In this issue of Blood, Higgins and Sloan use clinical data to develop and test mathematical models of human red-cell alloimmunization.
Among the most common risks of red-blood cell (RBC) transfusion is the development of RBC alloantibodies. The incidence of RBC alloimmunization is not insignificant, ranging from 4% to as high as 60% in some patient populations.1 Clinically, RBC alloimmunization can result in delays in patient care, hemolytic transfusion reactions, hemolytic disease of the fetus and newborn, and possibly increased morbidity following organ transplantation.2,3 In addition, RBC alloimmunization has a significant negative impact on laboratory and institutional resources associated with a need for increased laboratory testing difficulties in identification and procurement of compatible blood units for transfusion, obstetrical management, and with the evaluation and management of transfusion reactions. Proposals to minimize RBC alloimmunization through extended antigen matching for all patients are logistically impractical and would add significant costs to the health care system.4
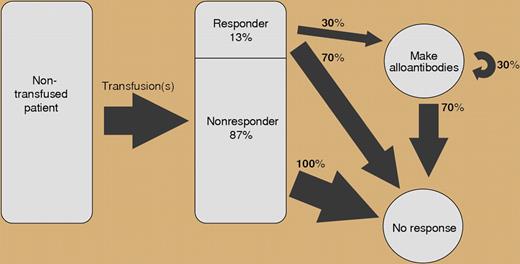
Schematic of the responder hypothesis. See the complete figure in the article beginning on page 2546.
Schematic of the responder hypothesis. See the complete figure in the article beginning on page 2546.
Given the clinical consequences of RBC alloimmunization, several retrospective studies have attempted to elucidate clinical and patient variables to help identify patients at increased risk for RBC alloimmunization. Sickle cell anemia and thalassemias are 2 patient populations with relatively high rates of RBC alloimmunization due, in part, to racial differences in the expression of specific RBC antigens. Patient age, sex, malignancy, diabetes, transplantation, autoimmune disease, and cumulative RBC transfusion history have also been linked to an increased risk of alloimmunization in univariate analysis.5 In murine models, concurrent inflammation appears to be a necessary cofactor for alloantibody formation.6
In this issue of Blood, Higgins and Sloan mined 15 years of patient records encompassing both adult and pediatric populations. Using this data, the authors identified and tested a novel mathematical model for RBC alloimmunization. Overall, the authors identified new RBC alloantibodies in approximately 4% of the general patient population. Contrary to common wisdom, there was only a weak correlation between the number of RBC alloantibodies formed and the total number of RBCs transfused (or transfusion count). Instead, alloimmunized patients appear to represent a distinct population with an inherent increased susceptibility for RBC sensitization. Furthermore, the risk of making additional alloantibodies in these patients is independent of the number of alloantibodies already preformed. Based on their data, the authors proposed a stochastic or “memoryless” model of RBC alloimmunization, in which additional alloantibodies are independently and randomly acquired. The authors calculated that approximately 13% of the general population are antibody responders. For these patients, there is a 30% alloimmunization risk, where the probability of forming n antibodies = (1-0.3)0.3n. The authors were able to confirm the potential validity of their model using their own data and that of others, showing a geometric probability distribution for RBC alloantibodies in both adult and pediatric populations.
The strength of their model is evident when alloimmunized patients were analyzed relative to underlying disease. Regardless of diagnostic subgroup, the same geometric probability distribution was identified—even among diagnostic categories previously identified as a risk factor in earlier studies.5 These results strongly suggest that genetic factor(s), not clinical factors, are primarily responsible for the responder phenotype. This appears to contradict recent murine models, which stress the importance of an inflammatory or danger signal.6 However, as noted by the authors, the 30% alloimmunization risk among responders may reflect the need for an inflammatory signal to stimulate an immune response. Their work should stimulate epidemiologic and genetic studies to define and characterize the responder phenotype. Earlier identification of antibody responders could lead to focused, cost-effective transfusion strategies for avoiding RBC alloimmunization in genetically susceptible patients.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal