Abstract
The role of the Ras/MEK/ERK pathway was examined in relation to DNA damage in human multiple myeloma (MM) cells exposed to Chk1 inhibitors in vitro and in vivo. Exposure of various MM cells to marginally toxic concentrations of the Chk1 inhibitors UCN-01 or Chk1i modestly induced DNA damage, accompanied by Ras and ERK1/2 activation. Interruption of these events by pharmacologic (eg, the farnesyltransferase inhibitor R115777 or the MEK1/2 inhibitor PD184352) or genetic (eg, transfection with dominant-negative Ras or MEK1 shRNA) means induced pronounced DNA damage, reflected by increased γH2A.X expression/foci formation and by comet assay. Increased DNA damage preceded extensive apoptosis. Notably, similar phenomena were observed in primary CD138+ MM cells. Enforced MEK1/2 activation by B-Raf transfection prevented R115777 but not PD184352 from inactivating ERK1/2 and promoting Chk1 inhibitor–induced γH2A.X expression. Finally, coadministration of R115777 diminished UCN-01–mediated ERK1/2 activation and markedly potentiated γH2A.X expression in a MM xenograft model, associated with a striking increase in tumor cell apoptosis and growth suppression. Such findings suggest that Ras/MEK/ERK activation opposes whereas its inhibition dramatically promotes Chk1 antagonist–mediated DNA damage. Together, these findings identify a novel mechanism by which agents targeting the Ras/MEK/ERK pathway potentiate Chk1 inhibitor lethality in MM.
Introduction
Checkpoint kinases (ie, Chk1 and Chk2) represent key components of the DNA damage checkpoint machinery, which monitors DNA breaks caused by endogenous/metabolic or environmental genotoxic insults or by replication stress.1,2 In response to DNA damage, cells activate checkpoint pathways, resulting in cell-cycle arrest, which permits the DNA repair machinery to rectify the damage. Depending on the nature of the DNA lesions and the context in which damage occurs, cells either survive and resume cell-cycle progression through a recovery mechanism when repair is successful or are eliminated by apoptosis if repair fails. Thus, checkpoints provide normal cells with critical surveillance machinery designed to promote genomic integrity and survival. Conversely, checkpoint dysfunction contributes to tumorigenesis by permitting cell proliferation in the face of genomic instability.3,4 Moreover, checkpoints are activated by numerous chemotherapeutic agents and ionizing radiation.5 This has prompted the development of anticancer strategies targeting checkpoint machinery.5,6 Among the diverse checkpoint pathway components, Chk1 represents a particularly attractive target for several reasons, that is, (1) Chk1 is functionally associated with all known checkpoints (eg, the G2-M transition, G1, intra-S,5 and, most recently, the mitotic spindle checkpoint7 ); (2) Chk1 is essential for maintenance of genomic integrity, whereas the role of Chk2 is conditional3 ; and (3) for multiple checkpoints, Chk2 function can be mimicked by Chk1, whereas Chk1 cannot be replaced by a functionally overlapping kinase such as Chk2.3
Chk1 inhibition (eg, by the Chk1 inhibitor UCN-01) results in abrogation of checkpoints induced by DNA-damaging chemotherapy and radiation, leading to enhanced tumor cell killing.8,9 Given these findings, a major emphasis has been placed on efforts to combine Chk1 inhibitors (eg, UCN-0110 or CHIR-12411 ) with diverse DNA-damaging agents. However, an alternative strategy is based on the concept that transformed cells may be ill-equipped to survive simultaneous interruption of both checkpoint machinery and prosurvival signaling. In this context, our group has reported that exposure of human leukemia and multiple myeloma (MM) cells to UCN-01 induces pronounced activation of MEK1/2 and ERK1/2,12,13 key components of the Ras/Raf/MEK/ERK cascade that plays a critical role in proliferation and survival of malignant cells.14 Significantly, disruption of ERK1/2 activation by pharmacologic MEK1/2 inhibitors,12,13 farnesyltransferase inhibitors (FTIs; eg, L744832)15,16 or HMG-CoA reductase inhibitors (ie, statins)17 results in a dramatic increase in apoptosis of hematopoietic malignant cells. Together, these findings suggest that activation of Ras/MEK/ERK signaling cascade may represent a compensatory response to Chk1 inhibitor lethality, and that interruption of this response lowers the death threshold.
Although the observation that MEK1/2 inhibitors or FTIs antagonize UCN-01–mediated ERK1/2 activation and potentiate lethality of this agent in various tumor cell types has been well documented,12,13,18,19 the mechanism by which interruption of the Ras/MEK/ERK pathway potentiates the lethality of Chk1 inhibitors remains to be fully elucidated. Recently, it has been found that Chk1 inhibition by either Chk1 inhibitors (eg, UCN-01 and CEP-3891) or Chk1 siRNA leads to formation of single-stranded DNA (ssDNA) and induction of DNA strand breaks20 (ie, manifested by increased expression of the phosphorylated form of the atypical histone H2A.X, referred to as γH2A.X9 ). Interestingly, ERK1/2 signaling has been implicated in attenuation of DNA damage through positive regulation of DNA repair mechanism.21 Such findings raise the possibility that interruption of Ras/MEK/ERK signaling may promote Chk1 inhibitor–mediated DNA damage, leading to enhanced lethality. To explore this possibility, we have examined the effects of the Ras/MEK/ERK pathway on Chk1 inhibitor–mediated DNA damage in MM cells. We report here for the first time that disruption of Ras/MEK/ERK signaling cascade, by either pharmacological agents (eg, the FTI R115777 or the MEK1/2 inhibitor PD184352) or genetic approaches (eg, dominant-negative S17N Ras or MEK1 shRNA) results in markedly increased DNA damage prior to massive apoptosis in MM cells. Conversely, enforced MEK1/2 activation by B-Raf attenuates the ability of R115777 but not PD184352 to promote Chk1 inhibitor–related DNA damage and lethality. Moreover, in an in vivo murine xenograft model of MM cells, coadministration of R115777 diminishes UCN-01–induced ERK1/2 activation, markedly increases γH2A.X expression and foci formation, strikingly induces apoptosis, and suppresses tumor growth. These findings argue strongly for a functional role of Ras/MEK/ERK signaling in the regulation of Chk1 inhibitor–mediated DNA damage both in vitro and in vivo. Moreover, they provide a novel mechanistic basis for understanding interactions between agents targeting the DNA damage checkpoint (eg, Chk1 inhibitors) and prosurvival signaling pathways (eg, farnesyltransferase or MEK1/2 inhibitors).
Methods
Cells and reagents
Human MM U266 (wild-type Ras), RPMI8226 (activated K-Ras), H929 (activated N-Ras),22 MM.1S, and MM.1R cells were maintained as reported previously.13 All experiments used logarithmically growing cells (3-5 × 105 cells/mL). Bone marrow samples were obtained with informed consent from 2 patients with MM undergoing routine diagnostic aspiration with IRB approval from Virginia Commonwealth University. Informed consent was provided in accordance with the Declaration of Helsinki. CD138+ myeloma cells were isolated as previously reported.13,16 UCN-01 was provided by Cancer Therapy Evaluation Program (CTEP), National Cancer Institute (NCI, Bethesda, MD). 4-(2-Phenyl)-9-hydroxypyrrolo[3,4-c]carbazole-1,3-(2H,6H)-dione (designated Chk1i throughout), a specific Wee1/Chk1 inhibitor (IC50 toward Chk1 vs Wee1 = 2.06-fold, determined by an in vitro assay),23 and PD184352 were purchased from Calbiochem (San Diego, CA) and Upstate Biotechnology (Lake Placid, NY), respectively. R115777 was provided by Johnson & Johnson Pharmaceuticals (La Jolla, CA) via CTEP. Agents were dissolved in DMSO and stored at −20°C. In all experiments, the final concentration of DMSO did not exceed 0.1%.
Plasmids and stable transfection
cDNAs for human H-Ras S17N mutant (dominant negative), full-length B-Raf, as well as MEK1 shRNA (pKD-MEK1-v2; GenBank no. NM 00275524 ) and its negative control (pKD-NegCon-v1) were obtained from Upstate Biotechnology. U266 cells were transfected with these constructs using Amaxa Nucleofector (Amaxa, Cologne, Germany) as per the manufacturer's instructions. Clones were selected by G418.
Flow cytometry
Apoptosis was evaluated by annexin V–FITC or 7AAD staining and flow cytometry as described previously.17 To analyze DNA damage, cells were stained with Alexa Fluor 488–conjugated phospho–histone H2A.X (Ser139) rabbit monoclonal antibody (Cell Signaling, Beverly, MA) as per the manufacturer's protocol, followed by flow cytometry to determine the percentage of cells with relatively increased γH2A.X expression. The results for each condition were normalized to values for cells stained with rabbit IgG (Southern Biotech, Birmingham, AL) as the primary antibody.
Comet assay
Single-cell gel electrophoresis assays were performed to assess both single- and double-stranded DNA breaks in cells using a Comet Assay Kit (Trevigen, Gaithersburg, MD) as per the manufacturer's instructions. Images were captured using fluorescence microscopy at 20×/0.50. Tail moment was determined25 using TriTek CometScore v1.5, a free software program downloaded from http://www.tritekcorp.com.
Western blot
Samples from whole-cell pellets were prepared and 30 μg protein for each condition was subjected to Western blot following previously described procedures.12 Blots were reprobed with antibodies against β-actin or α-tubulin to ensure equal loading and transfer of proteins. The following primary antibodies were used: phospho-p44/42 MAPK (ERK1/2, Thr202/Tyr204) and p44/42 MAPK (Cell Signaling); H-Ras, B-Raf, and MEK1 (Santa Cruz, Santa Cruz, CA); phospho–histone H2A.X (Ser139), and Ras (clone RAS10) that recognizes H-, K-, and N-Ras (Upstate Biotechnology); and PARP (Biomol, Plymouth Meeting, PA).
Ras activation assay
Ras activity was detected using a Ras Activation Assay Kit (Upstate Biotechnology) as described previously17 using 400 μg protein for each condition. Ras activity was reflected by the amount of Ras-GTP pulled down by Raf-1 RBD (Ras-binding domain).
Membrane distribution of Ras
Following treatment, cells were lysed and membrane proteins fractioned using Mem-PER Eukaryotic Membrane Protein Extraction Reagent Kit (Pierce, Rockford, IL) to assess membrane localization of Ras as per the manufacturer's instructions. Samples (5 μg protein for each condition) of membrane fractions were diluted 5-fold to prevent band distortion and subjected to Western blot analysis using Ras antibody (clone RAS10).
Animal studies
Animal studies were approved by the American Association for Accreditation of Laboratory Animal Care, and performed in accordance with current regulations and standards of the US Department of Agriculture, the US Department of Health and Human Services, and the National Institutes of Health. Female athymic NCr-nu/nu mice were purchased from The Jackson Laboratory (Bar Harbor, ME), and inoculated subcutaneously with 107 MM.1S cells into the right rear flank. Treatment was administrated intraperitoneally daily after tumors reached a volume of approximately 100 mm3. R115777 was freshly prepared in 2% B-cyclodextrin in 0.1 N HCl for a final dose of 25 mg/kg. UCN-01 in DMSO was diluted in 2% (wt/vol) Na citrate (pH 3.5) for a final dose of 0.5 mg/kg. Control animals were injected with equal volume of vehicle to that administered with R115777 and/or UCN-01. Tumors were measured every 2 days independently by 3 operators. Tumor volumes were calculated from the formula (L × W2)/2, where L and W represent longest and shortest lengths of the tumor, respectively.
Immunohistochemistry, immunofluorescence, and TUNEL staining
For animal studies, tumors were removed 30 minutes after the final dose. Tissue cryostat sections were prepared for immunohistochemical staining for phospho-ERK and Ki67, using phospho-p44/42 MAPK (Thr202/Tyr204) rabbit monoclonal antibody (20G11, IHC preferred; Cell Signaling) or Ki67 antibody (Oncogene, San Diego CA), respectively, as per the instructions of the manufacturer. To assess apoptosis, tumor sections were stained for terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick-end labeling (TUNEL) using In Situ Cell Death Detective Kit (fluorescein; Roche, Penzberg, Germany) as per the manufacturer's instructions. To monitor γH2A.X foci formation, samples were stained with Alexa Fluor 488–conjugated γH2A.X antibodies as per the manufacturer's instructions. Images were captured with Olympus BX40 fluorescence microscope (Olympus, Center Valley, PA) and a CE digital camera (Alpha Innotech, San Leandro, CA) with RS Image software (Roper Scientific Photometrics, Tucson, AZ).
Statistical analysis
For analysis of apoptosis, values represent the means plus or minus SD for at least 3 separate experiments performed in triplicate. Significance of differences between experimental variables was determined using the Student t test. Analysis of synergism was performed using median dose effect analysis using the Calcusyn software program (Biosoft, Ferguson, MO).17
Results
The FTI R115777 or the MEK1/2 inhibitor PD184352 disrupts Ras→ERK1/2 signaling in human MM cells exposed to Chk1 inhibitors, accompanied by increased γH2A.X expression and lethality
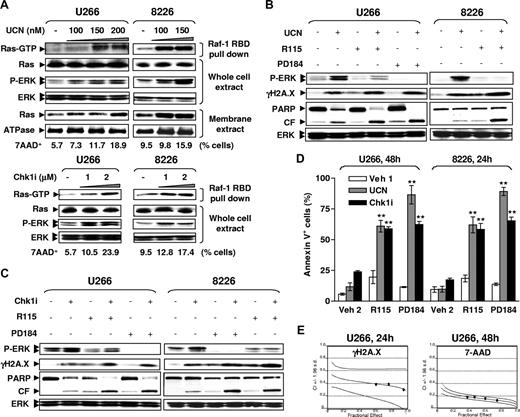
Previous studies indicated that exposure to UCN-01 induced ERK1/2 phosphorylation/activation in human leukemia and MM cells,12,13 and very recently, triggered Ras activation in MM cells.17 Consistent with those findings, exposure of U266 and RPMI8226 cells to marginally toxic concentrations of UCN-01 (ie, 100-200 nM; < 14% or 7% cell death in U266 [48 hours] or RPMI8226 [24 hours] cells, respectively) or Chk1i (1-2 μM; < 19% or 8% cell death in U266 [48 hours] or RPMI8226 [24 hours] cells, respectively) resulted in a clear increase in membrane localization and activation of Ras, accompanied by marked ERK1/2 phosphorylation (Figure 1A). In vitro loading with GTPγS rather than GDP in untreated cell lysates for each cell line resulted in a striking increase in the amount of active Ras-GTP pulled down by Raf-1 RBD-agarose beads (Figure S1A, available on the Blood website; see the Supplemental Materials link at the top of the online article), verifying specificity of the Ras activation assay. Similar results were obtained in MM.1S and its dexamethasone-resistant counterpart MM.1R (Figure S1B). These results indicate that pharmacologic inhibition of Chk1 triggers activation of Ras and ERK1/2 in various human MM cell lines, and also suggest that this represents a generalized phenomenon involving Chk1 inhibitors, rather than being restricted to the prototype UCN-01.
R115777 or PD184352 potentiates Chk1 inhibitor–induced γH2A.X expression and lethality in human MM cells in association with diminished Ras→ERK1/2 signaling. (A) U266 and RPMI8226 cells were exposed for 24 hours to UCN-01 (UCN, top panels) or Chk1i (bottom panels), after which Ras activation assays and Western blot analysis (WB) were performed to monitor Ras activation status and ERK1/2 phosphorylation, respectively. Ras activity was reflected by amount of Ras-GTP pulled down by Raf-1 RBD. Alternatively, membrane fractions were separated and subjected to WB. In parallel, the percentage of cell death (7AAD+) was assessed by flow cytometry to determine the toxicity of UCN-01 or Chk1i at the indicated concentrations in U266 (48h) or RPMI8226 cells (24h). (B,C) Cells were exposed to UCN-01 (RPMI8226, 100 nM for 24 hours; U266, 150 nM for 48 hours) or Chk1i (RPMI8226, 1 μM for 24 hours; U266, 2 μM for 48 hours) in the absence or the presence of either 5 μM R115777 (R115) or 5 μM PD184352 (PD184), after which cells were lysed and subjected to WB for phosphorylation of ERK1/2 and H2A.X (γH2A.X), as well as PARP degradation. For panels A through C, results of a representative experiment are shown; 2 additional studies yielded equivalent results. CF indicates cleavage fragment. (D) Alternatively, the percentage of apoptotic cells was determined by annexin V–FITC staining and flow cytometry. Veh indicates vehicle. Results represent the means plus or minus SD for 3 separate experiments performed in triplicate. ** indicates significantly greater than values for those treated with Chk1 inhibitors alone (P < .01). (E) U266 cells were treated with a range of R115777 and UCN-01 concentrations alone or in combination for 24 hours (for γH2A.X staining) or 48 hours (for 7AAD staining) at a fixed ratio (R115777/UCN-01, 50:1). At the end of this period, the percentage of cells with relative increases in γH2A.X expression (ie, drug treatment vs untreated controls, left panel) or 7AAD+ cells (right panel) was determined by flow cytometry, respectively. Median dose effect analysis was used to characterize the nature of the interaction. Two additional studies yielded equivalent results.
R115777 or PD184352 potentiates Chk1 inhibitor–induced γH2A.X expression and lethality in human MM cells in association with diminished Ras→ERK1/2 signaling. (A) U266 and RPMI8226 cells were exposed for 24 hours to UCN-01 (UCN, top panels) or Chk1i (bottom panels), after which Ras activation assays and Western blot analysis (WB) were performed to monitor Ras activation status and ERK1/2 phosphorylation, respectively. Ras activity was reflected by amount of Ras-GTP pulled down by Raf-1 RBD. Alternatively, membrane fractions were separated and subjected to WB. In parallel, the percentage of cell death (7AAD+) was assessed by flow cytometry to determine the toxicity of UCN-01 or Chk1i at the indicated concentrations in U266 (48h) or RPMI8226 cells (24h). (B,C) Cells were exposed to UCN-01 (RPMI8226, 100 nM for 24 hours; U266, 150 nM for 48 hours) or Chk1i (RPMI8226, 1 μM for 24 hours; U266, 2 μM for 48 hours) in the absence or the presence of either 5 μM R115777 (R115) or 5 μM PD184352 (PD184), after which cells were lysed and subjected to WB for phosphorylation of ERK1/2 and H2A.X (γH2A.X), as well as PARP degradation. For panels A through C, results of a representative experiment are shown; 2 additional studies yielded equivalent results. CF indicates cleavage fragment. (D) Alternatively, the percentage of apoptotic cells was determined by annexin V–FITC staining and flow cytometry. Veh indicates vehicle. Results represent the means plus or minus SD for 3 separate experiments performed in triplicate. ** indicates significantly greater than values for those treated with Chk1 inhibitors alone (P < .01). (E) U266 cells were treated with a range of R115777 and UCN-01 concentrations alone or in combination for 24 hours (for γH2A.X staining) or 48 hours (for 7AAD staining) at a fixed ratio (R115777/UCN-01, 50:1). At the end of this period, the percentage of cells with relative increases in γH2A.X expression (ie, drug treatment vs untreated controls, left panel) or 7AAD+ cells (right panel) was determined by flow cytometry, respectively. Median dose effect analysis was used to characterize the nature of the interaction. Two additional studies yielded equivalent results.
In view of earlier evidence that treatment with Chk1 inhibitors promotes DNA breaks, manifested by phosphorylation of histone H2A.X on Ser139 residues (designated γH2A.X),20 the possibility that interruption of ERK1/2 activation by pharmacological agents might affect Chk1 inhibitor–induced DNA damage was examined. Cotreatment with either the FTI R115777 or the MEK1/2 inhibitor PD184352 largely attenuated ERK1/2 phosphorylation in RPMI8226 or U266 cells exposed to UCN-01 or Chk1i (Figure 1B,C). Notably, exposure to UCN-01 or Chk1i induced a modest but discernible increase in γH2A.X, whereas coadministration of either R115777 or PD184352 dramatically increased Chk1 inhibitor–induced γH2A.X expression. Furthermore, both R115777 and PD184352 significantly potentiated apoptosis in MM cells exposed to either Chk1 inhibitor (Figure 1D; P < .01 in each case, compared with treatment with each Chk1 inhibitor individually), a phenomenon confirmed by increased PARP cleavage (Figure 1B,C). Similar results were obtained in MM.1S and MM.1R cells (Figure S1C,D), as well as H929 cells (Figure S1E,F). Finally, isobologram analysis revealed combination index values less than 0.4 (γH2A.X expression) or less than 0.2 (cell death, manifested by 7AAD+), indicating a highly synergistic interaction (Figure 1E). Together, these findings raise the possibility that simultaneous interference with compensatory Ras→ERK1/2 activation enhances Chk1 inhibitor–induced DNA damage, and that these events may contribute to synergism between Chk1 inhibitors and agents targeting the Ras/MEK/ERK pathway.
R115777 or PD184352 promotes DNA damage in MM cells exposed to Chk1 inhibitors, a phenomenon that occurs prior to induction of extensive apoptosis
Although γH2A.X expression is primarily induced by DNA double-stranded breaks (DSBs),26 DNA fragmentation during apoptosis may also lead to H2A.X phosphorylation,27 including phosphorylation on Ser139.28 To address this issue, 2 sensitive assays were used to determine whether increased γH2A.X expression is indeed related to enhanced DNA damage in the present settings. Notably, consistent with observations in established MM cell lines (ie, U266; Figure S2A), immunofluorescence analysis using Alexa Fluor 488–conjugated γH2A.X antibodies revealed that coadministration of Chk1 inhibitors (ie, UCN-01 or Chk1i) with R115777 markedly increased the number of nuclear foci, a phenomenon reflecting sustained colocalization of γH2A.X with diverse DNA damage mediator/repair factors at or near DNA break sites,29-31 in primary CD138+ MM cells (Figure 2A), but not in CD138− bone marrow cells (Figure S2B). Similar phenomena were observed when PD184352 was used instead of R115777 (data not shown). In addition, flow cytometric assays demonstrated that increased γH2A.X expression occurred in primary CD138+ MM cells as early as 6 hours following coexposure to Chk1 inhibitors with R115777 or PD184352 (Figure S3A), but this phenomenon was not observed in CD138− bone marrow cells even after a 24-hour exposure (Figure S3B). Moreover, after drug treatment (24 hours), U266 cells were analyzed by comet assay, an established method for evaluating both single- and double-stranded DNA breaks.32 In this assay, denatured, broken DNA fragments migrate out of the cell under the influence of an electric field, producing a comet tail, whereas undamaged DNA migrates more slowly and remains within the confines of the nucleus.33 As shown in Figure 2B, coadministration of R115777 or PD184352 with either UCN-01 or Chk1i induced a striking increase in the number of comet-positive cells compared with treatment with the agents individually. Notably, increased γH2A.X foci formation and the appearance of DNA comet tails in U266 cells coexposed to Chk1 inhibitors with R115777 or PD184352 occurred substantially before the induction of massive apoptosis (ie, 24 hours vs 48 hours). As shown in Figure 2C, quantification of data indicated that coadministration (24 hours) of R115777 or PD184352 with either UCN-01 or Chk1i induced approximately a 50% to 75% increase in comet tail moment, whereas only a modest increase in apoptosis (eg, ≤ 25% annexin V+ cells) occurred at this exposure interval. Together, these results confirm that coadministration of either R115777 or PD184352 markedly potentiates DNA damage in MM cells exposed to Chk1 inhibitors, and indicate that this event precedes induction of extensive apoptosis.
Cotreatment with R115777 and Chk1 inhibitors induces a pronounced increase in γH2A.X foci formation and DNA breaks. (A) Primary CD138+ cells were isolated from the bone marrow of a patient (no. 1) with MM. Cells were then either untreated or exposed (16 hours) to 150 nM UCN-01 (UCN) or 2 μM Chk1i in the absence or the presence of 5 μM R115777 (R115). After treatment, cells were harvested and stained with Alexa Fluor (AF) 488–conjugated phospho-H2A.X (Ser139) antibody for immunocytochemical analysis. Images were captured at 60×/1.40 oil. (B) U266 cells were treated with 150 nM UCN-01 or 2 μM Chk1i with or without 5 μM R115777 or 5 μM PD184352 for 24 hours, after which a comet assay was performed to assess DNA breaks. As control, U266 cells were treated with 100 μM hydrogen peroxide for 20 minutes. (C) Tail moment was calculated as the percentage of DNA in the tail and the distance between the means of head and tail distributions. Mean tail moment was determined by measuring at least 100 cells per sample. Results represent the means plus or minus SD for 3 separate experiments. In parallel, the percentage of annexin V+ cells was determined by flow cytometry. V indicates vehicle. Results represent the means plus or minus SD for 3 separate experiments performed in triplicate.
Cotreatment with R115777 and Chk1 inhibitors induces a pronounced increase in γH2A.X foci formation and DNA breaks. (A) Primary CD138+ cells were isolated from the bone marrow of a patient (no. 1) with MM. Cells were then either untreated or exposed (16 hours) to 150 nM UCN-01 (UCN) or 2 μM Chk1i in the absence or the presence of 5 μM R115777 (R115). After treatment, cells were harvested and stained with Alexa Fluor (AF) 488–conjugated phospho-H2A.X (Ser139) antibody for immunocytochemical analysis. Images were captured at 60×/1.40 oil. (B) U266 cells were treated with 150 nM UCN-01 or 2 μM Chk1i with or without 5 μM R115777 or 5 μM PD184352 for 24 hours, after which a comet assay was performed to assess DNA breaks. As control, U266 cells were treated with 100 μM hydrogen peroxide for 20 minutes. (C) Tail moment was calculated as the percentage of DNA in the tail and the distance between the means of head and tail distributions. Mean tail moment was determined by measuring at least 100 cells per sample. Results represent the means plus or minus SD for 3 separate experiments. In parallel, the percentage of annexin V+ cells was determined by flow cytometry. V indicates vehicle. Results represent the means plus or minus SD for 3 separate experiments performed in triplicate.
Dominant-negative Ras (S17N) prevents ERK1/2 activation and sensitizes MM cells to induction of γH2A.X expression and lethality mediated by Chk1 inhibitors
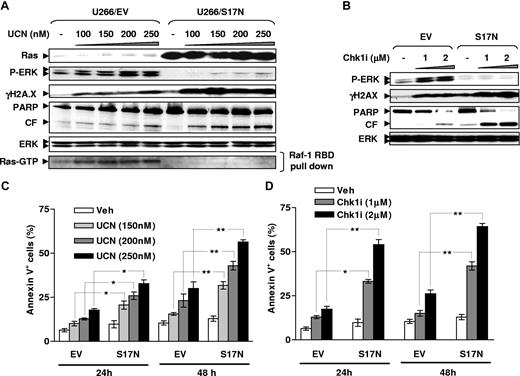
To gain further insights into the functional role of Ras→ERK1/2 signaling in Chk1 inhibitor–mediated DNA damage, U226 cells were stably transfected with a dominant-negative form (S17N) of H-Ras34,35 (Figure 3A top panel). As shown in Figure 3A (bottom panel), ectopic expression of S17N Ras substantially blocked Ras activation induced by UCN-01. Moreover, exposure to Chk1 inhibitors (ie, UCN-01 or Chk1i) failed to induce ERK1/2 phosphorylation in these cells (Figure 3A,B). Significantly, these events were accompanied by a pronounced increase in γH2A.X expression and PARP cleavage compared with EV controls. Consistent with these findings, cells expressing S17N Ras were significantly more sensitive to apoptosis induced by both Chk1 inhibitors than their EV counterparts (Figure 3C,D; P < .05 or P < .01). Collectively, these findings indicate that disruption of Ras signaling plays a significant functional role in potentiating Chk1 inhibitor–induced DNA damage and lethality.
Ectopic expression of dominant-negative Ras (S17N) prevents ERK1/2 activation and enhances γH2A.X expression following exposure to Chk1 inhibitors. (A,B) U266 cells were stably transfected with S17N H-Ras or its empty vector (EV), and ectopic expression of mutant protein was detected by WB (A, top panels). Cells were then exposed to UCN-01 or Chk1i for 24 hours, after which Ras activation assay and WB were performed to monitor Ras activity (A, bottom panel) and expression of phospho-ERK1/2 and γH2A.X, as well as PARP cleavage. The results of representative experiments are shown; 2 additional studies yielded equivalent results. CF indicates cleavage fragment. (C,D) Cells were incubated with UCN-01 or Chk1i for 24 hours and 48 hours, after which percentage of annexin V–FITC–positive cells was determined by flow cytometry. The results represent the means plus or minus SD for 3 separate experiments performed in triplicate. *P < .05; **P < .01.
Ectopic expression of dominant-negative Ras (S17N) prevents ERK1/2 activation and enhances γH2A.X expression following exposure to Chk1 inhibitors. (A,B) U266 cells were stably transfected with S17N H-Ras or its empty vector (EV), and ectopic expression of mutant protein was detected by WB (A, top panels). Cells were then exposed to UCN-01 or Chk1i for 24 hours, after which Ras activation assay and WB were performed to monitor Ras activity (A, bottom panel) and expression of phospho-ERK1/2 and γH2A.X, as well as PARP cleavage. The results of representative experiments are shown; 2 additional studies yielded equivalent results. CF indicates cleavage fragment. (C,D) Cells were incubated with UCN-01 or Chk1i for 24 hours and 48 hours, after which percentage of annexin V–FITC–positive cells was determined by flow cytometry. The results represent the means plus or minus SD for 3 separate experiments performed in triplicate. *P < .05; **P < .01.
MEK1 down-regulation by shRNA significantly enhances Chk1 inhibitor–mediated γH2A.X expression
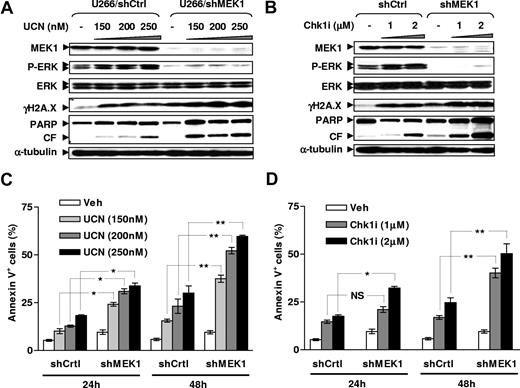
An shRNA approach targeting MEK1, a key mediator for Ras→ERK1/2 signaling,36 was then used to further examine functional relationships between MEK/ERK signaling and DNA damage. As shown in Figure 4, stable transfection of U266 cells with a construct encoding MEK1 shRNA resulted in marked down-regulation of MEK1 expression, accompanied by a pronounced reduction in basal levels of phospho-ERK1/2. Moreover, this approach dramatically diminished ERK1/2 phosphorylation induced by either UCN-01 or Chk1i, accompanied by more pronounced γH2A.X expression as well as PARP cleavage following exposure to either Chk1 inhibitor, compared with control counterparts transfected with a construct encoding a scrambled sequence (Figure 4A,B). Furthermore, MEK1 knockdown significantly sensitized U266 cells to Chk1 inhibitor lethality compared with controls (Figure 4C,D; P < .05 or P < .01). In conjunction with the preceding results, these findings argue strongly that activation of the Ras/MEK/ERK signaling cascade limits DNA damage induced by Chk1 inhibitors, and that blockade of these compensatory responses by targeting either Ras (eg, by FTIs or dominant-negative mutant Ras) or MEK (eg, by MEK1/2 inhibitors or MEK1 siRNA) results in dramatic potentiation of DNA damage and lethality.
MEK1 knockdown by shRNA blocks ERK1/2 activation and sensitizes MM cells to γH2A.X expression and lethality induced by Chk1 inhibitors. (A,B) U266 cells were stably transfected with constructs encoding MEK1 shRNA or a scrambled sequence as a control, and exhibited down-regulation of MEK1 expression by WB (top panels). Cells were then incubated with UCN-01 or Chk1i for 24 hours, after which WB analysis was performed to detect expression of phospho-ERK1/2 and γH2A.X, as well as PARP degradation. Blots were subsequently stripped and reprobed for expression of α-tubulin to ensure equivalent loading and transfer of protein. Additional 2 studies yielded equivalent results. CF indicates cleavage fragment. (C,D) Alternatively, after 24-hour and 48-hour treatment, flow cytometry was performed to monitor apoptosis (annexin V–FITC staining). Results represent the means plus or minus SD for 3 separate experiments performed in triplicate. *P < .05; **P < .01; NS indicates no significance (P > .05).
MEK1 knockdown by shRNA blocks ERK1/2 activation and sensitizes MM cells to γH2A.X expression and lethality induced by Chk1 inhibitors. (A,B) U266 cells were stably transfected with constructs encoding MEK1 shRNA or a scrambled sequence as a control, and exhibited down-regulation of MEK1 expression by WB (top panels). Cells were then incubated with UCN-01 or Chk1i for 24 hours, after which WB analysis was performed to detect expression of phospho-ERK1/2 and γH2A.X, as well as PARP degradation. Blots were subsequently stripped and reprobed for expression of α-tubulin to ensure equivalent loading and transfer of protein. Additional 2 studies yielded equivalent results. CF indicates cleavage fragment. (C,D) Alternatively, after 24-hour and 48-hour treatment, flow cytometry was performed to monitor apoptosis (annexin V–FITC staining). Results represent the means plus or minus SD for 3 separate experiments performed in triplicate. *P < .05; **P < .01; NS indicates no significance (P > .05).
Enforced activation of MEK1/2 by ectopic B-Raf expression prevents R115777 but not PD184352 from potentiating Chk1 inhibitor–mediated γH2A.X expression and lethality
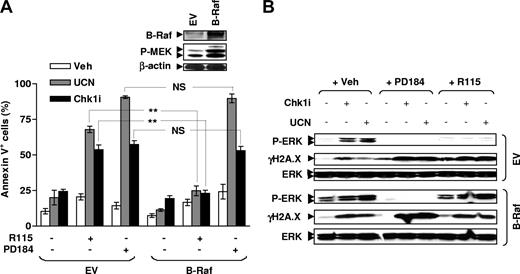
Attempts were made to determine whether enforced MEK1/2 activation at an intermediate level of the Ras/Raf/MEK/ERK signaling cascade (eg, by ectopic B-Raf expression) might differentially affect the ability of FTIs versus MEK1/2 inhibitors to potentiate Chk1 inhibitor–mediated DNA damage and lethality. To this end, U266 cells were stably transfected with a construct encoding full-length B-Raf. As anticipated, ectopic expression of B-Raf led to a marked increase in basal levels of phosphorylated MEK1/2 (Figure 5A inset) and ERK1/2 (Figure 5B).37,38 Notably, enforced activation of MEK1/2 by B-Raf substantially diminished the capacity of the FTI R115777, but not the MEK1/2 inhibitor PD184352, to block ERK1/2 phosphorylation and to potentiate γH2A.X expression induced by either Chk1 inhibitor (Figure 5B). Moreover, these cells also displayed significant resistance to the lethality of the Chk1 inhibitor/R115777 (P < .01 in each case) but not the Chk1 inhibitor/PD184352 (P > .05 in each case) regimen, compared with EV control cells (Figure 5A). Together, these findings suggest that FTIs target signaling events upstream of Raf to attenuate ERK1/2 activation and to promote DNA damage and lethality in MM cells exposed to Chk1 inhibitors, whereas MEK1/2 inhibitors act at a more distal site in the Ras/Raf/MEK/ERK cascade. These observations also provide further support for the notion that interruption of the Ras/Raf/MEK/ERK signaling pathway (eg, by FTIs and MEK1/2 inhibitors) potentiates Chk1 inhibitor–mediated DNA damage and lethality.
Enforced activation of MEK1/2 by B-Raf disables R115777 but not PD184352 to block ERK1/2 activation and to potentiate γH2A.X expression mediated by Chk1 inhibitors. (A) U266 cells were stably transfected with a construct encoding full-length B-Raf, and WB was performed to monitor B-Raf expression as well as resulting MEK1/2 phosphorylation (inset). Blots were subsequently stripped and reprobed using β-actin antibodies to ensure equivalent loading and transfer of proteins. Cells were then exposed to 150 nM UCN-01 or 2 μM Chk1i with or without 5 μM PD184352 or 5 μM R115777 for 48 hours, after which the percentage of annexin V+ cells was determined by flow cytometry. The results represent the means plus or minus SD for 3 separate experiments performed in triplicate. **P < .01; NS indicates no significance (P > .05). (B) Alternatively, after 24-hour exposure to drugs, WB was performed to monitor expression of phospho-ERK1/2 and γH2A.X. Results are representative of 3 separate experiments.
Enforced activation of MEK1/2 by B-Raf disables R115777 but not PD184352 to block ERK1/2 activation and to potentiate γH2A.X expression mediated by Chk1 inhibitors. (A) U266 cells were stably transfected with a construct encoding full-length B-Raf, and WB was performed to monitor B-Raf expression as well as resulting MEK1/2 phosphorylation (inset). Blots were subsequently stripped and reprobed using β-actin antibodies to ensure equivalent loading and transfer of proteins. Cells were then exposed to 150 nM UCN-01 or 2 μM Chk1i with or without 5 μM PD184352 or 5 μM R115777 for 48 hours, after which the percentage of annexin V+ cells was determined by flow cytometry. The results represent the means plus or minus SD for 3 separate experiments performed in triplicate. **P < .01; NS indicates no significance (P > .05). (B) Alternatively, after 24-hour exposure to drugs, WB was performed to monitor expression of phospho-ERK1/2 and γH2A.X. Results are representative of 3 separate experiments.
Coadministration of R115777 with UCN-01 attenuates ERK1/2 activation and increases γH2A.X expression/foci formation in vivo in a MM xenograft model, accompanied by striking induction of apoptosis and suppression of tumor growth
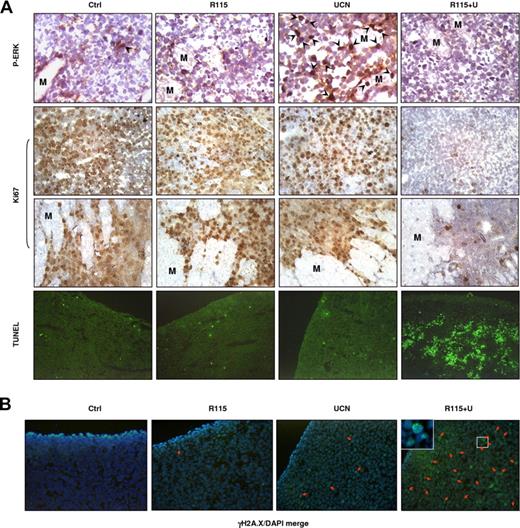
Finally, attempts were made to determine whether the preceding in vitro findings would be operative in vivo. To address this issue, athymic nude mice were inoculated in the flank with human myeloma MM.1S cells, and after tumors were measurable, treated daily intraperitoneally with either vehicle, UCN-01 (0.5 mg/kg), R115777 (25 mg/kg), or both R115777 and UCN-01 for 12 days. As shown in Figure 6, combined treatment with UCN-01 and R115777 resulted in a dramatic suppression of tumor growth during (Figure 6A) and following (Figure 6B) treatment, whereas agents administrated individually exerted only modest inhibitory effects. Similar interactions were observed in an RPMI8226 bioluminescence xenograft model (Figure S4). Moreover, immunohistochemical analysis of tumor sections excised 30 minutes after the final drug doses revealed that UCN-01 administered alone resulted in a clear increase in phospho-ERK1/2 expression in nuclei of tumor cells (arrows), an event dramatically diminished by coadministration of R115777 (Figure 7A top panels). In addition, combined treatment with UCN-01 and R115777 dramatically reduced expression of the cell proliferation marker Ki67 in tumor cells, as well as diminished infiltration of tumor cells into the surrounding muscle (M) tissue, whereas the agents administrated individually had little or no effect (Figure 7A middle panels). Moreover, coadministration of R115777 with UCN-01 resulted in a striking induction of apoptosis in tumor cells, manifested by TUNEL positivity, whereas the agents administered individually had minimal effects (Figure 7A bottom panels). Notably, treatment with UCN-01 alone resulted in only a modest increase in γH2A.X expression (reflected by increased fluorescent intensity of γH2A.X) as well as foci formation (Figure 7B arrows), whereas R115777 administered alone had no discernible effect. However, these phenomena were markedly potentiated when UCN-01 was coadministered with R115777 (Figure 7B). Collectively, these findings suggest that disruption of Ras→ERK1/2 signaling (eg, by the FTI R115777) may also contribute to promotion of DNA damage and lethality induced by Chk1 inhibitors (eg, UCN-01) in vivo.
Cotreatment with R115777 and UCN-01 results in marked tumor growth suppression in a murine xenograft model of MM.1S cells. (A,B) Nude mice were inoculated subcutaneously with 107 MM.1S cells into the right rear flank. After tumors were measurable, 25 mg/kg R115777 with or without 0.5 mg/kg UCN-01 were administrated intraperitoneally daily for 12 days. Tumor size was monitored every 2 days during drug treatment (days 1-12; panel A) and for an additional 12 days after cessation of drug treatment (days 13-24; panel B). Mean tumor volumes are shown (n = 9 per group).
Cotreatment with R115777 and UCN-01 results in marked tumor growth suppression in a murine xenograft model of MM.1S cells. (A,B) Nude mice were inoculated subcutaneously with 107 MM.1S cells into the right rear flank. After tumors were measurable, 25 mg/kg R115777 with or without 0.5 mg/kg UCN-01 were administrated intraperitoneally daily for 12 days. Tumor size was monitored every 2 days during drug treatment (days 1-12; panel A) and for an additional 12 days after cessation of drug treatment (days 13-24; panel B). Mean tumor volumes are shown (n = 9 per group).
R115777 diminishes UCN-01–induced ERK1/2 activation in association with an increase in γH2A.X expression/foci formation and a dramatic induction of apoptosis induced by UCN-01 in vivo. (A) Following the experiments described in Figure 6, tumors were excised after the final dose, and subjected to immunohistochemistry for ERK1/2 phosphorylation and Ki67 expression, as well as TUNEL staining.  indicates nuclear staining of phospho-ERK1/2 in tumor cells. M indicates muscle. (B) Alternatively, tumor tissue sections were stained with Alexa Fluor 488–conjugated γH2A.X antibodies to monitor γH2A.X expression and nuclear foci formation. Arrows indicate cells with γH2A.X foci. Results are representative of 3 separate sets of tumor sections.
indicates nuclear staining of phospho-ERK1/2 in tumor cells. M indicates muscle. (B) Alternatively, tumor tissue sections were stained with Alexa Fluor 488–conjugated γH2A.X antibodies to monitor γH2A.X expression and nuclear foci formation. Arrows indicate cells with γH2A.X foci. Results are representative of 3 separate sets of tumor sections.
R115777 diminishes UCN-01–induced ERK1/2 activation in association with an increase in γH2A.X expression/foci formation and a dramatic induction of apoptosis induced by UCN-01 in vivo. (A) Following the experiments described in Figure 6, tumors were excised after the final dose, and subjected to immunohistochemistry for ERK1/2 phosphorylation and Ki67 expression, as well as TUNEL staining.  indicates nuclear staining of phospho-ERK1/2 in tumor cells. M indicates muscle. (B) Alternatively, tumor tissue sections were stained with Alexa Fluor 488–conjugated γH2A.X antibodies to monitor γH2A.X expression and nuclear foci formation. Arrows indicate cells with γH2A.X foci. Results are representative of 3 separate sets of tumor sections.
indicates nuclear staining of phospho-ERK1/2 in tumor cells. M indicates muscle. (B) Alternatively, tumor tissue sections were stained with Alexa Fluor 488–conjugated γH2A.X antibodies to monitor γH2A.X expression and nuclear foci formation. Arrows indicate cells with γH2A.X foci. Results are representative of 3 separate sets of tumor sections.
Discussion
Chk1 plays a critical role in maintaining genomic integrity by regulating DNA damage–associated checkpoint responses, and represents an attractive therapeutic target.5 UCN-01, a first-generation Chk1 inhibitor, potently blocks Chk1 function at low, submicromolar concentrations,10 an action that can promote DNA damage by itself20 or in combination with DNA-damaging agents.39-41 As UCN-01 exerts pleiotropic actions, including inhibition of PKC, CDKs, and PDK1 in addition to Chk1,10 and because UCN-01's therapeutic effectiveness is limited by human alpha(1)-acid glycoprotein binding,42 efforts to develop newer generation, more specific Chk1 inhibitors are currently under way.43,44 These have focused primarily on disrupting checkpoint responses induced by DNA-damaging agents and ionizing radiation.5,6,43 However, UCN-01 also triggers compensatory activation of the prosurvival Ras/MEK/ERK cascade in various tumor cell types, including human MM cells.13,17 Significantly, interruption of the latter pathway (eg, by MEK1/2 inhibitors,12,13 as well as FTIs15,16 and statins17 ) dramatically increases UCN-01 lethality in human leukemia and MM cells. Although this phenomenon may stem from the pleiotropic actions of UCN-01, the present results demonstrate that a more selective Chk1 inhibitor (Chk1i)23 also triggered activation of both Ras and ERK1/2 in MM cells. Moreover, coadministration of either FTIs or MEK1/2 inhibitors dramatically potentiated Chk1i lethality in MM cells. Very recently, similar phenomena have been observed in the case of AZD7762,45 another specific and clinically relevant Chk1 inhibitor.44 Collectively, such findings argue that Ras/MEK/ERK pathway activation is not restricted to the prototypical Chk1 inhibitor UCN-01, but in all likelihood represent a more generalized response of tumor cells exposed to agents targeting Chk1.
Results of previous reports indicate that Chk1 inhibitors, including UCN-01, not only enhance genotoxic agent-mediated DNA damage,39-41,46 but also induce DNA breaks by themselves, manifested by increased phosphorylation of histone H2A.X at Serine 139 (γH2A.X).20 Thus, induction of DNA damage by Chk1 inhibitors may contribute to their antitumor activity. On the other hand, earlier findings suggested that Ras/MEK/ERK pathway activation represents a cytoprotective response of transformed cells to Chk1 inhibitors such as UCN-01.13,16 In this context, recent evidence that Raf/MEK/ERK signaling is critical to DNA damage responses and DNA repair21 prompted us to examine whether disruption of the Ras/Raf/MEK/ERK signaling cascade might enhance Chk1 inhibitor–induced DNA damage. Notably, coadministration of the FTI R115777 or the MEK1/2 inhibitor PD184352 dramatically increased DNA damage, reflected by both increased γH2A.X expression/foci formation and DNA comet tail appearance, in UCN-01– or Chk1i-treated cells. Significantly, these events closely correlated with blockade of Chk1 inhibitor–induced ERK1/2 activation by FTIs or MEK1/2 inhibitors, and occurred prior to massive apoptosis. It is tempting to speculate that even in the absence of exogenous DNA damage stimuli (eg, DNA damaging agents or radiation), Chk1 plays an important role in protecting cells from DNA breaks or stresses occurring during DNA replication. Indeed, interruption of Chk1 function (ie, by Chk1 siRNA or Chk1 inhibitors) induces DNA breaks, possibly a consequence of aberrant events related to increased initiation and/or elongation of DNA replication.20,41 The finding that interruption of Ras→ERK1/2 signaling dramatically promotes Chk1 inhibitor–mediated DNA damage in MM cells has not, to the best of our knowledge, been previously described, and may provide new insights into the basis for synergism between these agents.
The mechanism(s) responsible for potentiation of Chk1 inhibitor lethality by Ras/MEK/ERK pathway disruption may reflect multiple interrelated events. For example, ERK1/2 activation exerts cytoprotective actions through posttranslational modification of several components of the apoptotic pathway (eg, phosphorylation/inactivation of caspase-947 ) or proapoptotic Bcl-2 family members (eg, Bad48 ). Notably, interruption of the Ras/ERK pathway by pharmacologic or genetic approaches (ie, cells expressing S17N Ras) increased Chk1 inhibitor–mediated cdc2 activation (Y.D. and S.G., unpublished results, May 2008), an event associated with apoptosis.47 In addition, the ability of MEK1/2 inhibitors to potentiate UCN-01 lethality in MM cells has very recently been linked to diminished BimEL phosphorylation and degradation.49 Interestingly, MEK1/2 inhibitors administered alone at concentrations that substantially increase Bim expression are minimally lethal to MM cells.49 This suggests that Bim up-regulation by itself may be insufficient to trigger apoptosis, but instead lowers the lethal threshold for other events, such as DNA damage and/or cdc2 activation. Studies designed to test this hypothesis are under way.
The present findings argue strongly that the Ras/Raf/MEK/ERK pathway plays an important functional role in regulating Chk1 inhibitor–related DNA damage in MM cells. For example, ectopic expression of dominant-negative Ras (S17N) or MEK1 knockdown by shRNA largely recapitulated the capacity of R115777 and PD184352 to prevent Chk1 inhibitor–mediated ERK1/2 activation, thereby sensitizing MM cells to DNA damage and lethality. Such findings provide genetic evidence that both Ras and MEK1 are required for Chk1 inhibitor–mediated ERK1/2 activation and attenuation of DNA damage. Moreover, enforced MEK1/2 activation by B-Raf substantially diminished the ability of FTIs to block Chk1 inhibitor–induced ERK1/2 activation and protected MM cells from R115777/Chk1 inhibitor–induced DNA damage and lethality. In marked contrast, PD184352, by targeting MEK1/2, which acts directly downstream of Raf,36 circumvented the protective effects of ectopic B-Raf expression. Together, such findings suggest that activation of Ras/Raf/MEK/ERK signaling represents a self-protective response limiting DNA damage in Chk1 inhibitor–treated cells. Currently, the mechanism(s) by which Ras/MEK/ERK pathway interruption potentiates DNA damage by Chk1 inhibitors or by other genotoxic stimuli remains to be determined. In this context, it has been observed that Chk1 inhibition by either Chk1 inhibitors or Chk1 siRNA promotes DNA breaks through an ATR-dependent mechanism.20 Moreover, Chk1 is required for the repair of DNA double-stranded breaks.50,51 In addition, the Raf/MEK/ERK pathway has been linked to DNA repair through an ATM-dependent process.21 Thus, one plausible explanation for the present findings is that interference with the Ras/Raf/MEK/ERK signaling cascade disrupts DNA repair processes, thereby potentiating DNA damage induced by Chk1 inhibitors. Whatever the basis for this interaction, the observation that disruption of Ras→ERK1/2 signaling (eg, by R115777) dramatically potentiated UCN-01–mediated DNA damage and apoptosis in murine xenograft MM models argues that such mechanisms do not simply reflect in vitro phenomena, but are also operative in vivo.
Despite encouraging preclinical results, the clinical promise of FTIs remains to be fulfilled,14,52 and the lack of correlation between FTI responsiveness and Ras mutational status raises the possibility of alternative targets (eg, Rho proteins53 ). In the present study, genetic disruption of Ras function (eg, by dominant-negative S17N Ras) largely prevented Chk1 inhibitor–induced ERK1/2 activation and sensitized MM cells to these agents. Moreover, ectopic expression of B-Raf largely prevented R115777 from blocking ERK1/2 activation and enhancing DNA damage in MM cells exposed to Chk1 inhibitors. Such genetic evidence indicates that Chk1 inhibitors induce ERK1/2 activation via a Ras-dependent mechanism, and that FTIs act through interruption of the Ras/Raf/MEK/ERK cascade to promote Chk1 inhibitor–mediated DNA damage and lethality. In this context, members of the Ras family are frequently mutated/activated in human cancers,14,54 including MM (ie, 40%, increasing to 70% at relapse).55,56 A question arises whether Ras mutational status might influence MM cell responses to strategies combining Chk1 inhibitors with agents targeting the Ras→ERK1/2 signaling cascade, particularly inhibitors of upstream (eg, FTIs) versus downstream (eg, MEK1/2 inhibitors) events. However, the observations that the R115777/ and PD184352/Chk1 inhibitor regimens displayed similar activity toward MM cells with disparate Ras status, for example, RPMI8226 (activated K-Ras), U266 (wild-type Ras), and H929 (activated N-Ras),22 argue against this possibility.
In summary, the present findings suggest that activation of the Ras→ERK1/2 signaling cascade plays an important cytoprotective role in limiting Chk1 inhibitor–mediated DNA damage in MM cells. They also indicate that interruption of this pathway by FTIs or MEK1/2 inhibitors enhances Chk1 inhibitor lethality through a novel mechanism (ie, potentiation of DNA damage). Previous studies implicating the MEK/ERK pathway in regulating checkpoint responses have largely focused on DNA damage–related stimuli (eg, DNA-damaging agents or ionizing radiation).21,57,58 However, it is noteworthy that even absent such noxious stimuli, interruption of Chk1 function (eg, by Chk1 inhibitors or Chk1 siRNA) induces DNA breaks.20 The present results suggest that Ras→ERK1/2 activation provides a cellular defense permitting cells to survive the lethal consequences of Chk1 inhibition (eg, DNA damage), possibly by promoting DNA repair. Such findings provide a mechanistic basis for an alternative Chk1 inhibitor–based strategy that targets this defensive response. Finally, defective cell- cycle checkpoints characteristic of transformed cells3 may render them uniquely susceptible to such a strategy. In this context, the frequency of genetic abnormalities involving cell-cycle regulatory proteins in MM,59,60 as well as the genetic instability of such cells,60,61 may make the use of checkpoint antagonists particularly attractive in MM. Analogously, the Ras/MEK/ERK signaling cascade is a key pathway implicated in MM cell survival signaling mediated by IL-6,62,63 IGF-1,64 and stromal cells,65 and represents a promising candidate for therapeutic intervention.66,67 Importantly, simultaneous disruption of Chk1 and the Ras/Raf/MEK/ERK pathway (eg, by either FTIs or MEK1/2 inhibitors) has demonstrated selective toxicity toward neoplastic cells, including primary MM cells, in vitro.13,15,16 The in vivo activity of the R115777/UCN-01 regimen, as well as the preferential induction of γH2A.X in primary CD138+ MM cells versus CD138− normal cells, further supports this notion. Aside from providing insights into novel mechanisms (ie, potentiation of DNA damage) underlying interactions between agents targeting the checkpoint machinery and those blocking the Ras→ERK signaling cascade, such findings could have therapeutic implications.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by awards CA63753, CA93738, CA100866, CA88906, and CA72955 from the National Institutes of Health (NIH, Bethesda, MD), a Translational Research Award from the Leukemia & Lymphoma Society of America (New York, NY; 6045-03), an award from the Department of Defense (Washington, DC; DAMD-17-03-1-0209), an award from the V Foundation (Cary, NC), and the Universal Professorship (P.D.).
National Institutes of Health
Authorship
Contribution: Y.D. designed and performed the research, analyzed the data, and wrote the paper; S.C. designed and performed the research, and analyzed the data; X.-Y.P., J.A.A., L.B.K., and C.A.V. helped to perform the research; P.D. helped to design the research; and S.G. designed the research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Steven Grant, Division of Hematology/Oncology, Virginia Commonwealth University/Medical College of Virginia, MCV Station Box 230, Richmond VA, 23298; e-mail: stgrant@vcu.edu.