Abstract
We recently demonstrated that the 3-kb 5′-flanking region of the human ROBO4 gene directs endothelial cell–specific expression in vitro and in vivo. Moreover, a GA-binding protein (GABP)–binding motif at −119 was necessary for mediating promoter activity in vitro. The goal of the present study was to confirm the functional relevance of the −119 GABP-binding site in vivo. To that end, the Hprt locus of mice was targeted with a Robo4-LacZ transgenic cassette in which the GABP site was mutated. In other studies, the GABP mutation was introduced into the endogenous mouse Robo4 locus in which LacZ was knocked-in. Compared with their respective controls, the mutant promoters displayed a significant reduction in activity in embryoid bodies, embryos, and adult animals. Together, these data provide strong support for the role of the GABP-binding motif in mediating Robo4 expression in the intact endothelium.
Introduction
Roundabout (Robo) is a member of the neural cell adhesion molecule family. A total of 4 members of the Robo have been identified. Of these, Robo4 is expressed primarily in the endothelium,1,2 where it has been shown to play a role in stabilizing the vasculature.3 We recently demonstrated that the 3-kb 5′-flanking region of human ROBO4 contains information for endothelial cell–specific expression in vitro and in vivo.4 In vitro studies revealed the functional importance of an ETS consensus motif at −119 in mediating expression in endothelial cells. Based on electrophoretic mobility shift and chromatin immunoprecipitation assays, this motif was shown to bind to the heterodimeric member of the ETS family, GA-binding protein (GABP). The role for this ETS family member was further evidenced by the ability of siRNA against GABP to down-regulate expression of Robo4 in cultured endothelial cells. In the current study, we employed a combination of Hprt-locus and endogenous Robo4-locus targeting to determine whether the −119 motif regulates Robo4 expression in vivo
Methods
The generation of Hprt-targeted and knock-in mice with and without a mutation of the −119 ETS motif, β-galactosidase activity assays, embryoid body preparations, generation of tumor xenografts and detailed methods for other experiments are described in Document S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article).
The study was approved by Beth Israel Deaconess Medical Center Animal Care and Use Committee.
Results and discussion
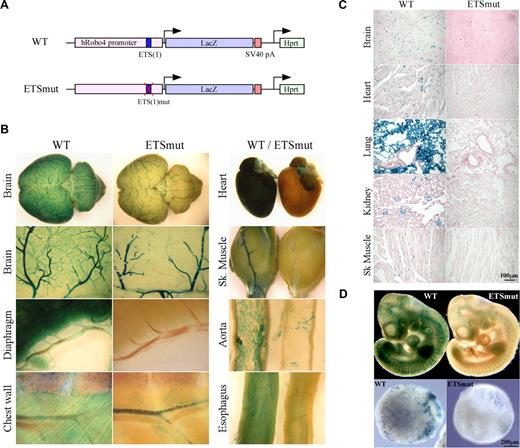
We have demonstrated a critical role for an ETS site at −119 (which we designated −119 ETS(1)) in mediating Robo4 expression in vitro (Okada et al4 and Figure S1). To determine the role of the −119 ETS(1) motif in directing expression of Robo4 in vivo, we generated a Robo4-LacZ transgenic cassette in which the ETS(1) of the human ROBO4 promoter was mutated (Figure 1A). A single copy of the transgenic cassette was targeted to the Hprt locus of mice using homologous recombination, as was previously described for the wild-type ROBO4 promoter.4 High-percentage male chimeras were bred to wild-type females. Resulting female agouti offspring were bred to generate stable lines. Reporter gene activity was assayed in 6- to 8-week-old F2 males. As shown in Figure 1B, whole-mount staining of the brain, diaphragm, chest wall, heart, skeletal muscle, aorta, and esophagus revealed reduced β-galactosidase activity in the vasculature. Tissue sections revealed markedly reduced LacZ staining in the endothelial lining of vessels in all organs examined (Figure 1C shows brain, heart, lung, kidney, and skeletal muscle). In whole mounts of embryonic day–10 (E10) embryos, the mutant promoter demonstrated significantly less LacZ expression compared with wild-type controls (Figure 1D top panel). The Hprt-targeted embryonic stem (ES) cells were used to generate day-10 embryoid bodies (EBs). EBs carrying the wild-type but not the mutant ROBO4 promoter demonstrated a vascular pattern of LacZ staining (Figure 1D bottom panel).
LacZ staining of whole organs from Hprt-Robo4-lacZ mice. (A) Schematic of the wild-type (WT) and ETSmut Hprt-targeted alleles. The 3-kb human ROBO4 promoter with or without the mutation in ETS(1) site, LacZ cDNA and SV40 polyadenylation signal (pA) in the upstream of the Hprt gene are indicated. (B) Organs were harvested from F2 wild-type (WT) or ETSmut Hprt-targeted mice, and processed for whole-mount staining with X-Gal. (C) Sections were prepared from organs of WT or ETSmut mice and stained with X-Gal. A scale bar is indicated in the bottom right panel. (D) Whole-mount E10 embryos (top panel) or day 10 embryoid bodies (bottom panels) from WT and ETSmut Hprt-targeted mice or ES clones were stained with X-Gal, respectively.
LacZ staining of whole organs from Hprt-Robo4-lacZ mice. (A) Schematic of the wild-type (WT) and ETSmut Hprt-targeted alleles. The 3-kb human ROBO4 promoter with or without the mutation in ETS(1) site, LacZ cDNA and SV40 polyadenylation signal (pA) in the upstream of the Hprt gene are indicated. (B) Organs were harvested from F2 wild-type (WT) or ETSmut Hprt-targeted mice, and processed for whole-mount staining with X-Gal. (C) Sections were prepared from organs of WT or ETSmut mice and stained with X-Gal. A scale bar is indicated in the bottom right panel. (D) Whole-mount E10 embryos (top panel) or day 10 embryoid bodies (bottom panels) from WT and ETSmut Hprt-targeted mice or ES clones were stained with X-Gal, respectively.
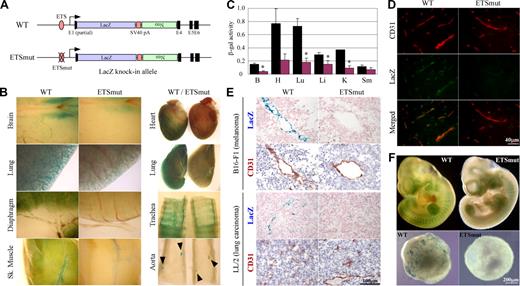
To provide further evidence for the in vivo role of the −119 ETS(1) site, we introduced the same mutation into the endogenous ROBO4 promoter and replaced the Robo4 gene with LacZ (Figure 2A). Reporter gene activity was assayed in 6- to 8-week-old F2 males and compared with that of age- and sex-matched mice in which the LacZ gene was knocked-in to the wild-type Robo4 locus. Expression of LacZ was greater in microvascular endothelium compared with endothelial cells of large arteries or veins. For example, whole-mount LacZ stains of the diaphragm revealed widespread microvascular expression, whereas the X-Gal reaction product in the aorta was limited to branch points (Figure 2B arrowheads). Similar to the results of the Hprt-targeted mice, a mutation of the GABP-binding site in the endogenous locus resulted in reduced expression in the blood vessels of all organs examined (Figure 2B). To quantitate these differences, the tissues were assayed for β-galactosidase activity. As shown in Figure 2C, β-galactosidase activity was significantly reduced in the brain (25% of wild-type), heart (28%), lung (25%), liver (52%), and kidney (24%). In immunofluorescent studies, LacZ expression colocalized with CD31 and was significantly reduced in the GABP mutant (Figure 2D shows the brain). In tumor xenografts, the wild-type promoter drove expression in endothelial cells, an effect that was markedly reduced in mice carrying the mutant promoter (Figure 2E). When corrected for VE-cadherin or CD31 expression, wild-type LacZ expression in tumors was similar to that of the liver, lung, heart, and skeletal muscle (data not shown). In whole mounts of E10 embryos, the mutant promoter demonstrated significantly less LacZ expression compared with wild-type controls (Figure 2F top panel). In keeping with these results, day-10 EBs derived from ES cells targeted with the wild-type but not mutant Robo4 promoter exhibited a vascular pattern of β-galactosidase activity (Figure 2E bottom panel).
Robo4-lacZ knock-in mouse. (A) Schematic of WT or ETSmut LacZ knock-in targeted alleles. The LacZ cDNA, SV40 polyadenylation signal (pA), neomycin resistant gene (Neo), and exons (E1, E4-E6) are indicated. (B) Organs were harvested from F1 wild-type (WT) or ETSmut male heterozygous mice carrying the LacZ gene, and processed for whole-mount staining with X-Gal. (C) β-galactosidase activity of protein extracts from various organs of WT (■) or ETSmut (red bars) knock-in mice. B indicates brain; H, heart; Lu, lung; Li, liver; K, kidney; Sm, skeletal muscle. Data represent mean plus or minus SE of 4 replicates. *P < .05 between organs from WT and ETSmut mice. (D) Serial sections were prepared from organs of WT or ETSmut knock-in mice and stained with CD31 or LacZ antibody. (E) Serial sections were prepared from tumor xenografts prepared in WT or ETSmut mice and stained with X-Gal. (F) Whole-mount E10 embryos (top panel) or day-10 embryoid bodies (bottom panels) from WT and ETSmut knock-in mice or ES clones were stained with X-Gal, respectively.
Robo4-lacZ knock-in mouse. (A) Schematic of WT or ETSmut LacZ knock-in targeted alleles. The LacZ cDNA, SV40 polyadenylation signal (pA), neomycin resistant gene (Neo), and exons (E1, E4-E6) are indicated. (B) Organs were harvested from F1 wild-type (WT) or ETSmut male heterozygous mice carrying the LacZ gene, and processed for whole-mount staining with X-Gal. (C) β-galactosidase activity of protein extracts from various organs of WT (■) or ETSmut (red bars) knock-in mice. B indicates brain; H, heart; Lu, lung; Li, liver; K, kidney; Sm, skeletal muscle. Data represent mean plus or minus SE of 4 replicates. *P < .05 between organs from WT and ETSmut mice. (D) Serial sections were prepared from organs of WT or ETSmut knock-in mice and stained with CD31 or LacZ antibody. (E) Serial sections were prepared from tumor xenografts prepared in WT or ETSmut mice and stained with X-Gal. (F) Whole-mount E10 embryos (top panel) or day-10 embryoid bodies (bottom panels) from WT and ETSmut knock-in mice or ES clones were stained with X-Gal, respectively.
Consensus ETS binding motifs have been identified within the promoters of several endothelial cell–restricted genes, including Flk-1, Flt-1, Tie1, Tie2, and VE-cadherin.5-11 In some cases, the functional relevance of ETS motifs in mediating endothelial cell gene expression in vivo has been demonstrated using standard transgenic mice. For example, ETS mutations of the Tie-1 gene resulted in decreased endothelial cell expression in most organs.7 Mutation of ETS sites in a 4.66-kb fragment of the Mef2C gene disrupted endothelial expression in embryos.12 In other cases, ETS sites have been shown to play a functional role in vitro, but not in transgenic mice.10,11 Such discordance underscores the importance of validating promoter activity in vivo. In standard transgenesis, variation in copy number and integration site may lead to significant line-to-line differences in expression. To control for these variables, we routinely employ a targeting approach in which a single copy of a transgene is inserted to a defined locus (the Hprt locus) via homologous recombination (reviewed in Minami and Aird13 ). Using this approach, we previously demonstrated that mutation of several ETS motifs in the Tie-2 promoter reduced promoter activity in adult endothelium of all organs except the brain.8 Here, we have used the Hprt-targeting strategy to demonstrate an important role for the −119 ETS(1) motif in mediating Robo4 expression in multiple vascular beds.
One drawback of both standard transgenic and Hprt-locus targeting approaches is that a predefined fragment of the promoter is introduced into a foreign genomic locus, where it is subject to the influence of heterologous chromatin. Although the data from these studies provide powerful proof of principle for vascular bed–specific gene regulation, ultimate proof for the involvement of one or another cis-regulatory element (or DNA promoter fragment) in mediating lineage- or cell subtype–specific regulation of a given gene requires selective deletion or mutation of that element from the endogenous locus. To that end, we mutated the −119 ETS(1) motif in the context of the endogenous Robo4 locus. Consistent with the results of the Hprt-targeted mice, the mutant knock-in animals displayed significantly reduced LacZ expression when compared with wild-type controls. To our knowledge, this is the first study to introduce a mutation into an endogenous endothelial cell–specific promoter.
Commercially available antibodies against Robo4 perform poorly in histochemical assays. Thus, we chose to knock-in LacZ as a marker for Robo4 expression. The knock-in strategy involved deletion of Robo4 DNA sequences, including potential regulatory elements in the first 3 introns. It is formally possible that LacZ expression does not precisely reflect expression of the endogenous Robo4 gene. That caveat notwithstanding, our data strongly support the importance of a GABP-binding motif in mediating Robo4 expression in the intact endothelium.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Takahiro Sato for help with statistical analyses.
This work was supported by National Institutes of Health grant HL076540.
National Institutes of Health
Authorship
Contribution: Y.O. and E.J. designed and performed research, analyzed data, and wrote the paper; K.Y., J.L., V.N.-K. designed research and performed research, and analyzed data; D.B., K.S., M.K., N.F., and L.J. designed and performed research; T.D. analyzed data; T.M. designed research and analyzed data; and P.O. and W.C.A. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: William C. Aird, Molecular and Vascular Medicine, Beth Israel Deaconess Medical Center, RW-663, 330 Brookline Avenue, Boston, MA 02215; e-mail: waird@bidmc.harvard.edu.
References
Author notes
*Y.O. and E.J. contributed equally to this work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal