Abstract
Neonates exhibit an increased risk of sepsis mortality compared with adults. We show that in contrast to adults, survival from polymicrobial sepsis in murine neonates does not depend on an intact adaptive immune system and is not improved by T cell–directed adaptive immunotherapy. Furthermore, neonates manifest an attenuated inflammatory and innate response to sepsis, and have functional defects in their peritoneal CD11b+ cells. Activation of innate immunity with either a Toll-like receptor 4 (TLR4) or TLR7/8 agonist, but not a TLR3 agonist, increased the magnitude, but abbreviated the early systemic inflammatory response, reduced bacteremia, and improved survival to polymicrobial sepsis. TLR4 agonist pretreatment enhanced peritoneal neutrophil recruitment with increased oxidative burst production, whereas the TLR7/8 agonist also enhanced peritoneal neutrophil recruitment with increased phagocytic ability. These benefits were independent of the adaptive immune system and type I interferon signaling. Improving innate immune function with select TLR agonists may be a useful strategy to prevent neonatal sepsis mortality.
Introduction
Sepsis causes profound defects in innate and acquired immunity. In septic adults, circulating leukocytes fail to mount an attenuated inflammatory response, monocytes have defective antigen presentation in part due to reduced MHC class II expression, and dendritic cells and lymphocytes exhibit increased apoptosis.1-4 These deficiencies contribute to a failure to clear primary pathogens, an increased propensity to develop superinfections, and an inability to mount adaptive immune responses. Considerable progress has been made in understanding the pathogenesis of and identifying potential immunomodulatory therapies for treating sepsis in adult animals. For example, MyD88 and type I interferon signaling pathways5,6 are important requisites for innate and inflammatory host defense responses to pathogens.7,8 Stimulating the innate immune system with Toll-like receptor (TLR) agonists improves survival in adult animal models of sepsis.9,10 Similarly, absence of the adaptive immune system11 or an inability of B cells to produce antibodies12 predisposes adult mice to a poor outcome in sepsis. Correction of adaptive immune dysfunction by prevention of lymphocyte apoptosis or treatment with agonistic glucocorticoid-induced tumor necrosis factor (TNF) receptor antibody (anti-GITR) to stimulate effector T-cell function, improves survival in animal models of adult sepsis.11,13 These studies highlight the importance of both the innate and adaptive immune systems in eliminating invading pathogens in adult mammals. However, the mechanisms of protective immunity in neonates that do not possess a fully intact immune system, and who develop sepsis at increased rates,14 are less clear.
More than 1 million babies die each year worldwide within the first 4 weeks of life from sepsis.15 Neonatal sepsis mortality is higher than in children and adults,16,17 peaking in premature infants, where rates can approach 50%.18 Neonates have well-described deficits in adaptive and innate immune function that place them at risk for the development of a serious bacterial infection. Among these are decreased production of T-helper 1 (TH1) polarizing cytokines (or bias to TH2-type responses), type I interferons, and MHC class II expression on antigen-presenting cells; impaired amplification, mobilization, and function of neutrophils; an immature dendritic cell system quantitatively and qualitatively; and decreased plasma concentrations of complement components, as well as delayed, shortened, and decreased antibody responses from B cells.19,20 In addition, as a result of underdeveloped splenic architecture20 and limited follicular development and antigen exposure, the trapping of bacteria via marginal zones,21 which normally occurs in the adult with bacteremia,22 is not present in the neonate. Thus, neonates are at significant risk for developing and succumbing to sepsis.
To better understand the neonatal immunologic response and its capabilities in vivo, we have used a murine model of polymicrobial sepsis induced by generalized peritonitis.23 Using this model, we have previously observed that neonates have increased susceptibility to sepsis and exhibit an attenuated inflammatory response as compared with adults.23 Few studies have evaluated the role of the innate or adaptive immune system in neonatal polymicrobial sepsis and specifically which immune responses are important for clearance of pathogens from within the neonatal peritoneum. Here, we show for the first time that sepsis survival in neonatal mice, in stark contrast to adults, is not affected by absence or specific targeting of the adaptive immune system with anti-GITR pretreatment. Neonates rely primarily on innate immunity for their protection from polymicrobial sepsis. Moreover, improving neonatal innate immune function can protect against sepsis mortality. These results demonstrate key differences between the adult and neonatal immune responses to pathogens, and suggest innate immunomodulatory therapies may be warranted to stimulate immunity in high-risk neonates.
Methods
Mice and monitoring
All studies were approved by the Institutional Animal Care and Use Committee at the University of Florida College of Medicine prior to their initiation. Specific pathogen–free male and female C57BL/6 (B6) mice, RAG-1 deficient mice (homozygous) on a B6 background, and C3H/HeJ breeding pairs were purchased from The Jackson Laboratory (Bar Harbor, ME) between 6 and 10 weeks of age and allowed a minimum of 7 days to equilibrate to their environment before any experimental use. Breeding pairs of IFN-αβR/A129 (IFNAR)–null mice on the 129S6/SvEv background (H-2b) and wild-type Sv129 mice were a kind gift from Dr Westley Reeves (University of Florida, Gainesville, FL) and were originally purchased from B&K Universal (Grimston, United Kingdom). Mice were maintained on standard rodent food and water ad libitum. Adults used in experiments were between 8 and 14 weeks of age. To generate neonatal mice, paired matings were established twice weekly; females were isolated from males as soon as they were visually identified as pregnant, and followed closely thereafter so an accurate date of birth was recorded. Litters aged 4 to 6 days were used for experimental procedures, and were defined as neonates.20,24
TLR agonists
Pups aged 4 to 6 days old received either TLR7/8 agonist (resiquimod 5 μg/g birth weight [BW]; 3M Pharmaceuticals, Minneapolis, MN), TLR3 agonist (poly I:C 10 μg/g BW; InvivoGen, San Diego, CA), or TLR4 agonist (lipopolysaccharide [LPS] 1 μg/g BW; Escherichia coli O26:B6; Sigma-Aldrich, St Louis, MO) in physiologic saline vehicle via an intraperitoneal injection in a volume less than 30 μL 24 hours prior to the initiation of sepsis using the fecal peritonitis method as described. Control animals received an intraperitoneal injection of physiologic saline in an identical volume.
Fecal-induced generalized peritonitis
Polymicrobial sepsis was induced in neonatal mice using the fecal peritonitis method as previously described.23
Bacteremia and cytokine analyses
Whole blood was obtained and evaluated for bacterial colonization and plasma cytokine concentrations as previously described.23
Splenocyte and peritoneal cell phenotypes
Spleens were harvested, processed, and stained for flow cytometry as previously described.23 In addition, splenocytes were stained for CD21–fluorescein isothiocyanate (FITC) and CD23-phycoerythrin (PE) and IgM peridinin chlorophyll protein–cyanine5.5 (PerCP-Cy5.5). Neonatal splenocytes were stained with anti-GITR conjugated to allophycocyanin (APC), anti-CD3 conjugated to Pacific Blue, anti-CD4 conjugated to FITC, and anti-CD25 conjugated to PE (BD Biosciences, San Jose, CA). Peritoneal washes were obtained from neonates killed by isoflurane induction with subsequent decapitation 24 hours after TLR agonist administration. Immediately after death, approximately 1 mL physiologic saline was injected into the peritoneal cavity and was lavaged repeatedly. The fluid obtained from the lavage was pooled from multiple animals (n = 7), as peritoneal washes from individual animals contained insufficient cells for analysis. Cells were washed in phosphate-buffered saline (PBS), pelleted, and subsequently stained for flow cytometry. Peritoneal cells were characterized using anti–GR-1 conjugated to PerCP-Cy5.5, anti-B220 conjugated to APC–AlexaFluor 750, anti-CD11c conjugated to APC, and anti-CD11b conjugated to Pacific Blue. Cell samples were acquired and analyzed on either an LSRII flow cytometer with FACSDiva software or a FACSCalibur flow cytometer with CellQuest software (all from BD Biosciences). At least 3 × 104 nondebris live cells (Sytox-blue negative or 7-aminoactinomycin D negative) were used for analysis.
Phagocytosis measurement
At 2 hours after stimulation in vivo using an LD70 of cecal slurry given intraperitoneally, neonatal peritoneal lavage samples were centrifuged, washed, and treated with and without liposome-encapsulated DiI (Invitrogen, Carlsbad, CA) prepared as previously described,25 incubated at 37°C for 30 minutes, collected by centrifugation, washed 3 times to remove extracellular liposomes, and stained as described for flow cytometry analysis.25 No serum was used. Liposomal Dil was used instead of beads to reduce false positives secondary to pinocytosis. The concentration of Dil was optimized by flow cytometry; the optimal concentration was 2 μL Dil/mL liposomes. Phagocytosis was subsequently determined by measuring mean fluorescence intensity in various cell types by flow cytometry using an LSRII flow cytometer. Cells were considered Dil “positive” when the mean fluorescence intensity (MFI) was greater than baseline (< 1000 MFI).
ROS detection
Following stimulation with an LD70 of cecal slurry given intraperitoneally 30 minutes, 2 hours, and 6 hours prior to peritoneal cell isolation, reactive oxygen species (ROS) production in peritoneal cellular populations was determined using dihydrorhodamine 123 (Invitrogen).26 The stock DHR123 was prepared by diluting the DHR123 1 mg:1 mL in DMSO, stored in 50-μL aliquots at −80°C. Before flow cytometry, 20 μL of stock was dissolved in 650 μL of PBS to a concentration of 30 μg/mL. A total of 25 μL of working DHR solution was added to peritoneal cell isolates suspended in 200 μL PBS and incubated at 37°C for 5 minutes. Cells were then spun and washed prior to flow cytometry analysis. Cell types were not sorted or selected prior to flow cytometry analysis in order to examine the ROS production from all cell types acquired from the total peritoneal wash at the time of collection. Cell populations were determined using cell surface markers. In particular, polymorphonuclear leukocytes (PMNs) were identified as GR-1+Ly6ClowCD11bhigh cells within the total cells obtained from the peritoneal wash, were verified to be PMNs using forward/side scatter back gating, and were the only population of cells that increased ROS production. ROS production by different cell types was determined by measuring MFI by flow cytometry using an LSRII flow cytometer. Cells were considered positive when the MFI was greater than baseline (greater than 100 MFI).
GITR antibody
Rat anti-mouse GITR agonistic (DTA-1) purified antibody was purchased from BioExpress (West Lebanon, NH). Rat anti–human IL-4 was used as an IgG2b isotype control antibody. Based on methods by Scumpia et al,13 the neonates were given an intraperitoneal injection of 20 μg/g of anti-GITR agonistic antibody or isotype control antibody 24 hours prior to administration of cecal slurry (LD70). Female C57BL/6 (B6) mice aged 6 to 8 weeks old were evaluated for sepsis-survival benefit following anti-GITR treatment 24 hours prior to creating sepsis via the cecal slurry method (LD70). Survival was followed for 5 days after sepsis.
NP-KLH immunization
Neonates were immunized via an intraperitoneal injection with 4-hydroxy-3-nitrophenylacetyl hapten conjugated to keyhole limpet hemocyanin (NP-KLH) at a 28:1 ratio (25 μg/animal; Biosearch Technologies, Novato, CA) and alum (1:1) in 20 μL, concurrently with either anti-GITR agonistic antibody (20 μg/g) or isotype control antibody at 2 days of life and bled 2 weeks after injection. Serum titers of NP-specific antibodies were determined by enzyme-linked immunosorbent assay (ELISA) as previously described.27
Statistics
Survival was compared using the Fisher exact test. Values were considered significant if the 2-tailed confidence level was at a P level less than .05. Cytokine concentrations, bacteremia levels, and leukocyte numbers and percentages from all TLR agonist-treated and sham-treated animals were compared using one-way analysis of variance (ANOVA). If the descriptive analyses passed normality and equal variance, then a post-hoc Tukey multiple range test was used to compare all groups. If the descriptive analyses failed normality or tests of equal variance, a comparison using Kruskal-Wallis analysis of variance on ranks and the Dunn method was performed versus sham-treated controls. A Student t test or a Wilcoxon signed-rank test was used to compare results from 2 groups. Values were considered significant if P values were less than .05. Because most of the plasma cytokine data did not pass tests of normality or equal variance, the results are reported as the medians with quartiles (75%/25%).
Results
Neonatal mice exhibit defects in both innate and adaptive immunity that affect systemic and local pathogen clearance
Using histologic staining and flow cytometry, we confirmed that neonatal mice do not yet possess splenic and, specifically, marginal zone architecture, and have a diminished proportion of marginal zone B cells (B220+IgMhighCD21highCD23low) compared with adults (data not shown).20 Compared with adults, the numbers and percentage of follicular B cells (data not shown) and CD4+ T cells23 were considerably lower in neonates, whereas the number and percentage of immature monocytic cells (GR-1lowCD11bhighLy6Chighc-kit+) was considerably higher (data not shown). Taken together, these data suggest that the neonatal spleen is acting more as a hematopoietic organ than as a means to trap microorganisms from the systemic circulation.
Since we use a model of generalized, polymicrobial peritonitis, we examined whether there were any cellular differences between neonates and adults in the peritoneum. The normal cellular composition of the adult peritoneal cavity includes mainly B1 cells and macrophages, with low numbers of monocytes and neutrophils (PMNs) (Figure 1A; data not shown). The percentage and phagocytic activity of B220+CD11b+ B1 cells were all dramatically reduced in the healthy, neonatal mice when compared with young adults (Figure 1A,B; regarding Dil+ PMNs and B1 cells, the percentage designation refers to the percentage of that cell population that registered greater than the baseline mean fluorescence intensity). B1 cells and GR-1+Ly6ClowCD11bhigh PMNs were the only cells demonstrating phagocytic activity (Dil+) in the resting adult peritoneum, whereas the neonate was virtually devoid of phagocytic activity in any of their peritoneal cell populations (Figure 1B; data not shown). Combined with data from our previous publication showing a markedly diminished inflammatory response to bacterial peritonitis,23 these studies confirm that relative to adults, neonatal mice have multiple defects in bacterial clearance, including inflammation, marginal zone entrapment, and phagocytic function.
Neonatal mice demonstrate altered peritoneal cell content and function compared with adult mice. Percentages (A) and phagocytic activity (B) (Dil+) of B220+CD11b+ peritoneal B1 cells and GR-1+Ly6ClowCD11bhigh neutrophils (PMN) from neonates versus young adults. Percentage of Dil+ cells refers to percentage of that cell population that registered greater than the baseline MFI (expressed as greater than 1000 MFI). Data shown are representative of 4 separate identical experiments. Bar graphs represent means with error bars representing standard deviations (*P < .05 by Student t test).
Neonatal mice demonstrate altered peritoneal cell content and function compared with adult mice. Percentages (A) and phagocytic activity (B) (Dil+) of B220+CD11b+ peritoneal B1 cells and GR-1+Ly6ClowCD11bhigh neutrophils (PMN) from neonates versus young adults. Percentage of Dil+ cells refers to percentage of that cell population that registered greater than the baseline MFI (expressed as greater than 1000 MFI). Data shown are representative of 4 separate identical experiments. Bar graphs represent means with error bars representing standard deviations (*P < .05 by Student t test).
The adaptive immune system does not participate in the protective host response to sepsis in neonatal mice
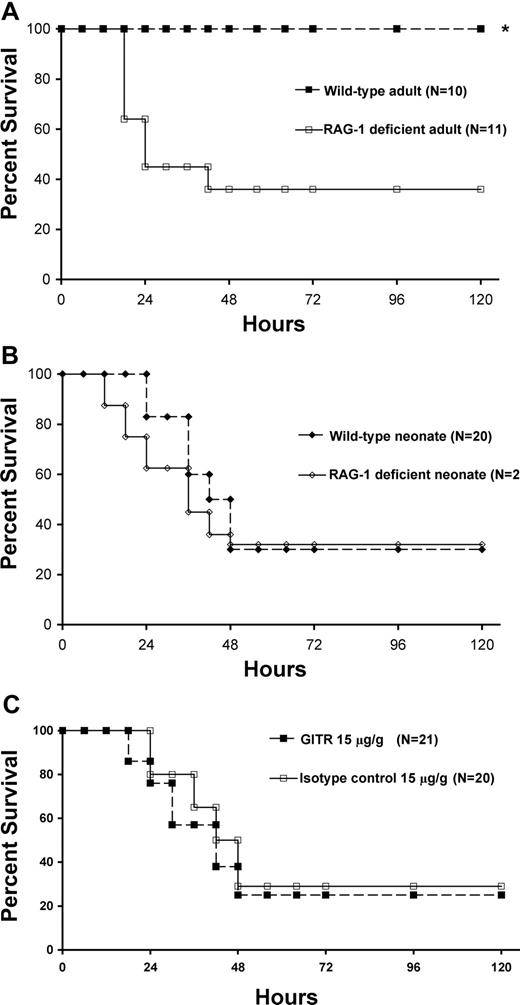
Although there is adaptive immune suppression during the response to sepsis, particularly in CD4+ T cells, the adaptive immune system undoubtedly participates in host immunity to sepsis in adult animals.11,12,28,29 To determine the contribution of the adaptive immune system in neonatal sepsis, we studied RAG-1–deficient neonates, which do not develop functional B or T cells. We anticipated that there would be a significant increase in mortality in RAG-1–deficient adults versus wild-type animals, and therefore adjusted the dose of cecal slurry in the adult animals accordingly to produce minimal mortality in the wild-type mice, and to allow any potential survival differences in the RAG-1–deficient adults to be recognized. When the studies were performed in adult animals, RAG-1–deficient mice had a markedly worsened outcome (Figure 2A). In striking contrast, the absence of adaptive immunity in RAG-1–deficient neonates did not worsen or attenuate sepsis mortality compared with wild-type mice given an LD70 dose (Figure 2B) or an LD10 dose (data not shown), suggesting that in neonates, the increased susceptibility to sepsis that we and others have seen is not exacerbated by the absence of cells of the adaptive immune system. Since the neonatal adaptive immune system functions distinctly from that of adults, we determined whether activating the adaptive immune system could protect neonates from sepsis mortality. We previously showed that adult mice pretreated with an anti-GITR antibody had improved outcome to polymicrobial sepsis that was dependent on CD4+ effector cells.13 When neonates were pretreated with identical weight-based doses of anti-GITR antibody, they did not exhibit either improved survival in comparison with an isotype control antibody (Figure 2C) or improved measures of adaptive immune function, as no increase in antigen-specific (NP) IgG1, IgG2a, IgG3, or IgM production occurred over isotype control–treated animals (data not shown). In order to verify that the difference in sepsis models between our present study (cecal slurry) and our previous study (cecal ligation and puncture [CLP]) did not affect the benefit seen with anti-GITR treatment in adults, we repeated the study in young adult female mice using cecal slurry as the mode of sepsis after anti-GITR treatment and found a similar survival benefit (approximately 25% over isotype control; data not shown). We found that neonatal splenic CD4+ T cells express similar levels of GITR compared with adult CD4+ T cells (data not shown). In the neonatal spleen, where 6% to 8% of the total splenic CD4+ T cells are CD4+CD25high, more than 90% of these cells are GITRhigh cells (data not shown).
Adaptive immunity does not contribute to neonatal sepsis survival. Kaplan-Meier sepsis survival curves. (A) Sepsis survival in C57BL/6 and RAG-1–deficient adult mice following administration of cecal slurry (1 mg/g LD10). Adult sepsis survival in RAG-1–null mice (■) was significantly less (36%; P < .05 by Fisher exact test) than C57BL/6 wild-type adults (□; 100%). (B) Sepsis survival in C57BL/6 and RAG-1–deficient neonatal mice following administration of cecal slurry (1.3 mg/g LD70). Neonatal sepsis survival was not statistically different in C57BL/6 wild-type (♦) or RAG-1–deficient (◇) mice. (C) Sepsis survival in wild-type C57BL/6 neonates following anti-GITR (■) or isotype control (□) pretreatment followed by administration of cecal slurry (1.3 mg/g LD70). Neonatal sepsis survival was not statistically different in anti-GITR (■) or isotype control (□) pretreatment groups.
Adaptive immunity does not contribute to neonatal sepsis survival. Kaplan-Meier sepsis survival curves. (A) Sepsis survival in C57BL/6 and RAG-1–deficient adult mice following administration of cecal slurry (1 mg/g LD10). Adult sepsis survival in RAG-1–null mice (■) was significantly less (36%; P < .05 by Fisher exact test) than C57BL/6 wild-type adults (□; 100%). (B) Sepsis survival in C57BL/6 and RAG-1–deficient neonatal mice following administration of cecal slurry (1.3 mg/g LD70). Neonatal sepsis survival was not statistically different in C57BL/6 wild-type (♦) or RAG-1–deficient (◇) mice. (C) Sepsis survival in wild-type C57BL/6 neonates following anti-GITR (■) or isotype control (□) pretreatment followed by administration of cecal slurry (1.3 mg/g LD70). Neonatal sepsis survival was not statistically different in anti-GITR (■) or isotype control (□) pretreatment groups.
A second approach to improve adaptive immunity could be the use of caspase inhibitors, which reduce sepsis-associated losses of T cells due to apoptosis and improve survival.11 However, in contrast to adults, neonatal T-cell numbers increase initially following sepsis in both humans30 and in our mouse model,23 which does not support a therapy designed at abrogating T-cell death. Taken together, these data indicate that outcome to polymicrobial sepsis in the murine neonate, in stark contrast to the adult, is not affected by either specific targeting of the adaptive immune system (with anti-GITR pretreatment) or absence of the adaptive immune system.
Activation of innate immunity with select TLR agonists improves sepsis survival, reduces bacteremia, and augments the systemic inflammatory response in neonatal mice
TLR agonists activate innate immunity in vivo,31 and have been shown to improve cellular function of neonatal immune cells. In particular, Levy et al showed that stimulation of human cord blood monocytes with resiquimod, an adjuvant that can activate immune cells through TLR7 and/or TLR8,32,33 resulted in TNF-α production equivalent to levels produced by adult blood monocytes,34 and also increased activation of antigen-presenting cells.35 Resiquimod induces the expression of both interferon (primarily IFN-α) and other cytokines through the activation of monocytes and dendritic cells, as well as promotes a TH1 response in a MyD88-dependent fashion.36-38 Poly I:C is also a strong inducer of type I interferons as well as a TH1 response,39 and signals through TLR3 and the adaptor TRIF (Toll/IL-1 receptor domain adapter inducing interferon β).40 LPS, often used to study cellular function and modulate innate immunity, signals through TLR4 and subsequently through MyD88 and/or TRIF, leading to increased expression of inflammatory mediators. We evaluated the effects of these 3 TLR agonists in neonates because of their diverse downstream signaling pathways.
To determine whether TLR agonist priming may improve host defense against sepsis, neonates were pretreated with the TLR agonist or vehicle control (sham) 24 hours prior to the initiation of sepsis (time point selected as cytokine elevations following TLR agonist administration in preliminary data had returned to baseline) and followed for 5 days after initiation of sepsis.23
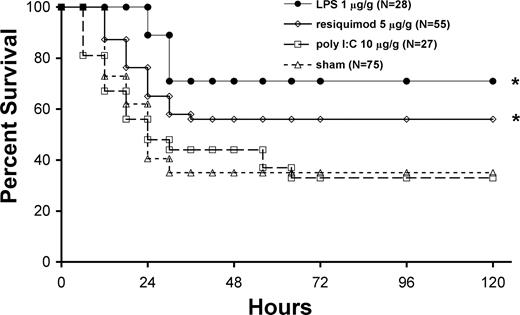
Survival was increased by 21% in neonatal mice pretreated with the TLR7/8 agonist over sham pretreatment (P < .02) and was associated with reduced bacteremia (Figures 3,4). Lower doses (1.5 μg/g BW) did not produce any improvement in survival over sham, and larger doses (15 μg/g BW) did not further improve the survival advantage (data not shown). Pretreatment with TLR4 agonist caused 40% improvement in survival in neonates and dramatically reduced bacteremia (Figures 3,4). The effects of TLR4 agonist on survival were dependent on TLR4, as C3H/HeJ mice (hypomorphic TLR4 mutation) did not experience the survival benefit following LPS pretreatment (data not shown). Furthermore, similar survival benefits were observed when an identical dose of E coli 0111:B4 LPS highly purified by ion exchange chromatography (Sigma-Aldrich) was used (data not shown). Doses of TLR4 agonist were reduced to 10 ng/g BW and were given up to 48 hours prior to sepsis without significant loss of survival benefits, and increasing the dose (10 μg/g BW) did not improve further improve the survival advantage (data not shown). In contrast, the TLR3 agonist had no effect on survival or bacteremia (Figures 3,4). Sepsis survival was significantly worsened (0% survival) with larger doses (30 μg/g BW), and smaller doses (3 and 10 μg/g BW) had no effect on survival over sham-treated septic mice (data not shown). These findings demonstrate that TLR7/8 or TLR4 agonist pretreatment significantly improves both the ability to clear pathogens and neonatal sepsis survival.
Pretreatment with TLR4 (LPS) or TLR7/8 (resiquimod) agonists enhances survival of neonatal mice with polymicrobial sepsis. Kaplan-Meier survival curve for neonates following TLR agonist pretreatment and subsequent administration of an approximate LD70 quantity of cecal slurry (1.3 mg/g LD70). LPS (●), resiquimod ([diao), poly I:C (□), and sham (normal saline; ▵). Survival was significantly (*P < .05 by Fisher exact test) improved in the LPS (61%)– and resiquimod (56%)–treated groups as compared with sham (35%) and poly I:C (33%).
Pretreatment with TLR4 (LPS) or TLR7/8 (resiquimod) agonists enhances survival of neonatal mice with polymicrobial sepsis. Kaplan-Meier survival curve for neonates following TLR agonist pretreatment and subsequent administration of an approximate LD70 quantity of cecal slurry (1.3 mg/g LD70). LPS (●), resiquimod ([diao), poly I:C (□), and sham (normal saline; ▵). Survival was significantly (*P < .05 by Fisher exact test) improved in the LPS (61%)– and resiquimod (56%)–treated groups as compared with sham (35%) and poly I:C (33%).
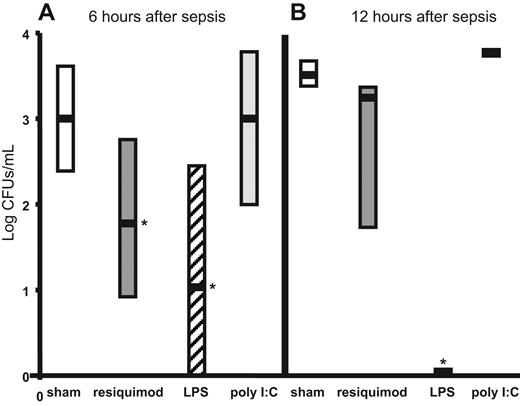
TLR4 (LPS) and TLR7/8 (resiquimod) pretreatment reduces bacteremia during polymicrobial sepsis. Bacteremia (log of colony forming units/mL) in sham (normal saline, □) and TLR agonist–(resiquimod-,  ; LPS-, ▨; poly I:C–,
; LPS-, ▨; poly I:C–,  ) pretreated neonates (n = 7) at 6 and 12 hours after sepsis. Medians, are indicated by ▬ and quartiles (25th and 75th percentiles) by the surrounding rectangles. Statistical significance (*P < .05 by 1-way ANOVA) was present for resiquimod versus sham (normal saline), resiquimod versus poly I:C, LPS versus sham, and LPS versus poly I:C at 6 hours after sepsis as well as LPS versus resiquimod, sham, and poly I:C at 12 hours after sepsis.
) pretreated neonates (n = 7) at 6 and 12 hours after sepsis. Medians, are indicated by ▬ and quartiles (25th and 75th percentiles) by the surrounding rectangles. Statistical significance (*P < .05 by 1-way ANOVA) was present for resiquimod versus sham (normal saline), resiquimod versus poly I:C, LPS versus sham, and LPS versus poly I:C at 6 hours after sepsis as well as LPS versus resiquimod, sham, and poly I:C at 12 hours after sepsis.
TLR4 (LPS) and TLR7/8 (resiquimod) pretreatment reduces bacteremia during polymicrobial sepsis. Bacteremia (log of colony forming units/mL) in sham (normal saline, □) and TLR agonist–(resiquimod-,  ; LPS-, ▨; poly I:C–,
; LPS-, ▨; poly I:C–,  ) pretreated neonates (n = 7) at 6 and 12 hours after sepsis. Medians, are indicated by ▬ and quartiles (25th and 75th percentiles) by the surrounding rectangles. Statistical significance (*P < .05 by 1-way ANOVA) was present for resiquimod versus sham (normal saline), resiquimod versus poly I:C, LPS versus sham, and LPS versus poly I:C at 6 hours after sepsis as well as LPS versus resiquimod, sham, and poly I:C at 12 hours after sepsis.
) pretreated neonates (n = 7) at 6 and 12 hours after sepsis. Medians, are indicated by ▬ and quartiles (25th and 75th percentiles) by the surrounding rectangles. Statistical significance (*P < .05 by 1-way ANOVA) was present for resiquimod versus sham (normal saline), resiquimod versus poly I:C, LPS versus sham, and LPS versus poly I:C at 6 hours after sepsis as well as LPS versus resiquimod, sham, and poly I:C at 12 hours after sepsis.
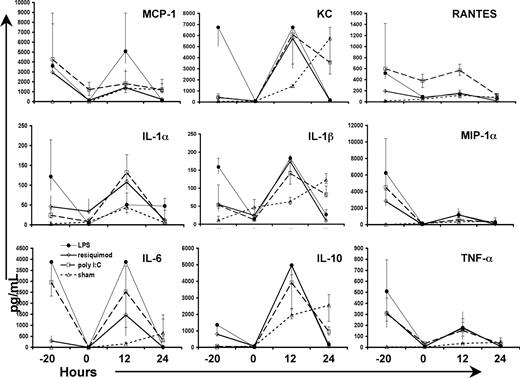
One mechanism by which TLR4 or TLR7/8 agonist pretreatment might enhance neonatal sepsis survival might relate to improving the normally impaired systemic inflammatory response.23 We measured plasma cytokine and chemokine concentrations at various time points after TLR agonist treatment and/or sepsis initiation. TLR agonist administration produced an initial systemic inflammatory response at 4 hours that returned to baseline values by 24 hours (Figure 5). The magnitude of the inflammatory response suggested that the doses used were adequate to elicit systemic inflammation. The plasma cytokine response to sepsis was dramatically affected by the pretreatment with the TLR agonists. TLR7/8 agonist–pretreated animals exhibited elevated levels of IL-1α/β, IL-6, keratinocyte-derived chemokine (KC), IL-10, IL-17 (Figure 5), and MIP-1α over sham at 12 hours after sepsis (P < .05). Levels of IL-1β, IL-6, IL-10, KC, MIP-1α, and MCP-1 (as well as granulocyte-macrophage colony-stimulating factor [GM-CSF], IL-12p40/70, IL-13, IL-17, and IFN-γ; data not shown) were significantly elevated at 12 hours after sepsis in the TLR4 agonist–pretreated group as compared with sham (P < .05). In contrast, by 24 hours, many of the cytokines measured after sepsis were not elevated, but were attenuated in the groups that received TLR7/8 or TLR4 agonist pretreatment. Specifically, IL-6, KC, MCP-1, TNF-α, RANTES, IFN-γ (data not shown), IL-1β, MIP-1α, and IL-10 were significantly reduced at 24 hours as compared with sham (P < .05). The temporal response of the plasma cytokines to polymicrobial sepsis in the animals pretreated with TLR agonists was therefore consistent with an earlier and more robust systemic inflammatory response than that seen in sham mice, and one that was shorter in duration. However, similar changes in the early inflammatory response were also seen with poly I:C pretreatment, which was not associated with an improvement in survival. Thus, it is unlikely that the restoration of a more normal systemic inflammatory response by pretreatment with any of these TLR agonists contributed directly to the selective improvements in outcome.
TLR pretreatment increases and abbreviates the early neonatal inflammatory response following sepsis. Plasma cytokine levels and chemokine levels (pg/mL) at 4 and 24 hours after TLR agonist treatment (time points −20 and 0 hours, respectively) and at 12 and 24 hours after sepsis. LPS (●), resiquimod (◇), poly I:C (□), and sham (normal saline; ▵). The time point “zero” refers to the time sepsis was initiated. Values plotted represent medians, with error bars representing quartiles (75%/25%). Values that reached statistical significance (P < .05 by 1-way ANOVA) were too numerous to represent and are reported in “Results.”
TLR pretreatment increases and abbreviates the early neonatal inflammatory response following sepsis. Plasma cytokine levels and chemokine levels (pg/mL) at 4 and 24 hours after TLR agonist treatment (time points −20 and 0 hours, respectively) and at 12 and 24 hours after sepsis. LPS (●), resiquimod (◇), poly I:C (□), and sham (normal saline; ▵). The time point “zero” refers to the time sepsis was initiated. Values plotted represent medians, with error bars representing quartiles (75%/25%). Values that reached statistical significance (P < .05 by 1-way ANOVA) were too numerous to represent and are reported in “Results.”
TLR7/8 agonist increases phagocytosis, whereas TLR4 agonist increases oxygen free radical production of recruited peritoneal cells in neonatal mice
As neonatal mice demonstrated quantitative and qualitative defects in the cells of the peritoneum, we examined whether the TLR agonist pretreatments could correct this dysfunction by inducing the recruitment of functional phagocytes. Recruitment of GR-1+Ly6ClowCD11bhigh peritoneal PMNs was modestly increased following TLR7/8 agonist treatment as compared with sham (P < .05; Figure 6A). Importantly, phagocytosis by both peritoneal B1 and GR-1+Ly6ClowCD11bhigh peritoneal PMNs was greatly improved following TLR7/8 pretreatment compared with sham, TLR4, or TLR3 pretreatment (P < .05; Figure 6B). TLR7/8 agonist similarly increased phagocytosis in the peritoneal F4/80highCD11bhigh macrophage and GR-1lowLy6ChighCD11bhigh monocyte populations (data not shown). TLR4 agonist induced greater recruitment of GR-1+Ly6ClowCD11bhigh peritoneal PMNs to the peritoneum compared with sham, TLR3, and TLR7/8 agonists (P < .05; Figure 6A), and dramatically increased their production of ROS (P < .05; Figure 6C), but had no effect on their phagocytic activity (P < .05; Figure 6B). TLR4 agonist pretreatment caused no improvement over sham on B1 cell recruitment or phagocytic function (Figure 6A). TLR3 pretreatment was associated with a small increase in GR-1+Ly6ClowCD11bhigh peritoneal PMN ROS production (40% of cells had baseline MFI > 100; average MFI, 985) compared with sham (8%, average MFI, 195) and TLR7/8 agonist (25% average MFI, 587; P < .05; Figure 6C) and had no effect on phagocytic function of PMNs or B1 cells (Figure 6B) or PMN recruitment (Figure 6A). To more accurately define the time course of ROS production from peritoneal PMNs from LPS-primed neonatal mice, we examined peritoneal washes at additional time points. We found that PMN ROS production from LPS-pretreated peritoneal cells was significantly elevated (100% of cells over baseline; MFI > 100; average MFI, 89 905) over cells from sham-pretreated (8% of cells over baseline; average MFI, 195) 2 hours after cecal slurry administration (P < .05; Figure 6D and Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). There were no significant differences in ROS production in cells obtained at 30 minutes or at 6 hours after cecal slurry administration. While recruitment of PMNs remained higher in the LPS-pretreated group compared with sham-pretreatment at 30 minutes and 2 hours after sepsis (Figure 6E), the difference in activation was also present at the 2-hour postsepsis time point.
TLR7/8 agonist (resiquimod) enhances peritoneal B1 and neutrophil recruitment and phagocytosis, whereas TLR4 agonist (LPS) enhances neutrophil recruitment and ROS production. (A) Percentages of neonatal peritoneal neutrophils (PMN) and peritoneal B1 cells from nonseptic sham (normal saline)–pretreated versus poly I:C–pretreated, resiquimod-pretreated, and LPS-pretreated neonatal mice at 24 hours after pretreatment. (B) Percentage (percentage of that cell population that registered greater than the baseline mean fluorescence intensity [expressed <1000 MFI]) of Dil+ peritoneal PMN and B1 cells from sham (normal saline)–pretreated versus poly I:C–pretreated, resiquimod-pretreated, and LPS-pretreated neonatal mice 24 hours after pretreatment and 2 hours after cecal slurry adminstration. (C) Percentage (percentage of PMNs that registered greater than the baseline mean fluorescence intensity [expressed <100 MFI]) of ROS production in PMNs from sham (normal saline)–pretreated versus poly I:C–pretreated, resiquimod-pretreated, and LPS-pretreated neonatal mice 24 hours after pretreatment and 2 hours after cecal slurry administration. (D) Percentage (percentage of PMNs that registered greater than the baseline mean fluorescence intensity [greater than 100 MFI]) of ROS production in PMNs from sham (normal saline)–pretreated versus TLR4(LPS)-pretreated neonatal mice 24 hours after pretreatment and measured 30 minutes, 2 hours, and 6 hours after cecal slurry administration. (E) Time course of recruitment of PMNs following TLR4 (LPS) pretreatment measured at 30 minutes, 2 hours, and 6 hours after cecal slurry as compared with sham pretreatment (normal saline). Data shown are representative of 3 separate experiments. Bar graphs represent means with standard deviation (*P < .05 compared with sham; ‡P < .05 as compared with sham and TLR7/8; †P < .05 as compared with all, by Student t test or 1-way ANOVA). In panels D and E, means are shown, with error bars representing SD.
TLR7/8 agonist (resiquimod) enhances peritoneal B1 and neutrophil recruitment and phagocytosis, whereas TLR4 agonist (LPS) enhances neutrophil recruitment and ROS production. (A) Percentages of neonatal peritoneal neutrophils (PMN) and peritoneal B1 cells from nonseptic sham (normal saline)–pretreated versus poly I:C–pretreated, resiquimod-pretreated, and LPS-pretreated neonatal mice at 24 hours after pretreatment. (B) Percentage (percentage of that cell population that registered greater than the baseline mean fluorescence intensity [expressed <1000 MFI]) of Dil+ peritoneal PMN and B1 cells from sham (normal saline)–pretreated versus poly I:C–pretreated, resiquimod-pretreated, and LPS-pretreated neonatal mice 24 hours after pretreatment and 2 hours after cecal slurry adminstration. (C) Percentage (percentage of PMNs that registered greater than the baseline mean fluorescence intensity [expressed <100 MFI]) of ROS production in PMNs from sham (normal saline)–pretreated versus poly I:C–pretreated, resiquimod-pretreated, and LPS-pretreated neonatal mice 24 hours after pretreatment and 2 hours after cecal slurry administration. (D) Percentage (percentage of PMNs that registered greater than the baseline mean fluorescence intensity [greater than 100 MFI]) of ROS production in PMNs from sham (normal saline)–pretreated versus TLR4(LPS)-pretreated neonatal mice 24 hours after pretreatment and measured 30 minutes, 2 hours, and 6 hours after cecal slurry administration. (E) Time course of recruitment of PMNs following TLR4 (LPS) pretreatment measured at 30 minutes, 2 hours, and 6 hours after cecal slurry as compared with sham pretreatment (normal saline). Data shown are representative of 3 separate experiments. Bar graphs represent means with standard deviation (*P < .05 compared with sham; ‡P < .05 as compared with sham and TLR7/8; †P < .05 as compared with all, by Student t test or 1-way ANOVA). In panels D and E, means are shown, with error bars representing SD.
The protective effects of TLR7/8 and TLR4 agonists in neonatal sepsis are independent of the adaptive immune system or type I IFN signaling
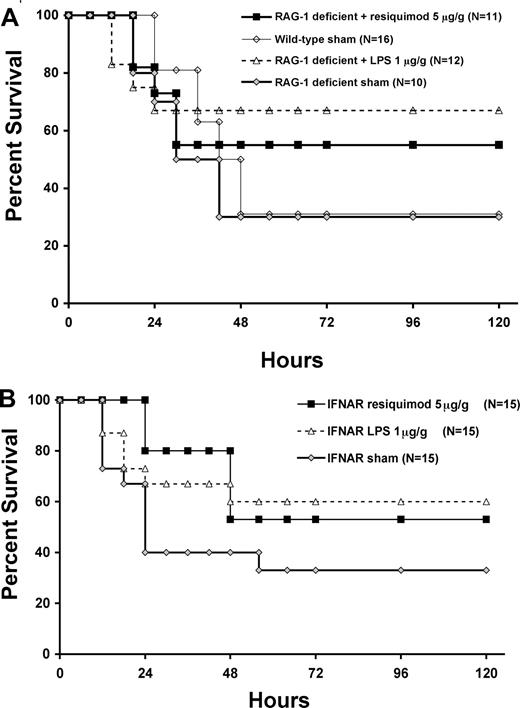
As TLR agonists have been shown to activate the adaptive immune system,41,42 we examined the effects of the TLR7/8 and TLR4 agonists on sepsis survival in RAG-1–deficient neonates to see if the survival benefit was maintained. RAG-1–deficient neonates pretreated with TLR7/8 or TLR4 agonists showed improved sepsis survival similar to that seen in wild-type mice (Figure 7A), supporting the conclusion that the benefits come through innate immunity alone.
TLR7/8 (resiquimod) and TLR4 (LPS) agonist–enhanced survival in neonatal mice with polymicrobial sepsis is independent of the adaptive immune system and type I IFN signaling. Kaplan-Meier survival curves for RAG-1–deficient (A) and IFNAR-null (B) neonates following sham (♦; normal saline) or TLR agonist pretreatment (resiquimod 5 μg/gm BW; ■) or LPS (1 μg/gm BW; ▵) versus wild-type sham (normal saline; ◇) and subsequent administration of cecal slurry (1.3 mg/g LD70).
TLR7/8 (resiquimod) and TLR4 (LPS) agonist–enhanced survival in neonatal mice with polymicrobial sepsis is independent of the adaptive immune system and type I IFN signaling. Kaplan-Meier survival curves for RAG-1–deficient (A) and IFNAR-null (B) neonates following sham (♦; normal saline) or TLR agonist pretreatment (resiquimod 5 μg/gm BW; ■) or LPS (1 μg/gm BW; ▵) versus wild-type sham (normal saline; ◇) and subsequent administration of cecal slurry (1.3 mg/g LD70).
Since type I IFN induction by resiquimod is an important mechanism of induction of antimicrobial immunity,38 and resiquimod is a more potent inducer of type I IFN than LPS,43 we examined whether type I IFN signaling was required for TLR7/8 agonist-mediated improvements in phagocytosis and sepsis survival. In fact, both the improved survival seen in wild-type mice (Figure 3) and the induction of phagocytosis in response to TLR7/8 agonist (Figure 6B) were both preserved in the IFNα/β receptor (IFNAR)–deficient mice (data not shown; Figure 7B), suggesting that the beneficial effects were not dependent on type I IFN signaling. TLR4 agonist–pretreated IFNAR-deficient neonates also maintained the enhanced recruitment of peritoneal PMNs and production of oxygen-free radicals (data not shown) as well as the improvement in sepsis survival over sham as seen in wild-type mice (Figure 7B).
Discussion
Despite considerable advances in our understanding of the adult response to sepsis, the neonatal immune system's responsiveness to polymicrobial sepsis is just now being elucidated. The neonate's capabilities differ in a number of important ways, as both the innate and adaptive immune systems are functionally impaired compared with the adult.19,20 These functional impairments put the neonate at increased risk for developing and succumbing to a serious infection. The focus of this report was to determine those facets of the neonatal immune system essential for protection against severe infection that can be successfully targeted to improve resistance to sepsis. We confirmed that neonates exhibit specific immunologic limitations in vivo that reduce their ability to clear pathogens, including a decreased inflammatory response, decreased percentages of adaptive immune cells including splenic marginal zone and follicular B cells, and the lack of physiologic barriers such as functional marginal zones that trap bacteria. We observed that the percentages of peritoneal B1 cells, the main phagocytic cell in the adult peritoneal cavity that recognizes self- and common bacterial antigens, and secretes antibodies,20 are dramatically reduced in resting neonates, and the few cells present in the neonatal peritoneum are largely devoid of phagocytic function and the ability to generate oxygen-free radicals. These deficiencies most likely participate in the inability of neonates to control a local peritoneal infection, such as that which develops in necrotizing enterocolitis or intestinal perforation,44 and may lead to decompensation due to rapid systemic bacterial proliferation.
RAG-1–deficient murine neonates do not exhibit an increased or decreased susceptibility to sepsis compared with wild-type mice. These data suggest that the neonatal adaptive immune system does not participate significantly in the clearance of severe microbial infections in the peritoneum. Since the adaptive immune system does not function optimally in neonates, and adaptive immunomodulatory therapies such as anti-GITR treatment can improve outcome in adult mice,13 we subsequently determined whether anti-GITR pretreatment could improve murine neonatal sepsis outcome. We found that anti-GITR treatment did not alter neonatal sepsis survival and did not result in an improved adaptive immune response (data not shown). These results showing no effect following elimination of the adaptive immune system are contrary to recently published data showing that CD5+ B cells, a component of the neonatal adaptive immune system, are important in preventing lethal shock responses to CpG DNA in neonates through an anti-inflammatory pathway involving type I IFN and IL-10.45 However, their model did not involve an infectious pathogen, whereas we used a model of severe polymicrobial sepsis with clinical relevance. Furthermore, since neonates exhibit an attenuated inflammatory response and increased sepsis mortality,23 these cells may be contributing to the poor bacterial clearance and increased mortality induced by the infection.
Priming with TLR agonists improves outcome in adult infection and sepsis models.9,10 Recently, TLR9 agonist therapy with CpG DNA has been evaluated in neonatal animals with experimental infection with either lethal neurotropic arenavirus46 or Listeria monocytogenes,47 resulting in reduced microbial burden and improved survival in both models. The TLR7/8 agonist resiquimod has not been evaluated in any sepsis model, and no TLR agonist has been previously evaluated in vivo in neonatal mice with polymicrobial sepsis.
The beneficial survival effects of TLR4 and TLR7/8 stimulation on neonatal sepsis were independent of type I IFN signaling. Type I interferons are important for host defense following TLR stimulation, and help prime naive cells for subsequent infection. Poly I:C is a strong inducer of type I IFN, and both LPS and resiquimod stimulate innate immunity through either NF-κB–dependent signaling or through type I IFN.40 It is therefore important to decipher the contribution of each pathway to the observed increases in peritoneal function. In this study, the TRIF-dependent TLR3 agonist poly I:C had no effect on neonatal sepsis survival or bacteremia. The alterations in cellular function following TLR7/8 or TLR4 pretreatment associated with increased sepsis survival were decreased or absent in the TLR3 pretreated mice and may help to explain why TLR3 pretreatment did not improve survival (Figure 6). The lack of positive effects following TLR3 agonist pretreatment could potentially be attributed to a lack of TLR3 expression on the cellular population responsible for the survival improvements. However, TLR3 expression has been documented in multiple murine innate cell types, including macrophages and PMNs,48 which were the cell types that exhibited increased recruitment and functional improvements following TLR4 or TLR7/8 pretreatment. Furthermore, IFNAR-deficient mice that received TLR7/8 agonist pretreatment still demonstrated peritoneal recruitment of PMNs with increased phagocytic activity and protection from sepsis mortality. Taken together, these data suggest that the beneficial effects of TLR4 and TLR7/8 agonist pretreatment were likely through a MyD88-dependent response, not involving TRIF or type I IFN signaling. These results argue against an endotoxin tolerance–like phenomenon, as endotoxin tolerance to the lipid A component of endotoxin has recently been shown to involve down-regulation of MyD88-dependent gene expression, but induction of a TRIF- and IFNα/β-dependent pathway.49 In contrast, we saw an increase in MyD88-dependent cytokines after sepsis following TLR4 or TLR7/8 agonist pretreatment. Our in vivo findings of TLR7/8 agonist pretreatment increasing peritoneal cell phagocytosis correlate with the report by Doyle et al, where in vitro TLR7/8 agonist treatment of RAW 264.7 macrophages was shown to increase phagocytosis via a MyD88-IRAK4-p38–dependent pathway.50 Foster et al showed that the TLR4-mediated responses, increased inflammation and antimicrobial effectors, are differentially regulated, suggesting that the potential for targeting only the beneficial effects of improved innate cellular responses following TLR stimulation is present.51 Further studies examining the effects of TLR pretreatment on murine neonatal sepsis survival in TRIF- and MyD88-null mice may further elucidate the mechanisms of benefit and are currently under way in our laboratory.
In summary, we observed that murine neonates have reduced percentages and decreased phagocytic and oxidative activity in resident peritoneal cells compared with adults. We show for the first time that unlike adults, the absence of B and T cells or specific adaptive immunotherapy to stimulate T-cell function does not affect neonatal sepsis survival. In addition, we describe here for the first time that enhancing innate immunity improves neonatal sepsis survival through improved clearance of bacteria. The TLR7/8 agonist (resiquimod) induced an increased but more transient early inflammatory response, and improved peritoneal phagocytic function, bacterial clearance, and survival to a subsequent septic challenge in a type I IFN–independent manner. The TLR4 agonist (LPS) also increased and abbreviated the early inflammatory response, substantially increased recruitment of peritoneal GR-1+Ly6ClowCD11bhigh PMNs, and enhanced their production of ROS, resulting in decreased bacteremia and improved sepsis survival. Sepsis survival benefits following TLR agonist–mediated improvement of innate immunity were recapitulated in RAG-1–deficient and IFNAR-null neonatal mice, demonstrating that the benefits were independent of B- and T-cell involvement and type I interferon signaling. The effects of pretreating newborns with innate immune adjuvants based on the risk and severity of sepsis morbidity and mortality warrants further evaluation in animal models. These findings are an important step toward improving human neonatal sepsis outcomes through directed therapy at neonatal-specific immune deficits.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by grants from the Children's Miracle Network (to J.L.W.) and the National Institute of General Medical Sciences (GM-40586 to L.L.M.). M.J.D. is supported by a National Institute of General Medical Sciences training grant in burns, trauma, and inflammation (T32 GM-08721-07); and R.D.W. is supported by a National Cancer Institute training grant in Surgical Oncology (T32 CA-106493-03).
National Institutes of Health
Authorship
Contribution: J.L.W. and P.O.S. created the concept, designed the experiments, performed data collection and analysis, and authored the paper; R.D.W., M.J.D., K.K.-S., and R.U. assisted with data collection; T.B. contributed reagents; O.L. provided intellectual input and reviewed paper; and L.L.M. was the project manager and senior advisor.
Conflict-of-interest disclosure: The authors declare no com-peting financial interests.
Correspondence: Lyle L. Moldawer, Department of Surgery, University of Florida College of Medicine, Rm 6116, Shands Hospital, P.O. Box 100286, Gainesville, FL 32610-0286; e-mail: moldawer@surgery.ufl.edu.


![Figure 6. TLR7/8 agonist (resiquimod) enhances peritoneal B1 and neutrophil recruitment and phagocytosis, whereas TLR4 agonist (LPS) enhances neutrophil recruitment and ROS production. (A) Percentages of neonatal peritoneal neutrophils (PMN) and peritoneal B1 cells from nonseptic sham (normal saline)–pretreated versus poly I:C–pretreated, resiquimod-pretreated, and LPS-pretreated neonatal mice at 24 hours after pretreatment. (B) Percentage (percentage of that cell population that registered greater than the baseline mean fluorescence intensity [expressed <1000 MFI]) of Dil+ peritoneal PMN and B1 cells from sham (normal saline)–pretreated versus poly I:C–pretreated, resiquimod-pretreated, and LPS-pretreated neonatal mice 24 hours after pretreatment and 2 hours after cecal slurry adminstration. (C) Percentage (percentage of PMNs that registered greater than the baseline mean fluorescence intensity [expressed <100 MFI]) of ROS production in PMNs from sham (normal saline)–pretreated versus poly I:C–pretreated, resiquimod-pretreated, and LPS-pretreated neonatal mice 24 hours after pretreatment and 2 hours after cecal slurry administration. (D) Percentage (percentage of PMNs that registered greater than the baseline mean fluorescence intensity [greater than 100 MFI]) of ROS production in PMNs from sham (normal saline)–pretreated versus TLR4(LPS)-pretreated neonatal mice 24 hours after pretreatment and measured 30 minutes, 2 hours, and 6 hours after cecal slurry administration. (E) Time course of recruitment of PMNs following TLR4 (LPS) pretreatment measured at 30 minutes, 2 hours, and 6 hours after cecal slurry as compared with sham pretreatment (normal saline). Data shown are representative of 3 separate experiments. Bar graphs represent means with standard deviation (*P < .05 compared with sham; ‡P < .05 as compared with sham and TLR7/8; †P < .05 as compared with all, by Student t test or 1-way ANOVA). In panels D and E, means are shown, with error bars representing SD.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/112/5/10.1182_blood-2008-01-130500/5/m_zh80180824130006.jpeg?Expires=1767709725&Signature=sEuVIuzm6bGXGMNbQNgrwj22AKMBTigRX6kyZ6rqOfaqy~jY64SYsTxYPWX0Xy6HuQ0cK7SQPYQ5kP4pWLWaU7EYgclgbM0szRP~GIP5rHUMcLjPYTfC0Xdl8O2ms1zani5RAWuhtdNM9mHCPWdS5zg-ziWp2HkLEF5rvlUMlsgevxydI2fGfGW0nX3nWw8vZRAoqE4Q8Q0nzDB5B6ZLfLfvJgMpBU~EnutEV7f5SVK55r8UqS2k2PPoywAJh~zgi6-XF-~606tIk9xW2qnoAJUrSMdIN9n5ol605mkmvOS22HAhv3dWmWrATgj3njx-DcF~ebMoWIwAm-apuO~hwQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal