Abstract
Interactions between chemokines and enzymes are vital in immunoregulation. Structural protein citrullination by peptidylarginine deiminase (PAD) has been associated with autoimmunity. In this report, we identified a novel naturally occurring posttranslational modification of chemokines, that is, the deimination of arginine at position 5 into citrulline of CXC chemokine ligand 10 (CXCL10) by rabbit PAD and human PAD2. Citrullination reduced (≥ 10-fold) the chemoattracting and signaling capacity of CXCL10 for CXC chemokine receptor 3 (CXCR3) transfectants; however, it did not affect CXCR3 binding. On T lymphocytes, though, citrullinated CXCL10 remained active but was again weaker than authentic CXCL10. PAD was also able to convert CXCL11, causing an impairment of CXCR3 signaling and T-cell activation, though less pronounced than for CXCL10. Similarly, receptor binding properties of CXCL11 were not altered by citrullination. However, deimination decreased heparin binding properties of both CXCL10 and CXCL11. Overall, chemokines are the first immune modulators reported of being functionally modified by citrullination. These data provide new structure-function dimensions for chemokines in leukocyte mobilization, disclosing an anti-inflammatory role for PAD. Additionally because citrullination has severe consequences for chemokine biology, this invites to reassess the involvement and impact of PAD and citrullinated peptides in inflammation, autoimmunity, and hematologic disorders.
Introduction
Chemokines are a family of chemoattractive cytokines that regulate the recruitment of leukocytes toward inflammatory sites. Chemokines also exert homeostatic properties in cell migration during development and immunosurveillance and affect angiogenesis as well as tumor growth.1,2 Based on the pattern of conserved cysteine residues in their amino acid structure, chemokines are classified into CXC, CC, CX3C, and C subfamilies.1,3,4 Chemokines exhibit redundancy in their binding and signaling capacities in that the ligands can interact with more than one chemokine receptor and vice versa.5 CXC chemokine ligand 10 (CXCL10) or interferon-γ–inducible protein-10 (IP-10) is a potent attractant of lymphocytes and natural killer cells, recognized by CXC chemokine receptor 3 (CXCR3), a 7-transmembrane spanning G protein–coupled receptor (GPCR).6 Interestingly, CXCL10 also exerts angiostatic properties depending on CXCR3; however, the exact mechanism is still unclarified.7 It was also suggested that CXCL10 binds to another unidentified receptor that does not interact with other CXCR3 ligands, that is, interferon T cell α-chemoattractant (I-TAC/CXCL11) and monokine induced by interferon-γ (Mig/CXCL9).8 CXCL11, on the other hand, was found to bind a second receptor, that is, CXCR7.9 CXCR7 was reported to be expressed in various transformed cells and tumor development of human lymphoma or carcinoma in mice was diminished by treatment with a CXCR7 antagonist.9-11 Recently, a function in the coordination of cell migration was also appointed to CXCR7 in cooperation with CXCR4 in zebrafish development.12,13
Chemokines generate the release of proteases but also act as substrates, resulting in an altered chemokine response.14 NH2-terminal cleavage of CXCL10 and CXCL11 by dipeptidyl peptidase IV (DPP-IV/CD26) impairs their CXCR3 signaling, and chemoattracting capacity, however, does not interfere with their antiangiogenic character.15 Moreover, on further cleavage by aminopeptidase N/CD13, the angiostatic properties of CXCL11 were strongly reduced.16 In addition, tyrosine sulfation of CXCR3 was described as a posttranslational receptor modification required for binding and activation of all 3 CXCR3 ligands.17
CXCR3 and its ligands have been shown to be of importance in many severe diseases, such as rheumatoid arthritis (RA), type I diabetes, multiple sclerosis (MS), and cancer.2,18-20 However, elevated chemokine protein levels or coexpression of chemokines together with disease markers is more difficult to interpret if posttranslational modifications of these chemokines can be fast and alter the biologic activity. In experiments designed to identify multiple chemokine variants, we encountered a novel naturally occurring chemokine modification, that is, the deimination of arginine (Arg) at position 5 of CXCL10 into citrulline (Cit). This conversion was confirmed to be initiated by peptidylarginine deiminase (PAD), which colocalizes with citrullinated peptides in the synovium in RA and for which in epidemiologic studies an association with susceptibility for RA was determined.21,22 Moreover, analysis of anticitrullinated peptide antibodies (ACPAs) is applied for RA diagnosis, and these ACPAs are even detectable in serum of RA patients up to 9 years before disease onset.23 Furthermore, protein levels of CXCL10 were described to be significantly elevated in synovial fluid of autoimmune arthritis patients.18,24 Here, we show that CXCL10 was rapidly deiminated by human PAD2, which is considered to play an imperative role in the pathogenesis of MS.25 These observations suggest an important role for citrullinated CXCL10 in inflammation and in the development of autoimmunity because CXCL10 is an angiostatic predominant T helper 1 chemoattractant. To assess the universality and physiologic relevance of this novel chemokine modification in the inflammatory response, we investigated the effects of citrullination on a series of biologic activities of both CXCL10 and CXCL11 and found rather severe and diverse consequences emerging in a chemokine- and cell type–dependent regulation by PAD.
Methods
Reagents and cell lines
Recombinant human interferon-γ (IFN-γ), CXCL10, and IL-2 were obtained from PeproTech (Rocky Hill, NJ). Double-stranded (ds) RNA polyriboinosinic:polyribocytidylic acid (polyrI:rC) and PAD purified from rabbit skeletal muscle (200 units/mg) were purchased from Sigma-Aldrich (St Louis, MO). Recombinant human PAD2 was from ModiQuest Research (Nijmegen, The Netherlands). Recombinant human CXCL11 was from R&D Systems (Minneapolis, MN). Chinese hamster ovary cells transfected with CXCR3A (CHO-CXCR3) or CXCR7 (kindly provided by M. Parmentier) and human astroglioma U87 cells transfected with human CXCR3 (U87-CXCR3; kindly provided by D. Schols) were cultured as previously described.15,26
Leukocyte isolation and production of natural chemokines
Leukocytes were isolated from fresh human buffy coats (blood transfusion center of Leuven) as previously described.27 In brief, erythrocytes were removed by sedimentation in hydroxyethyl-starch (Plasmasteril; Fresenius, Bad Homburg, Germany). Mononuclear cells and granulocytes were segregated by gradient centrifugation on Ficoll-sodium metrizoate (Lymphoprep; Nycomed, Oslo, Norway). Activated T cells were obtained by stimulating the purified peripheral blood mononuclear cells (PBMCs) from individual donors with 2 μg/mL phytohemoagglutinin (PHA; Sigma-Aldrich) for 2 to 5 days in RPMI 1640 (Lonza Verviers SPRL, Verviers, Belgium) enriched with 10% fetal bovine serum (FBS) and 0.05% (wt/vol) gentamycin (Invitrogen, Carlsbad, CA). Subsequently, cells were washed with medium and cultured for 10 days with 50 U/mL IL-2. T-cell chemotaxis, calcium signaling, and receptor binding experiments were performed 2 to 3 weeks after PHA activation of the PBMCs and 2 days after the last IL-2 stimulation. Alternatively, for chemokine production, PBMCs from 24 buffy coats were pooled (11.4 × 109 cells) and induced at 5 × 106 cells/mL with 10 μg/mL polyrI:rC and 20 ng/mL IFN-γ in RPMI 1640 containing 2% FBS. The conditioned media were stored at −20°C until purification.
Purification of natural chemokines
A 4-step purification procedure was performed to purify natural human chemokines to homogeneity. In brief, leukocyte-derived conditioned media were first concentrated to controlled pore glass by adsorption.27 Next, the concentrated proteins were further purified by heparin-Sepharose affinity chromatography and Mono S cation exchange chromatography (GE Healthcare, Little Chalfont, United Kingdom). A specific sandwich enzyme-linked immunosorbent assay (ELISA) was carried out to detect CXCL10 in column fractions as previously described.18 Finally, the CXCL10-containing fractions were subjected to reversed-phase high-performance liquid chromatography (RP-HPLC; 2.1 × 220 mm Brownlee C8 Aquapore RP-300 column; PerkinElmer Life and Analytical Sciences, Waltham, MA) to acquire homogeneously purified chemokine. Proteins were eluted in an acetonitrile gradient in 0.1% trifluoroacetic acid and UV absorption was monitored at 214 nm. The RP-HPLC column effluent containing natural CXCL10 was split (1/150) online to an electrospray ion trap mass spectrometer (Esquire LC; Bruker, Bremen, Germany) and collected in 400-μL fractions. Purity of the fractions was evaluated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis under reducing conditions on Tris/tricine gels, and proteins were visualized by silver staining.28
Identification of posttranslational modifications
During the elution from the RP-HPLC column, profile mass spectra were collected every 0.1 seconds and averaged profile spectra were calculated over the UV absorption peaks that contained CXCL10 immunoreactivity. The measured average relative molecular mass (Mr) was compared with the theoretical average Mr of CXCL10 calculated using the known primary structure of CXCL10. In addition, the NH2-terminal sequence of the isolated chemokines was determined by Edman degradation (491cLC Procise protein sequencer; Applied Biosystems, Foster City, CA).
In vitro citrullination of CXCL10 by PAD
CXCL10 (100 pmol) was incubated with rabbit PAD or human PAD2 corresponding to an enzyme-substrate molar ratio (E/S) of 1:20 or 1:200 in 40 mM Tris (pH 7.4) supplemented with 2 mM CaCl2 for 2, 5, 10, and 30 minutes at 37°C. Deimination was stopped with 0.1% trifluoroacetic acid, and samples were split and desalted on C4 ZipTip (Millipore, Billerica, MA) before mass spectrometry or in parallel spotted on polyvinylidene difluoride membranes (ProSorb; Applied Biosystems) before Edman degradation. For use in bioassays, recombinant CXCL10 was incubated with rabbit PAD for 90 minutes at an E/S of 1:20 and citrullinated CXCL10 was purified by RP-HPLC on a C8 Aquapore RP-300 column (50 mm) where 2% of the flow was converted online to the mass spectrometer.
In vitro chemotaxis assays
CHO-CXCR3 cells or T cells were suspended at 2 × 106 cells/mL in Hanks balanced salt solution (HBSS; Invitrogen) supplemented with 0.1% (wt/vol) human serum albumin and added to the upper wells of a Boyden microchamber (NeuroProbe, Gaithersburg, MD).16 Test samples were applied to the lower wells and separated from the upper compartments by a polyvinylpyrrolidone-free polycarbonate filter (Nuclepore, Pleasanton, CA) with a pore size of 8 μm for CHO cells or 5 μm for T cells. For T cells, the filters were precoated with 20 μg/mL fibronectin (Invitrogen). The microchambers were incubated for 2 hours at 37°C for T cells or at 32°C for CHO cells followed by fixation and staining of the membranes with Hemacolor staining solutions (Merck, Darmstadt, Germany). The cells were counted microscopically at 500× magnification in 10 oil-immersion fields. The chemotactic index was determined by dividing the number of migrated cells with the number of spontaneously migrated cells toward the sample dilution buffer (HBSS + 0.1% human serum albumin). The lowest concentration that is required for statistically significant chemotaxis is called the minimal effective concentration.
Signal transduction assays
Phosphorylation of extracellular signal-regulated kinase 1 and 2 (ERK1/2) and protein kinase B (PKB)/AKT in response to chemokine treatment was measured as previously described.18 In brief, CHO-CXCR3 or CHO-CXCR7 cells were stimulated with test samples for 5 minutes followed by cell lysis and subsequent centrifugation. The protein concentration in the supernatant was determined by the bicinchoninic acid protein assay (Pierce Chemical, Rockford, IL), and the amount of ERK1/2 and PKB/AKT phosphorylation was examined by a specific ELISA for phosphorylated ERK1 and 2 (R&D Systems; pg phospho-ERK1/2 per mg of total protein) and for phospho-AKT (R&D Systems).
Changes in intracellular calcium concentration ([Ca2+]i) were measured by fluorescence spectrometry on an LS50B spectrofluorimeter (PerkinElmer Life and Analytical Sciences) as previously described.16 In brief, cells were loaded with the ratiometric fluorescent dye Fura-2/AM (Invitrogen) for 30 minutes at 37°C in the presence (for CHO and T cells) of 125 μM probenecid (MP Biomedicals, Irvine, CA). The cells were washed and resuspended in HBSS containing 1 mM Ca2+ (+Mg2+), 0.1% FBS, 10 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, pH 7.0, and 125 μM probenecid (only for CHO and T cells) at a concentration of 106 CHO or U87 cells/mL or 10 × 106 T cells/mL. Subsequently, the cells were equilibrated at a temperature of 37°C for lymphocytes and U87 cells or at 32°C for CHO cells. Fura-2 fluorescence was measured at 510 nm on excitation at 340 nm and 380 nm.
Peptide synthesis
Intact CXCL11 and CXCL11 with a citrulline residue on position 6 were synthesized by solid phase peptide synthesis with fluorenylmethoxycarbonyl protected α-amino groups using a 433A peptide synthesizer (Applied Biosystems) as previously described.16 The synthetic peptides were purified on a 4.6 × 150 mm Source 5RPC column (GE Healthcare) and detected by UV absorption at 220 nm. The average Mr was determined by online ion trap mass spectrometry. The peptides with the correct Mr were folded overnight, repurified, and analyzed by online mass spectrometry. The NH2-terminal sequence was confirmed by Edman degradation, and the protein concentration was determined using the bicinchoninic acid protein assay.
Binding assays
Receptor binding properties were determined by competition for 125I-labeled CXCL10 or CXCL11 binding on CHO-CXCR3, CHO-CXCR7, or T cells as previously described.15 In brief, 2 × 106 cells were incubated for 2 hours at 4°C with 125I-labeled (GE Healthcare) and unlabeled chemokine. Subsequently, the cells were centrifuged and washed 3 times, and radioactivity was measured.
Heparin binding was assessed following the manufacturer's instructions by immobilizing 25 μg/mL of low-molecular-weight heparin (Sigma-Aldrich) diluted in phosphate-buffered saline overnight at room temperature on EpranEx plates (Plasso Technology, Sheffield, United Kingdom). The plates were then washed 3 times with standard assay buffer (100 mM NaCl, 50 mM NaAc, 0.2% (vol/vol) Tween-20, pH 7.2) and blocked at 37°C with standard assay buffer enriched with 0.2% (wt/vol) gelatin. The heparin-captured chemokines were detected with 0.16 μg/mL biotinylated antihuman CXCL10 or CXCL11 (PeproTech) and peroxidase conjugated streptavidin (Jackson ImmunoResearch Laboratories, West Grove, PA). Peroxidase activity was quantified by measuring the conversion of 3,3′,5,5′-tetramethylbenzidine (Sigma-Aldrich) at 450 nm. No difference in specificity of the biotinylated antibodies for the chemokines and their citrullinated isoforms was uncovered. Percentage binding was calculated by subtracting the mean optical density (OD) of the negative control (blocking buffer) from the measured OD of the sample, subsequent division with the average OD of the highest concentration of intact chemokine, and finally multiplying by 100. This calculation grants a value of 100% binding to the highest concentration of intact chemokine and 0% binding to the negative control.
Results
Identification of naturally citrullinated isoforms of CXCL10
Natural CXCL10 was purified from stimulated PBMCs by heparin affinity chromatography, cation exchange, and C8 RP-HPLC.27 At each chromatographic step, CXCL10 immunoreactivity was determined by specific ELISA; and pure proteins corresponding to CXCL10 immunoreactivity were identified by Edman degradation. Because Cys residues were not alkylated before amino acid sequencing, the phenyl thiohydantoin (PTH)–Cys residues were not detected. On NH2-terminal sequencing of natural CXCL10, an unidentified compound eluted from the RP-HPLC column in between PTH-Thr and PTH-Gly instead of the PTH-Arg, predicted from the CXCL10 sequence at position 5 (PTH-Arg5; Figure 1A). This compound was only detected in certain CXCL10 fractions, whereas the PTH-Arg8 was present in all proteins corresponding to CXCL10 immunoreactivity. Surprisingly, the experimentally determined Mr of this naturally modified CXCL10 did not significantly differ from the theoretical Mr of CXCL10 (data not shown). A possible posttranslational modification of Arg, which could result in such insignificant alteration in Mr (1 mass unit), is the conversion of Arg into Cit. Edman degradation on pure L-citrulline resulted in a PTH derivative that eluted at exactly the same position as the unidentified amino acid in the purified CXCL10 fractions, that is, in between PTH-Thr (T) and PTH-Gly (G; Figure 1B). Thus, the unidentified amino acid present in the sequence of natural PBMC-derived CXCL10 is indeed Cit. No further citrullination was detected for natural CXCL10 as this would result in a detectable increase in Mr of 2 or more mass units by ion trap mass spectrometry. In parallel, citrullination of natural PBMC-derived CXCL10 was confirmed using an anticitrulline (modified) antibody (Millipore; data not shown).29
Identification of naturally citrullinated CXCL10. Natural CXCL10 was subjected to Edman degradation. Overlays are shown of the RP-HPLC chromatograms detected at 270 nm (mAU, milliabsorption units) of 2 pmol of the 19 PTH amino acids (indicated by their 1-letter code on the top chromatograms) and of the 5th amino acid in the CXCL10 sequence (A) or of PTH-citrulline (Cit; B). ↙ indicates the major signal in these chromatograms.
Identification of naturally citrullinated CXCL10. Natural CXCL10 was subjected to Edman degradation. Overlays are shown of the RP-HPLC chromatograms detected at 270 nm (mAU, milliabsorption units) of 2 pmol of the 19 PTH amino acids (indicated by their 1-letter code on the top chromatograms) and of the 5th amino acid in the CXCL10 sequence (A) or of PTH-citrulline (Cit; B). ↙ indicates the major signal in these chromatograms.
Modification of CXCL10 by peptidylarginine deiminase
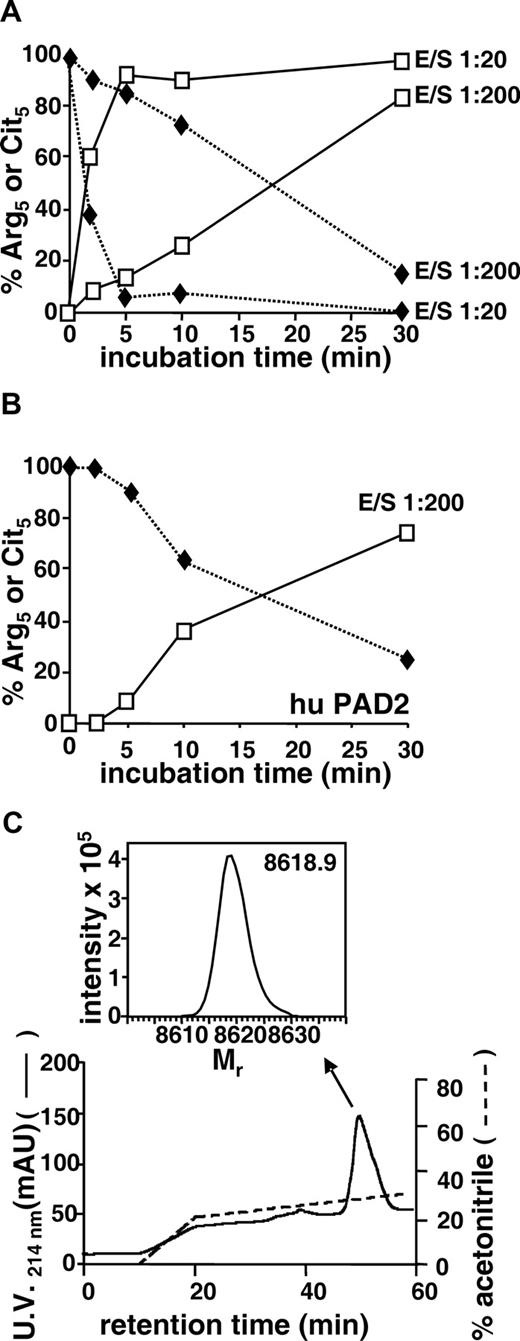
The enzymatic hydrolysis of the guanidino group of Arg in proteins yielding a Cit incorporated in the primary structure of these proteins can be generated by PADs.30 Recombinant CXCL10 was therefore incubated with rabbit PAD at a 1:20 or 1:200 E/S. PAD rapidly and efficiently deiminated CXCL10 (Figure 2A). Indeed, after 2 minutes at an E/S of 1:200, PTH-Cit was already detectable by Edman degradation at position 5 in the CXCL10 sequence, and after 30 minutes 85% of the detected CXCL10 was deiminated. Moreover, at an E/S of 1:20, already 62% of the Arg5 was converted into Cit5 after 2 minutes and nearly total citrullination (95%) occurred after 5 minutes (Figure 2A). Very recently, recombinant human PAD became commercially available. CXCL10 was therefore also incubated with human PAD2 under equal conditions as used for rabbit PAD kinetics experiments. Human PAD2 was shown to deiminate CXCL10 at a comparable rate as rabbit PAD (Figure 2B). Indeed, at a 1:200 E/S, 37% of the Arg5 was modified into Cit5 after 10 minutes, and after 30 minutes 75% of Arg5 was deiminated into Cit5 as detected by Edman degradation (Figure 2B). In all incubations, Arg8 was fully preserved as identified by Edman degradation; and in parallel mass spectrometry measurements, no additional increase of the Mr was detected, indicating that no further citrullination occurred (data not shown).
Modification of CXCL10 by peptidylarginine deiminase (PAD) and RP-HPLC purification of citrullinated CXCL10. Recombinant CXCL10 (100 pmol) was incubated with rabbit PAD (A) or human PAD2 (B) at an enzyme-substrate molar ratio (E/S) of 1:20 or 1:200 for different time periods. Samples were applied on polyvinylidene difluoride membranes for Edman degradation and in parallel were desalted on a C4 ZipTip before examination on an ion trap mass spectrometer to determine the presence of Arg (♦) or Cit (□) at position 5. The percentage conversion of Arg5 into Cit5 and the conservation of Arg8 in the sequence were calculated from the amount of PTH-Arg and PTH-Cit that were detected by RP-HPLC after 5 and 8 cycles of Edman degradation. (C) Recombinant CXCL10 was incubated with rabbit PAD for 90 minutes at an enzyme-substrate molar ratio of 1:20, purified by C8 RP-HPLC, eluted in an acetonitrile gradient, and detected at 214 nm (mAU). Part of the column effluent (0.67%) was split on-line to an ion trap mass spectrometer, and the averaged spectra for the chromatographic peaks were deconvoluted to obtain the Mr of the proteins (inset indicated by ↖).
Modification of CXCL10 by peptidylarginine deiminase (PAD) and RP-HPLC purification of citrullinated CXCL10. Recombinant CXCL10 (100 pmol) was incubated with rabbit PAD (A) or human PAD2 (B) at an enzyme-substrate molar ratio (E/S) of 1:20 or 1:200 for different time periods. Samples were applied on polyvinylidene difluoride membranes for Edman degradation and in parallel were desalted on a C4 ZipTip before examination on an ion trap mass spectrometer to determine the presence of Arg (♦) or Cit (□) at position 5. The percentage conversion of Arg5 into Cit5 and the conservation of Arg8 in the sequence were calculated from the amount of PTH-Arg and PTH-Cit that were detected by RP-HPLC after 5 and 8 cycles of Edman degradation. (C) Recombinant CXCL10 was incubated with rabbit PAD for 90 minutes at an enzyme-substrate molar ratio of 1:20, purified by C8 RP-HPLC, eluted in an acetonitrile gradient, and detected at 214 nm (mAU). Part of the column effluent (0.67%) was split on-line to an ion trap mass spectrometer, and the averaged spectra for the chromatographic peaks were deconvoluted to obtain the Mr of the proteins (inset indicated by ↖).
For a detailed biologic characterization of citrullinated chemokine, recombinant CXCL10 was enzymatically converted into CXCL10-Cit5 by incubation with rabbit PAD for 90 minutes at a 1:20 PAD/CXCL10 molar ratio. HPLC-purified PAD-treated CXCL10 had a Mr of 8618.9 corresponding to CXCL10-Cit5 (theoretical Mr of 8618.3; Figure 2C). Complete conversion of Arg5 into Cit5 and full retention of Arg8 were confirmed by Edman degradation (data not shown).
Citrullination impairs in vitro biologic activity of CXCL10 on CXCR3-tranfected cells
The CXCR3-dependent chemotactic activity of recombinant CXCL10 and citrullinated CXCL10-Cit5 were compared on CHO-CXCR3 cells. CXCL10 provoked a typical bell-shaped dose-response with significant chemotaxis compared with buffer starting from 0.3 nM onward (Figure 3A), whereas a maximal chemotactic index of 10 was reached at 1 nM CXCL10. Citrullinated CXCL10-Cit5, however, was unable to induce significant chemotaxis for CHO-CXCR3 cells. Moreover, a highly significant difference existed between the chemotactic indices obtained with CXCL10 and CXCL10-Cit5 for each chemokine concentration.
In vitro biologic activity of citrullinated CXCL10 in CHO-CXCR3 cells. (A) The chemotactic activity of CXCL10 and CXCL10-Cit5 for CHO-CXCR3 cells was measured using a Boyden microchamber (5 or more independent experiments). (B) The amount of phosphorylated ERK1/2 or PKB/AKT (pg/mg total protein) was measured by specific ELISAs after stimulation of serum-starved CHO-CXCR3 cells for 5 minutes with CXCL10, CXCL10-Cit5, or medium (control). Results represent the percentage ERK1/2 (□) and PKB/AKT (■) phosphorylation (mean ± SEM) compared with medium-treated cells (100%) (3 or more independent experiments). (C) The increase of [Ca2+]i in CHO-CXCR3 cells was measured on stimulation with CXCL10 (♦) or CXCL10-Cit5 (□). Values represent the mean (± SEM) increase of [Ca2+]i of 3 or more independent experiments with a detection limit at 10 nM (…). (D) Desensitization experiments were performed by rechallenging the CHO-CXCR3 cells with 3 nM of CXCL10 100 seconds after the first stimulus. Results (mean ± SEM) represent the percentage inhibition of the second agonist by the first stimulus in comparison with buffer as first stimulus. Significant differences were calculated using the Mann-Whitney test on paired values (*P < .05, **P < .01 for comparison with buffer; ‡P < .05, ‡‡P < .01 for comparison of CXCL10 with CXCL10-Cit5) for the corresponding chemokine concentration as depicted in panels A through D.
In vitro biologic activity of citrullinated CXCL10 in CHO-CXCR3 cells. (A) The chemotactic activity of CXCL10 and CXCL10-Cit5 for CHO-CXCR3 cells was measured using a Boyden microchamber (5 or more independent experiments). (B) The amount of phosphorylated ERK1/2 or PKB/AKT (pg/mg total protein) was measured by specific ELISAs after stimulation of serum-starved CHO-CXCR3 cells for 5 minutes with CXCL10, CXCL10-Cit5, or medium (control). Results represent the percentage ERK1/2 (□) and PKB/AKT (■) phosphorylation (mean ± SEM) compared with medium-treated cells (100%) (3 or more independent experiments). (C) The increase of [Ca2+]i in CHO-CXCR3 cells was measured on stimulation with CXCL10 (♦) or CXCL10-Cit5 (□). Values represent the mean (± SEM) increase of [Ca2+]i of 3 or more independent experiments with a detection limit at 10 nM (…). (D) Desensitization experiments were performed by rechallenging the CHO-CXCR3 cells with 3 nM of CXCL10 100 seconds after the first stimulus. Results (mean ± SEM) represent the percentage inhibition of the second agonist by the first stimulus in comparison with buffer as first stimulus. Significant differences were calculated using the Mann-Whitney test on paired values (*P < .05, **P < .01 for comparison with buffer; ‡P < .05, ‡‡P < .01 for comparison of CXCL10 with CXCL10-Cit5) for the corresponding chemokine concentration as depicted in panels A through D.
Subsequently, authentic CXCL10 and CXCL10-Cit5 were examined for their signaling capacities. In intracellular phosphorylation assays on CHO-CXCR3 cells, 10 nM of CXCL10 induced a significantly increased amount of phosphorylated ERK1/2 and PKB/AKT compared with medium-treated control (Figure 3B). In contrast, CXCL10-Cit5 was incapable of provoking enhanced phosphorylation of these kinases in CHO-CXCR3 cells, resulting in a significant difference between the 2 isoforms. On the other hand, CXCL10-Cit5 still caused a significant increase in [Ca2+]i in CHO-CXCR3 cells but starting at a dose of 10 nM (Figure 3C). Nonetheless, authentic CXCL10 was more potent (at least 10-fold) than CXCL10-Cit5 because a significant increase in [Ca2+]i was obtained at 1 nM. A similar pattern emerged in receptor desensitization experiments (Figure 3D). Indeed, on rechallenging CHO-CXCR3 cells with chemokine, CXCL10-Cit5 was observed to be approximately 10 times less effective at inhibiting the intracellular calcium release of authentic CXCL10 in comparison with autodesensitization. Taken together, these data demonstrate that, although CXCL10-Cit5 fails to exert chemotactic activity and to induce phosphorylation, it is still capable of binding CXCR3 and to some extent preventing calcium signal transduction by authentic CXCL10.
Chemical synthesis of CXCL11-Cit6 and its in vitro biologic activity in CXCR3-transfected cells
To assess whether related chemokines are substrates for PAD and are affected by citrullination, we tested another potent CXCR3 ligand and T-cell chemoattractant, that is, CXCL11. Recombinant CXCL11 was also found to be citrullinated by rabbit PAD into CXCL11-Cit6 as confirmed by Edman degradation (data not shown). To evaluate the effects of citrullination on the biologic activities of CXCL11, both authentic CXCL11 and CXCL11 with a Cit incorporated at position 6 were chemically synthesized using fluorenylmethoxycarbonyl chemistry as described.16 After deprotection and purification by RP-HPLC, the Mr was verified by online ion trap mass spectrometry and by NH2-terminal sequencing (data not shown). Consequently, the chemokines were folded in buffer containing oxidized and reduced glutathione. The folded peptides were purified by RP-HPLC, and the formation of the disulfide bridges was confirmed by online mass spectrometry (data not shown). Synthetic and recombinant intact CXCL11 were equally potent in chemotaxis and calcium signaling assays, indicating a successful peptide synthesis and folding procedure (see “Citrullination reduces T-cell activities of CXCL10 and CXCL11” and data not shown).
Although authentic CXCL11 seemed to be more effective at phosphorylating ERK1/2 and PKB/AKT in CHO-CXCR3 cells compared with CXCL11-Cit6, no statistical difference was detected between the 2 isoforms (Figure 4A,B). However, CXCL11 was significantly more potent than CXCL11-Cit6 (∼3-fold) at inducing an increase in [Ca2+]i in U87-CXCR3 cells (Figure 4C). These data suggest an important role for citrullination on the biologic effect of CXCL11 through CXCR3, however, less pronounced than for CXCL10.
In vitro biologic activity of citrullinated CXCL11 in CXCR3 transfectants. Phosphorylated ERK1/2 (A) or PKB/AKT (B) were measured by specific ELISAs after stimulation of serum-starved CHO-CXCR3 cells for 5 minutes with CXCL11, CXCL11-Cit6, or medium (control). Results represent the percentage ERK1/2 (□) and PKB/AKT (■) phosphorylation (mean ± SEM) compared with medium-treated cells (100%) from 3 or more independent experiments. Statistical analysis was performed using the Mann-Whitney test on paired values (*P < .05, **P < .01, ***P < .001 for comparison with control). (C) The increase of [Ca2+]i (nM) in U87-CXCR3 cells was measured on stimulation with different doses (nM) of CXCL11 (♦) or CXCL11-Cit6 (□). Values represent the mean (± SEM) increase of [Ca2+]i (3-6 independent experiments) with a detection limit of 10 nM (…). Statistical analysis was performed using the Mann-Whitney test (‡P < .05, ‡‡P < .01 for comparison of authentic with citrullinated chemokine).
In vitro biologic activity of citrullinated CXCL11 in CXCR3 transfectants. Phosphorylated ERK1/2 (A) or PKB/AKT (B) were measured by specific ELISAs after stimulation of serum-starved CHO-CXCR3 cells for 5 minutes with CXCL11, CXCL11-Cit6, or medium (control). Results represent the percentage ERK1/2 (□) and PKB/AKT (■) phosphorylation (mean ± SEM) compared with medium-treated cells (100%) from 3 or more independent experiments. Statistical analysis was performed using the Mann-Whitney test on paired values (*P < .05, **P < .01, ***P < .001 for comparison with control). (C) The increase of [Ca2+]i (nM) in U87-CXCR3 cells was measured on stimulation with different doses (nM) of CXCL11 (♦) or CXCL11-Cit6 (□). Values represent the mean (± SEM) increase of [Ca2+]i (3-6 independent experiments) with a detection limit of 10 nM (…). Statistical analysis was performed using the Mann-Whitney test (‡P < .05, ‡‡P < .01 for comparison of authentic with citrullinated chemokine).
Citrullination does not interfere with binding properties of CXCL10 and CXCL11 on CXCR3 and CXCR7
To further characterize the properties of citrullinated CXCL10 and CXCL11, receptor binding studies were performed. Surprisingly, CXCL10 and CXCL10-Cit5 were equally potent at dislocating 125I-labeled CXCL10 from CHO-CXCR3 cells (Figure 5A). Similarly, authentic CXCL11 and CXCL11-Cit6 competed equally well for 125I-labeled CXCL11 in binding to CXCR3-transfected cells (Figure 5B). CXCL11 and CXCL11-Cit6 were also alike in displacing the radiolabeled CXCL11 from CXCR7 transfectants (Figure 5C). In addition, the capacity to compete for 125I-labeled CXCL11 binding on T cells was similar for CXCL11 and CXCL11-Cit6 (Figure 5D). This indicates that the Arg at position 5 or 6 in, respectively, CXCL10 and CXCL11 and thus the change from a basic to a neutral amino acid are not implicated in the receptor binding competition. Nevertheless, CXCL10-Cit5 and, to some extent, CXCL11-Cit6 were weaker at provoking biologic activities through CXCR3 (Figures 3,4).
Effect of citrullination on the receptor binding properties of CXCL10 and CXCL11 on CXCR3 and CXCR7 transfectants and on PHA-activated T cells. (A) The receptor binding properties of CXCL10 (♦) and CXCL10-Cit5 (□) on CHO-CXCR3 cells were determined by competition for 125I-labeled CXCL10. (B-D) The receptor binding properties of CXCL11 (♦) and CXCL11-Cit6 (□) on CHO-CXCR3 (B) or CHO-CXCR7 (C) cells and on PHA-activated T cells (D) were determined by competition for 125I-labeled CXCL11. Results represent the mean (± SEM) percentage bound labeled chemokine compared with the maximal amount of bound labeled chemokine when no cold ligand was added (3-6 independent experiments).
Effect of citrullination on the receptor binding properties of CXCL10 and CXCL11 on CXCR3 and CXCR7 transfectants and on PHA-activated T cells. (A) The receptor binding properties of CXCL10 (♦) and CXCL10-Cit5 (□) on CHO-CXCR3 cells were determined by competition for 125I-labeled CXCL10. (B-D) The receptor binding properties of CXCL11 (♦) and CXCL11-Cit6 (□) on CHO-CXCR3 (B) or CHO-CXCR7 (C) cells and on PHA-activated T cells (D) were determined by competition for 125I-labeled CXCL11. Results represent the mean (± SEM) percentage bound labeled chemokine compared with the maximal amount of bound labeled chemokine when no cold ligand was added (3-6 independent experiments).
Citrullination reduces T-cell activities of CXCL10 and CXCL11
The biologic activities of citrullinated and unmodified CXCR3 ligands were compared under more physiologic conditions, that is, on activated T lymphocytes. CXCL10-Cit5 was less potent (minimal effective concentration of 20 nM) at provoking chemotaxis of T cells (Figure 6A). In contrast to the chemotactic response observed on CHO-CXCR3 cells (Figure 3), however, CXCL10-Cit5 remained active on T cells. In calcium signaling experiments, CXCL10 already induced an increase in [Ca2+]i starting from 3 nM onward, whereas CXCL10-Cit5 only exceeded the detection limit at 30 nM (Figure 6B), resulting in a statistically significant difference between the 2 isoforms, as was observed for signaling in CHO-CXCR3 transfectants (Figure 3). CXCL11-Cit6 was also shown to stimulate T-cell chemotaxis (Figure 6C), but the minimal effective concentration (3 nM) was slightly (3-fold) higher than for authentic CXCL11. For comparison, synthetic and recombinant CXCL11 did not differ in chemoattraction of T cells, confirming a successful chemical synthesis. Furthermore, in the calcium signaling experiments, T cells responded significantly less to CXCL11-Cit6 than to authentic CXCL11 (Figure 6D). Indeed, unmodified CXCL11 induced increases in [Ca2+]i from 1 nM onward, whereas for CXCL11-Cit6 10 nM was required. Similarly, CXCL11 desensitized the calcium response more efficiently than CXCL11-Cit6 (Figure 6E). These data are in agreement with the observation that the calcium signaling response of CXCL11-Cit6 in CXCR3 cells was reduced compared with authentic CXCL11 (Figure 4C). Thus, although no differences for these CXCL11 forms could be observed in their binding properties to CXCR3 or CXCR7 transfected cells and to T cells (Figure 5) or in their CXCR3 dependent phosphorylation (Figure 4A,B), these molecules do differ in chemoattraction and calcium signaling in CXCR3-transfected cells and in T lymphocytes. Moreover, no CXCR7 mRNA could be detected on T cells by reverse-transcribed polymerase chain reaction (data not shown). CXCR3 expression on T cells, however, was confirmed by fluorescence-activated cell sorter analysis (data not shown).
Effect of citrullination on the in vitro biologic activity in T cells of CXCL10 and CXCL11. (A,C) The chemotactic activity of CXCL10, CXCL10-Cit5, recombinant (rec) CXCL11, synthetic (synth) CXCL11, and synthetic CXCL11-Cit6 for PHA-activated T cells was measured using a Boyden microchamber (5-20 independent experiments). Statistical analysis was performed using the Mann-Whitney test on paired values (*P < .05, **P < .01, ***P < .001 for comparison with buffer). (B,D) The increase of [Ca2+]i in PHA-activated T cells was measured on stimulation with CXCL10 (♦) or CXCL10-Cit5 (□) as shown in panel B or with CXCL11 (♦) or CXCL11-Cit6 (□) as depicted in panel D. Values represent the mean (± SEM) increase of [Ca2+]i of 3 or more independent experiments with a detection limit at 5 nM (…). Significant differences were calculated using the Mann-Whitney test on paired values (‡P < .05, ‡‡P < .01 for comparison of authentic with citrullinated chemokine). (E) Desensitization experiments were performed by rechallenging the T cells with 1 nM of synthetic CXCL11 100 seconds after the first stimulus. Results (mean ± SEM) represent the percentage inhibition of the second agonist by the first stimulus in comparison with buffer as first stimulus (3-5 independent experiments). Significant differences were calculated using the Mann-Whitney test (‡P < .05 comparison between CXCL11 and CXCL11-Cit6).
Effect of citrullination on the in vitro biologic activity in T cells of CXCL10 and CXCL11. (A,C) The chemotactic activity of CXCL10, CXCL10-Cit5, recombinant (rec) CXCL11, synthetic (synth) CXCL11, and synthetic CXCL11-Cit6 for PHA-activated T cells was measured using a Boyden microchamber (5-20 independent experiments). Statistical analysis was performed using the Mann-Whitney test on paired values (*P < .05, **P < .01, ***P < .001 for comparison with buffer). (B,D) The increase of [Ca2+]i in PHA-activated T cells was measured on stimulation with CXCL10 (♦) or CXCL10-Cit5 (□) as shown in panel B or with CXCL11 (♦) or CXCL11-Cit6 (□) as depicted in panel D. Values represent the mean (± SEM) increase of [Ca2+]i of 3 or more independent experiments with a detection limit at 5 nM (…). Significant differences were calculated using the Mann-Whitney test on paired values (‡P < .05, ‡‡P < .01 for comparison of authentic with citrullinated chemokine). (E) Desensitization experiments were performed by rechallenging the T cells with 1 nM of synthetic CXCL11 100 seconds after the first stimulus. Results (mean ± SEM) represent the percentage inhibition of the second agonist by the first stimulus in comparison with buffer as first stimulus (3-5 independent experiments). Significant differences were calculated using the Mann-Whitney test (‡P < .05 comparison between CXCL11 and CXCL11-Cit6).
Citrullination impairs GAG binding of CXCL10 and CXCL11
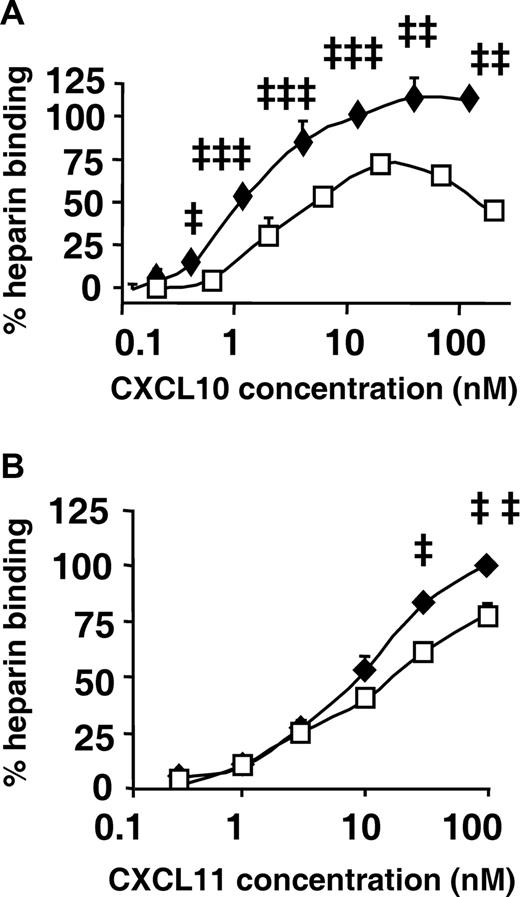
As a final approach to obtain more insight in the discrepancies observed in biologic activities between authentic and citrullinated CXCL10 and CXCL11, we investigated glycosaminoglycan (GAG)–binding properties. GAGs are sulfated polysaccharides that form part of the glycocalyx on cell surfaces and are imperative in the sequestration of chemokines as well as the formation of a chemokine gradient.31 GAG-chemokine binding may also modulate the presentation of the chemokine to its receptor and hence affect its biologic activity.32-35 Interestingly, the heparin binding capacity of CXCL10-Cit5 was significantly diminished by citrullination in comparison with authentic CXCL10 (Figure 7A). In addition, citrullination decreased the heparin binding capacity of CXCL11 (Figure 7B). These data reveal a high degree of dissimilarity on GAG-binding because of citrullination of a single Arg.
Effect of citrullination on heparin binding properties of CXCL10 and CXCL11. (A,B) GAG binding was evaluated by immobilizing heparin on EpranEx plates, followed by adding a series of dilutions of CXCL10 (♦) or CXCL10-Cit5 (□) from 6 to 12 independent experiments depicted in panel A, or CXCL11 (♦) or CXCL11-Cit6 (□) from 3 to 6 independent experiments shown in panel B. GAG binding (mean ± SEM) was detected by chemokine-specific biotinylated antibodies and shown as the percentage binding of 10 nM of CXCL10 in panel A (SEM control was 1.07%) and of 100 nM of CXCL11 in panel B (SEM control was 1.24%). Statistical analysis was performed using the Mann-Whitney test (‡P < .05, ‡‡P < .01, ‡‡‡P < .001 for comparison of authentic with citrullinated chemokine).
Effect of citrullination on heparin binding properties of CXCL10 and CXCL11. (A,B) GAG binding was evaluated by immobilizing heparin on EpranEx plates, followed by adding a series of dilutions of CXCL10 (♦) or CXCL10-Cit5 (□) from 6 to 12 independent experiments depicted in panel A, or CXCL11 (♦) or CXCL11-Cit6 (□) from 3 to 6 independent experiments shown in panel B. GAG binding (mean ± SEM) was detected by chemokine-specific biotinylated antibodies and shown as the percentage binding of 10 nM of CXCL10 in panel A (SEM control was 1.07%) and of 100 nM of CXCL11 in panel B (SEM control was 1.24%). Statistical analysis was performed using the Mann-Whitney test (‡P < .05, ‡‡P < .01, ‡‡‡P < .001 for comparison of authentic with citrullinated chemokine).
Discussion
The interaction between chemokines and proteases is a key process in the regulation of the immune response.14,36 Chemokines not only trigger the release of proteases but also act as substrates. Several natural posttranslational modifications have been reported to affect the biologic nature of the chemokine substrate. For example, the conversion of CXCL8(1-77) into CXCL8(6-77) by plasmin or thrombin potentiates its biologic activity, facilitating a positive feedback loop.37,38 Moreover, posttranslational modifications seem to complicate the pattern of apparent redundancy in chemokine-receptor interactions. Indeed, after processing by DPP-IV/CD26, the CC chemokine CCL5/RANTES discloses an enhanced receptor interaction with CCR5, parallel though with a decreased affinity for CCR1 and CCR3. This NH2-terminal truncation of CCL5 does not reduce lymphocyte attraction but results in a loss of monocyte chemotaxis.14 Thus, posttranslational proteolysis influences the selectivity of the attracted leukocyte subset during inflammation or infection. In addition, various tumor cells constitutively express chemokines, for example, granulocyte chemotactic protein-2 (GCP-2/CXCL6), provoking leukocyte infiltration from blood vessels into the tumor tissue and thereby leaving a pathway of degraded extracellular matrix open for metastasis.27,39 On the other hand, proteases can also influence other properties of chemokines, for example, DPP-IV/CD26-processed CCL5 was more potent at protecting CD4+ cells against HIV-1 infection because of the increased affinity for CCR5.14 NH2-terminal cleavage of CXCL10 by DPP-IV/CD26 impairs its CXCR3 signaling and chemoattractive capacity; however, it does not interfere with its antiangiogenic character.15 In contrast, the subsequent cleavage of CXCL11 by DPP-IV/CD26 and aminopeptidase N/CD13 not only weakens its receptor binding, signaling, and chemoattractive characteristics but also reduces its angiostatic nature.16
In this manuscript, we describe a nonproteolytic though enzymatic posttranslational modification of chemokines with profound effect on the biologic activity. Indeed, we purified a novel natural CXCL10 isoform from stimulated pooled PBMCs, ie, citrullinated CXCL10. We confirmed the conversion of arginine at position 5 into citrulline to be exerted by rabbit PAD but with the retention of arginine at position 8, the latter being probably because of steric hinder effects.40 Because PAD is expressed in inflamed tissue and is coexpressed with citrullinated peptides in the synovium of RA patients, it is probable that CXCL10-Cit5 also occurs in vivo, as protein levels of CXCL10 were enhanced in synovial fluids of RA and spondylarthropathy patients.18,21,24,41 More recent studies report that only PAD2 and 4 are expressed in the inflamed synovium of RA and other arthritides, but no other human isoforms, that is, PAD1, 3, or 6.42 Moreover, PAD2 is being extensively studied for its possible implications in MS pathogenesis.25 In this manuscript, human PAD2 was also shown to deiminate CXCL10 nearly completely within 30 minutes and at a practically identical rate as rabbit PAD. This posttranslational modification of a positively charged amino acid into a neutral amino acid by PAD revealed to have important biologic consequences because CXCR3-transfected cells did not respond to CXCL10-Cit5 in a standard chemotaxis assay and only weakly in signaling experiments, although CXCR3 binding properties were preserved. In addition, PAD was shown to deiminate CXCL11. This conversion of CXCL11 resulted in a diminished capacity to provoke intracellular calcium mobilization through CXCR3 although less pronounced than for citrullinated CXCL10. These results point to a fine-tuned regulation by PAD of CXCR3-mediated immune responses: a chemokine-specific modulation.
Further investigation in a more physiologic context confirmed the reduced response toward citrullinated CXCL10 and CXCL11. In T cells, a decrease in biologic activity was observed in chemotaxis and calcium signaling assays in response to CXCL10-Cit5. In contrast to CHO-CXCR3 cells, where CXCL10-Cit5 was completely unable to induce chemotaxis, CXCL10-Cit5 remained slightly active in T cells. In addition, CXCL11-Cit6 was also 10-fold less potent at inducing calcium signaling in CXCR3 transfectants and T lymphocytes. This impaired behavior of citrullinated CXCL10 and CXCL11 may result from the implication of additional cell-associated mediators, such as GAGs, by augmenting local chemokine concentration or by inducing chemokine oligomerization. Indeed, citrullination of CXCL10 and CXCL11 significantly diminished its heparin-binding properties. These dissimilarities between authentic and citrullinated CXCL10 and CXCL11 support the idea that PAD regulates the local immune responses through chemokine modification. Moreover, it indicates that specific GAG binding is localized at multiple spots on chemokines and that the loss of one positive charge because of citrullination already has a considerable impact on GAG-binding domains localized at the NH2-terminus of the chemokine. Indeed, these data demonstrate that structurally distinct regions of CXCL10 and CXCL11 are responsible for different functions, such as calcium signaling and chemoattraction, as previously reported in structure-function studies through NH2-terminal truncation and alanine mutagenesis experiments.15-17,43,44
Overall, the effects of citrullination observed in this manuscript reveal a new dimension in the fine regulation of chemokine activity in the immune response. For CXCL10, this posttranslational modification by PAD causes a negative feedback loop, possibly implicated in anti-inflammatory processes by decreased lymphocyte recruitment. Because our results show both chemokine receptor-dependent responses and decreased heparin binding of citrullinated CXCL10 and CXCL11, it is probable that PAD acts rather locally. This immunomodulating and potential protective role for PAD is new because previous reports suggest a PAD function in apoptosis because of its calcium-dependent character. Indeed, it is thought that during apoptosis caspases cleave calcium channels, causing an intracellular calcium increase. As a consequence, this activates PAD, which deiminates abundantly present peptides, possibly making them more prone for degradation through the loss of positive charges. Alternatively, during inflammation chemokine-receptor binding also promotes intracellular calcium increase, which can facilitate PAD activation and citrullination of chemokines. Up until this study, structural and commonly distributed proteins, rather than immune mediators, have been reported of being citrullinated, among others, keratin, fillagrin, vimentin, α-enolase, the protease inhibitor antithrombin, myelin basic protein, and some nuclear proteins, eg, nucleophosmin, p300, and histones.29,45-52 It has been postulated that, in the pathogenesis of RA, the citrullinated peptides are a target for the ACPAs resulting in an altered T- and B-cell response. Klareskog et al53 proficiently overview the current ideas concerning the extent of requirement of ACPAs in the pathogenesis of RA, proposing a 3-stage etiologic model wherein environmental and genetic factors collaborate. The finding that inflammatory mediators are also biologically affected by citrullination invites to reconsider or to extend this model.
Taken as a whole, our study identifies and categorizes citrullination of an immune mediator. This novel posttranslational modification of the cytokine CXCL10 is not only naturally occurring, but it also has substantial consequences for the biologic activities of this PAD substrate and provides us with new structure-function information on chemokines.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank René Conings for technical assistance. The CHO-CXCR3 and CHO-CXCR7 cells were kindly provided by Prof Marc Parmentier (Institut de Recherche Interdisciplinaire en Biologie Humaine et Moléculaire, Université Libre de Bruxelles, Brussels, Belgium). The U87-CXCR3 cells were kindly provided by Prof Dominique Schols (Laboratory of Virology and Chemotherapy, Rega Institute, K U Leuven, Leuven, Belgium).
This work was supported by the Center of Excellence (Credit no. EF/05/15) of the K U Leuven, the Concerted Research Actions (GOA) of the Regional Government of Flanders, the Fund for Scientific Research of Flanders (FWO-Vlaanderen), the Interuniversity Attraction Poles Program (Belgian State) Belgian Science Policy, and the European Union 6FP EC contract INNOCHEM (grant LSHB-CT-2005-518167). A.M. is a research assistant and M.G. is a senior research assistant of the FWO-Vlaanderen.
Authorship
Contribution: T.L., A.M., M.G., I.R., W.P., J.-P.L., and P.P. performed research and collected data; and T.L., J.V.D., and P.P. designed research, analyzed the data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Paul Proost, Laboratory of Molecular Immunology, Rega Institute, KU Leuven, Minderbroedersstraat 10, 3000 Leuven, Belgium; e-mail: paul.proost@rega.kuleuven.be.


![Figure 3. In vitro biologic activity of citrullinated CXCL10 in CHO-CXCR3 cells. (A) The chemotactic activity of CXCL10 and CXCL10-Cit5 for CHO-CXCR3 cells was measured using a Boyden microchamber (5 or more independent experiments). (B) The amount of phosphorylated ERK1/2 or PKB/AKT (pg/mg total protein) was measured by specific ELISAs after stimulation of serum-starved CHO-CXCR3 cells for 5 minutes with CXCL10, CXCL10-Cit5, or medium (control). Results represent the percentage ERK1/2 (□) and PKB/AKT (■) phosphorylation (mean ± SEM) compared with medium-treated cells (100%) (3 or more independent experiments). (C) The increase of [Ca2+]i in CHO-CXCR3 cells was measured on stimulation with CXCL10 (♦) or CXCL10-Cit5 (□). Values represent the mean (± SEM) increase of [Ca2+]i of 3 or more independent experiments with a detection limit at 10 nM (…). (D) Desensitization experiments were performed by rechallenging the CHO-CXCR3 cells with 3 nM of CXCL10 100 seconds after the first stimulus. Results (mean ± SEM) represent the percentage inhibition of the second agonist by the first stimulus in comparison with buffer as first stimulus. Significant differences were calculated using the Mann-Whitney test on paired values (*P < .05, **P < .01 for comparison with buffer; ‡P < .05, ‡‡P < .01 for comparison of CXCL10 with CXCL10-Cit5) for the corresponding chemokine concentration as depicted in panels A through D.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/112/7/10.1182_blood-2008-04-149039/6/m_zh80200824960003.jpeg?Expires=1765900396&Signature=Wr2fP81q~kcTJpTNKm0WKEmaqLpcMBtbYtbN-9z4aa7mukH0M1uL4ZeRDPtK2SJgYhzAF8eXLpfCc99Iy55~zh888axbS97i-~wfXYgnDE9RucqxXyVwMwJj6frdpxxJVrCXiR7PSudpXRAxMQAAhs7XUQUjPnpto8Jb79SR1A1~xnV-7w0zVzLwvhweMIAXiwZAyW68FkkOWnwA5ZJN9NjBRkSbpG40wExrf7JDzYtcA6OEQKjcn0KDwz96Ji6koqHrsi9gAjeODw82Vrfrio0gEWCZVVTtWPTwcojCKAPht-PFQYCKk9NGPLP0cpuuhuCH9vjL~E8HtSOjqNeyfA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. In vitro biologic activity of citrullinated CXCL11 in CXCR3 transfectants. Phosphorylated ERK1/2 (A) or PKB/AKT (B) were measured by specific ELISAs after stimulation of serum-starved CHO-CXCR3 cells for 5 minutes with CXCL11, CXCL11-Cit6, or medium (control). Results represent the percentage ERK1/2 (□) and PKB/AKT (■) phosphorylation (mean ± SEM) compared with medium-treated cells (100%) from 3 or more independent experiments. Statistical analysis was performed using the Mann-Whitney test on paired values (*P < .05, **P < .01, ***P < .001 for comparison with control). (C) The increase of [Ca2+]i (nM) in U87-CXCR3 cells was measured on stimulation with different doses (nM) of CXCL11 (♦) or CXCL11-Cit6 (□). Values represent the mean (± SEM) increase of [Ca2+]i (3-6 independent experiments) with a detection limit of 10 nM (…). Statistical analysis was performed using the Mann-Whitney test (‡P < .05, ‡‡P < .01 for comparison of authentic with citrullinated chemokine).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/112/7/10.1182_blood-2008-04-149039/6/m_zh80200824960004.jpeg?Expires=1765900396&Signature=KmgqlhvvtGiSRFu6uyihMAOq64L6O-Wb3vztM6E6ZT4hNa3X4R9bjR1ino~KLischYUNI43AaTwvNS3j6OiUhXB8jYxHDv5DG-gwOFmfYs6CBPwAdod6JIuvHKVKKkkBoPhsLXEZhC8U9oKV88pEYlIOluwHY8i4Ao91kwQ3NtMs4n4yOofX7dgA3Py51LyP3utIIpJmqjIyfqMm8~jQSKpwUvSrXHMY42h~96sX5DFryEKGP6enY2MCAdwjLq21U~lUiYI2l8gINDSZD~9rFWLB~ej26Mmrph-l-2GISRy~mNHpVXA2sJupLyVtW0myXVp1~NXsaeL-gYTUUxbjlA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 6. Effect of citrullination on the in vitro biologic activity in T cells of CXCL10 and CXCL11. (A,C) The chemotactic activity of CXCL10, CXCL10-Cit5, recombinant (rec) CXCL11, synthetic (synth) CXCL11, and synthetic CXCL11-Cit6 for PHA-activated T cells was measured using a Boyden microchamber (5-20 independent experiments). Statistical analysis was performed using the Mann-Whitney test on paired values (*P < .05, **P < .01, ***P < .001 for comparison with buffer). (B,D) The increase of [Ca2+]i in PHA-activated T cells was measured on stimulation with CXCL10 (♦) or CXCL10-Cit5 (□) as shown in panel B or with CXCL11 (♦) or CXCL11-Cit6 (□) as depicted in panel D. Values represent the mean (± SEM) increase of [Ca2+]i of 3 or more independent experiments with a detection limit at 5 nM (…). Significant differences were calculated using the Mann-Whitney test on paired values (‡P < .05, ‡‡P < .01 for comparison of authentic with citrullinated chemokine). (E) Desensitization experiments were performed by rechallenging the T cells with 1 nM of synthetic CXCL11 100 seconds after the first stimulus. Results (mean ± SEM) represent the percentage inhibition of the second agonist by the first stimulus in comparison with buffer as first stimulus (3-5 independent experiments). Significant differences were calculated using the Mann-Whitney test (‡P < .05 comparison between CXCL11 and CXCL11-Cit6).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/112/7/10.1182_blood-2008-04-149039/6/m_zh80200824960006.jpeg?Expires=1765900396&Signature=rijruoqEf~bQn-BO3mseWUIOWLyWzsE94z1hoaeFfzKebrai1ZWNF-trc7Kn2EKsgbXPzGZlLN2nXPCkm45VQUVvGpgnY5sFHqw7LCEFLO20PyVjGyrJ91LVHKOTqIz6y-Ph8xhmI32ZiUbxK0dT49NsCSxl23tSu3OBkNX0XOUfF49MtldBuCKtI1z0oEsQcv~mx6TxHYsZXombNME-67egbDsZKZHM~efCpmntj~xy2spZ7Bl0kFNC2jRzgzJ44ENmBexsU4K-ljQ5aJCUSgBPeiN2jr10SMZXyWp5ArIlxGO8aipvzdFT~~NShJVLqiRPrOBfi8Mr8Y5D8tioiw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal