Abstract
To address the role of chromatin structure in the establishment of hematopoietic stem cell (HSC) multilineage potential and commitment to the lymphoid lineage, we have analyzed histone modifications at a panel of lymphoid- and myeloid-affiliated genes in multipotent and lineage-committed hematopoietic cells isolated from human cord blood. Our results show that many B- and T-lymphoid genes, although silent in HSCs, are associated with acetylated histones H3 and H4. We also detected histone H3 lysine 4 methylation but not repressive lysine 9 or 27 methylation marks at these loci, indicative of an open chromatin structure. Interestingly, the relative level of H3 lysine 4 dimethylation to trimethylation at B-specific loci was high in multipotent CD34+CD38lo progenitors and decreased as they become actively transcribed in B-lineage cells. In vitro differentiation of CD34+ cells toward the erythroid, granulocyte, and T-cell lineages resulted in a loss of histone acetylation at nonlineage-associated genes. This study provides evidence that histone modifications involved in chromatin decondensation are already in place at lymphoid-specific genes in primary human HSCs, supporting the idea that these genes are “primed” for expression before lineage commitment. This permissive chromatin structure is progressively lost as the stem cell differentiates.
Introduction
All blood cells are derived from multipotent hematopoietic stem cells (HSCs) via a series of differentiation steps during which the multilineage potential of the stem cell is gradually lost as HSCs differentiate into lymphoid- or myeloid-committed cells.1 These differentiation steps require the coordinated activation of characteristic sets of genes and the silencing of others. Increasing evidence indicates that epigenetic modifications play an important role in cell fate decision-making processes by regulating the chromatin state of genes and, hence, their potential to be expressed.2,3 Remodeling of chromatin can be achieved in several distinct but interconnected ways, including covalent modifications of histones such as acetylation, phosphorylation, methylation, and ubiquitinylation, often at N-terminal residues.4,5 These modifications play an important role in the formation and maintenance of active and repressive chromatin states.6 Acetylation of N-terminal lysine residues of histones H3 and H4 and methylation of lysine 4 of histone H3 have been associated with active chromatin conformations, whereas methylation of histone H3 lysines 9 and 27 are hallmarks of repressive chromatin states.7-13
Recent studies of histone modifications in embryonic stem (ES) cells have revealed novel epigenetic features, which are thought to contribute to the maintenance of pluripotency and cell lineage determination in these stem cells.14-18 To date, much less is known about the chromatin structure of multipotent adult stem cells, such as HSCs. Despite the fact that HSCs are probably the best characterized stem cells, the role of histone modifications in regulating their multilineage potential is poorly understood. Many studies have shown that HSCs and early hematopoietic progenitors express multiple lineage-associated genetic programs at low levels.19-22 This phenomenon, known as lineage priming, is thought to reflect alterations in the chromatin conformation of genes expressed in specific hematopoietic lineages, rendering them accessible to transcription factors in multipotent cells before lineage commitment.23,24 Interestingly, promiscuous expression of myeloid- but not lymphoid-affiliated genes was observed in HSCs,19,21 suggesting that opening of B- and T-lymphoid loci may occur later during hematopoiesis.
In this study, we have investigated the chromatin structure at a panel of lymphoid- and myeloid-affiliated genes in HSCs and lineage-committed cells isolated from human umbilical cord blood by analyzing their pattern of histone modifications. We show that lymphoid-affiliated genes are associated with a combination of active histone modifications, including histone acetylation and histone H3 lysine 4 dimethylation, but very low levels of negative marks in HSCs. This accessible chromatin conformation precedes gene expression and lineage commitment during HSC differentiation. Further active histone modifications, notably H3 lysine 4 trimethylation, are added at these loci in lymphoid-committed cells expressing high levels of the genes, whereas the histone marks present in HSCs are replaced by repressive H3 lysine 9 or 27 methylation on commitment to other lineages. These results show that active epigenetic marks are already present at lymphoid loci in HSCs and highlight the role of chromatin structure in the maintenance of multilineage potential in these stem cells.
Methods
Cell isolation
Umbilical cord blood samples were collected from normal full-term deliveries, after obtaining informed consent of the mothers according to the approved institutional guidelines of GHU Nord (Assistance Publique Hopitaux de Paris, Paris, France). After Ficoll density gradient separation (Lymphocytes Separation Medium, Laboratories Eurobio, Les Ulis, France), CD34+ cells were purified by immunomagnetic selection (StemCell Technologies, Vancouver, BC). CD34+ cells (purity ≥ 95%) were used immediately or after storage in liquid nitrogen. CD34+CD38lo cells, which comprise up to 10% of the CD34+ cell population, were routinely isolated from pools of 5 to 10 cord blood samples by fluorescence-activated cell sorter using a Vantage SE-DiVa (BD Biosciences, San Jose, CA). Isolation of cord blood B cells was performed on CD34− cell fraction based on CD19 expression (Miltenyi Biotec, Auburn, CA).
Human muscle satellite cells (a generous gift from Dr V. Mouly, Institut de Myologie, Paris, France) were isolated and cultured as described.25
Chromatin immunoprecipitation assays
Purified hematopoietic cells were cross-linked with 0.5% formaldehyde in RPMI medium containing 10% fetal calf serum for 10 minutes at 37°C, then washed in 10 volumes of RPMI medium. Cells (4 × 105-106) were then lysed in 400 μL 1% sodium dodecyl sulfate, 10 mM ethylenediaminetetraacetic acid, 50 mM Tris-HCl, pH 8.0, lysis buffer, and sonicated with a Branson Sonifier in 1.5-mL Eppendorf tubes. Chromatin immunoprecipitation (ChIP) experiments were performed on solubilized chromatin diluted 10-fold in ChIP dilution buffer (#20-153, Upstate Biotechnology, Charlottesville, VA) using 2 × 105 cells per histone modification. All immunoprecipitation and washing steps were performed in Eppendorf tubes at 4°C. Chromatin was precleared by incubating with 80 μL salmon sperm DNA/protein A agarose beads for 3 hours and then incubated overnight with antibodies against anti-acetyl histone H4, anti-acetyl histone H3, anti-dimethyl histone H3K4, anti-trimethyl histone H3K4, anti-trimethyl histone H3K9, or anti-trimethyl histone H3K27 (all from Upstate Biotechnology). Immune complexes were collected by incubating with 60 μL salmon sperm DNA/protein A agarose beads, washed as described (Upstate Biotechnology) using 900 μL of each buffer, and modified histone/DNA complexes eluted with 500 μL 1% sodium dodecyl sulfate, 0.1 M NaHCO3. After reversal of cross-link, DNA was purified by phenol extraction. An aliquot of the sonicated chromatin was treated identically for use as input. ChIP experiments for the 6 histone modifications were performed on the same chromatin sample. Chromatin from 1.2 × 106 CD34+CD38lo cells isolated from 20 to 30 cord blood samples was pooled for each complete experiment.
Quantitative polymerase chain reaction (PCR) was performed to determine the relative enrichment of gene segments in ChIP compared with input DNA. Reactions were performed in triplicate using SYBR Green and the ABI 7000 Sequence Detection System (Applied Biosystems, Foster City, CA). Percent immunoprecipitation was calculated by dividing the average value of the immunoprecipitate by the average value of the corresponding input normalized by the dilution factor. To compare histone modifications in different cell types, results were routinely normalized with respect to the promoter of the ubiquitously expressed β2-microglobulin gene for histone H3 and H4 acetylation and histone H3 lysine 4 methylation or the nonhematopoietic THP gene for histone H3 lysine 9 and 27 methylation.
PCR primers were designed within the promoter region or known regulatory sequences using the Primer Express (Applied Biosystems) software (Table S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). For some loci, because of particular base sequence or containing repeats, the amplified fragment was located within coding regions, essentially in the first exon.
In vitro differentiation
Freshly isolated CD34+CD38lo cells were differentiated toward the T lineage by in vitro culture on OP9 stromal cells expressing human Notch ligand Delta-1 (OP9-hDelta1) as described.26,27 Differentiation was monitored by fluorescence-activated cell sorter analysis based on phenotypic changes in CD34, CD7, CD4, and CD8 cell surface expression. In vitro differentiation of CD34+ progenitors toward the erythroid and granulocyte lineages was performed as described.28
Results
Lymphoid genes are associated with acetylated histone H3 and H4 in cord blood CD34+CD38lo cells
We focused our study on a panel of more than 20 lymphoid- and myeloid-specific genes (Table S1) that are not expressed or very poorly expressed in HSCs but are expressed at later stages of HSC lineage commitment and differentiation (C.G., J.M., M.M., M.G., and C.F., manuscript submitted).29-31 As controls, we also analyzed nonhematopoietic genes, such as the neuronal Pax6 and kidney-specific THP genes, as well as genes that are highly expressed in HSCs, such as TAL132 and the ubiquitous β2-microglobulin and GAPDH genes.
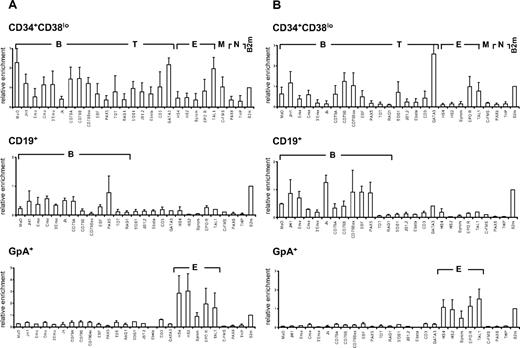
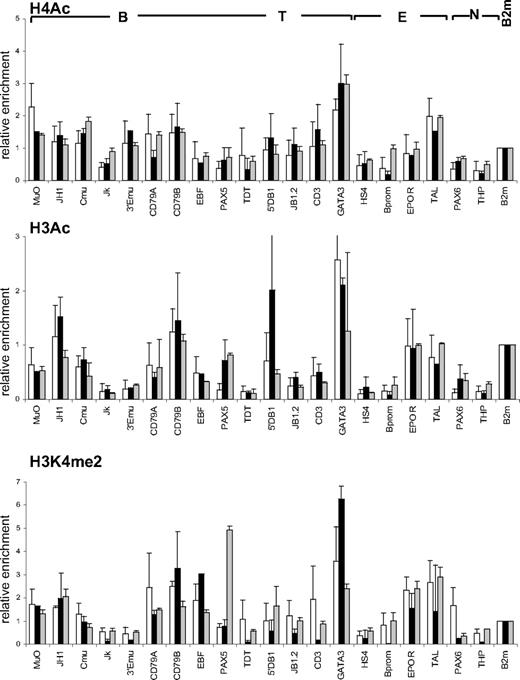
We first investigated the pattern of histone H3 and H4 acetylation by ChIP experiments in lineage-committed and in CD34+CD38lo cord blood cells enriched in HSCs and multipotent progenitors. To compare histone modifications in different cell types, results were normalized with respect to the promoter of the ubiquitously expressed β2-microglobulin or GAPDH gene. Similar modification patterns were observed with the 2 control genes (Figure S1). Results are presented relative to the β2-microglobulin control. In CD19+ B-lineage cells, we found that histone H3 and H4 modifications were lineage specific with only the set of B-specific genes marked by acetylated H3 and H4 at levels similar to that of β2-microglobulin (Figure 1 middle panels). Similarly, histone acetylation was only observed at erythroid loci in glycophorin-positive (GpA+) erythroid precursors and at T-specific genes in CD4+ T cells isolated from cord blood (Figure 1 bottom panels and data not shown).
Pattern of histone H3 and H4 acetylation at lymphoid- and myeloid-affiliated genes in cord blood cells. Chromatin from multipotent CD34+CD38lo, B-committed CD19+, and erythroid GpA+ cells was analyzed by ChIP using antibodies to acetylated histone H4 (A) and H3 (B) followed by real-time PCR with primers specific for B-lymphoid (B), T-lymphoid (T), erythroid (E), myeloid (M), nonhematopoietic (N), and β2-microglobulin (B2m) genes. Histogram shows enrichment values (bound/input) normalized to B2m control (set at 1). Results are means and standard deviations of 3 to 5 independent ChIP experiments analyzed in triplicate.
Pattern of histone H3 and H4 acetylation at lymphoid- and myeloid-affiliated genes in cord blood cells. Chromatin from multipotent CD34+CD38lo, B-committed CD19+, and erythroid GpA+ cells was analyzed by ChIP using antibodies to acetylated histone H4 (A) and H3 (B) followed by real-time PCR with primers specific for B-lymphoid (B), T-lymphoid (T), erythroid (E), myeloid (M), nonhematopoietic (N), and β2-microglobulin (B2m) genes. Histogram shows enrichment values (bound/input) normalized to B2m control (set at 1). Results are means and standard deviations of 3 to 5 independent ChIP experiments analyzed in triplicate.
A distinct acetylation profile was observed in multipotent CD34+CD38lo cord blood cells, with many B- and T-lymphoid genes associated with acetylated histones H3 and H4 (Figure 1 top panels). The level of H4 acetylation was similar or even greater than that of the expressed β2-microglobulin or TAL1 genes for most of the loci analyzed. Although fairly low levels of acetylated histone H4 were found at the β-globin locus, the EPOR and c-fms genes were clearly acetylated, indicating that erythroid and myeloid genes are also acetylated in CD34+CD38lo cord blood progenitors, consistent with results from previous studies.24,33 Acetylated histone H3 was detected at a more restricted number of genes in CD34+CD38lo cells. Nevertheless, similar levels of histone H3 acetylation were observed at most B-specific genes in CD34+CD38lo and in CD19+ B cells, indicating that this modification is already fully present at certain lymphoid loci in HSCs. Interestingly, histone H4 acetylation levels at these genes were somewhat higher in CD34+CD38lo than in B-committed cells, suggesting that H4 acetylation is the prominent mark at transcriptionally silent B-lymphoid genes in HSCs.
For the B-specific CD79B gene, we assayed histone H3 and H4 acetylation at DNaseI-hypersensitive (HS) sites throughout the locus (Figure S2). High levels of both acetylated H3 and H4 were found at the promoter and 0.7-kb HS sites in CD34+CD38lo cells. Acetylation of histone H3 decreased at 3′ HS sites, whereas levels of H4 acetylation remained high throughout the locus.
Altogether, our results show that multipotent CD34+CD38lo cells have a much broader histone acetylation profile than lineage-committed cells with many lymphoid- and myeloid-affiliated genes associated with acetylated histones. The genes analyzed are either not transcribed or are expressed at very low levels in CD34+CD38lo cells. This indicates that histone acetylation at these loci occurs before transcription and lineage commitment in HSCs and may be associated with lineage potential rather than gene expression per se.
Lymphoid loci are not associated with repressive histone methylation marks in cord blood CD34+CD38lo cells
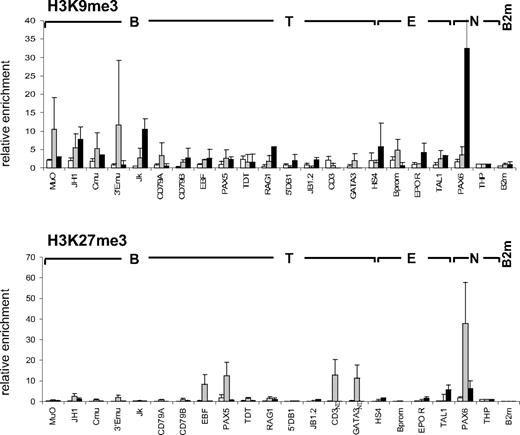
Methylation of lysines 9 and 27 of histone H3 (H3K9me3 and H3K27me3) have been described as negative marks mediating gene repression and chromatin condensation via the recruitment of heterochromatin protein 1 (HP1) and polycomb complexes, respectively.7,8,10 We therefore examined the presence of these repressive modifications at the panel of lymphoid and myeloid genes in CD34+CD38lo cells. All loci analyzed had low levels of H3K9me3 in CD34+CD38lo cells (Figure 2). In cord blood CD19+ cells, however, H3K9me3 was observed at the nonhematopoietic gene PAX6, as well as the Ig kappa locus, consistent with previous reports of H3K9me3 and HP1 binding at Ig kappa genes in murine B cells, implicated in heterochromatin recruitment and allelic exclusion.34,35 Similarly, we did not detect appreciable levels of H3K27me3 in CD34+CD38lo cells at the lymphoid and myeloid loci analyzed, although H3K27me3 was observed at the nonhematopoietic genes PAX6 and THP (Figure 2). A notable exception was for the B-cell transcription factor PAX5, although the level of H3K27me3 detected at the PAX5 gene in CD34+CD38lo cells was 10-fold lower than in GpA+ erythroid cells. In these erythroid cells, high H3K27me3 levels were also observed at genes coding for neuronal (PAX6) and other lymphoid (EBF and GATA-3) transcription factors. Together, these results show that most lymphoid and myeloid genes are associated with very low levels of repressive histone marks in CD34+CD38lo cells, supporting the idea that these loci have an open chromatin conformation in HSC.
Histone H3K9me3 and H3K27me3 modifications at lymphoid genes in CD34+CD38lo cells. ChIP analyses of multipotent CD34+CD38lo (▭), erythroid GpA+ ( ), and B-committed CD19+ (
), and B-committed CD19+ ( ) cells with antibodies to H3K9me3 and H3K27me3. Histone modifications at B-lymphoid (B), T-lymphoid (T), erythroid (E), and nonhematopoietic (N) genes were normalized to the nonhematopoietic THP gene (set at 1). Results are means and standard deviations of 2 to 5 independent ChIP experiments analyzed in triplicate. ND indicates not determined.
) cells with antibodies to H3K9me3 and H3K27me3. Histone modifications at B-lymphoid (B), T-lymphoid (T), erythroid (E), and nonhematopoietic (N) genes were normalized to the nonhematopoietic THP gene (set at 1). Results are means and standard deviations of 2 to 5 independent ChIP experiments analyzed in triplicate. ND indicates not determined.
Histone H3K9me3 and H3K27me3 modifications at lymphoid genes in CD34+CD38lo cells. ChIP analyses of multipotent CD34+CD38lo (▭), erythroid GpA+ ( ), and B-committed CD19+ (
), and B-committed CD19+ ( ) cells with antibodies to H3K9me3 and H3K27me3. Histone modifications at B-lymphoid (B), T-lymphoid (T), erythroid (E), and nonhematopoietic (N) genes were normalized to the nonhematopoietic THP gene (set at 1). Results are means and standard deviations of 2 to 5 independent ChIP experiments analyzed in triplicate. ND indicates not determined.
) cells with antibodies to H3K9me3 and H3K27me3. Histone modifications at B-lymphoid (B), T-lymphoid (T), erythroid (E), and nonhematopoietic (N) genes were normalized to the nonhematopoietic THP gene (set at 1). Results are means and standard deviations of 2 to 5 independent ChIP experiments analyzed in triplicate. ND indicates not determined.
B-specific genes are associated with higher levels of H3K4 dimethylation to trimethylation in CD34+CD38lo than in CD19+ cells
We also analyzed the methylation status of histone H3 lysine 4 (H3K4me), which has been reported to be an activating modification, facilitating transcription by the recruitment of remodeling complexes or by preventing transcriptional repressors from binding to chromatin.36 Both dimethylation and trimethylation forms are associated with active loci, although H3K4me3 is found at promoters of actively transcribed genes, whereas H3K4me2 can be present on poised, inactive genes.11,37,38 Methylated H3K4, especially H3K4me2, was detected at lymphoid loci in CD34+CD38lo cells but not at the nonhematopoietic THP gene or in GpA+ cells (Figure 3; and data not shown), again suggesting that lymphoid loci have an accessible chromatin conformation in multipotent hematopoietic progenitors. As expected, B- but not T-lymphoid genes were associated with methylated H3K4 in CD19+ B-committed cells. A direct comparison of absolute levels of dimethylation to trimethylation cannot be made, as the efficiency of the 2 antibodies used in the ChIP experiments may not be the same. However, the relative level of H3K4me2 to H3K4me3 was higher at B-specific loci in CD34+CD38lo progenitors, where they are not yet transcribed, than in CD19+ B cells, expressing high levels of these genes (Figure 3). The presence of high levels of H3K4me2 in CD34+CD38lo cells suggests that it may be a marker of transcriptional or developmental “competence” in human HSCs, as previously reported for yeast and avian cells,11,38 whereas H3K4me3 appears to be more directly related to transcriptional activity.
Histone H3K4 dimethylation and trimethylation at B-specific genes in CD34+CD38lo and CD19+ cells. Multipotent CD34+CD38lo and B-committed CD19+ cells were subjected to ChIP analyses with antibodies to H3K4me2 (▭) and H3K4me3 ( ). Results were calculated as in Figure 1 and are means and standard deviations of at least 2 independent ChIP experiments analyzed in triplicate.
). Results were calculated as in Figure 1 and are means and standard deviations of at least 2 independent ChIP experiments analyzed in triplicate.
Histone H3K4 dimethylation and trimethylation at B-specific genes in CD34+CD38lo and CD19+ cells. Multipotent CD34+CD38lo and B-committed CD19+ cells were subjected to ChIP analyses with antibodies to H3K4me2 (▭) and H3K4me3 ( ). Results were calculated as in Figure 1 and are means and standard deviations of at least 2 independent ChIP experiments analyzed in triplicate.
). Results were calculated as in Figure 1 and are means and standard deviations of at least 2 independent ChIP experiments analyzed in triplicate.
Muscle satellite cells and HSCs have different histone modification profiles
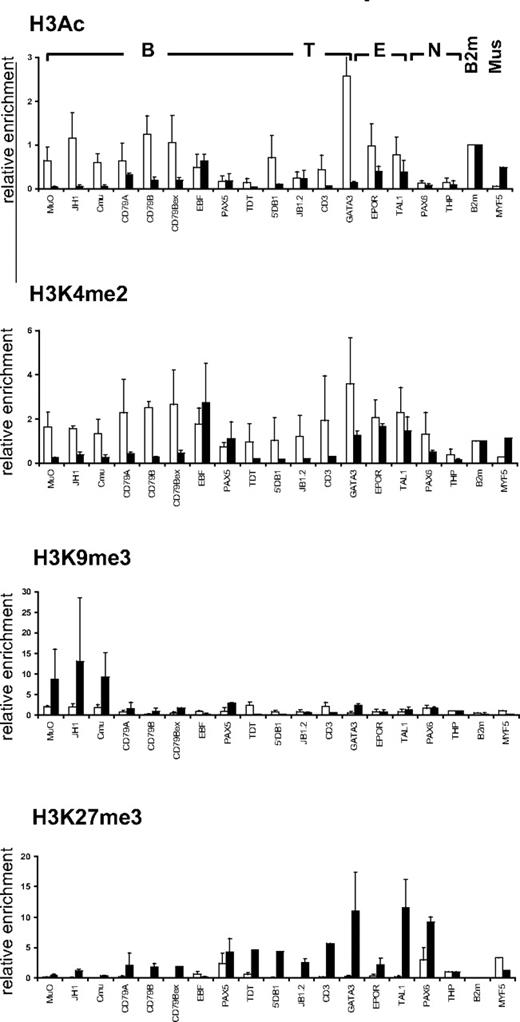
To investigate whether the chromatin structure observed in CD34+CD38lo HSCs at lymphoid loci is related to the differentiation potential of the stem cell, we compared the histone modification profile in HSCs and in human muscle satellite cells, which are responsible for regeneration of postnatal skeletal muscle.25 Unlike CD34+CD38lo cells, hematopoietic genes were not marked by active histone modifications in primary muscle satellite cells (Figure 4). We found very low levels of histone H3 acetylation and H3K4me2 at most B, T, and erythroid genes in the muscle satellite cells, with the exception of hematopoietic transcription factors that retained significant H3K4me2 marks (Figure 4). Furthermore, high levels of repressive H3K9me3 and H3K27me3 marks were detected at lymphoid and erythroid loci in the muscle satellite cells. Thus, we found high levels of H3K9me3 at the IgH locus, whereas transcription factors expressed in hematopoietic and neuronal cells were associated with high levels of H3K27me3. In contrast, the muscle determining myogenic factor-5 (MYF5) was marked with acetylated histone H3 and H3K4me2 but not with repressive H3K9me3 or H3K27me3 modifications in muscle satellite cells; whereas in CD34+CD38lo cells, MYF5 is associated with high levels of H3K27me3 marks but not H3K4me2 or histone H3 acetylation (Figure 4). The histone modification profile observed in HSCs and muscle satellite cells therefore seems to reflect the differentiation potential of the stem cells.
Comparison of histone modifications in HSC and muscle satellite cells. ChIP analyses of CD34+CD38lo HSC (▭) and human muscle satellite cells ( ) with antibodies to histone H3Ac, H3K4me2, H3K9me3, and H3K27me3 at representative B-lymphoid (B), T-lymphoid (T), erythroid (E), nonhematopoietic (N), β2-microglobulin (B2m), and muscle specific (Mus) genes. Results are shown as enrichment values (bound/input) relative to the B2m gene (for H3Ac and H3K4me2) or to the THP gene (for H3K9me3 and H3K27me3) and are means and standard deviations of 2 to 5 independent ChIP experiments analyzed in triplicate.
) with antibodies to histone H3Ac, H3K4me2, H3K9me3, and H3K27me3 at representative B-lymphoid (B), T-lymphoid (T), erythroid (E), nonhematopoietic (N), β2-microglobulin (B2m), and muscle specific (Mus) genes. Results are shown as enrichment values (bound/input) relative to the B2m gene (for H3Ac and H3K4me2) or to the THP gene (for H3K9me3 and H3K27me3) and are means and standard deviations of 2 to 5 independent ChIP experiments analyzed in triplicate.
Comparison of histone modifications in HSC and muscle satellite cells. ChIP analyses of CD34+CD38lo HSC (▭) and human muscle satellite cells ( ) with antibodies to histone H3Ac, H3K4me2, H3K9me3, and H3K27me3 at representative B-lymphoid (B), T-lymphoid (T), erythroid (E), nonhematopoietic (N), β2-microglobulin (B2m), and muscle specific (Mus) genes. Results are shown as enrichment values (bound/input) relative to the B2m gene (for H3Ac and H3K4me2) or to the THP gene (for H3K9me3 and H3K27me3) and are means and standard deviations of 2 to 5 independent ChIP experiments analyzed in triplicate.
) with antibodies to histone H3Ac, H3K4me2, H3K9me3, and H3K27me3 at representative B-lymphoid (B), T-lymphoid (T), erythroid (E), nonhematopoietic (N), β2-microglobulin (B2m), and muscle specific (Mus) genes. Results are shown as enrichment values (bound/input) relative to the B2m gene (for H3Ac and H3K4me2) or to the THP gene (for H3K9me3 and H3K27me3) and are means and standard deviations of 2 to 5 independent ChIP experiments analyzed in triplicate.
Comparison of histone modification profiles in CD34+ and CD34+CD38lo cord blood cells
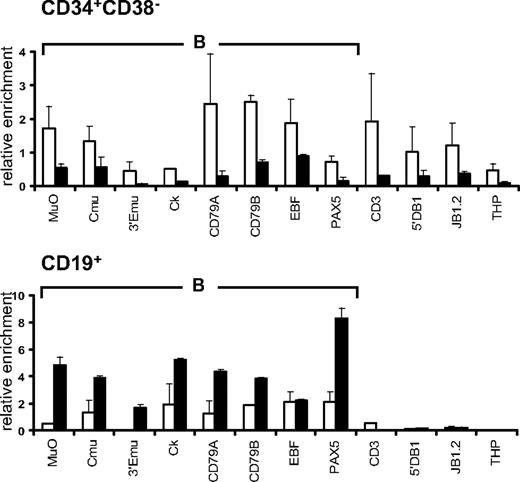
We compared histone modification profiles in CD34+CD38lo cells and in total CD34+ cord blood population, containing multipotent and lineage-committed progenitors. To our surprise, the pattern of histone H4 and H3 acetylation and H3K4me2 in CD34+ cells was similar to that observed in CD34+CD38lo cells (Figure 5). These results suggest that active histone modifications are maintained in CD34+ progenitor cells at lineage-inappropriate loci. To test this, we isolated CD34+CD38+CD19−CD10− cells to remove multipotent and B-lineage–committed CD34+ progenitors and analyzed histone modifications at B-specific loci. The pattern of histone acetylation and H3K4me2 marks in these cells was similar to that observed in total CD34+ and CD34+CD38lo cells (Figure 5). This shows that B-specific genes are associated with active histone modifications in CD34+ progenitors committed to lineages other than the B-cell lineage, suggesting that Lin+CD34+ progenitors retain a certain degree of lineage plasticity.
Comparison of histone modifications in CD34+CD38lo, CD34+, and CD34+38+10−19− cord blood progenitors. ChIP analyses of multipotent CD34+CD38lo (▭), total CD34+ ( ), and B-lymphoid–depleted CD34+38+10−19− (
), and B-lymphoid–depleted CD34+38+10−19− ( ) progenitors with antibodies to histone H4Ac, H3Ac, and H3K4me2 at B-lymphoid (B), T-lymphoid (T), erythroid (E), nonhematopoietic (N), and β2-microglobulin (B2m) genes. Results are shown as enrichment values (bound/input) relative to the B2m control and are means and standard deviations of 2 to 5 independent ChIP experiments analyzed in triplicate.
) progenitors with antibodies to histone H4Ac, H3Ac, and H3K4me2 at B-lymphoid (B), T-lymphoid (T), erythroid (E), nonhematopoietic (N), and β2-microglobulin (B2m) genes. Results are shown as enrichment values (bound/input) relative to the B2m control and are means and standard deviations of 2 to 5 independent ChIP experiments analyzed in triplicate.
Comparison of histone modifications in CD34+CD38lo, CD34+, and CD34+38+10−19− cord blood progenitors. ChIP analyses of multipotent CD34+CD38lo (▭), total CD34+ ( ), and B-lymphoid–depleted CD34+38+10−19− (
), and B-lymphoid–depleted CD34+38+10−19− ( ) progenitors with antibodies to histone H4Ac, H3Ac, and H3K4me2 at B-lymphoid (B), T-lymphoid (T), erythroid (E), nonhematopoietic (N), and β2-microglobulin (B2m) genes. Results are shown as enrichment values (bound/input) relative to the B2m control and are means and standard deviations of 2 to 5 independent ChIP experiments analyzed in triplicate.
) progenitors with antibodies to histone H4Ac, H3Ac, and H3K4me2 at B-lymphoid (B), T-lymphoid (T), erythroid (E), nonhematopoietic (N), and β2-microglobulin (B2m) genes. Results are shown as enrichment values (bound/input) relative to the B2m control and are means and standard deviations of 2 to 5 independent ChIP experiments analyzed in triplicate.
Differentiation of CD34+ cells is associated with decreased histone acetylation of non–lineage-associated genes
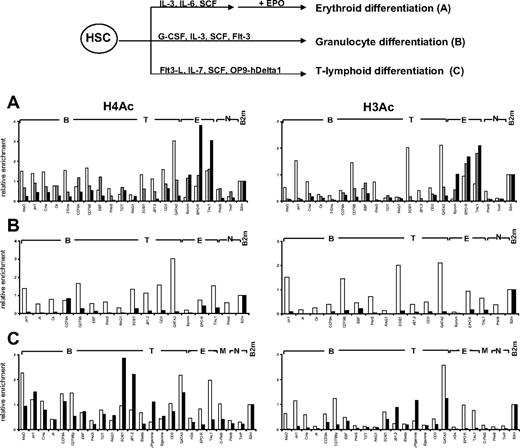
We next looked at histone modifications at lineage-affiliated genes during in vitro differentiation of HSCs. Given the low numbers of CD34+CD38lo cells and the similar chromatin structure to CD34+ cells, histone H3 and H4 acetylation was analyzed after in vitro differentiation of CD34+ cells toward the erythroid, granulocyte, and T-cell lineages (Figure 6). For erythroid differentiation, CD34+ cells were first cultured in vitro in the presence of stem cell factor, interleukin-3 (IL-3), and IL-6 for 7 days, after which CD36+ erythroid progenitors were purified and cultured for a further 5 days in the presence of erythropoietin (Epo), which promotes the differentiation of CD34−CD36+GpA+ erythroid cells.28 We observed a decrease in histone acetylation at all B- and T-affiliated genes after erythroid differentiation, whereas acetylation levels at erythroid genes were increased or remained the same (Figure 6A). Interestingly, loss of histone H3 acetylation at lymphoid loci occurs in CD36+ progenitors before addition of Epo, whereas the decrease in H4 acetylation is more gradual and is only marked after 5 days of Epo treatment. Differentiation of CD34+ cells toward the granulocyte lineage was associated with a marked decrease in the level of histone acetylation at both lymphoid and erythroid genes (Figure 6B).
Changes in histone acetylation during in vitro differentiation of cord blood CD34+ progenitors. (Top) Scheme of in vitro differentiation. (A) Differentiation toward the erythroid lineage. Freshly isolated CD34+ cells (▭) were cultured in the presence of IL-3, IL-6, and stem cell factor; and after 7 days, CD36+ cells ( ) were isolated and cultured for a further 5 days in the presence of Epo to give GpA+ cells (
) were isolated and cultured for a further 5 days in the presence of Epo to give GpA+ cells ( ). (B) Differentiation toward the granulocyte lineage. Freshly isolated CD34+ cells (▭) were cultured in the presence of G-CSF, IL-3, stem cell factor, and Flt3-L for 14 days to give CD11b+ cells (
). (B) Differentiation toward the granulocyte lineage. Freshly isolated CD34+ cells (▭) were cultured in the presence of G-CSF, IL-3, stem cell factor, and Flt3-L for 14 days to give CD11b+ cells ( ). (C) Differentiation toward the T-lymphoid lineage. In vitro culture of CD34+CD38lo cells (▭) was performed on OP9-hDelta1 stromal cells in the presence of Flt3-L, stem cell factor, and IL-7. After 21 days, more than 90% of cells were CD7+ committed T-cell precursors (
). (C) Differentiation toward the T-lymphoid lineage. In vitro culture of CD34+CD38lo cells (▭) was performed on OP9-hDelta1 stromal cells in the presence of Flt3-L, stem cell factor, and IL-7. After 21 days, more than 90% of cells were CD7+ committed T-cell precursors ( ). ChIP experiments were performed with antibodies to acetylated histones H3 and H4 at B-lymphoid (B), T-lymphoid (T), erythroid (E), myeloid (M), nonhematopoietic (N), and β2-microglobulin (B2m) genes. Representative results of 2 to 4 independent experiments are shown.
). ChIP experiments were performed with antibodies to acetylated histones H3 and H4 at B-lymphoid (B), T-lymphoid (T), erythroid (E), myeloid (M), nonhematopoietic (N), and β2-microglobulin (B2m) genes. Representative results of 2 to 4 independent experiments are shown.
Changes in histone acetylation during in vitro differentiation of cord blood CD34+ progenitors. (Top) Scheme of in vitro differentiation. (A) Differentiation toward the erythroid lineage. Freshly isolated CD34+ cells (▭) were cultured in the presence of IL-3, IL-6, and stem cell factor; and after 7 days, CD36+ cells ( ) were isolated and cultured for a further 5 days in the presence of Epo to give GpA+ cells (
) were isolated and cultured for a further 5 days in the presence of Epo to give GpA+ cells ( ). (B) Differentiation toward the granulocyte lineage. Freshly isolated CD34+ cells (▭) were cultured in the presence of G-CSF, IL-3, stem cell factor, and Flt3-L for 14 days to give CD11b+ cells (
). (B) Differentiation toward the granulocyte lineage. Freshly isolated CD34+ cells (▭) were cultured in the presence of G-CSF, IL-3, stem cell factor, and Flt3-L for 14 days to give CD11b+ cells ( ). (C) Differentiation toward the T-lymphoid lineage. In vitro culture of CD34+CD38lo cells (▭) was performed on OP9-hDelta1 stromal cells in the presence of Flt3-L, stem cell factor, and IL-7. After 21 days, more than 90% of cells were CD7+ committed T-cell precursors (
). (C) Differentiation toward the T-lymphoid lineage. In vitro culture of CD34+CD38lo cells (▭) was performed on OP9-hDelta1 stromal cells in the presence of Flt3-L, stem cell factor, and IL-7. After 21 days, more than 90% of cells were CD7+ committed T-cell precursors ( ). ChIP experiments were performed with antibodies to acetylated histones H3 and H4 at B-lymphoid (B), T-lymphoid (T), erythroid (E), myeloid (M), nonhematopoietic (N), and β2-microglobulin (B2m) genes. Representative results of 2 to 4 independent experiments are shown.
). ChIP experiments were performed with antibodies to acetylated histones H3 and H4 at B-lymphoid (B), T-lymphoid (T), erythroid (E), myeloid (M), nonhematopoietic (N), and β2-microglobulin (B2m) genes. Representative results of 2 to 4 independent experiments are shown.
In vitro culture of HSCs on OP9 stromal cells expressing human notch ligand Delta-1 (OP9-hDelta1) leads to cellular expansion and differentiation toward the T-cell lineage.27 Analysis of histone acetylation after 21 days of culture showed a decrease in histone H3 acetylation at all non–T-specific genes (Figure 6C). Of note, acetylated histone H4 was still observed at B-lineage loci but not at erythroid or myeloid genes, perhaps reflecting the proximity of B- and T-lymphoid lineages.
Taken together, these results show that histone modifications involved in chromatin decondensation are already in place at lymphoid-affiliated genes in HSCs and that commitment to one of the myeloid or lymphoid lineages is associated with loss of active histone marks at lineage inappropriate loci.
Discussion
In this study, we have investigated the chromatin structure of primary HSCs and lineage-committed cells isolated from human cord blood. By pooling cord blood samples and improving the sensitivity of conventional ChIP assays, we have analyzed 6 histone modifications specific of active or repressive chromatin at a panel of more than 20 lymphoid- and myeloid-affiliated genes. Although the results presented here are not based on a genome-wide analysis, clear trends are observable. Unlike lineage-committed cells, which have a tissue-specific histone acetylation profile, multipotent cord blood CD34+CD38lo cells have a much broader profile with many lymphoid- and myeloid-affiliated genes associated with acetylated H4 and to a lesser extent H3 acetylation. We found that these genes are also marked by histone H3K4me2, another modification associated with chromatin decondensation and transcriptional activation, but not with repressive H3K9me3 or H3K27me3 marks. This indicates that many lymphoid- and myeloid-specific genes have an open/accessible chromatin conformation in human HSCs, consistent with the notion that lymphoid, like myeloid, genes are “primed” for expression before lineage commitment in multipotent hematopoietic progenitors.19-21
The histone modification profile in HSCs appears to reflect the differentiation potential of the stem cell and is different from that of other adult stem cells. Thus, in human muscle satellite cells, active histone modifications were found at the muscle-specifying MYF5 gene, whereas the set of lymphoid and myeloid genes analyzed were marked by high levels of repressive histone modifications. H3K9me3 was present at the IgH locus, suggesting that silencing of Ig genes in muscle satellite cells is regulated by interaction with HP1 and recruitment to heterochromatin, as previously reported in terminally differentiated mouse B cells.34,35,39 Neuronal and lymphoid transcription factors were associated with H3K27me3, indicating that lineage-inappropriate transcription factors are repressed via a polycomb protein–mediated pathway in these cells.6
We found that differentiation of multipotent hematopoietic progenitors toward one of the myeloid or lymphoid lineages leads to loss of histone H3 and H4 acetylation and addition of repressive H3K9 or H3K27 methylation marks at lineage-inappropriate genes. These results show that progressive lineage restriction and cell fate specification of HSCs are accompanied by epigenetic silencing of lineage-inappropriate genes, as originally proposed by transcription profiling studies.20,21,40 Interestingly, lineage-committed CD34+ progenitors still have a broad histone acetylation profile, suggesting that early CD34+ precursors retain a certain degree of lineage plasticity. Differentiation of HSCs was also associated with a change in the relative levels of H3K4 dimethylation to trimethylation. We found that B-lymphoid genes are marked by H3K4me2 but relatively low levels of H3K4me3 in HSCs. As the genes become expressed in B committed cells, H3K4me3 levels increased, whereas the levels of H3K4me2 were maintained. Similarly, Attema et al found lower levels of H3K4me3 in murine HSCs than in more mature progenitors at GATA 1, GATA 3, c-fms, and Ptcrα promoters.41 Although histone H3K4me2 and H3K4me3 are found at promoters of transcriptionally active and “poised” inactive genes, H3K4me3 correlates closely with transcription rate and polymerase II occupancy.11,37,42 Consistent with this, our results indicate that high H3K4me2/H3K4me3 ratios mark potentially active lymphoid-specific genes in HSCs and may therefore define a “permissive” chromatin conformation, whereas transcription is associated with increased H3K4me3 levels that may allow a more fully active chromatin state.
Recent studies mapping histone modifications in ES cells have considerably advanced our understanding of the relationship between chromatin state and cell fate decisions. These studies revealed that a set of genes, including key developmental and lineage-specifying genes, are marked by opposing H3K4me3 and H3K27me3 modifications in ES cells.14-18 Bivalent chromatin marks occur at promoters with high CpG island content and are proposed to keep genes transcriptionally silent in ES cells but “poised” for lineage-specific activation or repression later during development. We find that this bivalent chromatin is largely resolved in cord blood HSCs. Nonhematopoietic transcripion factors, such as Pax6 and Myf5, lose H3K4me3 but retain the H3K27me3 mark, indicating that they become stably repressed in a polycomb-dependent manner in hematopoietic cells. In contrast, we found low levels of both H3K4me3 and H3K27me3 marks at lymphoid-specific transcription factors in HSCs. The lack of H3K27me3 suggests that these genes are kept silent in HSCs by a polycomb protein–independent pathway. This chromatin structure resembles that reported for a second category of transcriptionally silent genes in ES cells, which are marked by neither H3K4me3 nor H3K27me3 marks. This set of genes have a low CpG island content and contain tissue-specific genes involved in responses to external stimuli, including immune responses. Our results suggest that, in HSCs, transcriptionally silent hematopoietic genes with both high and low CpG content are devoid of both H3K4me3 and H3K27me3. The presence of high H4 acetylation and H3K4 dimethylation in the absence of active H3K4me3 or repressive H3K27me3 or H3K9me3 marks appears to describe a transcriptional competent “ground state” for these genes in human HSCs keeping them silent but poised for expression at later stages of differentiation. A key question that remains to be resolved is how these epigenetic states are established during hematopoiesis. Notably, activating histone modifications are already in place at B-affiliated genes in CD34+CD38lo HSCs before expression of the major B-cell transcription factors E2A, EBF, and PAX5. The hematopoietic factor PU.1 that is expressed in multipotent progenitors may play a role in initiating these early epigenetic marks because there is evidence to suggest that PU.1 interacts with the promoter of the myeloid c-fms gene in murine HSCs and is implicated in chromatin reorganization of the c-fms and the B-specific λ5-VpreB locus.33,43,44 Further characterization of the epigenetic states of hematopoietic cells at different stages of differentiation and studies aimed at identifying nonhistone proteins associated with lymphoid loci in HSC should help elucidate this question.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Drs Vincent Mouly and Serge Fichelson for the human satellite muscle cells and help with in vitro erythrocyte differentiation experiments and Dr Christine Dosquet for critical reading of the manuscript.
This work was supported by research funding from Inserm and grants from Association pour la Recherche sur le Cancer “ARECA” network (M.G., C.F.) and Inserm Adult Stem Cell AIP A03187DS (M.G., M.C.-C., F.P.) and AIP A03203DS (M.G., C.F.).
Authorship
Contribution: J.M. and M.M. performed research, analyzed and interpreted data, and drafted the manuscript; C.G. and E.S. performed research; F.P. contributed vital new analytical tools; I.A.-S. analyzed and interpreted data; M.C.-C. contributed vital new analytical tools; D.C. contributed to research strategy; C.F. analyzed and interpreted data and drafted the manuscript; and M.G. designed research, analyzed and interpreted data, and drafted the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Michele Goodhardt, Inserm U662, Institut Universitaire d'Hématologie, Hôpital Saint-Louis, 1 av. Claude Vellefaux, 75010 Paris, France; e-mail: michele.goodhardt@univ-paris-diderot.fr.
References
Author notes
*J.M. and M.M. contributed equally to this work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal