Abstract
Although interaction between programmed death-1 (PD-1) and the ligand PD-L1 has been shown to mediate CD8 cell exhaustion in the setting of chronic infection or the absence of CD4 help, a role for this pathway in attenuating early alloreactive CD8 cell responses has not been identified. We demonstrate that the PD-1/PD-L1 pathway is needed to rapidly tolerize alloreactive CD8 cells in a model that requires CD4 cells and culminates in CD8 cell deletion. This protocol involves allogeneic bone marrow transplantation (BMT) following conditioning with low-dose total body irradiation and anti-CD154 antibody. Tolerized donor-reactive T-cell receptor transgenic CD8 cells are shown to be in an abortive activation state prior to their deletion, showing early and prolonged expression of activation markers (compared with rejecting CD8 cells) while being functionally silenced by day 4 after transplantation. Although both tolerized and rejecting alloreactive CD8 cells up-regulate PD-1, CD8 cell tolerance is dependent on the PD-1/PD-L1 pathway. In contrast, CD4 cells are tolerized independently of this pathway following BMT with anti-CD154. These studies demonstrate a dichotomy between the requirements for CD4 and CD8 tolerance and identify a role for PD-1 in the rapid tolerization of an alloreactive T-cell population via a deletional mechanism.
Introduction
The balance between stimulatory and inhibitory signals following T-cell receptor (TCR) engagement critically regulates the outcome of the immune response and can lead to T-cell activation or tolerance. In the past decade, costimulation blockade and activation of inhibitory pathways have been investigated as approaches to controlling T-cell reactivity in autoimmunity and transplantation. CD8 T cells have been shown to be more resistant to costimulation blockade than CD4 T cells.1 Indeed, in a model of allogeneic bone marrow transplantation (BMT), a conditioning regimen involving anti-CD154 and 3 Gy total body irradiation (TBI) on day 0 reliably led to tolerance of donor-reactive CD4 T cells but not CD8 T cells.2 Moving the TBI to day −1 more reliably allowed tolerance induction of donor-reactive CD8 cells in addition to CD4 cells.3 This CD8 T-cell tolerance is dependent on CD4 cells and is associated with specific deletion of donor-reactive CD8 cells within about 10 days after transplantation.4 Thus, the pathways involved in CD4 and CD8 tolerance with BMT, low-dose TBI, and anti-CD154 are linked but different. Whereas blocking CD154 rapidly tolerizes alloreactive CD4 T cells by inducing anergy, which is followed by gradual deletion by a non–Fas-dependent mechanism,5-7 the pathways involved in CD8 tolerance prior to their more rapid deletion have not been identified.
The programmed cell death-1 (PD-1, CD279) receptor is up-regulated after activation on T or B lymphocytes and inhibits T-cell intracellular signaling, proliferation, and cytokine production.8 It has also been shown to play an important role in CD8 T-cell exhaustion in infectious disease.9-11 PD-1 has 2 ligands, PD ligand-1 (PD-L1; B7-H1; CD274) and PD-L2 (B7-DC; CD273). PD-L1 is expressed on hematopoietic cells and can be up-regulated when they are activated. In addition, PD-L1 is expressed on nonhematopoietic cells (placenta, small intestine, endothelium, heart, and pancreatic islets).8 PD-L2 expression is inducible on dendritic cells (DCs), macrophages, and cultured bone marrow–derived mast cells.12
PD-1–deficient mice of some strains develop autoimmune diseases with increasing age, indicating a role for this pathway in the control of autoreactive cells.13 Blockade of the PD-1 inhibitory pathway while the TCR is engaged leads to an increase in autoreactive CD4 and CD8 effector T-cell functions.14,15 Activation of the PD-1 inhibitory pathway with a PD-L1.Ig molecule was shown to exert synergistic activity with anti-CD154 to prolong graft survival in transplantation models. However, tolerance was not induced.16,17 In this study, we investigated the mechanism involved in the tolerization of alloreactive CD8 T cells after BMT with nonmyeloablative, anti-CD154–based conditioning. We demonstrate that donor-specific alloreactive CD8 T cells have an activated phenotype, but are functionally silenced as early as day 4 after BMT. Whereas both tolerized and activated alloreactive CD8 T cells express high levels of PD-1, CD8 but not CD4 T-cell tolerance is dependent on the PD-1/PD-L1 pathway after BMT with anti-CD154. Together with previous results,4,18 data presented here suggest a model wherein interactions of recipient CD4 cells with recipient B cells and DCs presenting alloantigen under cover of anti-CD154 up-regulate APC PD-L1 expression, which then provides the requisite ligand to PD-1 on CD8 cells, promoting their tolerance.
Methods
Animals
All studies were performed under an institutionally approved animal protocol in accordance with guidelines from the National Institutes of Health (NIH, Bethesda, MD). Female C57BL/6 (H-2b), B10.A (H-2a; Ld+), A.SW (H-2s; Ld−), B10.S (H-2s; Ld−), and B10.RIII (H-2r) mice were purchased from Frederick Cancer Research Center (Frederick, MD) or from The Jackson Laboratory (Bar Harbor, ME). C57BL/6-2C-TCR transgenic (TCR-Tg) (H-2b), C57BL/6-PD-1 KO (H-2b), and C57BL/6-PD-L1 KO mice were bred in our animal facility. All mice were housed in a specific pathogen-free microisolator environment.
Conditioning
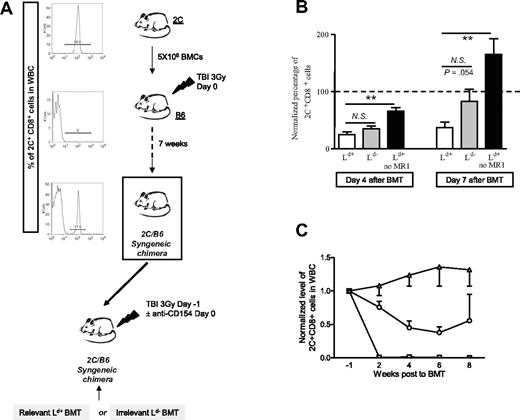
Age-matched (7-14 weeks old) mice received a low dose of TBI (3 Gy) from a 137cesium irradiator on day −1 with respect to BMT. When indicated, anti-CD8 mAb (2.43; 1.44 mg/mouse) was administered intraperitoneally on day −1. Blocking anti–PD-1 mAb (29F1A12, rat IgG2a, 200 μg/mouse), blocking anti–PD-L1 mAb (10F9G2, rat IgG2b, 200 μg/mouse), irrelevant rat IgG2a or rat IgG2b mAbs (200 μg/mouse) (BioExpress, West Lebanon, NH) were administered intraperitoneally on days −1, 2, 5, 8, and 11 with respect to BMT. Antimouse CD154 mAb (MR1; 2 mg/mouse; National Cell Culture Center, Minneapolis, MN) was administered intraperitoneally on day 0 prior to transplantation with 20 to 25 × 106 allogeneic bone marrow cells (BMCs) by tail vein injection. C57BL/6 mice with a traceable donor-reactive transgenic CD8 T-cell (2C TCR-Tg CD8 cells) population were prepared as previously described4 (Figure 1A). These mixed 2C/wild-type C57BL/6 chimeras are referred to as 2C/B6 mice.
Flow cytometric analysis
Multilineage chimerism in white blood cells.
Four-color flow cytometric (FCM) analysis was performed on white blood cells (WBCs) to analyze the development of multilineage chimerism.19 Donor-derived cells were identified in the live cell population (propidium iodide negative) using fluorescein isothiocyanate (FITC)–conjugated anti–H-2Dd mAb 34-2-12. Cells were counterstained with phycoerythrin (PE)–conjugated anti-CD4 (Becton Dickinson [BD]/PharMingen, San Diego, CA) or MAC-1 (Caltag, San Francisco, CA) and with allophycocyanin (APC)–conjugated anti-CD8 or anti-B220 mAb (BD/PharMingen), respectively. Negative control mAbs included HOPC1-FITC (prepared in our laboratory) and rat antimouse IgG2a-PE or -APC. A mouse was considered chimeric when it demonstrated 5% or more of donor cells in all lineages tested.
Activation markers on splenocytes.
2C CD8+ as well as non-2C CD8+ T cells were analyzed by FCM on live splenocytes using an anticlonotypic mAb 1B2 (specific for the 2C TCR20 ) revealed by FITC-conjugated anti–mouse IgG1 mAb and APC-conjugated anti-CD8. PE-conjugated anti-CD69, anti-CD25, anti-CD44, and anti–PD-1 mAbs (BD/PharMingen) were used to detect the surface expression of activation markers. Negative control mAbs included HOPC1-FITC and rat anti–mouse IgG2a-PE or -APC. Ten thousand CD8+ splenocytes were acquired for each analysis.
For the analysis of dendritic cells, spleens were flushed with 1 mL warm collagenase D in RPMI then cut into small pieces and incubated for 30 minutes at 37°C in 6% CO2. The reaction was stopped by adding 10% EDTA. The small pieces of spleen were mashed and washed, and red blood cells (RBCs) were then lysed in ACK. The enriched DC population was stained with 34.2.12 FITC, anti–PD-L1 PE (BD/PharMingen), and anti-CD19 APC (BD/PharMingen) or anti-CD11c APC (BD/PharMingen). Negative control mAbs included HOPC1-FITC and rat antimouse IgG2a-PE or -APC.
Cell-mediated lympholysis assay
Cell-mediated lympholysis (CML) assay was performed as described.2 Briefly, responders and stimulators were cocultured at a 1:1 ratio for 5 days. Cells were then serially diluted and coincubated with 51Cr-labeled ConA blast target cells for 4 hours.
Statistical analysis
Statistical analysis was performed using the Mann-Whitney U test. P values less than .05 were considered to be significant.
Results
Prolonged up-regulation of activation markers upon specific antigen recognition by CD8 T cells tolerized in vivo
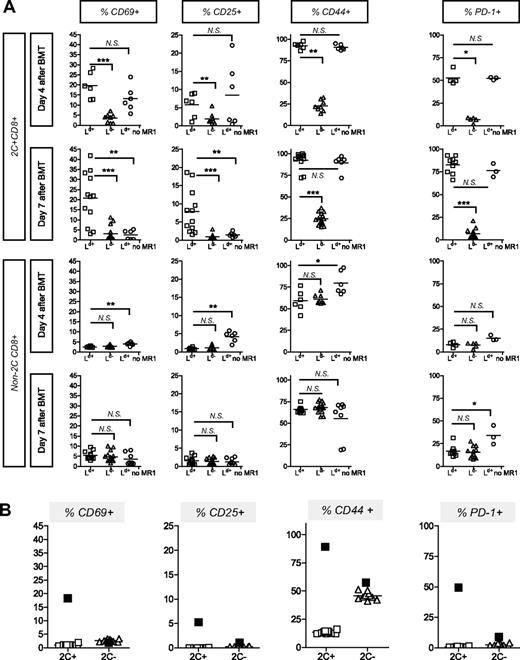
To specifically track donor-reactive CD8+ T cells in mice undergoing allogeneic BMT, we generated mice containing a majority of wild-type cells and a minority of traceable donor-reactive CD8 cells expressing the transgenic 2C TCR, which recognizes the MHC class I molecule Ld (Figure 1A).21 We compared the expression level of activation markers on 2C CD8+ T cells in 2C/B6 mice that received 3 Gy TBI and 5 × 106 2C BMCs 7 weeks prior to conditioning (3 Gy TBI on day −1, 2 mg anti-CD154 intraperitoneally) followed by either Ld+ (B10.A; H-2a) or Ld− (A.SW or B10.S; H-2s) bone marrow (BM) transplant. A 2C/B6 control group received TBI and Ld+ BM transplant without anti-CD154. Such treatment does not permit development of chimerism.2 2C CD8+ cells are rapidly deleted within 2 weeks after BMT in recipients of Ld+ BM transplant with this tolerizing regimen.4 However, 4 and 7 days after allogeneic BMT, the spleen contained a measurable 2C CD8+ population (Figure 1B). The percentage of 2C CD8+ cells was much lower in the chimeric (B10.A BM transplant) than in the rejecting mice at both time points (Figure 1B). By day 7, rejecting animals showed evidence of expansion of 2C cells, whereas tolerant animals showed partial deletion and recipients of irrelevant marrow contained near baseline 2C levels (Figure 1B). By 2 weeks after BMT, tolerant animals showed complete deletion of 2C alloreactive CD8 cells in the WBCs (Figure 1C). Figure 2A shows that 2C CD8+ cells expressed activation markers (ie, CD69, CD25, CD44) only when they were exposed in vivo to relevant Ld+ BM and not when they were exposed to irrelevant Ld− marrow. The levels of these activation markers on 2C and non-2C CD8+ cells were negligible prior to allogeneic BMT, as shown in Figure 2B. Surprisingly, tolerizing treatment with anti-CD154 mAb in mice receiving relevant Ld+ BM did not prevent the activation of donor-reactive 2C CD8+ cells. As shown in Figure 2A, similar levels of CD69, CD25, and CD44 activation markers were detected on 2C CD8+ cells 4 days after Ld+ BMT, regardless of whether the recipient was treated with anti-CD154. However, CD69 and CD25 were no longer detectable 7 days after BMT on 2C CD8+ cells from rejecting animals not treated with anti-CD154, whereas CD44 was still highly expressed. In contrast, high levels of expression of CD69, CD25, and CD44 were maintained 7 days after BMT on tolerized 2C CD8+ cells (Figure 2A). This prolonged up-regulation of activation markers in the tolerized population is associated with the persistence of donor antigen, which disappears in the control mice due to the rejection of donor BMCs (data not shown).
Generation of 2C/B6 syngeneic chimeras. (A) B6 mice received 3 Gy TBI and 5 × 106 syngeneic 2C BMCs. Seven weeks later, the presence of 2C cells among peripheral blood CD8 cells of the recipient mice was analyzed by FCM analysis using anticlonotypic mAb 1B2. Histograms show 1B2 staining on gated CD8+ WBCs. These 2C/B6 syngeneic chimeras then received 3 Gy TBI on day − 1 and allo-BM transplant on day 0. 2C/B6 mice received a transplant of either Ld+ (B10.A) or Ld− (A.SW or B10.S) BM and were injected with anti-CD154 on day 0 or received Ld+ (B10.A) BM without anti-CD154 (MR1). (B) Normalized mean (± SEM) percentage of 2C cells among splenic CD8 cells at 4 and 7 days after allogeneic BMT. A value of 100% was given to the percentage of 2C CD8+ T cells in the blood 1 week prior to allogeneic BMT, and the percentage of 2C CD8+ T cells in the spleen on days 4 and 7 was normalized to this value. Statistical analyses were performed with a Mann-Whitney U test to compare the “Ld+ group” with the “Ld− group” or the “Ld+ group” with the “Ld+ no MR1 group”: ** indicates P < .005; NS, not significant. Two experiments are shown for the day-4 analysis (n = 2-5 animals/group/experiment) and 3 experiments are shown for the day-7 analysis (n = 3-5 animals/group/experiment). (C) Time course of 2C deletion in peripheral WBCs. A value of 1 was given to the percentage of 2C CD8+ T cells in the blood 1 week prior to allogeneic BMT. The percentage of 2C+CD8+ cells among WBC CD8 cells was then analyzed every 2 weeks and normalized to the value before BMT. The mean (± SEM) is presented. □ indicates recipients of Ld+ (B10.A); ○, recipients of Ld− (B10.S); and ▵, recipients of Ld+ (B10.A) BMCs without anti-CD154.
Generation of 2C/B6 syngeneic chimeras. (A) B6 mice received 3 Gy TBI and 5 × 106 syngeneic 2C BMCs. Seven weeks later, the presence of 2C cells among peripheral blood CD8 cells of the recipient mice was analyzed by FCM analysis using anticlonotypic mAb 1B2. Histograms show 1B2 staining on gated CD8+ WBCs. These 2C/B6 syngeneic chimeras then received 3 Gy TBI on day − 1 and allo-BM transplant on day 0. 2C/B6 mice received a transplant of either Ld+ (B10.A) or Ld− (A.SW or B10.S) BM and were injected with anti-CD154 on day 0 or received Ld+ (B10.A) BM without anti-CD154 (MR1). (B) Normalized mean (± SEM) percentage of 2C cells among splenic CD8 cells at 4 and 7 days after allogeneic BMT. A value of 100% was given to the percentage of 2C CD8+ T cells in the blood 1 week prior to allogeneic BMT, and the percentage of 2C CD8+ T cells in the spleen on days 4 and 7 was normalized to this value. Statistical analyses were performed with a Mann-Whitney U test to compare the “Ld+ group” with the “Ld− group” or the “Ld+ group” with the “Ld+ no MR1 group”: ** indicates P < .005; NS, not significant. Two experiments are shown for the day-4 analysis (n = 2-5 animals/group/experiment) and 3 experiments are shown for the day-7 analysis (n = 3-5 animals/group/experiment). (C) Time course of 2C deletion in peripheral WBCs. A value of 1 was given to the percentage of 2C CD8+ T cells in the blood 1 week prior to allogeneic BMT. The percentage of 2C+CD8+ cells among WBC CD8 cells was then analyzed every 2 weeks and normalized to the value before BMT. The mean (± SEM) is presented. □ indicates recipients of Ld+ (B10.A); ○, recipients of Ld− (B10.S); and ▵, recipients of Ld+ (B10.A) BMCs without anti-CD154.
Up-regulation of activation markers on CD8 cells from chimeric and rejecting mice upon specific stimulation in vivo. (A) 2C/B6 mice were prepared 7 weeks before allo-BMT and received 3 Gy TBI on day −1 and allo-BM transplant on day 0. 2C/B6 mice received a transplant of either Ld+ (B10.A; H-2a) or Ld− (A.SW or B10.S; H-2s) BM and were injected with anti-CD154 on day 0 or received Ld+ (B10.A) BM without anti-CD154 (MR1). Activation markers were assessed on days 4 and 7 on 2C CD8+ and non-2C CD8+ splenocytes by FCM. Statistical analyses were performed with a Mann-Whitney U test to compare the “Ld+ group” with the “Ld− group” or the “Ld+ group” with “Ld+ no MR1 group”: * indicates P < .05; **P < .01; ***P < .001; and NS, not significant. Two experiments are shown for the day-4 analysis (n = 2-5 animals/group per experiment) and 3 experiments are shown for the day-7 analysis (n = 3-5 animals/group per experiment). (B) Expression of activation/memory markers on 2C CD8+ and non-2C CD8+ splenocytes by FCM is analyzed in 2C/B6 mice that did not receive conditioning or allogeneic BM transplant. The mean percentage of cells expressing the indicated markers 4 days after Ld+ BMT (with anti-CD154) in another experiment is represented by ■. One experiment is shown with 10 animals.
Up-regulation of activation markers on CD8 cells from chimeric and rejecting mice upon specific stimulation in vivo. (A) 2C/B6 mice were prepared 7 weeks before allo-BMT and received 3 Gy TBI on day −1 and allo-BM transplant on day 0. 2C/B6 mice received a transplant of either Ld+ (B10.A; H-2a) or Ld− (A.SW or B10.S; H-2s) BM and were injected with anti-CD154 on day 0 or received Ld+ (B10.A) BM without anti-CD154 (MR1). Activation markers were assessed on days 4 and 7 on 2C CD8+ and non-2C CD8+ splenocytes by FCM. Statistical analyses were performed with a Mann-Whitney U test to compare the “Ld+ group” with the “Ld− group” or the “Ld+ group” with “Ld+ no MR1 group”: * indicates P < .05; **P < .01; ***P < .001; and NS, not significant. Two experiments are shown for the day-4 analysis (n = 2-5 animals/group per experiment) and 3 experiments are shown for the day-7 analysis (n = 3-5 animals/group per experiment). (B) Expression of activation/memory markers on 2C CD8+ and non-2C CD8+ splenocytes by FCM is analyzed in 2C/B6 mice that did not receive conditioning or allogeneic BM transplant. The mean percentage of cells expressing the indicated markers 4 days after Ld+ BMT (with anti-CD154) in another experiment is represented by ■. One experiment is shown with 10 animals.
The non-2C polyclonal CD8+ population in tolerant animals (ie, recipients of the full conditioning regimen and Ld+ or Ld− BM transplant) did not show any increase in CD69 or CD25 activation markers (Figure 2A). However, polyclonal CD8 T cells (ie, non-2C CD8 cells) in mice receiving BM transplant without anti-CD154 treatment expressed significantly higher levels of CD69, CD25, and CD44 4 days after BMT compared with mice receiving BM transplant with the tolerizing regimen. The increased polyclonal CD8 activation on day 4 in animals receiving BM transplant without anti-CD154 is consistent with the rejection process, which not only involves 2C CD8+ T cells but also polyclonal CD8 cells in the 2C/B6 recipients. Since polyclonal donor-reactive CD8 cells, like the 2C cells (Figure 1B,C), survived and/or expanded in the nontolerant group when they were presumably beginning to be deleted in the tolerant group (like 2C in Figure 1B,C), there is an increase in the total percentage of activated polyclonal CD8 cells in the nontolerant compared with the tolerant group on day 4 (Figure 2A). By 7 days, when animals receiving BM transplant without anti-CD154 mAb had rejected their grafts, polyclonal CD8 cells still expressed high levels of CD44 but had lost the CD69 and CD25 activation markers (Figure 2A).
Up-regulation of PD-1 upon specific antigen recognition in vivo by tolerized and nontolerized CD8 T cells
Inhibitory molecules that control the immune response, including CTLA-4 and PD-1, are up-regulated after lymphocyte activation (see Greenwald et al11 for review). Thus, we investigated the level of PD-1 expression on 2C and non-2C CD8+ T cells 4 and 7 days after BMT. PD-1 was found to be highly expressed on 2C CD8+ cells in mice receiving Ld+ relevant allogeneic marrow, regardless of whether they were treated with anti-CD154 (Figure 2A). PD-1 expression on 2C CD8+ cells was significantly greater when mice received the tolerizing regimen with Ld+ relevant BM than in mice receiving the irrelevant control marrow (Figure 2A), indicating that PD-1 up-regulation is antigen driven. PD-1 expression was significantly greater on non-2C CD8+ cells 7 days after Ld+ BMT without anti-CD154 treatment (ie, in rejecting mice) than in tolerized mice (Figure 2A), consistent with the expansion of activated cells in rejecting mice (Figure 1B,C).
Tolerized CD8 T cells are in an abortive activation state
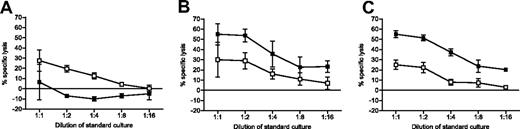
Despite persistently high levels of activation markers on donor-specific CD8 cells and expression of the inhibitory PD-1 receptor, these animals accepted allogeneic BM when treated with anti-CD154. We hypothesized that these CD8 T cells were already tolerized within the first week after BMT. Consistent with this interpretation, Figure 3A shows that restimulated CD8 cells obtained from 2C/B6 mice receiving Ld+ BM with anti-CD154 were unable to kill donor target (B10.A) cells, while maintaining CTL activity against third-party cells 4 days after allogeneic BMT. As shown in Figure 3B, 2C/B6 mice receiving Ld− BM (B10.S) with anti-CD154 were not tolerant to B10.A targets and therefore were able to kill B10.A as well as targets from another allogeneic strain (B10.RIII). Finally, 2C/B6 mice receiving Ld+ BM without anti-CD154 were not tolerant to the B10.A marrow, as they were able to kill donor target (B10.A) cells (Figure 3C). Thus, donor-reactive CD8+ cells from chimeras underwent abortive activation that culminated in effector-CTL tolerance within 4 days of BMT with anti-CD154. Similar results were obtained when splenocytes were analyzed 8 days after BMT (data not shown).
Early tolerance of CD8 T cells. Cytolytic capacity of tolerized donor-reactive CD8 T cells was analyzed with a 51Cr release assay following a 5-day restimulation in vitro. 2C/B6 mice were prepared 7 weeks before allo-BMT and received 3 Gy TBI on day −1 and allo-BM transplant on day 0. 2C/B6 mice received a transplant of either Ld+ (B10.A) (A) or Ld− (A.SW or B10.S) BM (B) and were injected with anti-CD154 on day 0 or received Ld+ (B10.A) BM without anti-CD154 (MR1) (C). Four days after BMT, splenocytes were tested against donor (B10.A) stimulator and target cells (■) and against third-party (B10.RIII) stimulator and target cells (□). One representative experiment of 3 is shown (n = 2-3 animals per group; mean ± SEM shown).
Early tolerance of CD8 T cells. Cytolytic capacity of tolerized donor-reactive CD8 T cells was analyzed with a 51Cr release assay following a 5-day restimulation in vitro. 2C/B6 mice were prepared 7 weeks before allo-BMT and received 3 Gy TBI on day −1 and allo-BM transplant on day 0. 2C/B6 mice received a transplant of either Ld+ (B10.A) (A) or Ld− (A.SW or B10.S) BM (B) and were injected with anti-CD154 on day 0 or received Ld+ (B10.A) BM without anti-CD154 (MR1) (C). Four days after BMT, splenocytes were tested against donor (B10.A) stimulator and target cells (■) and against third-party (B10.RIII) stimulator and target cells (□). One representative experiment of 3 is shown (n = 2-3 animals per group; mean ± SEM shown).
The PD-1/PD-L1 pathway is essential for CD8 but not CD4 tolerance in recipients of allogeneic BM transplant with anti-CD154
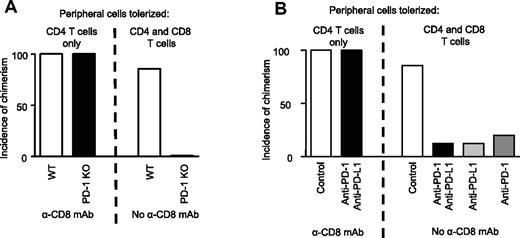
We compared the requirement for the PD-1 pathway in 2 similar models in which the only difference is that only peripheral CD4 (and not CD8) T-cell tolerance is required in one model because CD8 cells are depleted with mAb. Whereas wild-type (WT) control mice successfully achieved multilineage mixed chimerism with or without CD8 depletion,3,4 PD-1 KO mice failed to develop mixed chimerism unless the recipients were depleted of CD8 cells (Figure 4A; Table S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). The failure to achieve even initial engraftment of B10.A marrow in PD-1 KO mice receiving anti-CD154 and 3 Gy TBI while chimerism was achieved in CD8-depleted PD-1 KO mice suggested that PD-1 may be required for the peripheral tolerance of CD8 but not CD4 T cells. To further address the role of the PD-1 pathway, we evaluated marrow engraftment in wild-type recipient mice treated with blocking mAbs targeting PD-1 and PD-L1. Blocking the PD-1 pathway with these mAbs prevented the development of mixed chimerism in 7 of 8 mice that were not depleted of CD8 cells (Figure 4B; Table S2). In contrast, blocking mAbs against the PD-1 pathway did not impair the establishment of multilineage mixed chimerism in mice that were initially depleted of CD8 T cells. When mAbs against either the PD-L1 or the PD-1 molecule were used in recipients of BM transplant, 3 Gy TBI, and anti-CD154, allogeneic BMCs were rejected, whereas mice receiving control mAb showed a high incidence of mixed chimerism (Figure 4B). Therefore, the PD-1/PD-L1 pathway is critical in tolerizing alloreactive CD8 and not CD4 T cells in this model.
Requirement for the PD-1/PD-L1 pathway for CD8 but not CD4 T-cell tolerance. (A) C57BL/6 or PD-1 KO mice (on a C57BL/6 background), CD8 depleted or not, received 20 to 25 × 106 B10.A BM cells with anti-CD154 and 3 Gy TBI. Incidence of chimerism is shown for the B-cell lineage 6 weeks after BMT and is representative of all lineages analyzed. Multilineage chimerism 6 weeks after BMT is shown in Table S1. One representative experiment of 3 total is shown (n = 5-8 animals/group per experiment). (B) B6 mice, CD8 depleted or not, received 20 to 25 × 106 B10.A BM cells with PD-1 and PD-L1 blocking mAbs, anti–PD-L1 alone, anti–PD-1 alone, or control irrelevant IgG2a and/or IgG2b mAbs, anti-CD154, and 3 Gy TBI. Incidence of chimerism is shown for the B-cell lineage 6 weeks after BMT and is representative of all lineages analyzed. Multilineage chimerism 6 weeks after BMT is shown in Table S2. Incidence of chimerism was similar in control group treated with both irrelevant mAbs or with each irrelevant mAb alone. One representative experiment of 2 in total is shown (n = 5-8 animals/group per experiment).
Requirement for the PD-1/PD-L1 pathway for CD8 but not CD4 T-cell tolerance. (A) C57BL/6 or PD-1 KO mice (on a C57BL/6 background), CD8 depleted or not, received 20 to 25 × 106 B10.A BM cells with anti-CD154 and 3 Gy TBI. Incidence of chimerism is shown for the B-cell lineage 6 weeks after BMT and is representative of all lineages analyzed. Multilineage chimerism 6 weeks after BMT is shown in Table S1. One representative experiment of 3 total is shown (n = 5-8 animals/group per experiment). (B) B6 mice, CD8 depleted or not, received 20 to 25 × 106 B10.A BM cells with PD-1 and PD-L1 blocking mAbs, anti–PD-L1 alone, anti–PD-1 alone, or control irrelevant IgG2a and/or IgG2b mAbs, anti-CD154, and 3 Gy TBI. Incidence of chimerism is shown for the B-cell lineage 6 weeks after BMT and is representative of all lineages analyzed. Multilineage chimerism 6 weeks after BMT is shown in Table S2. Incidence of chimerism was similar in control group treated with both irrelevant mAbs or with each irrelevant mAb alone. One representative experiment of 2 in total is shown (n = 5-8 animals/group per experiment).
CD4 depletion impairs up-regulation of PD-L1 on APC
Recipient B cells and dendritic cells (DCs) are needed for CD8 tolerance in this protocol.18 Since CD4 cells and PD-L1 are also involved in CD8 tolerance, we analyzed the effect of CD4 cells on PD-L1 expression on recipient B cells (CD19+) and dendritic cells (CD11c+) in mice that received B10.A BM transplant with our regimen, with or without anti-CD4 treatment. Mice receiving BM transplant without anti-CD154 showed a significant increase in PD-L1 expression on DCs relative to mice receiving anti-CD154. A lesser, statistically insignificant increase was observed on B cells of mice receiving no MR1 (Figure 5A). These data suggest that recipient DCs were activated to a greater extent when anti-CD154 treatment was omitted from the conditioning regimen. On the other hand, when fully conditioned recipients were also CD4 depleted, PD-L1 expression on both DCs and B cells was significantly lower than when CD4 cells were not depleted (Figure 5B). These results are consistent with the observation that PD-L1 on APC is partially independent of CD40 signaling.22 They suggest that, despite blockade of the CD154/CD40L interaction, CD4 T cells still provide some activating signal to recipient APCs, allowing sufficient up-regulation of PD-L1 for interactions with PD-1 on CD8 T cells that promote CD8 tolerance.
Modulation of PD-L1 expression on B cells and dendritic cells. C57BL/6 mice received 3 Gy TBI on day −1 followed by 20 to 25 × 106 B10.A BM cells with or without anti-CD154 (MR1) on day 0 (A); or anti-CD154 with or without CD4 depletion (B). Four days later, dendritic cells (CD11c+) and B cells (CD19+) were extracted from the spleen as described in “Activation markers on splenocytes” and then stained and analyzed by FCM. Dotted line represents a normal control mouse. Statistical analyses were performed with a Mann-Whitney U test. * indicates P < .05; **P < .005; and NS, not significant. One experiment is shown for panel A and one experiment is shown for panel B. Each symbol represents an individual animal.
Modulation of PD-L1 expression on B cells and dendritic cells. C57BL/6 mice received 3 Gy TBI on day −1 followed by 20 to 25 × 106 B10.A BM cells with or without anti-CD154 (MR1) on day 0 (A); or anti-CD154 with or without CD4 depletion (B). Four days later, dendritic cells (CD11c+) and B cells (CD19+) were extracted from the spleen as described in “Activation markers on splenocytes” and then stained and analyzed by FCM. Dotted line represents a normal control mouse. Statistical analyses were performed with a Mann-Whitney U test. * indicates P < .05; **P < .005; and NS, not significant. One experiment is shown for panel A and one experiment is shown for panel B. Each symbol represents an individual animal.
Discussion
Mixed hematopoietic chimerism reflecting CD4 and CD8 T-cell tolerance is achieved when 3 Gy TBI is given on day −1 and anti-CD154 mAb is given with allogeneic BM transplant on day 0.3 We previously showed that initial peripheral CD8 T-cell tolerance is dependent on the presence of CD4 T cells when this conditioning regimen is used.4 In the present study, we report that CD8 but not CD4 T-cell tolerance in this model is dependent on the PD-1/PD-L1 pathway.
CD154-CD40 interactions between helper CD4 T cells and APC license the APC to prime CD8 T cells.23-25 CD8 T cells themselves also express CD154, which may license APC in the absence of CD4 T-cell help.26 Therefore, selective blockade of this interaction with anti-CD154 mAb might be sufficient to block CD4-independent CD8 priming. However, our results show that CD154-CD40 interactions are not absolutely required to prime CD4-independent CD8 T cells, since CD8 cells reject marrow in CD4-depleted mice receiving BM transplant with anti-CD154.4 In thymectomized and partially T cell–depleted mice, treatment with anti-CD154 without BMT did not prevent the activation of alloreactive Tg-CD8 T cells, but this treatment was also insufficient to prevent the rejection of allogeneic heart transplants.1 We now report that: (1) anti-CD154 treatment does not prevent the antigen-driven activation of donor-specific CD8+ T cells; (2) this activation is prolonged and maintained at a higher level 7 days after BMT in tolerized 2C CD8+ T cells compared with nontolerized 2C CD8+ T cells; and (3) despite this activated phenotype, CD8+ T cells do not reject donor BM when the mice receive the tolerizing regimen and in fact are specifically tolerant of the donor within 4 days of BMT. We also demonstrate that no correlation exists between high expression of activation markers and cytolytic activity of the tolerized CD8 T cells, since “activated” CD8 T cells in tolerant mice were not able to kill donor target cells. In contrast to our results, combined treatment with anti–LFA-1 and anti-CD154 in CD4 KO recipients of allogeneic hepatocytes impaired the up-regulation of CD69 on infiltrating CD8 T cells.27
Consistent with a previous study,28 we found that only antigen-driven activation induces the expression of PD-1 on CD8 T cells. Of note, we found similar levels of PD-1 expression by day 4 on tolerized and rejecting allospecific CD8 cells, and this expression persisted at 7 days. Exhausted virus-specific CD8 T cells, which are impaired in their effector function, maintain a high level of PD-1 expression during chronic viral infection, and the PD-1/PD-L1 pathway mediates exhaustion.9-11 Therefore we analyzed the cytolytic function of tolerized CD8 T cells and found that despite early and prolonged expression of activation markers (compared with rejecting CD8 cells), tolerized CD8 T cells were functionally silenced by day 4 after transplantation. Since these cells are fully deleted by 10 days after BMT,4 our data show a role for PD-1 in the rapid tolerization of a CD8 cell population by a mechanism that, in contrast to the slower “exhaustion” process, culminates in rapid deletion.
Our results show, for the first time, a dichotomy between polyclonal alloreactive CD4 and CD8 T cells in their requirement for the PD-1 pathway for tolerance induction with a treatment that tolerizes both subsets. Although PD-1 is known to act on both CD4 and CD8 T-cell populations,8 previous studies assessed only one of these T-cell subsets at a time. Blocking the PD-1 pathway while infusing autoreactive BDC2.5 Tg CD4 T cells to nonobese diabetic (NOD) mice receiving a tolerogenic treatment prevented and reversed tolerance.15 PD-1 blockade accelerated diabetes when OT-1 Tg CD8 T cells were given to ligand-bearing RIP-mOVA recipient mice.14 Treatment with PD-L1.Ig, a fusion protein that stimulates the PD-1 pathway, has been shown to synergize with low doses of cyclosporine A to significantly enhance heart allograft survival in mice.16 However, this study did not identify the relevant T-cell population(s) targeted or the physiologically important ligand of PD-1 that could enhance allograft survival. Human PD-L1.Ig (hPD-L1.Ig) fusion protein has also been shown to synergize with anti-CD154 in achieving long-term islet allograft survival in mice.17 However, the dichotomy of the PD-1 requirement for CD8 but not CD4 tolerance that we have detected in vivo has not been previously described.
Our studies show that blockade of the PD-L1 molecule alone is sufficient to prevent CD8 cell tolerance. Whereas a recent study reports that blocking PD-L2 was predominant in limiting alloreactive CD8+ CD28KO T-cell proliferation,29 blockade of PD-L1 but not PD-L2 abrogated tolerance induced by CTLA-4.Ig treatment in a fully allogeneic heart transplant model and induced expansion of effector CD8 T cells in another study.30 In another model, PD-L1 was shown to be involved in the deletion of activated CD8 T cells specifically in the liver.31 Our data show a critical role for the PD-L1 molecule in tolerizing alloreactive CD8 and not CD4 T cells in vivo, in a model in which CD8 T-cell tolerance paradoxically depends on the presence of CD4 cells.2,4
Our observation that anti-CD154 treatment did not modify the expression of PD-L1 on B cells but decreased the expression of PD-L1 on DCs is consistent with a previous study showing that stimulation with agonistic anti-CD40 mAb slightly enhanced PD-L1 expression on DCs but not on B cells.22 However, our data show that PD-L1 is still up-regulated to some extent on recipient B cells and DCs in mice receiving allogeneic BM transplant with anti-CD154, and that this up-regulation is dependent on the presence of recipient CD4 cells. Given that CD8 T-cell tolerance is dependent on recipient CD4 T cells,4 DCs, and B cells18 in this model, our current data suggest a pathway wherein recipient CD4 cells are required to up-regulate PD-L1 expression on recipient APC, providing the critical PD-L1 interaction with PD-1 on CD8 cells that are thereby tolerized. Studies are in progress to further dissect this pathway.
Thus, we demonstrate the following in recipients of BM transplant with anti-CD154: (1) rapid tolerance induction of donor-reactive cytotoxic CD8 cells; (2) a crucial role for the PD-1/PD-L1 pathway for CD8 allotolerance induction; and (3) a lack of requirement for the PD-1 pathway for CD4 allotolerance. We hypothesize that when CD8 T cells first encounter alloantigen in the absence of CD40-mediated signals to APC, activation of the PD-1 pathway by PD-L1 on the APC impairs the proliferation, cytotoxic differentiation, cytokine production, and survival of allospecific CD8 T cells. These findings are of clinical relevance since alloreactive CD8 T cells have been shown to prevent tolerance induction in primates and humans receiving costimulatory blockade.32 Activating the PD-1 pathway could provide a new strategy to specifically promote tolerance of alloreactive CD8 T cells. The cell population expressing the PD-L1 leading to the tolerization of CD8 T cells is currently under investigation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Drs Gilles Benichou and Nicolas Degauque for critical review of this paper. In addition, we thank Mr Orlando Moreno for outstanding animal husbandry and technical assistance and Ms Kelly Walsh for expert assistance with the paper.
This work was supported by National Institutes of Health (NIH); Bethesda, MD) grant R01 HL49915 (M.S.) and P01AI56299 (G.J.F.). F.H. was supported by a research fellowship of the FRM (Fondation pour la Recherche Medicale, Paris, France) and a research fellowship of the AST/ASBMT (American Society of Transplantation/American Society of Blood and Marrow Transplantation). T.F. was supported by a research fellowship of the Swiss Foundation for Medical and Biological Grants (with support from Novartis Switzerland, Basel, Switzerland) and a research fellowship of the Walter and Gertrud Siegenthaler Foundation (Medical Faculty, University of Zurich, Zurich, Switzerland).
National Institutes of Health
Authorship
Contribution: F.H. designed, performed, analyzed, and interpreted experiments and wrote the paper; T.F. designed, performed, analyzed, and interpreted experiments and contributed to the paper; C.G. designed, performed, analyzed, and interpreted experiments; G.Z. and T. Hogan performed experiments; T. Honjo provided PD-1 KO mice and contributed to the paper; G.J.F. provided the anti–PD-1 and anti–PD-L1 blocking mAb and contributed to the paper; and M.S. designed experiments, interpreted results, wrote the paper, and oversaw all aspects of the work.
Conflict-of-interest disclosure: G.J.F. has patents in the PD-1/PD-1 ligand pathway and receives royalty payments. The remaining authors declare no competing financial interests.
Correspondence: Megan Sykes, Transplantation Biology Research Center, Massachusetts General Hospital, MGH East Bldg 149-5102, 13th Street, Boston, MA 02129; e-mail: megan.sykes@tbrc.mgh.harvard.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal