Abstract
Exposure of human monocytic cells to herpes simplex virus type 1 (HSV-1) results in immediate up-regulation of interleukin (IL)–15 gene expression. However, the receptor involved in this induction is not known. Here, we provide evidence that this induction depends on TLR2-mediated signaling pathway. Through the use of small interfering RNAs (siRNAs), we demonstrate that HSV-1–induced up-regulation of IL-15 gene expression in monocytic THP1 cells requires the presence of the adaptors MyD88, IRAK1, and TRAF6. Interestingly, TIRAP/Mal, an adaptor molecule specifically recruited to TLR2 and TLR4, was also required for maximal up-regulation of IL-15. This response was completely abrogated by anti-TLR2, but not anti-TLR4, blocking mAbs in both primary monocytes and THP1 cells. Furthermore, THP1 cells rendered defective in TLR2 expression by disrupting the expression of Sp1, a major transcription factor involved in TLR2 promoter activity, were unable to up-regulate IL-15 gene expression in response to HSV-1. In addition, HSV-1–induced NF-κB activation was significantly reduced after neutralization of TLR2 and the adaptor proteins. Altogether, these results unequivocally show that HSV-1 induces TLR2-dependent activation of IL-15 gene expression, which requires the recruitment of both MyD88 and TIRAP/Mal and the activation of IRAK1 and TRAF6 leading to NF-κB translocation to the nucleus.
Introduction
Herpes simplex virus type 1 (HSV-1) is a ubiquitous human pathogen that is most often acquired in early childhood and establishes lifelong latent infection in the peripheral nervous system. However, HSV-1 infections can periodically be reactivated after environmental or physical stress. Most reactivation events are controlled by the host's innate and adaptive immune system. In the first line of defense against herpesvirus infection, monocytes/macrophages and natural killer (NK) cells play a crucial role in mounting a strong innate immune response.1,2 Part of the macrophage defense mechanisms is mediated by production of cytokines and chemokines, some of which serve to shape and regulate NK cell–mediated responses to a variety of infectious agents. Indeed, increased NK activity after HSV-1 infection has been demonstrated in both in vitro and in vivo studies,3,4 and the molecular mechanism underlying this enhanced NK activity was shown to be related to cytokine production. One such cytokine is interleukin (IL)–15. This cytokine is produced by a variety of cell types; but among the immunocytes, the monocyte/macrophage subpopulation is its main source.5 IL-15 is a pleiotropic cytokine involved in a wide range of biologic activities. It has been shown to be essential for the generation, activation, and proliferation of NK and NKT cells.6,7 Studies in mice with targeted disruption of IL-15,7 IL-15 receptor subunits,8-10 or IL-15 signaling components11,12 have all shown impaired NK-cell production and function. Importantly, recent emerging data suggest that IL-15 may serve as an effective therapeutic target for an array of diseases.13 The role of IL-15 in host defense against viral infections is well documented.3,14-17 The antiviral activity of IL-15 is primarily mediated via the activation of NK and NKT cells.3,15-18 We have earlier shown that different viruses, including HSV-1, as well as bacteria and yeasts, induce robust IL-15 responses, which in turn lead to the enhancement of NK cytolytic activity.19-21 However, the exact molecular mechanism, including the receptor via which HSV-1 induced the up-regulation of IL-15 gene expression, remained to be elucidated
Substantial progress has been made in understanding the molecular mechanisms underlying the recognition and triggering of the initial nonspecific host immune responses to viral infections. In this regard, toll-like receptors (TLRs) have been shown to play an important role in the host's innate immune responses to viral infections through the induction of proinflammatory cytokines, chemokines, and type I interferons by macrophages and dendritic cells.22 Some members of the TLR family, namely, TLR2 and TLR9, have been shown to be critical in the initiation of innate immune responses to HSV-1 infection in the mouse model through the induction of proinflammatory cytokines by macrophages and dendritic cells.23-25 All TLRs share an intracellular signaling motif with the IL-1 receptor, called the TIR (toll-IL-1-R) domain. Recognition of a microbial invasion through the TLRs triggers the activation of signaling pathways, resulting in the recruitment of several adaptor proteins to the TIR domain. The most well known of these adaptors is MyD88 (myeloid differentiation factor 88). It is a key adaptor common to almost all TLRs except TLR3.26 Once recruited to the TLR TIR domain, MyD88 activates in turn IRAK (IL-1 receptor–associated kinases) family members and TRAF6 (tumor necrosis factor-alpha receptor–associated factor 6).27,28 These adaptor proteins are required for signaling the NF-κB/mitogen-activated protein kinase pathways.29-32 The transcription factor NF-κB is a critical regulator of the expression of key proinflammatory cytokines that contribute to the immune and inflammatory responses.33 Because monocytes/macrophages highly express TLR2 molecules and produce high levels of IL-15 after HSV-1 infection, we sought to investigate whether the TLR2 signaling pathway plays a role in the production of IL-15 after HSV-1 infection. To this end, we used the monocytic cell line THP1, which we had found to display a strong IL-15 response after exposure to HSV-1. We thus found that, among all the TLRs expressed on the surface of THP1 cells, TLR2 is exclusively used by HSV-1 to up-regulate IL-15 gene expression; indeed, we show that THP-1 cells defective in TLR2 expression cannot up-regulate IL-15 gene expression in response to HSV-1. Moreover, by knocking down the genes of the key regulatory adaptor proteins involved in TLR2 signaling pathways, we demonstrate that the HSV-1–induced IL-15 expression through TLR2 requires the activation of MyD88- and TIRAP/Mal-dependent pathway leading to the activation of NF-κB.
Methods
Reagents and antibodies
WP631 (Sp1 inhibitor) was purchased from Calbiochem (San Diego, CA). Sp1 rabbit polyclonal antibody (pAb) was purchased from Geneka Biotechnology (Carlsbad, CA). Rabbit pAbs for IRAK1, MyD88 were obtained from ProSci (Poway, CA). TRAF6 rabbit pAb was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). TIRAP rabbit pAb was bought from GeneTex (San Antonio, TX). Goat anti–rabbit IgG HRP-linked antibody was purchased from Cell Signaling Technology (Danvers, MA). Dimethyl sulfoxide (DMSO), mithramycin, and antiβ-actin monoclonal antibodies (mAbs) were purchased from Sigma-Aldrich (Oakville, ON).
Cell culture and viral treatment
The THP1 cell line was maintained in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum, 2 mM l-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin, and 0.25 μg amphotericin B. Primary monocytes were purified from peripheral blood mononuclear cells (PBMCs) obtained from healthy donors by using monocyte enrichment columns (StemCell Technologies, Vancouver, BC) as per the manufacturer's instructions. Because of the ease of obtaining large numbers of cells from THP1 cell lines compared with the difficulty in obtaining many primary monocyte preparations from numerous donors, we opted for the use of THP1 cells for the detailed analysis of molecular pathways. For all experiments, a cell concentration of 106 cells/mL was used. HSV-1 (McIntyre strain) was produced from infected Vero cells, and the viral preparations were titrated by plaque-forming assay on Vero cells as described.34 Noninfectious HSV-1 was obtained by UV treatment as previously described.35 The efficiency of the inactivation procedure was assessed by the inability of the inactivated viral preparations to produce plaque-forming units on Vero cells. Based on results from preliminary experiments to determine optimal viral dose, unless otherwise specified, all the virus treatment tests were performed using UV-inactivated HSV-1 (equivalent to a preinactivation dose of 3 pfu/cell) for 1 hour at 37°C with mixing every 15 minutes. The control consisted of treatment with mock at similar conditions. The cells were then washed with phosphate-buffered saline (PBS) to remove unadsorbed virions, resuspended in culture medium, and incubated for a total of 6 hours after treatment time.
Flow cytometry
Following the different treatments indicated, cells were washed once with cold PBS and then incubated with anti-TLR2 mAb in PBS with 0.2% fetal bovine serum (staining buffer) in the dark at 4°C for 40 minutes. Cells were then washed twice with cold staining buffer and incubated with fluorescein isothiocyanate-conjugated goat anti–mouse secondary Ab (R&D Systems, Minneapolis, MN) for 30 minutes at 4°C. Cells were then washed twice, resuspended in 1% paraformaldehyde solution, and analyzed on a FACScalibur flow cytometer (BD Biosciences, San Jose, CA). An isotype-matched Ab of irrelevant specificity was used as control.
Reverse-transcribed polymerase chain reaction and real-time reverse-transcribed polymerase chain reaction
Total RNA was extracted using Rneasy Mini kit (Qiagen, Mississauga, ON). Reverse-transcribed polymerase chain reaction (RT-PCR) was performed with 0.5 μg of total RNA using the OneStep RT-PCR kit (QIAGEN). The primer pairs used were: IL-15Fwd (5′-GGATTTACCGTGGCTTTGAGTAATGAG-3′) and IL-15Rev (5′-GAATCAATTGCAATCAAGAAGTG-3′)36 and 18S rRNA Fwd (5′-TGCATGTCTAAGTACGCACGGCC-3′) and 18S rRNA Rev 5′-GATAGGGCAGACGTTCGAATGGG-3′ (used as housekeeping gene). The RNA was reverse-transcribed at 50°C for 30 minutes and the reverse transcriptase inactivated at 95°C for 15 minutes, then the PCR was run at the following conditions: 35 cycles of 94°C for 1 minute, 50°C for 1 minute, 72°C for 1 minute, and a final extension step at 72°C for 10 minutes. For quantitative RT-PCR, mRNA was quantified using SYBR Green One-Step qRT-PCR Master Mix kit (Stratagene, La Jolla, CA), and human IL-15 or β-2-microglobulin (housekeeping gene) QuantiTect Primer Assays (Qiagen). Amplifications were run in Mx3000 Thermal Cycler (Stratagene). Thermocycling conditions were 50°C for 30 minutes, 95°C for 10 minutes, then 40 cycles of (95°C/30 seconds; 55°C/1 minute; 72°C/30 seconds). Relative mRNA expression was determined using the ΔCt method. Ct values were normalized relative to Rox (a reference dye). Data were also normalized against the housekeeping gene data (calculated using 2−ΔΔCt). Data are means plus or minus SE.
Gene silencing
Cells were transfected with either the gene-targeted small interfering RNA (siRNAs; 200 nM) or their related controls as indicated via modified calcium phosphate precipitation method for cells in suspension. Briefly, cells were incubated with siRNA-calcium phosphate precipitate for 18 minutes and plated in serum-containing medium without antibiotics for 3 hours. Cells were washed with PBS and allowed to recover for 36 hours in complete medium before the treatment with mock or HSV-1. The following siRNAs were used: Sp1 and MyD88 siRNAs and negative mismatched control siRNAs (Invitrogen, Carlsbad, CA), IRAK1 and TIRAP siRNAs, and the control siRNAs (Ambion, Austin, TX), TRAF6 siRNA (Santa Cruz Biotechnology).
Western blot analysis
For all Western blot analyses, cell pellets were collected 48 hours after transfection after the cells had been subjected to the different indicated treatments. Cells were lysed with cell lysis buffer (1% Triton X-100, 20 mM Tris, pH 7.5, 10% glycerol, 1 mM dithiothreitol, and 10 μL/mL protease inhibitor cocktail; Sigma-Aldrich). An equal amount (20 μg) of protein was analyzed by Western blotting to measure respective protein levels and assess the gene knock-down efficiencies.
Electrophoretic mobility shift assay
THP-1 cells were treated with 200 nM of the pharmacologic inhibitors (WP631 or mithramycin) or transfected with control siRNAs or gene-specific siRNAs. Then, cells were exposed to mock or HSV-1 for 1 hour. Nuclear cell proteins were isolated 1 hour after treatment by NucBuster Protein Extraction kit (Novagen, Madison, WI) and subjected to electromobility shift assay (EMSA) as described previously.35 In brief, nuclear cell extracts (7-10 μg) were incubated with γ-[32P]ATP end-labeled consensus NF-κB oligonucleotide (5′-AGT TGAGGGGACTTTCCCAGG-3′; Promega, Madison, WI) in the presence of unlabeled NF-κB consensus oligonucleotide (competitor) or unlabeled IRF3 consensus oligonucleotide (noncompetitor). DNA-protein complexes were resolved on 4% nondenatured polyacrylamide gel, and NF-κB binding was visualized by autoradiography.
Statistical analysis
Statistical analysis was performed using a one-way analysis of variance followed by the Newman-Keuls multiple comparison test. The results were expressed as mean plus or minus SE. All values were derived from the measurements of 3 independently performed experiments. A P value less than .05 was considered statistically significant.
Results
MyD88, IRAK1, and TRAF6 are involved in HSV-1–induced IL-15 up-regulation in THP1 cells
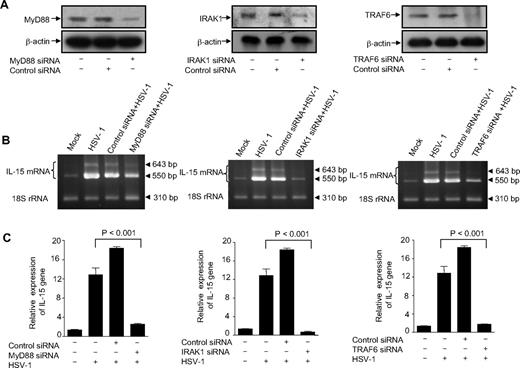
We found earlier that viral transcription and replication were not required for virus-mediated induction of IL-15 production in human monocytic cells and that only viral interaction with a yet unknown cellular receptor on the target cells was responsible for inducing this effect. Indeed, UV-inactivated virus was as efficient as the infectious virus in up-regulating IL-15 gene expression.35 Thus, in an attempt to identify the receptor probably involved in this event, we carried out this study using UV-inactivated HSV-1. To determine whether HSV-1–induced IL-15 production is mediated via toll-dependent mechanisms, we used siRNAs to knock down the expression of the adaptor molecules MyD88, IRAK-1, and TRAF6. THP1 cells were transiently transfected with either a pool of unrelated siRNA oligonucleotides (control siRNAs) or gene-specific siRNAs. Immunoblotting analysis revealed the effective suppression of constitutive MyD88, IRAK1, and TRAF6 protein expression in THP1 cells transfected with specific gene-targeted siRNA (Figure 1A). In contrast, nonspecific control siRNA did not inhibit the protein expression of these adaptor molecules (Figure 1A). These results demonstrated the effectiveness of the knockdown assays. On exposure of cells to HSV-1, there was a remarkable up-regulation of IL-15 gene expression as determined by both RT-PCR and real-time RT-PCR (Figure 1B,C). The inducing effect of HSV-1 was completely abrogated in cells transfected with MyD88-specific siRNA (Figure 1B,C). These results demonstrate the involvement of the MyD88-dependent TLR signaling in the up-regulation of IL-15 gene expression on exposure of monocytes to HSV-1. To further determine the components of the signaling pathway downstream of MyD88, we achieved a functional knockdown of IRAK1 and TRAF6 using adaptor-targeted siRNAs. As shown in Figure 1B and C, cells deficient in either IRAK1 or TRAF6 failed to respond to HSV-1 treatment for up-regulating the IL-15 gene expression, which clearly indicates the absolute requirement of these adaptor molecules for the TLR signaling pathway leading to IL-15 gene expression in monocytes. The presence of IL-15 amplicons in HSV-1–treated MyD88-deficient monocytic cells appears to be an indication of the presence of residual MyD88 molecules rather than the involvement of a secondary MyD88-independent signaling pathway.
MyD88, IRAK1, and TRAF6 are involved in HSV-1–induced IL-15 up-regulation in THP1 cells. (A) THP1 cells were transfected with either adaptor-specific siRNAs or control siRNA. Cells were harvested 48 hours later, and protein lysates were analyzed for MyD88, IRAK1, TRAF6, and β-actin expression by immunoblotting. Representative Western blots are shown. (B) Wild-type THP1 cells as well as MyD88-, IRAK1-, or TRAF6-deficient cells were incubated with mock or HSV-1. Cells were harvested 6 hours after treatment for total RNA extraction, and IL-15 gene expression was examined by RT-PCR. The PCR products were 550 and 640 bp for IL-15 (representing the 2 isoforms) and 310 bp for the internal control 18S rRNA and are representative of 3 independent experiments. (C) Total RNA was also used to determine IL-15 gene expression by real-time RT-PCR. Data are expressed as the ratio of IL-15 mRNA in treated samples to the untreated sample (after normalization with reference dye and housekeeping gene).
MyD88, IRAK1, and TRAF6 are involved in HSV-1–induced IL-15 up-regulation in THP1 cells. (A) THP1 cells were transfected with either adaptor-specific siRNAs or control siRNA. Cells were harvested 48 hours later, and protein lysates were analyzed for MyD88, IRAK1, TRAF6, and β-actin expression by immunoblotting. Representative Western blots are shown. (B) Wild-type THP1 cells as well as MyD88-, IRAK1-, or TRAF6-deficient cells were incubated with mock or HSV-1. Cells were harvested 6 hours after treatment for total RNA extraction, and IL-15 gene expression was examined by RT-PCR. The PCR products were 550 and 640 bp for IL-15 (representing the 2 isoforms) and 310 bp for the internal control 18S rRNA and are representative of 3 independent experiments. (C) Total RNA was also used to determine IL-15 gene expression by real-time RT-PCR. Data are expressed as the ratio of IL-15 mRNA in treated samples to the untreated sample (after normalization with reference dye and housekeeping gene).
MyD88, IRAK1, and TRAF6 are required for the transactivation of NF-κB in response to HSV–1 leading to IL-15 production
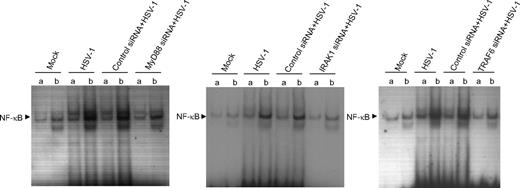
Because the IL-15 promoter is regulated in part by an NF-κB–responsive sequence, we sought to assess the role of MyD88, IRAK1, and TRAF6 in NF-κB transactivation. We generated MyD88-, IRAK1-, and TRAF6-deficient THP-1 cells using siRNA oligonucleotides in transient transfections. The cells were treated with mock or HSV-1 for 1 hour and nuclear cell extracts were harvested for EMSA to detect NF-κB activation. In agreement with IL-15 gene expression results, we found that the transient transfection of THP1 cells with MyD88-, IRAK1-, or TRAF6-specific siRNA significantly inhibited HSV-1–induced DNA binding of NF-κB to a level comparable with that obtained for mock-treated cells (Figure 2). These effects were not seen in THP1 cells transfected with control siRNAs. These results indicate that HSV-1–induced up-regulation of IL-15 gene expression in monocytic cells through NF-κB is through a mechanism that depends on the activation of MyD88, IRAK1, and TRAF6. Therefore, we can conclude that HSV-1–induced IL-15 production is a consequence of the primary recognition of HSV-1–associated molecular pattern by a specific Toll-like receptor.
MyD88, IRAK-1, and TRAF6 are required for HSV-1–induced NF-κB leading to IL-15 gene expression. Wild-type THP1 cells or MyD88-, IRAK1-, TRAF6-deficient cells were treated with mock or HSV-1. After 1 hour of incubation, cells were collected and nuclear extracts were subjected to EMSA analysis with radiolabeled NF-κB consensus oligonucleotide in the presence of unlabeled competitor (a lanes) or nonspecific competitor (b lanes). NF-κB binding was visible by autoradiography.
MyD88, IRAK-1, and TRAF6 are required for HSV-1–induced NF-κB leading to IL-15 gene expression. Wild-type THP1 cells or MyD88-, IRAK1-, TRAF6-deficient cells were treated with mock or HSV-1. After 1 hour of incubation, cells were collected and nuclear extracts were subjected to EMSA analysis with radiolabeled NF-κB consensus oligonucleotide in the presence of unlabeled competitor (a lanes) or nonspecific competitor (b lanes). NF-κB binding was visible by autoradiography.
TLR2 is required for the recognition of HSV-1 and the release of IL-15
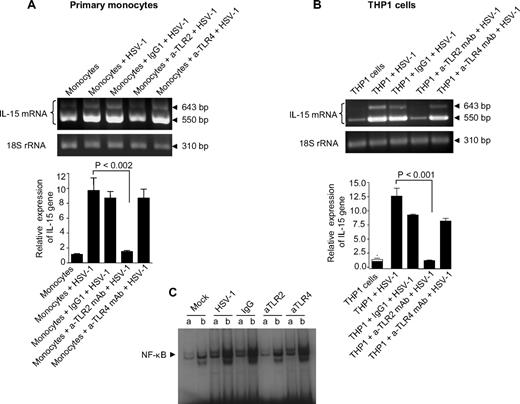
TLR2 has been documented to be involved in the production of some cytokines in HSV-infected mice, cell lines, and human PBMCs,23,24,37,38 and based on the results described in Figures 1 and 2, we hypothesized that TLR2 was involved in the up-regulation of the IL-15 gene expression in human monocytic cells after exposure to HSV-1. We therefore examined the effects of the functional inactivation of TLR2 on HSV-1–induced up-regulation of IL-15 gene expression. This was accomplished by exposing adult primary monocytes, as well as THP1 cells, to an anti-TLR2-neutralizing monoclonal antibody (a-TLR2 mAb) or a control isotype (IgG1). The cells were then treated with HSV-1 and evaluated for IL-15 gene expression. Neutralization of TLR2, with an anti-TLR2 mAb, led to a complete inhibition HSV-1–induced IL-15 gene up-regulation in both primary monocytes (Figure 3A) and THP1 cells (Figure 3B). In this condition, IL-15 gene expression levels were similar to those seen in nontreated cells, whereas there was no change in IL-15 expression in cells treated with the control isotype antibody. Interestingly, neutralization of TLR4 had no inhibitory effect on the up-regulation of the IL-15 gene in response to HSV-1 (Figure 3A,B), which ruled out the involvement of TLR4. Moreover, neutralization of TLR2, but not of TLR4, also significantly abrogated HSV-1–induced DNA binding of NF-κB (Figure 3C) in THP1 cells. Altogether, these results demonstrate the absolute requirement of TLR2-mediated signaling for IL-15 gene up-regulation by HSV-1 in monocytic cells.
TLR2 is required for the recognition of HSV-1 and the release of IL-15 by monocytic cells. (A) Primary human monocytes were treated separately with 1 μg/mL of anti-TLR2 (neutralizing) mAb, anti-TLR4 (neutralizing) mAb, or isotype-matched control (IgG1) for 30 minutes. Nontreated cells as well as antibody-treated cells were exposed to mock or HSV-1. Cells were harvested 6 hours after treatment for isolation of total RNA to assess IL-15 gene expression by RT-PCR (top panel) and real-time RT-PCR (bottom panel). (B) THP1 cells were treated similarly to primary monocytes, and IL-15 gene expression was assessed with RT-PCR (top panel) and real-time RT-PCR (bottom panel). In both cases, RT-PCR values show that IL-15 mRNA and 18S rRNA are representative of 3 independent experiments; real-time RT-PCR values are expressed as the ratio of IL-15 mRNA in treated samples to the untreated sample (after normalization with reference dye and housekeeping gene). (C) THP1 cells pretreated as indicated were incubated with mock or HSV-1 for 1 hour. Nuclear extracts were subjected to EMSA analysis with radiolabeled NF-κB consensus oligonucleotide in the presence of unlabeled competitor (a lanes) or nonspecific competitor (b lanes). NF-κB binding was visible by autoradiography.
TLR2 is required for the recognition of HSV-1 and the release of IL-15 by monocytic cells. (A) Primary human monocytes were treated separately with 1 μg/mL of anti-TLR2 (neutralizing) mAb, anti-TLR4 (neutralizing) mAb, or isotype-matched control (IgG1) for 30 minutes. Nontreated cells as well as antibody-treated cells were exposed to mock or HSV-1. Cells were harvested 6 hours after treatment for isolation of total RNA to assess IL-15 gene expression by RT-PCR (top panel) and real-time RT-PCR (bottom panel). (B) THP1 cells were treated similarly to primary monocytes, and IL-15 gene expression was assessed with RT-PCR (top panel) and real-time RT-PCR (bottom panel). In both cases, RT-PCR values show that IL-15 mRNA and 18S rRNA are representative of 3 independent experiments; real-time RT-PCR values are expressed as the ratio of IL-15 mRNA in treated samples to the untreated sample (after normalization with reference dye and housekeeping gene). (C) THP1 cells pretreated as indicated were incubated with mock or HSV-1 for 1 hour. Nuclear extracts were subjected to EMSA analysis with radiolabeled NF-κB consensus oligonucleotide in the presence of unlabeled competitor (a lanes) or nonspecific competitor (b lanes). NF-κB binding was visible by autoradiography.
Defective expression of TLR2 on THP1 cells reduced the HSV-1–mediated IL-15 gene activation
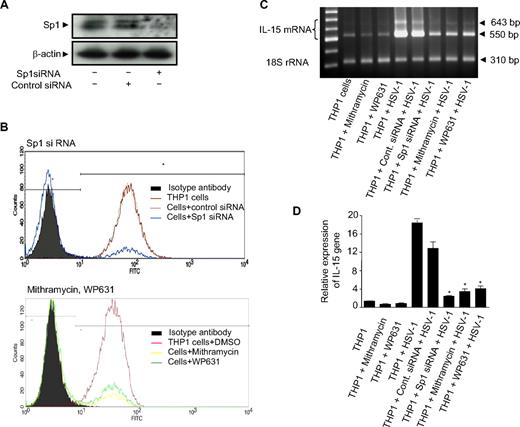
Given that the TLR2 promoter contains the Sp1 binding site and that Sp1 regulates TLR2 promoter activity in THP1 cells,39 the loss of the Sp1 transcription factor may lead to reduced TLR2 gene expression. In light of these observations, using Sp1 siRNA, we blocked Sp1 expression and hence rendered the THP1 cells defective in TLR2 expression. In addition, we also blocked TLR2 expression by treating the cells with 2 different pharmacologic inhibitors, mithramycin and bisanthracycline WP631, which have been shown to inhibit Sp1-induced transcription in vitro.40,41 In both treatments, cells were collected and TLR2 expression was examined by flow cytometry. As shown in Figure 4A, Sp1 siRNA almost completely abrogated the intracellular protein expression of Sp1 as determined by immunoblotting, whereas the expression of β-actin was not affected. As for the expression of TLR2, it was inhibited in Sp1 siRNA-transfected cells but not with control siRNA (Figure 4B). Similarly, both mithramycin and WP631 significantly reduced the expression of TLR2 on THP1 cells in comparison to the control (DMSO) (Figure 4B). The cells defective in TLR2 expression were then exposed to HSV-1, and IL-15 gene up-regulation was assessed by RT-PCR and real-time RT-PCR (Figure 4C,D). As expected, wild-type cells and control siRNA-transfected cells exposed to HSV-1 showed the highest expression levels of IL-15 (lanes 4 and 5). These levels were significantly reduced in HSV-1–treated TLR2-deficient cells (lanes 6-8). Thus, the functional knockdown of Sp1 induced the loss of TLR2 surface expression, which in turn led to the inhibition of specific HSV-1–mediated gene activation. Overall, these genetic and pharmacologic lines of evidence strongly support the assumption that TLR2 is the major, if not the only, mediator of HSV-1–induced activation of IL-15 gene expression.
Defective expression of TLR2 resulted in abrogation of HSV-1–induced IL-15 gene expression. (A) THP-1 cells were transfected with Sp1 specific siRNA or its control siRNA. After 48 hours, the cell lysates were examined for Sp1 protein expression by immunoblotting. A representative Western blot is shown. (B) Sp1 siRNA-transfected THP1 cells (top panel) and THP1 cells pretreated with mithramycin, WP631, or DMSO as control (bottom panel) were assessed for their expression of TLR2. THP-1 surface expression of TLR2 was determined by flow cytometry as described in “Flow cytometry.” (C) THP-1 cells subjected to the different treatments (as indicated) were incubated with mock or HSV-1. Cells were harvested 6 hours after incubation, and total RNA was analyzed for IL-15 gene expression by RT-PCR. The figure shows IL-15 mRNA (top panel) and 18S rRNA (bottom panel) and is representative of 3 independent experiments. (D) Similarly, real-time RT-PCR was performed and data are expressed as the ratio of IL-15 mRNA in treated samples to the untreated sample (after normalization with reference dye and housekeeping gene). *Observed difference is statistically significant (P < .001) comparing gene expression with wild-type cells exposed to HSV-1.
Defective expression of TLR2 resulted in abrogation of HSV-1–induced IL-15 gene expression. (A) THP-1 cells were transfected with Sp1 specific siRNA or its control siRNA. After 48 hours, the cell lysates were examined for Sp1 protein expression by immunoblotting. A representative Western blot is shown. (B) Sp1 siRNA-transfected THP1 cells (top panel) and THP1 cells pretreated with mithramycin, WP631, or DMSO as control (bottom panel) were assessed for their expression of TLR2. THP-1 surface expression of TLR2 was determined by flow cytometry as described in “Flow cytometry.” (C) THP-1 cells subjected to the different treatments (as indicated) were incubated with mock or HSV-1. Cells were harvested 6 hours after incubation, and total RNA was analyzed for IL-15 gene expression by RT-PCR. The figure shows IL-15 mRNA (top panel) and 18S rRNA (bottom panel) and is representative of 3 independent experiments. (D) Similarly, real-time RT-PCR was performed and data are expressed as the ratio of IL-15 mRNA in treated samples to the untreated sample (after normalization with reference dye and housekeeping gene). *Observed difference is statistically significant (P < .001) comparing gene expression with wild-type cells exposed to HSV-1.
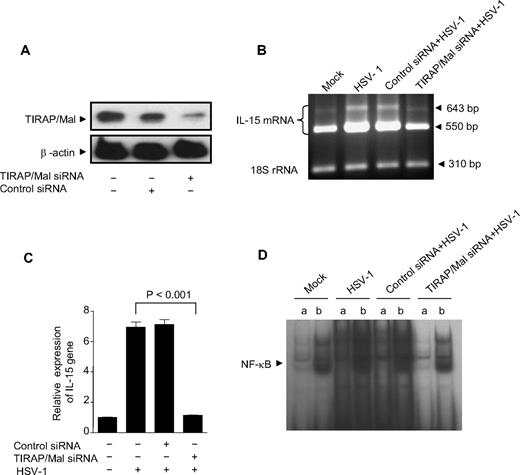
TIRAP/Mal is required in HSV-1–induced up-regulation of IL-15 gene expression
Emerging evidence suggests that the activation of the MyD88-dependent pathway requires the participation of an additional TIR domain-containing adaptor protein (TIRAP/Mal) downstream of TLR2 and TLR4.42,43 We therefore examined whether TIRAP/Mal is involved in HSV-1–induced TLR2 signaling pathway leading to IL-15 production. To test this hypothesis, we generated TIRAP/Mal deficient THP1 cells using siRNA. As shown in Figure 5A, immunoblot analysis of TIRAP siRNA-transfected THP1 cells revealed the abolition of the TIRAP protein expression because of the disruption of the TIRAP gene, in contrast to control siRNA-transfected cells and wild-type cells. Wild-type THP1 cells and transfected THP1 cells were subjected to mock or virus stimulation and subsequently assessed for IL-15 gene expression. Interestingly, we found that HSV-1–induced up-regulation of IL-15 gene expression was completely abrogated in HSV-1–treated TIRAP-deficient THP1 cells compared with HSV-1–treated control siRNA-transfected cells or wild-type THP1 cells (Figure 5B,C). Therefore, we decided to further assess the ability of TIRAP-deficient cells to activate NF-κB in response to HSV-1 stimulation. Wild-type THP1 cells or THP1 cells transfected with either control siRNA or TIRAP-specific siRNA were exposed to mock or virus for 1 hour, and nuclear cell extracts were harvested for EMSA to detect NF-κB binding. We found that HSV-1–induced DNA binding of NF-κB in TIRAP-deficient cells was completely inhibited, and the levels of NF-κB activation observed were similar to the levels observed in nontreated wild-type cells. In contrast, in wild-type cells or control siRNA-transfected cells, virus stimulation significantly increased the binding of NF-κB to the IL-15 promoter compared with the mock-treated THP1 cells (Figure 5D). Taken together, these findings demonstrate that TIRAP plays a critical role in the signaling cascade emanating from TLR2 that is activated by HSV-1 and leads to IL-15 up-regulation in monocytic cells.
TIRAP/Mal is required in HSV-1–induced up-regulation of IL-15 gene expression. (A) THP-1 cells were transfected with TIRAP/Mal siRNA or control siRNA for a total period of 48 hours, and protein lysates were analyzed for TIRAP and β-actin expression levels by immunoblotting. (B) TIRAP/Mal-deficient THP-1 cells were treated with HSV-1, and IL-15 gene expression was determined by RT-PCR. IL-15 mRNA (top panel) and 18S rRNA (bottom panel) are shown and are representative of 3 independent experiments. (C) IL-15 gene expression was also analyzed by real-time RT-PCR. Data are expressed as the ratio of IL-15 mRNA in treated samples to the untreated sample (after normalization with reference dye and housekeeping gene). (D) TIRAP/Mal-deficient THP1 cells were incubated with HSV-1 for 1 hour. Nuclear extracts were subjected to EMSA analysis with radiolabeled NF-κB consensus oligonucleotide in the presence of unlabeled competitor (a lanes) or nonspecific competitor (b lanes). NF-κB binding was visible by autoradiography.
TIRAP/Mal is required in HSV-1–induced up-regulation of IL-15 gene expression. (A) THP-1 cells were transfected with TIRAP/Mal siRNA or control siRNA for a total period of 48 hours, and protein lysates were analyzed for TIRAP and β-actin expression levels by immunoblotting. (B) TIRAP/Mal-deficient THP-1 cells were treated with HSV-1, and IL-15 gene expression was determined by RT-PCR. IL-15 mRNA (top panel) and 18S rRNA (bottom panel) are shown and are representative of 3 independent experiments. (C) IL-15 gene expression was also analyzed by real-time RT-PCR. Data are expressed as the ratio of IL-15 mRNA in treated samples to the untreated sample (after normalization with reference dye and housekeeping gene). (D) TIRAP/Mal-deficient THP1 cells were incubated with HSV-1 for 1 hour. Nuclear extracts were subjected to EMSA analysis with radiolabeled NF-κB consensus oligonucleotide in the presence of unlabeled competitor (a lanes) or nonspecific competitor (b lanes). NF-κB binding was visible by autoradiography.
Discussion
TLRs have been identified as a subset of pattern-recognition receptors. Once activated, these receptors initiate intracellular signaling events and generate a coordinated innate cytokine response that, in turn, contributes to direct and shape an effective acquired antiviral immune response.22,44 Indeed, studies of HSV-1–induced secretion of cytokines have demonstrated that HSV-1 activates murine macrophages through TLR224 and signals TLR9 in murine natural interferon-producing cells.45 TLR2 signaling has also been shown to be required for the production of some major proinflammatory cytokines and chemokines in HSV-1–treated murine microglial cells.23 In addition, TLR2 plays a critical role in HSV-1–induced pathogenesis in a mouse model of HSV-1 infection24 and in HSV-1–mediated production of IL-6 and IL-8 in PBMCs from neonates.37
With regard to the host's immune response to herpesviruses, previous reports have described the activation of IL-15 gene expression by different viruses and have shown the unique role of this cytokine in the control of viral infection through the activation of NK and NKT cells.3,18 However, there has not yet been any work to identify the receptor and the related molecular mechanism involved in virus-induced IL-15 production. In the present study, using HSV-1, we sought to investigate and identify the TLR signaling components via which this virus induces the up-regulation of IL-15 gene expression in human monocytic cells.
Identification of TLR signal transduction cascade components for a particular cytokine provides information about the receptor probably used by the virus to induce its inflammatory response. Therefore, it was important to determine whether IL-15 up-regulation by HSV-1 was mediated by MyD88-dependent mechanisms. Using the RNA interference approach, we clearly show here that HSV-1–induced up-regulation of IL-15 gene expression in monocytic cells operates through a MyD88-dependent signaling pathway. These results clearly show the involvement of TLRs as sensors of HSV-1 constituents, which initiate the MyD88-dependent signaling cascade that ultimately leads to the activation of IL-15 gene expression. Studies in MyD88-deficient mice have revealed that this protein plays a critical role in the production of proinflammatory cytokines in response to TLR ligands.28,46 Accordingly, MyD88-deficient mice were found to be highly susceptible to viral infections and displayed a high rate of mortality.47-50
The interaction of MyD88 with the TLR receptor TIR domain usually induces the formation of MyD88 homodimers, which promote the recruitment of IRAK1 and TRAF6. Consistent with the importance of these adaptor proteins in TLR responses, we also show here that inhibition of IRAK1 and TRAF6 expression results in complete suppression of HSV-1–induced up-regulation of IL-15 gene transcription. We have also demonstrated that MyD88, IRAK1, and TRAF6 are key regulatory adaptor molecules for HSV-1–induced NF-κB activation. In this regard, we have recently shown the major involvement of NF-κB in the regulation of IL-15 gene transcription in THP1 cells on exposure to HSV-1.35 Altogether, the present results define the signaling pathway emanating from MyD88 and involving IRAK1 and TRAF6 that is involved in transactivation of IL-15 gene by NF-κB in response to HSV-1 in THP1 monocytic cells.
The interaction of different viruses with TLR2 has been shown to be decisive in the outcome of immune responses and viral pathogenesis. For instance, measles virus hemagglutinin protein has been shown to activate TLR2 signaling in dendritic cells and monocytes, resulting in IL-6 and IL-1β production.51 Studies using monocytes and dendritic cells derived from hepatitis C virus–infected patients have indicated that the TLR2 system is involved in hepatitis C virus–induced immunopathogenesis.52-55 Other studies have also shown that TLR2 participates in HSV-1–induced neuropathogenesis, which suggests that disease outcome is probably determined by the response of the host, rather than by the infectious agent alone.37 In the present study, to demonstrate that TLR2 participates in the induction of IL-15 by HSV-1, we achieved a functional knockdown of cellular responses to the TLR2 ligand HSV. We show here that monocytic cells rendered defective in TLR2 expression by using Sp1 siRNA or Sp1 inhibitors, such as mithramycin and WP631, were no longer responsive to HSV-1 challenge for up-regulating IL-15 gene expression. This was also the case when cells were treated with anti-TLR2, but not with anti-TLR4, neutralizing mAbs. These results clearly demonstrate that HSV-1 signals specifically through TLR2 to up-regulate IL-15 gene expression in monocytes and that no other TLR is required for this response. Although HSV-1 may be recognized by multiple TLRs, there are differences in the ultimate gene expression profile that result from activation of an individual TLR. It is noteworthy in this regard that the discovery of a second set of the TIR domain-containing adaptor, MyD88 adaptor-like (Mal), also known as TIRAP, has provided evidence for a molecular basis of this specificity.56,57 Mal has been shown to be recruited specifically to TLR2 and TLR4. This was later confirmed in Mal-deficient mice, which have an impaired response to ligands for TLR2 and TLR4, but have normal responses to ligands for other TLRs.58-60 With regard to IL-15, it is well known that, in contrast to other proinflammatory cytokines, it exerts unique antiviral functions; therefore, its production needs to be highly regulated. Thus, to further rule out the possibility of the involvement of other TLRs in the HSV-1–induced IL-15 response, we generated Mal/TIRAP-deficient THP1 cells using siRNA knockdown. Here we show that responses to TLR2 ligand (HSV-1), including NF-κB activation and IL-15 up-regulation in THP1 cells, were completely abolished in cells deficient in Mal/TIRAP. Our findings imply that Mal is an essential adaptor protein for TLR2 signaling and that HSV-1 induces the recruitment of Mal to induce a specific immune response. Surprisingly, the response to TLR2 signaling was also completely abolished in MyD88-deficient cells, which suggests that Mal, although essential to TLR2 signaling pathway, cannot compensate for the lack of MyD88. Indeed, for the virus to induce IL-15 up-regulation in monocytic cells, it is necessary that both adaptors be activated and recruited to TLR2. Therefore, one may postulate, at this point, that TLR2 signaling in this particular response cannot be driven via homodimers of MyD88 or Mal.57 This finding was further exemplified by the inability of MyD88 homodimerization inhibitory peptides to block the transcription of IL-15 gene expression and NF-κB activation in HSV-1–treated THP1 cells (data not shown). Mal and MyD88 differ markedly in terms of biochemical structure and surface charge.57 It is then possible that homodimers of Mal or MyD88 will signal differently from heterodimers, which may disrupt the specificity of the response and hence the subsequent antiviral immune response.
In conclusion, our results demonstrate, for the first time, that HSV-1–induced IL-15 gene expression is mediated via a TLR2-associated signaling pathway. To this end, we presented evidence obtained at 3 levels (ie, receptor, adapter molecules, and transcription) using both genetic and pharmacologic approaches. Our data further show that this up-regulation of IL-15 gene expression occurs through the activation of a unique combination of adapter proteins. This distinctiveness in the molecular mechanisms of the signaling events warrants tight regulation and allows for specificity in the innate immune response to viral infections. Overall, the present results clearly show the intricacy of the very early immune response to HSV-1 infection. Further research on this aspect will provide new insights into the molecular mechanisms controlling the innate immune response to a viral infection, such as HSV-1, which, in turn, should contribute substantially to a better understanding of viral pathogenesis.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Carolina Alfieri for her comments on the manuscript. S.E. thanks the Fonds de la Recherche en Santé du Québec for studentship.
This work was supported by a grant from the Canadian Institutes of Health Research.
Authorship
Contribution: R.A. and S.E. designed and performed research and wrote the paper; P.C. contributed to some of the experiments; and J.M. participated in designing the research, analyzing the data, and in writing and editing the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: José Menezes, Laboratory of Immunovirology, Ste-Justine Hospital, 3175 Cote Ste-Catherine, Montreal, Quebec, H3T 1C5, Canada; e-mail: jose.menezes@recherche-ste-justine.qc.ca.
References
Author notes
*R.A. and S.E. contributed equally to this work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal