the VEGF-DKD tadpoles are intact and comparable with those of the control tadpole. DA indicates dorsal aorta; DLAV, dorsal longitudinal anastomosing vessel; ISV, intersomitic vessel; PCV, posterior cardinal vein; and VCLV, ventral caudal lymph vessel. Bars represent 250 μm (C-F), 1 mm (G,H), 100 μm (I,J) and 50 μm insets (I,J).
Lymph vessel development in the posterior trunk in xVEGF-D knockdown tadpoles is impaired moderately. (A,B) Morphometric measurement of area 1 (LEC commitment; A) and area 2 (migration; B) in xProx1-stained tadpoles at the indicated stage after morpholino knockdown of xVEGF-D (75 ng morpholino). *P < .05 versus control. The number of tadpoles (n) is indicated. Error bars represent SEM. (C,D) Whole-mount xProx1 in situ hybridization of stage-37/38 tadpoles, revealing the presence of abundant xProx1+ LECs in area 1 but fewer cells in area 2 in the posterior trunk in VEGF-DKD (D) than in control (C) tadpoles. Black dashed line indicates dorsal margin of the endoderm; yellow dashed line, floor plate of the neural tube. (E,F) Lymphangiography in stage-45 control (E) or VEGF-DKD tadpoles (F), revealing normal filling of the VCLV. (G,H) Normal appearance of VEGF-DKD tadpoles at stage 45 (H), comparable with control (G). (I,J) Angiography in stage-45 control (I) and VEGF-DKD tadpoles (J), revealing normal blood vessel development. The insets in panels I and J represent larger magnifications, showing that the ISVs in the VEGF-DKD tadpoles are intact and comparable with those of the control tadpole. DA indicates dorsal aorta; DLAV, dorsal longitudinal anastomosing vessel; ISV, intersomitic vessel; PCV, posterior cardinal vein; and VCLV, ventral caudal lymph vessel. Bars represent 250 μm (C-F), 1 mm (G,H), 100 μm (I,J) and 50 μm insets (I,J).
xVEGF-D and xVEGF-C coregulate lymph vessel development in the posterior trunk. (A-D) Whole-mount xProx1 in situ hybridization of stage-37/38 tadpoles. Single or simultaneous knockdown of xVEGF-D (50 ng morpholino) or of xVEGF-C (35 ng morpholino) did not affect lymphatic commitment (area 1). In contrast, the numbers and distance of migrating cells (area 2) in single xVEGF-D or xVEGF-C knockdowns were, respectively, normal (B) and partially reduced (C), but further reduced significantly in xVEGF-C/D double knockdowns (D). Black dashed line indicates dorsal margin of the endoderm. (E) Morphometric measurement of area 2 in xProx1-stained tadpoles at stage 37/38 using single and combined morpholino injection at the indicated concentrations. *P < .05 versus control. The number of tadpoles (n) is indicated. Error bars represent SEM. (F,G) Representative picture of lymphangiography of VEGF-C/DKD tadpoles showing failure of the DCLV to drain injected dye (G) as opposed to normal uptake and drainage in control tadpoles (F). The arrow in panel G indicates the distal site of DCLV filling. (H,I) Significant lymphedema in VEGF-C/DKD tadpoles (35 ng xVEGF-C/50 ng xVEGF-D morpholino) at stage 45 (I), in contrast to normal appearance of the controls (H). (J,K) Angiography of control (J) and VEGF-C/DKD tadpoles (K) showing normal appearance of the vasculature in a VEGF-C/DKD tadpole that displayed lymph vessel dysfunction (G). DA indicates dorsal aorta; DCLV, dorsal caudal lymph vessel; DLAV, dorsal longitudinal anastomosing vessel; ISV, intersomitic vessel; MO, morpholino; and PCV, posterior cardinal vein. Bars represent 250 μm (A-D), 100 μm (F,G,J,K), and 1 mm (H,I).
xVEGF-D and xVEGF-C coregulate lymph vessel development in the posterior trunk. (A-D) Whole-mount xProx1 in situ hybridization of stage-37/38 tadpoles. Single or simultaneous knockdown of xVEGF-D (50 ng morpholino) or of xVEGF-C (35 ng morpholino) did not affect lymphatic commitment (area 1). In contrast, the numbers and distance of migrating cells (area 2) in single xVEGF-D or xVEGF-C knockdowns were, respectively, normal (B) and partially reduced (C), but further reduced significantly in xVEGF-C/D double knockdowns (D). Black dashed line indicates dorsal margin of the endoderm. (E) Morphometric measurement of area 2 in xProx1-stained tadpoles at stage 37/38 using single and combined morpholino injection at the indicated concentrations. *P < .05 versus control. The number of tadpoles (n) is indicated. Error bars represent SEM. (F,G) Representative picture of lymphangiography of VEGF-C/DKD tadpoles showing failure of the DCLV to drain injected dye (G) as opposed to normal uptake and drainage in control tadpoles (F). The arrow in panel G indicates the distal site of DCLV filling. (H,I) Significant lymphedema in VEGF-C/DKD tadpoles (35 ng xVEGF-C/50 ng xVEGF-D morpholino) at stage 45 (I), in contrast to normal appearance of the controls (H). (J,K) Angiography of control (J) and VEGF-C/DKD tadpoles (K) showing normal appearance of the vasculature in a VEGF-C/DKD tadpole that displayed lymph vessel dysfunction (G). DA indicates dorsal aorta; DCLV, dorsal caudal lymph vessel; DLAV, dorsal longitudinal anastomosing vessel; ISV, intersomitic vessel; MO, morpholino; and PCV, posterior cardinal vein. Bars represent 250 μm (A-D), 100 μm (F,G,J,K), and 1 mm (H,I).
Possible functional consequences of this sprouting defect on lymph vessel formation were analyzed by lymphangiography in stage-45 tadpoles. Upon subcutaneous injection of FITC-dextran,12 the major lymph vessels in the posterior trunk (ie, dorsal and ventral caudal lymph vessels) appeared morphologically grossly normal and drained the dye in all tadpoles analyzed (N = 6; Figure 1E,F; not shown). In accordance, no increased occurrence of lymphedema was observed upon live screening of morphant tadpoles compared with controls (5.7% of 157 controls versus 5.3% of 75 VEGF-DKD tadpoles; P = NS by chi-square; Figure 1G,H). Thus, the sprouting defect observed in the absence of VEGF-D did not result in lymphatic malfunctioning.
Human VEGF-D binds not only VEGFR-3 but also VEGFR-2, although with lower affinity.26,27 Even though the binding properties of Xenopus VEGF-D to VEGFR-2 or VEGFR-3 are unknown, we analyzed blood vascular development in VEGF-DKD embryos. Blood flow in the major vessels in the trunk and head, as well as in the intersomitic vessels (ISVs), in VEGF-DKD embryos appeared normal by microscopic inspection. Whole-mount xMsr staining (not shown) and angiography with TRITC-dextran (Figure 1I,J) revealed normal blood vessel development in VEGF-DKD embryos. Thus, absence of xVEGF-D affected only lymphatic sprouting.
VEGF-D is a modifier of VEGF-C–driven lymphatic development
To examine whether VEGF-C and VEGF-D cooperatively regulate embryonic lymphangiogenesis, we simultaneously knocked down their expression by coinjecting suboptimal doses of xVEGF-C morpholino (25 ng or 35 ng) and xVEGF-D morpholino (35 or 50 ng), which, when injected alone, affected lymphangiogenesis only moderately or not at all (Figure 2A-C,E; and Ny et al12 ). Histologic analysis and morphometric measurements of xProx1+ areas in the posterior trunk at stage 37/38 revealed that, similar to single VEGF-CKD and VEGF-DKD tadpoles, lymphatic commitment via transdifferentiation of venous xMsr+ BECs into xProx1+ LECs in area 112 was normal in double VEGF-C/DKD tadpoles (Figure 2A,D). In contrast, sprouting and migration of LECs was impaired more in VEGF-C/DKD compared with single knockdown tadpoles. Indeed, area 2 was reduced by 70% in double knockdowns (Figure 2A-E). Of note, LEC migration was already slightly impaired when using lower morpholino doses (25 ng of each morpholino), which, when injected alone, were ineffective (Figure 2E).
Lymphangiography of VEGF-C/DKD morphants at stage 45 revealed that in all double morphants (N = 17), both the VCLV (not shown) and DCLV appeared hypoplastic, underdeveloped, and irregular, and the majority of them (14/17) failed to drain the fluorescent dye to the LH (Figure 2F,G), indicating dysfunctional lymph vessels. (Figure 2F,G). This was in accordance with the higher percentage of double morphant tadpoles with lymphedema at stage 45 (11% of 188 controls, 24% of 69 VEGF-DKD tadpoles, 42% of 102 VEGF-CKD tadpoles, and 80% of 152 VEGF-C/DKD tadpoles with morpholino doses of 35 ng and 50 ng for xVEGF-D and xVEGF-C, respectively; P < .005 for VEGF-C/DKD vs control and vs VEGF-CKD by chi-square analysis; Figure 2H,I; Document S1 Note 4).
Angiography of VEGF-C/DKD morphants at stage 45 revealed normal perfusion and blood vasculature development in 9 of 23 tadpoles examined. In the remaining 14, the ISVs and the dorsal longitudinal anastomosing vessel (DLAV) were malformed, with occasional incomplete perfusion of the ISVs (4 tadpoles) and apparent vessel fragility (suggested by the diffuse appearance of the angiogram) (9 tadpoles) (not shown). These vascular defects might be attributable to direct effects of reduced xVEGFR-2 and xVEGFR-3 signaling upon knockdown of the ligands (see “Discussion”). Nevertheless, 7 of 17 double KD tadpoles with dysfunctional lymphatics had no obvious blood vessel abnormalities (Figure 2J,K), indicating that the VEGF-C/DKD lymphangiogenesis impairment and lymphatic dysfunction was likely a primary effect. Thus, both VEGF-D and VEGF-C, together, regulated LEC sprouting.
Knockdown of xVEGFR-3 impairs lymphatic development and drainage
Thus far, a role of VEGFR-3 in lymphatic development in mouse embryos has not been conclusively documented, as loss of VEGFR-3 in mice causes lethal blood vessel defects before lymphatic vessels arise.28 We therefore investigated the role of VEGFR-3 in early lymphatic development in the tadpole using 2 approaches, morpholino knockdown and chemical inhibition of receptor signaling. Morpholino knockdown of xVEGFR-3 (75-100 ng) did not impair lymphatic commitment in the posterior trunk as indicated by similar area 1 values in the tail of knockdowns and controls (× 103 μm2: 29.8 ± 0.97 for control and 31.3 ± 0.90, 31.9 ± 0.82, and 28.3 ± 0.94 for VEGFR-3KD at 75, 87.5, and 100 ng morpholino, respectively; mean ± SEM, N = 51-72, P = NS). This indicates that xVEGFR-3, which is expressed by the PCV throughout the commitment stages (stage 32-34; Document S1 Note 5 and Figure S3A), is not critical for transdifferentiation to LECs.
However, sprouting and migration were impaired dose-dependently, with a pronounced reduction at the highest morpholino dose in stage 35/36 tadpoles (4-fold reduction; P < .05) (Figure 3A). By stage 42, when the VCLV and DCLV are fully formed and present along the whole length of the trunk in control tadpoles (Figure 3B), only fragments of these vessels were formed in VEGFR-3KD tadpoles (Figure 3C). Compared with the DCLV, the formation of the VCLV was impaired less, and fewer segments were missing. Nonetheless, the VCLV segment, formed in the morphant tadpoles, was irregular and parts of it were slightly enlarged (Figure 3B,C). In accordance with impaired lymphangiogenesis, VEGFR-3KD (87.5 or 100 ng morpholino) resulted in dysfunctional lymph vessels. Indeed, in 50% of the tadpoles tested (N = 11), both the VCLV and DCLV failed to drain injected dye toward the LH, with occasional backward filling of these vessels upon lymphangiography (Figure 3D,E).
Morpholino knockdown of xVEGFR-3 causes lymphatic defects. (A) Morphometric measurement of area 2 in the posterior trunk of xProx1-stained tadpoles at stage 35/36, showing dose-dependent inhibition of migration after xVEGFR-3 knockdown. The number of tadpoles is indicated within each bar. *P < .05 versus control (control morpholino) for pairwise dose analysis. The percentage of tadpoles exhibiting a lower value of the morphometric parameter than the average value in the control group (a measure of the penetrance of the phenotype) and the P value of the chi-square analysis comparing this percentage with the control group are indicated below the bar graphs. Note that the phenotype as well as its penetrance increased dose-dependently. Error bars represent SEM. (B,C) Whole-mount in situ hybridization for xProx1 of stage-42 control or xVEGFR-3 knockdown tadpoles (100 ng morpholino), showing only fragmentary formation of the ventral (VCLV) and dorsal (DCLV) caudal lymph vessels in the VEGFR-3KD tadpoles (C) in contrast to completely formed VCLV and DCLV in the control (B). Arrows in panel C denote missing vessel segments. (D-I) Functionality of the lymphatic and blood vasculature in control and VEGFR-3KD tadpoles. (D,F,H) Normal filling of the VCLV in control tadpole (stage 45) upon lymphangiography (D), with normal appearance of the vasculature by angiography (F) and no signs of edema (H). (E,G,I) In contrast, VEGFR-3KD tadpoles at stage 45 showed dysfunctional lymph vessels upon lymphangiography, occasionally with reverse filling (E), and edema formation (I). Angiography revealed a normal overall architecture of the blood vasculature, however, the diffuse angiogram suggests vessel fragility (G). DA indicates dorsal aorta; DCLV, dorsal caudal lymph vessel; DLAV, dorsal longitudinal anastomosing vessel; ISV, intersomitic vessel; PCV, posterior cardinal vein; and VCLV, ventral caudal lymph vessel. Bars represent 500 μm (B,C), 100 μm (D-G), and 1 mm (H,I).
Morpholino knockdown of xVEGFR-3 causes lymphatic defects. (A) Morphometric measurement of area 2 in the posterior trunk of xProx1-stained tadpoles at stage 35/36, showing dose-dependent inhibition of migration after xVEGFR-3 knockdown. The number of tadpoles is indicated within each bar. *P < .05 versus control (control morpholino) for pairwise dose analysis. The percentage of tadpoles exhibiting a lower value of the morphometric parameter than the average value in the control group (a measure of the penetrance of the phenotype) and the P value of the chi-square analysis comparing this percentage with the control group are indicated below the bar graphs. Note that the phenotype as well as its penetrance increased dose-dependently. Error bars represent SEM. (B,C) Whole-mount in situ hybridization for xProx1 of stage-42 control or xVEGFR-3 knockdown tadpoles (100 ng morpholino), showing only fragmentary formation of the ventral (VCLV) and dorsal (DCLV) caudal lymph vessels in the VEGFR-3KD tadpoles (C) in contrast to completely formed VCLV and DCLV in the control (B). Arrows in panel C denote missing vessel segments. (D-I) Functionality of the lymphatic and blood vasculature in control and VEGFR-3KD tadpoles. (D,F,H) Normal filling of the VCLV in control tadpole (stage 45) upon lymphangiography (D), with normal appearance of the vasculature by angiography (F) and no signs of edema (H). (E,G,I) In contrast, VEGFR-3KD tadpoles at stage 45 showed dysfunctional lymph vessels upon lymphangiography, occasionally with reverse filling (E), and edema formation (I). Angiography revealed a normal overall architecture of the blood vasculature, however, the diffuse angiogram suggests vessel fragility (G). DA indicates dorsal aorta; DCLV, dorsal caudal lymph vessel; DLAV, dorsal longitudinal anastomosing vessel; ISV, intersomitic vessel; PCV, posterior cardinal vein; and VCLV, ventral caudal lymph vessel. Bars represent 500 μm (B,C), 100 μm (D-G), and 1 mm (H,I).
Blood vessel abnormalities were observed in several VEGFR-3KD tadpoles (vessel fragility as demonstrated by diffuse appearance of the angiograms; Figure 3G). This might be explained by the finding that, similar to mice and humans,29 xVEGFR-3 is expressed in blood vessels in early tadpole stages and becomes more restricted to the lymphatics only at later stages (Document S1 Note 5; Figure S3). However, blood vessel abnormalities did not correlate with defective lymph vessel function (eg, 2 of 5 tadpoles with dysfunctional caudal lymph vessels had normal angiograms, while 2 of 6 tadpoles with normal functional lymph vessels showed diffuse angiograms). Furthermore, apart from the leakiness and tortuosity of some vessels (eg, DLAV; Figure 3G), the overall architecture of the blood vasculature appeared normal in all tadpoles analyzed by angiography (N = 11; Figure 3F,G), allowing normal commitment of BECs of the PCV to LECs (normal area 1). Together, this suggests that the observed lymphatic defects were largely a primary effect of VEGFR-3KD. The majority of the VEGFR-3KD tadpoles injected with 100 ng morpholino developed edema at stage 45 (78% of 65 VEGFR-3KD tadpoles vs 9.7% of 145 controls; P < .001 by chi-square analysis; Figure 3H,I).
Inhibition of xVEGFR-3 impairs lymphangiogenesis
To further minimize possible effects on blood vessel development, which might secondarily confound our interpretation of lymphatic development, we also used the tyrosine kinase inhibitor MAZ51. Contrary to most other chemical VEGF receptor tyrosine kinase inhibitors, MAZ51 has a relatively high selectivity for VEGFR-3 and inhibits the tyrosine kinase activity of VEGFR-1 and VEGFR-2 only partially, at more than 10-fold higher concentrations than those used to inhibit VEGFR-3.22 Even though we cannot exclude the possibility that this compound inhibits other (unknown) targets, we tested whether MAZ51 would phenocopy the knockdown of xVEGFR-3. To minimize effects on the blood vasculature as much as possible, we supplemented MAZ51 from stage 32/33 onward, when BECs of the PCV have started to transdifferentiate to LECs.12
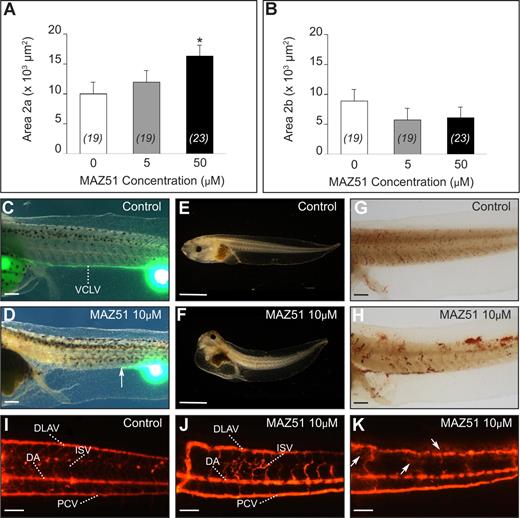
Quantification of the xProx1+ areas in stage 35/36 embryos treated with MAZ51 (5 or 50 μM)22 revealed no difference with control (DMSO) for area 1 (not shown), likely because commitment had already largely taken place at the start point of MAZ51 supplementation. However, MAZ51 (5-50 μM) dose-dependently impaired lymphatic migration, characterized by normal initiation of migration by committed LECs, but reduced the number of LECs that continued along the normal trajectory. This was revealed more accurately by quantification of xProx1+ area 2, subdivided into area 2a and 2b, located ventral or dorsal to the floor plate of the neural tube (denoted by the yellow line in Figure 1C), respectively. Compared with control vehicle, area 2a was increased whereas area 2b was decreased in MAZ51-treated tadpoles (Figure 4A,B). MAZ51 treatment at a concentration higher than 25 μM was associated with absence of blood flow or death by stage 45, impeding assessment of lymph vessel functionality by lymphangiography at these concentrations. Nonetheless, lymphangiography of tadpoles treated with 5 or 10 μM MAZ51 confirmed defective lymph vessel formation, as filling of the VCLV was partially or completely impaired in all 9 tadpoles tested (Figure 4C,D). MAZ51 dose-dependently caused edema by stage 45, while the control vehicle was ineffective (51% and 98% of tadpoles treated with 5 or 10 μM MAZ51, respectively [n = 66 and 51] vs none of 10 DMSO controls; Figure 4E,F).
Chemical inhibition of xVEGFR-3 signaling with MAZ51 causes lymphatic defects. (A,B) Morphometric measurement of area 2a and 2b in the posterior trunk of xProx1-stained tadpoles at stage 35/36, showing dose-dependent inhibition of migration by MAZ51, reflected by the stalling of the cells in area 2a. *P < .05 versus control (DMSO). The number of tadpoles is indicated within each bar. Error bars represent SEM. (C-K) Functionality of the lymphatic and blood vasculature in control and MAZ51-treated tadpoles. Lymphangiography showed normal filling of the VCLV in DMSO-treated control tadpole (stage 45) (C) without signs of edema (E) and displaying normal intact blood vessels upon microscopic inspection (G) and angiography (I). In contrast, stage-45 tadpoles treated with 10 μM MAZ51 showed dysfunctional lymph vessels upon lymphangiography (D) and edema formation (F). The arrow in panel D indicates the distal site of VCLV filling. Angiography revealed blood vessel defects, including irregularly shaped DLAVs and malformed or absent intersomitic vessels (J,K), and leakage of the fluorescent dye (arrows in K) suggesting vessel fragility. Widespread hemorrhaging throughout tadpoles treated with 10 μM MAZ51 was also suggested by o-dianisidine staining of erythrocytes, showing numerous patches of cells outside of the blood vasculature (H; compare with control in G). DA indicates dorsal aorta; DLAV, dorsal longitudinal anastomosing vessel; ISV, intersomitic vessel; PCV, posterior cardinal vein; and VCLV, ventral caudal lymph vessel. Bars represent 500 μm (C,D,G,H), 1 mm (E,F), and 100 μm (I-K).
Chemical inhibition of xVEGFR-3 signaling with MAZ51 causes lymphatic defects. (A,B) Morphometric measurement of area 2a and 2b in the posterior trunk of xProx1-stained tadpoles at stage 35/36, showing dose-dependent inhibition of migration by MAZ51, reflected by the stalling of the cells in area 2a. *P < .05 versus control (DMSO). The number of tadpoles is indicated within each bar. Error bars represent SEM. (C-K) Functionality of the lymphatic and blood vasculature in control and MAZ51-treated tadpoles. Lymphangiography showed normal filling of the VCLV in DMSO-treated control tadpole (stage 45) (C) without signs of edema (E) and displaying normal intact blood vessels upon microscopic inspection (G) and angiography (I). In contrast, stage-45 tadpoles treated with 10 μM MAZ51 showed dysfunctional lymph vessels upon lymphangiography (D) and edema formation (F). The arrow in panel D indicates the distal site of VCLV filling. Angiography revealed blood vessel defects, including irregularly shaped DLAVs and malformed or absent intersomitic vessels (J,K), and leakage of the fluorescent dye (arrows in K) suggesting vessel fragility. Widespread hemorrhaging throughout tadpoles treated with 10 μM MAZ51 was also suggested by o-dianisidine staining of erythrocytes, showing numerous patches of cells outside of the blood vasculature (H; compare with control in G). DA indicates dorsal aorta; DLAV, dorsal longitudinal anastomosing vessel; ISV, intersomitic vessel; PCV, posterior cardinal vein; and VCLV, ventral caudal lymph vessel. Bars represent 500 μm (C,D,G,H), 1 mm (E,F), and 100 μm (I-K).
MAZ51 treatment at a concentration of 10 μM was only partially associated with absence of blood flow in the major vessels of the tail (40% of 51 live screened tadpoles), absence of heartbeat (6%), and pronounced hemorrhaging at various locations (57%; Figure 4G,H). Similar defects were observed with MAZ51 concentrations of 5 μM but at lower frequencies (only 6% of 66 tadpoles had no blood flow in the major vessels of the tail; 14% of 66 showed some hemorrhaging). Angiography of stage-45 tadpoles treated with 5 or 10 μM MAZ51 confirmed blood vessel defects, including irregularly shaped DLAV, malformed or absent intersomitic vessels (ISVs) in all of 9 tested tadpoles (Figure 4I,J), and apparent fragility of the blood vasculature, as suggested by diffusion of the fluorescent dye from the vessels (Figure 4K). These effects might be due to interference with xVEGFR-2 signaling by MAZ51 and/or effects of reduced signaling via xVEGFR-3, which is also expressed on blood vessels during tadpole development (Document S1 Note 5). Nevertheless, the PCV appeared normally developed in the majority of the MAZ51-treated tadpoles (Figure 4J,K), allowing normal commitment in area 1. The impaired migration of committed LECs suggested a decreased responsiveness toward the migratory VEGFR-3 ligands, likely indicating a primary lymphangiogenesis defect.
Deficient VEGFR-3 function causes lymph heart dysfunction
A particular lymph heart (LH) phenotype was observed upon live screening at stage 39-45 in tadpoles with functional xVEGFR-3 blockage. In mammals, lymph fluid is drained, in part, by the rhythmic contractions of lymphatic smooth muscle cells around the collecting lymph ducts containing one-way valves.30,31 In contrast, in tadpoles, the lymph is drained by continuously pumping LHs.12 These LHs develop via transdifferentiation of xMsr+ venous BECs from the pronephric sinus into xProx1+ LECs, which become surrounded by muscle cells that display features of cardiac and skeletal muscle, but are of uncertain embryologic tissue origin.25,32,33 Of note, the LH expresses xVEGFR-3, as shown by in situ hybridization for xVEGFR-3 of stage-42 tadpoles (Document S1 Figure S3C). In 7% (5/66) and 70% (35/51) of tadpoles treated with 5 or 10 μM MAZ51, respectively, the LHs had ceased beating at stage 45 (among a collection of tadpoles with confirmed initial LH beating at stage 42). In addition, they were dilated and contained red blood cells (in 83% of the nonbeating LHs; Document S1 Figure S4A,B). Because this might be due to incomplete separation of the blood vascular and lymphatic systems (as observed with other genetic deficiencies34 ), we analyzed serial cross-sections of numerous embryos. However, this histologic analysis did not reveal obvious connections or shunts between the blood vasculature and lymphatic system (not shown), suggesting that separation of both systems occurred normally. This particular LH phenotype was also observed in VEGF-C/DKD and VEGFR-3KD tadpoles (10% of 152 VEGF-C/DKD tadpoles and 57% of 65 VEGFR-3KD tadpoles [100 ng] had enlarged LHs, of which 13% or 11% contained red blood cells, respectively; Document S1 Figure S4D,E,G,H and not shown), in the absence of severe hemorrhaging as seen in the MAZ51-treated tadpoles.
Quantification of the maximal cross-sectional area of the LH on a series of consecutive histologic sections of stage-45 tadpoles confirmed LH dilation, revealing up to 5.5-fold larger values in VEGF-C/DKD–, VEGFR-3KD–, or MAZ51-treated tadpoles compared with their respective controls (Document S1 Table S2). This impairment did not seem due to defective amounts of lymph heart muscle fibers (Document S1 Figure S4A,B; not shown). Dilation of LHs was only occasionally observed in single VEGF-CKD (35 ng morpholino) or VEGF-DKD (50 ng morpholino) tadpoles (circa 5% of 115 or of 69 tadpoles by live screening, respectively).
In VEGFR-3KD tadpoles with beating LHs, beating rates appeared to be normal (average beating rate in randomly selected tadpoles: 92 ± 8 bpm vs 104 ± 3 bpm for control tadpoles; N = 7-8; mean ± SEM; P = NS). In normal conditions, the beating LH secures forward flow of the lymph from the lymphatic to venous circulation; backflow from blood to the LH is prevented by the presence of valves between the LH and the pronephric sinus.35 Angiography, through injection of a dye in the blood heart, in tadpoles with dilated LHs (VEGFR-3KD, VEGF-C/DKD, or MAZ51 treated) invariably resulted in instantaneous filling of the LH with the dye (Document S1 Figure S4C,F,J), even when the LH was still beating. This suggested that dye reached the LH through backflow from the blood heart via the duct of Cuvier and pronephric sinus. Backflow of the dye to the LH was never observed in control tadpoles (N = 12; Document S1 Figure S4I). This phenotype seemed not to be due to failure of valve formation, as the efferent LH valves connecting the LH to the venous circulation were regularly detected on histologic sections (Document S1 Figure S4D,E,G,H; not shown). The valves occasionally appeared distended (Document S1 Figure S4E), which might be due to the LH dilation, although structural defects or dysfunction cannot be excluded (Document S1 Figure S4D,E,G,H and not shown).
Of note, enlargement of the LH area was observed at stage 39 in VEGFR-3KD tadpoles (100 ng morpholino), as revealed by the larger xProx1+ area in 86% of whole-mount xProx1-stained tadpoles analyzed (13 of 15 tadpoles), in contrast to normal LH areas in all control tadpoles analyzed (N = 20; Document S1 Figure S4K,L). At this stage, the LH still looks elongated on cross-sections, but slight dilation was already noticed in the VEGFR-3KD tadpoles (Document S1 Figure S4M,N). By stage 42, the LH had expanded and contained a lumen, and was considerably dilated in VEGFR-3KD tadpoles compared with controls (Document S1 Figure S4O,P). These observations indicated that LH abnormalities were present before complete formation of the large lymph vessels (VCLV, DCLV; Document S1 Figure S3, stage-39 panel) and that LH dilation occurred from the moment the LH starts beating (stage 42). Because no obvious anatomic structural defects were detectable, loss of xVEGFR-3 signaling might have caused developmental or functional LH defects, predisposing it to dilation and failure to pump.
We cannot exclude the possibility that the LH phenotype might also be, in part, related to the blood vessel defects, which likely increased the peripheral resistance. This is suggested by the finding that the occurrence of blood vessel defects (as indicated by blood flow arrest or reverted flow in specific vessels; not shown) upon xVEGFR-3 blockage partially correlated with the occurrence of LH dilation and presence of red blood cells in the LH (eg, of 51 tadpoles treated with 10 μM MAZ51, 40% showed blood flow defects and 57% had RBC-containing LHs). Thus, xVEGFR-3 dysfunction, by morpholino knockdown of xVEGFR-3, combined knockdown of its ligands, or tyrosine kinase inhibition, impaired not only lymph vessel formation but also LH function. The underlying mechanisms might involve functional LH defects caused by xVEGFR-3 inhibition and/or hydrodynamic deregulation, and remain to be further resolved.
Discussion
VEGF-D (also named c-fos–induced growth factor or FIGF36 ), which binds VEGFR-3 on LECs,26 is widely expressed throughout the embryo, including in areas where future lymphatic structures develop, such as the skin.37,38 VEGF-D stimulates lymphangiogenesis in adulthood and is involved in metastasis in experimental and human cancer.39-46 Phenotyping of VEGF-D knockout mice revealed a negligible role for VEGF-D in lymph vessel development. In adult mice, only a slight reduction in the number of bronchiolar lymphatics was observed, which did not affect normal pulmonary lymphatic function.17 Because VEGF-C and VEGF-D might have redundant activities, it remained outstanding whether the modest lymphatic defects in the VEGF-D–deficient mice might have resulted from rescue by VEGF-C. Furthermore, interactions of VEGF-D with other factors during lymphatic development had not been studied so far. In addition, the role of its receptor VEGFR-3 in lymphatic development in mouse embryos could not be evaluated to date, as loss of VEGFR-3 causes embryonic lethality before lymph vessels form.28 We therefore investigated the effect of xVEGF-D or xVEGFR-3 deficiency on lymphatic development using single and combined knockdown and chemical inhibition in Xenopus tadpoles.
The role of endogenous VEGF-D in embryonic lymphatic development has not been studied in detail yet. An advantage of the tadpole model is that it allows whole-mount visualization of LEC commitment and migration in the young developing embryo. Phenotyping of morphant tadpoles revealed that knockdown of xVEGF-D impaired sprouting and migration of transdifferentiated LECs. The role of xVEGF-D is, however, more subtle than that of VEGF-C,11,12 as the impaired sprouting in VEGF-DKD tadpoles did not prevent the formation of functional lymph vessels, explaining why most of them did not develop lymphedema. Thus, although exogenous VEGF-D is capable of partially rescuing the impaired sprouting of LECs in VEGF-Cnull mice,11 endogenous VEGF-D does not seem to play a predominant role in this process. Furthermore, recent data indicate that cooperative signaling of VEGFR-2 and VEGFR-3 is required for lymphatic migration and proliferation.47 Because VEGF-C binds both receptors, loss of this growth factor likely caused severe lymphatic defects.12 Unfortunately, we could not determine whether xVEGF-D also binds both xVEGFR-2 and xVEGFR-3, precluding us from drawing any firm conclusions that the more transient LEC migration defect in VEGF-DKD embryos might have been caused by the lack of binding of xVEGF-D to xVEGFR-2. Interestingly, angiography of VEGF-DKD tadpoles did not reveal any abnormalities in blood vessel formation (which is largely driven by VEGFR-2 signaling), consistent with the normal blood vascular development in VEGF-D knockout mice (Baldwin et al17 and M.K., P.C., and M.D., unpublished data, January 2008). In a recent study, zVEGF-D mRNA overexpression in zebrafish was reported to cause pronounced defects of the blood vasculature.48 However, this study did not address potential effects on the formation of the axial lymphatic vessel, which develops as the first lymphatic vessel in close proximity to the dorsal aorta.13,14
The Xenopus model also offered the opportunity of studying genetic interactions via coinjection of different morpholinos. Applying this approach, combined knockdown of VEGF-C and VEGF-D was performed, which revealed more severe defects in lymphatic development compared with each of the single knockdowns. This suggested that a combination of both VEGF-C and VEGF-D results in stronger or different signals than those induced by each factor alone, possibly via activation of VEGFR-3 homodimers or of VEGFR-2/VEGFR-3 heterodimers. Alternatively, in the single knockdowns, each factor might have compensated for the loss of the other, although, similar to findings in VEGF-D knockout mice (Baldwin et al17 and M.K., P.C., and M.D., unpublished data, January 2008), we could not find evidence for a compensatory up-regulation of VEGF-C in tadpoles in the absence of VEGF-D (not shown). Thus, endogenous VEGF-D, rather than being a key determinant in lymph vessel formation, appears to have a role as a modifier of other lymphangiogenic factors. Initial data from combined knockdown at suboptimal morpholino doses of VEGF-D and Prox1, a master switch determining the transdifferentiation of venous BECs into LECs,10,49 similarly resulted in more severe lymphatic defects than in corresponding single knockdowns (not shown). This result indicates that VEGF-D also modulates the effects of Prox1 in the tadpole and further highlights a role of VEGF-D in developmental lymphangiogenesis as a modifier.
The use of the Xenopus model also enables interference with molecular function via pharmacologic inhibition, allowing, unlike morpholino knockdown, conditional inhibition by exposure to the inhibitor at defined developmental stages. We took advantage of this approach to analyze the role of VEGFR-3 in embryonic lymphatic development, by supplementing the tyrosine kinase inhibitor MAZ51 to the developing tadpoles at a time point selected to minimize effects on the partially synchronously developing blood vasculature. Indeed, like in mice and humans,29 xVEGFR-3 in tadpoles is also expressed in the blood vasculature at early developmental stages, becoming partially restricted to the lymphatics only beyond stage 39. Treatment thus was initiated at stage 32/33, at the onset of heartbeat and beginning of early blood circulation.50 Even though MAZ51 has been reported to exhibit a much greater selectivity for VEGFR-3 than for VEGFR-1 or VEGFR-2,22 we realize that this compound might also inhibit other (unknown) targets. Nonetheless, MAZ51 and VEGFR-3KD remarkably phenocopied each other, suggesting that the lymphatic defects in the MAZ51-treated embryos are, at least in part, due to inhibition of xVEGFR-3. Indeed, both MAZ51 and xVEGFR-3 morpholino knockdown revealed a prominent role of xVEGFR-3 in migration of committed LECs in the tadpole tail and failure to develop functional lymphatic vessels. VEGFR-3 knockdown was previously studied in zebrafish, but the analysis focused on blood vessel development and lymphatic vessel formation was not assessed.51 In the tadpole, MAZ51 treatment and, less pronounced, knockdown of xVEGFR-3 or combined VEGF-C/D knockdown also caused blood vessel abnormalities or fragility (vessel malformations, hemorrhaging, leakage upon angiography), which presumably also contributed to edema formation. Possibly, these blood vessel abnormalities were caused by effects on xVEGFR-2 signaling (MAZ51 and combined VEGF-C/D knockdown) combined with direct effects of deficient xVEGFR-3 signaling on blood vessel development (as in zebrafish and mice14,28,51,52 ). Indeed, as shown in “Results” (Figure 3), xVEGFR-3 is also expressed in the developing blood vasculature, the expansion of which partially overlaps with lymphatic development. Importantly, however, dysfunction of the lymph vessels did not correlate in an absolute manner with dysfunction of the blood vessel system (dysfunctional lymph vessels were observed in tadpoles with normal blood flow, heartbeat, and angiograms in xVEGFR-3 or combined xVEGF-C/D knockdown tadpoles). This indicated that the lymphatic defects observed in the tadpoles with deficient xVEGFR-3 function were, at least in part, primary lymphangiogenesis defects. It will be interesting to see whether more selective VEGFR-3 inhibitors (once available) will phenocopy the lymphatic defects caused by MAZ51.
An additional observation in this study was that interference with xVEGFR-3 function caused dilation of the LHs, which express xVEGFR-3, in a substantial fraction of the treated tadpoles. This was observed after MAZ51 treatment, xVEGFR-3 knockdown, as well as combined xVEGF-C/D knockdown. These LHs frequently contained red blood cells and often had stopped beating. Red blood cells were, however, not detected elsewhere within the lymphatic system, nor were there obvious signs of incomplete separation of the blood and lymphatic vasculature, as observed in SLP-76– and Syk-deficient mice.34 LH enlargement and dilation already occurred before or at the start of beating. Furthermore, the incidence of the LH phenotype partially correlated with the incidence of blood flow defects. Together, these findings suggested that intrinsic LH developmental defects combined with indirect hemodynamic effects caused by blood vessel damage might underlie the LH phenotype. These potential mechanisms remain to be further explored.
In summary, this chemicogenetic study in Xenopus tadpoles indicated that endogenous xVEGF-D is only minimally involved in embryonic development, in accordance with previous findings in the mouse. Nonetheless, a novel finding, not previously recognized in mouse mutants, is that VEGF-D amplifies the effects of other lymphangiogenesis regulators, primarily via effects on LEC migration. Thus, the cooperation of endogenous xVEGF-D with xVEGF-C and xProx1 as revealed in the tadpole suggests a role of VEGF-D in lymphangiogenesis as a modifier. Furthermore, knockdown experiments documented a critical role of VEGFR-3 in developmental lymphangiogenesis indispensable for normal lymph vessel function in the tadpole.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank A. Ciau-Uitz of the Weatherall Institute of Molecular Medicine (Oxford, United Kingdom) for providing the xMsr and xFli cDNA; E. Chorianopoulos for helpful discussion; K. Brepoels, A. Carton, P. Chevron, A. Claeys, A. Cobut, S. Jansen, E. Janssens, H. Laisnez, S. Louwette, W. Y. Man, A. Manderveld, W. Martens, J. Souffreau, S. Terclavers, A. Van Nuffelen, B. Vanwetswinkel, V. Weerelds, and S. Wyns for their technical assistance; and L. Notebaert and M. Deprez for the artwork.
This work was supported in part by grant no. G.0567.05 from the Fund for Scientific Research Flanders (FWO; Brussels, Belgium), by an unrestricted grant to P.C. from Bristol-Myers-Squibb (Syracuse, NY), by grant no. GOA2001/09 from Concerted Research Activities (Leuven, Belgium), by grant no. LSHG-CT-2004-503573 of the European Union 6th Framework Programme, and by Belgian Science Policy (Brussels, Belgium; IAP no. P6-20). A.N. is sponsored by the European Union 6th Framework Programme and by a Marie Curie Intra-European Fellowship; M.K., by the Fundacao para a Ciencia e Tecnologia (Lisbon, Portugal; grant SFRH/BD/9349/2002); W.V. and I.G., by the Flemish Institute for the promotion of scientific research (IWT; Brussels, Belgium); M.S., by the Lymphatic Research Foundation (East Hills, NY); C.F., by the Deutsche Forschungsgemeinschaft (Bonn, Germany); A.D.-J., by Centro Nacional de Investigaciones Cardiovasculares (Madrid, Spain); and D.L., by the FWO.
Authorship
Contribution: A.N., P.C., and M.D. designed research; A.N., W.V., M.K., M.S., C.F., A.D.-J., E.N., I.G., S.M., and S.P. performed the experiments; A.N., M.K., W.V., M.S., C.F., E.N., I.G., L.M., S.P., D.L., and M.D. analyzed data; and A.N., P.C., and M.D. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mieke Dewerchin, Vesalius Research Center, VIB-KU Leuven, Campus Gasthuisberg O&N1, Herestraat 49 box 912, B-3000 Leuven, Belgium; e-mail: mieke.dewerchin@med.kuleuven.be.