Abstract
The partial tandem duplication of MLL (MLL-PTD) is found in 5% to 10% of patients with acute myeloid leukemia (AML) and normal cytogenetics. Its expression in leukemic blasts is coincident with a silenced wild-type (WT) MLL allele. We therefore generated mice expressing the Mll-PTD in the absence of Mll-WT. These MllPTD/− mice die at birth unlike the normal life expectancy of MllPTD/WT, MllWT/−, and MllWT/WT mice. Using MllWT/WT fetal liver cells (FLC) as baseline, we compared MllPTD/− with MllPTD/WT FLC and found both had increased HoxA gene expression and granulocyte-macrophage colony-forming progenitor cells (CFU-GM); in contrast, only MllPTD/WT FLC had increased pluripotent hemopoietic progenitors (CFU-GEMM). The similarities between MllPTD/WT and MllPTD/− mice suggest that the Mll-PTD mutation can up-regulate target genes in a dominant, gain-of-function fashion. The differences between these 2 genotypes suggest that in select tissues the Mll-PTD requires cooperation with the Mll-WT in the genesis of the observed abnormality.
Introduction
Approximately 5% to 10% of patients with acute myeloid leukemia (AML) and normal cytogenetics present with rearrangement of the mixed-lineage leukemia (MLL, also known as ALL1 or HRX) gene as the result of a partial tandem duplication (PTD) within a single MLL allele.1,2 In AML blasts harboring the somatic MLL PTD mutation, the MLL wild-type (WT) allele is not expressed and, when reexpressed, leukemic cell death was observed.3 We previously reported on the MllPTD/WT knockin mice that are fully viable with modest developmental defects, have aberrant gene expression and altered hematopoiesis, but do not develop leukemia.4 These mice express both the Mll PTD and WT Mll alleles. Thus, to partially recapitulate what is observed in human primary AML blasts regarding the MLL-PTD and absence of MLL-WT expression, we generated mice that harbor a Mll-PTD but lack Mll-WT (MllPTD/−) in the germ line. We then asked how loss of function of Mll-WT in the context of Mll-PTD would affect HoxA gene expression and hematologic abnormalities previously observed in MllPTD/WT mice and, eventually, occurrence of leukemia.
Methods
Generation of MllPTD/− mice
MllWT/− mice5 were generously provided by the late Dr Stanley Korsmeyer (Dana-Farber Cancer Institute, Boston, MA). These mice were maintained on a B6C3F1 background. To obtain MllPTD/− mice, F1 offspring were obtained by crossing the MllWT/− mice with the MllPTD/WT mice4 (maintained on a pure C57Bl/6J background). This work was performed with approval of The Ohio State University institution review board and under an Institutional Animal Care and Use Committee–approved proposal.
Comparative real-time RT-PCR
Total RNA was extracted from E17.5 fetal liver cells (FLC) from MllPTD/WT, MllPTD/−, MllWT/−, and MllWT/WT embryos. Comparative real- time reverse transcription–polymerase chain reaction (RT-PCR) assays on whole fetal liver and c-kit+- and CD11b+-sorted populations were performed as previously described.4,6
Chromatin immunoprecipitation
H3 (Lys4) dimethylation has been shown to occur as a direct result of MLL's SET domain methyltransferase activity.4,7 Therefore, chromatin immunoprecipitation (ChIP) assays were performed on 2 × 106 FLC using the EZ ChIP Assay Kit with the anti-dimethyl Histone H3 (Lys4) antibody (Millipore, Billerica, MA) according to the manufacturer's standard protocol. DNA was quantified using PCR and nested real-time quantitative PCR with SYBR green incorporation (Applied Biosystems, Foster City, CA) using previously described methods.4
Colony forming unit–progenitor assays
Single cell suspensions were plated at a density of 50 000 cells/dish in M3434 methylcellulose (StemCell Technologies, Vancouver, BC), and were performed according to the manufacturer's protocol (StemCell Technologies) and methods as previously described.4
Statistics
To evaluate whether significant differences in pluripotent hemopoietic progenitors (colony forming unit [CFU]–GEMM), CFU-GM, or burst forming unit-erythroid (BFU-E) existed between mouse genotypes as indicated in the Figure 1 legend, paired t tests were carried out using siblings.
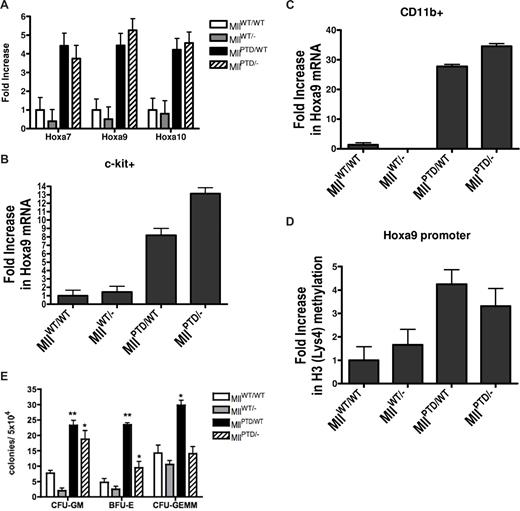
Phenotypic characterization of the MllPTD/− mice. (A) Increased HoxA gene expression in MllPTD/− E17.5 FLC. Using real-time RT-PCR, Hoxa7, Hoxa9, and Hoxa10 were shown to be overexpressed in E17.5 FLC from MllPTD/− and MllPTD/WT embryos compared with both MllWT/− and MllWT/WT littermate controls. Error bars show standard deviation. Equivalent numbers of (B) c-kit+ and (C) CD11b+ FLC were sorted with more than 95% purity from MllWT/WT, MllWT/−, MllPTD/WT, and MllPTD/− E17.5 embryos. Using real-time RT-PCR, increases in Hoxa9 were measured in MllPTD/WT and MllPTD/− cells but not in MllWT/WT and MllWT/− cells. Error bars show standard deviation. (D) ChIP experiments using an anti-H3 dimethylated antibody showed increases in the levels of H3 (Lys4) methylation at the Hoxa9 promoter in both MllPTD/WT and MllPTD/− FLC compared with MllWT/WT and MllWT/− controls. Error bars show standard deviation. (E) E17.5 fetal liver hematopoietic progenitor populations were assessed using CFU assays. MllPTD/WT mice showed increases in CFU-GM, BFU-E, and CFU-GEMM compared with MllWT/WT, while MllPTD/− mice showed increases in CFU-GM and BFU-E compared with MllWT/WT. Notably, MllPTD/− mice showed substantially lower increases in BFU-E compared with MllPTD/WT mice. Error bars represent standard error of the means. *P < .05, **P < .01.
Phenotypic characterization of the MllPTD/− mice. (A) Increased HoxA gene expression in MllPTD/− E17.5 FLC. Using real-time RT-PCR, Hoxa7, Hoxa9, and Hoxa10 were shown to be overexpressed in E17.5 FLC from MllPTD/− and MllPTD/WT embryos compared with both MllWT/− and MllWT/WT littermate controls. Error bars show standard deviation. Equivalent numbers of (B) c-kit+ and (C) CD11b+ FLC were sorted with more than 95% purity from MllWT/WT, MllWT/−, MllPTD/WT, and MllPTD/− E17.5 embryos. Using real-time RT-PCR, increases in Hoxa9 were measured in MllPTD/WT and MllPTD/− cells but not in MllWT/WT and MllWT/− cells. Error bars show standard deviation. (D) ChIP experiments using an anti-H3 dimethylated antibody showed increases in the levels of H3 (Lys4) methylation at the Hoxa9 promoter in both MllPTD/WT and MllPTD/− FLC compared with MllWT/WT and MllWT/− controls. Error bars show standard deviation. (E) E17.5 fetal liver hematopoietic progenitor populations were assessed using CFU assays. MllPTD/WT mice showed increases in CFU-GM, BFU-E, and CFU-GEMM compared with MllWT/WT, while MllPTD/− mice showed increases in CFU-GM and BFU-E compared with MllWT/WT. Notably, MllPTD/− mice showed substantially lower increases in BFU-E compared with MllPTD/WT mice. Error bars represent standard error of the means. *P < .05, **P < .01.
Results and discussion
Results of the comparative analysis among the MllWT/WT, MllWT/−, MllPTD/WT and MllPTD/− mice are summarized in Table 1. In terms of survival, the first 3 genotypes were all viable and born at expected Mendelian ratios, however, although present at normal Mendelian ratios, 100% of the pups having the MllPTD/− genotype died by postpartum day 1 (P1). Interestingly, Mll−/− mice are also nonviable, but die around embryonic day 10.5 (E10.5).5 These results indicate the Mll PTD itself provides some, albeit insufficient, compensation for embryonic development in the absence of both Mll WT alleles.
Comparison of Mll genotypes
| Characteristic . | MllPTD/WT . | MllPTD/− . | MllWT/− . | MllWT/WT . |
|---|---|---|---|---|
| Premature death | no | yes* | no | no |
| HoxA expression | increased | increased | decreased but NS | normal |
| Increased H3 (Lys4) | yes | yes | no | no |
| Increased BFU-E | yes | yes | no | — |
| Increased CFU-GEMM | yes | no | no | — |
| Increased CFU-GM | yes | yes | no | — |
| Characteristic . | MllPTD/WT . | MllPTD/− . | MllWT/− . | MllWT/WT . |
|---|---|---|---|---|
| Premature death | no | yes* | no | no |
| HoxA expression | increased | increased | decreased but NS | normal |
| Increased H3 (Lys4) | yes | yes | no | no |
| Increased BFU-E | yes | yes | no | — |
| Increased CFU-GEMM | yes | no | no | — |
| Increased CFU-GM | yes | yes | no | — |
NS indicates not significant; BFU-E, erythroid progenitors; and —, not applicable.
Pups died by postpartum day 1.
With regards to HoxA gene expression, we found that the Mll PTD is required for aberrant overexpression of HoxA genes in E17.5 FLC. MllPTD/WT and MllPTD/− mice showed nearly equivalent levels of HoxA over-expression in unsorted FLC, while MllWT/− cells expressed HoxA levels that were consistently lower but not significantly different from the expression levels found in MllWT/WT FLC (Figure 1A). To determine whether the overexpression of Hoxa9 was on a per cell basis rather than an increase in a Hox-expressing subpopulation within the unsorted FLC, we sorted out equivalent numbers of c-kit+ (Figure 1B) and CD11b+ (Figure 1C) cells from MllPTD/WT, MllPTD/−, MllWT/WT and MllWT/− E17.5 fetal livers. Significantly increased levels of Hoxa9 were found in the MllPTD/WT- and MllPTD/−-sorted FLC but not in MllWT/WT- or MllWT/−-sorted FLC. In fact, MllWT/− CD11b+ cells had no detectable levels of Hoxa9 transcript. These results support the notion that the overexpression of HoxA is occurring on a per cell basis within the fetal liver. Furthermore, ChIP assays demonstrate that the presence of the Mll PTD is associated with increased H3 (Lys4) methylation at the Hoxa9 promoter in the presence or absence of the Mll-WT allele (Figure 1D). This increased H3 (Lys4) methylation likely accounts for the observed HoxA gene overexpression in both MllPTD/WT and MllPTD/− FLC.
Although premature death of the MllPTD/− mice precluded assessment of leukemia development, we did examine fetal livers for any alterations in normal hematopoiesis. We performed CFU assays to assess fetal hematopoietic liver function in vitro using E17.5 FLC obtained from each of the 4 genotypes. Significant increases in the CFU-GM, BFU-E, and the more immature CFU-GEMM were seen in cells obtained from the MllPTD/WT mice compared with MllWT/WT mice (P < .01, P < .01, and P < .05, respectively), suggesting that the Mll PTD may cooperate with the Mll WT allele at an early stage of hematopoiesis such as the common myeloid progenitor cell. In contrast, FLC obtained from MllPTD/− mice had increases in the CFU-GM and BFU-E populations compared with MllWT/WT mice (P < .05 and P < .05, respectively), but not in the CFU-GEMM population (Figure 1E). Finally, FLC obtained from MllPTD/− mice had a significantly lower number of BFU-E progenitors compared with MllPTD/WT FLC. Together these results are consistent with the notion that the Mll PTD itself is required and sufficient for abnormal expansion at some stages of progenitor cell differentiation but may not be at other stages. These results also support 2 recent reports that showed the role of Mll WT in hematopoietic stem cells is distinct from its role in hematopoietic progenitor cells.8,9
Similar phenotypic abnormalities observed in MllPTD/− and MllPTD/WT genotypes support the notion that the Mll PTD by itself is capable of dysregulating downstream targets and can therefore behave as a dominant gain-of-function mutation in the absence of the Mll WT. Although these studies suggest a direct role for the Mll PTD, it is important to note that a more stable (lacZ fused) Mll protein containing several important N-terminal Mll functional motifs exists in the knockout model. Because our heterozygous animals exhibit a more severe phenotype than other (non-lacZ fused) Mll heterozygous knockout mice,8,9 we cannot exclude the possibility that the knockout allele acts in an interfering manner.
Mll has now been shown to have very different functions in different subpopulations in the hematopoietic compartment.10 Our results suggest that in some cases Mll function may have been lost and cannot be replaced by the Mll PTD allele, as in the case of MllPTD/− early lethality at P1. However, in some cases such as HoxA gene overexpression and CFU-GM expansion, the Mll PTD appears to behave more as a dominant gain-of-function mutation because the quantifications performed for these experiments were similar between the MllPTD/WT and MllPTD/− genotypes, ie, in the presence and absence of Mll WT, respectively. In contrast, differences in the number of BFU-E and CFU-GEMM progenitors seen between the MllPTD/WT and MllPTD/− genotypes reveal the lack of dominant activity by the Mll PTD allele. In this tissue-specific context, the Mll PTD may require interaction with the Mll WT in order to achieve the maximum manifestation of the abnormality.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
This work was funded in part by the Lady Tata Memorial Trust (London, United Kingdom) Award (to A.M.D.) and National Cancer Institute (Bethesda, MD) grant funding (R01 CA89341 to M.A.C.).
National Institutes of Health
Authorship
Contribution: A.M.D. and M.A.C. designed experiments, analyzed and interpreted data, and cowrote the manuscript; A.M.D., S.L., A.C., B.R.P., D.N., M.G., W.Y., and D.C. performed experiments; and S.P.W. and G.M. provided intellectual expertise and careful review and editing of the manuscript. All authors agreed on the final text version.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Michael A. Caligiuri, MD, 458A Starling-Loving Hall, West 10th Avenue, Columbus, OH, 43210; e-mail: Michael.caligiuri@osumc.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal