Abstract
Immunoreceptor tyrosine-based activation motif (ITAM)–containing proteins have recently been demonstrated in macrophages and neutrophils to be required for cell surface integrins to transmit activation signals into the cell. To identify ITAM-bearing proteins that mediate signaling via the platelet-specific integrin αIIbβ3, fibrinogen binding was induced by (1) allowing platelets to spread directly on immobilized fibrinogen, or (2) activating the PAR1 thrombin receptor on platelets in suspension. Both initiated strong, ligand binding–dependent tyrosine phosphorylation of the ITAM-bearing platelet Fc receptor, FcγRIIa, as well as downstream phosphorylation of the protein tyrosine kinase Syk and activation of phospholipase Cγ2 (PLCγ2). Addition of Fab fragments of an FcγRIIa-specific monoclonal antibody strongly inhibited platelet spreading on immobilized fibrinogen, as well as downstream tyrosine phosphorylation of FcγRIIa, Syk, and PLCγ2, and platelets from a patient whose platelets express reduced levels of FcγRIIa exhibited markedly reduced spreading on immobilized fibrinogen. Finally, fibrinogen binding–induced FcγRIIa phosphorylation did not occur in human platelets expressing a truncated β3 cytoplasmic domain. Taken together, these data suggest that ligand binding to platelet αIIbβ3 induces integrin cytoplasmic domain–dependent phosphorylation of FcγRIIa, which then enlists selected components of the immunoreceptor signaling cascade to transmit amplification signals into the cell.
Introduction
Exposure at sites of vascular injury of collagen, von Willebrand factor (VWF), laminin, and other components of the extracellular matrix results in rapid adherence of circulating platelets, which in turn initiates a complex series of platelet surface membrane glycoprotein-mediated signaling events that lead to platelet activation, granule secretion, and recruitment of additional platelets—processes that are critical for the maintenance of vascular integrity, blood flow, and hemostasis (for a review, see Jackson et al1 ). The combined actions of released or generated secondary platelet agonists such as ADP and thromboxane A2, as well as activation signals that result from the binding of fibrinogen and VWF to the major platelet integrin, αIIbβ3 (also known as the glycoprotein IIb-IIIa [GPIIb-IIIa] complex), generate important additional amplification signals that are necessary to support stable platelet-platelet interactions and thrombus formation
Immunoreceptor tyrosine-based activation motifs (ITAMs) are signaling motifs composed of the amino acid sequence YxxL, and are found within the cytoplasmic domains of T- and B-cell antigen receptor subunits,2-4 the adaptor molecule DAP12 expressed in lymphoid, myeloid, and natural killer cells,5,6 and the Fc receptor γ (FcRγ) chain homodimer that is noncovalently associated with the GPVI collagen receptor on platelets7,8 and with high affinity Fc receptors for IgG and IgE.9 An ITAM is also an intrinsic component of the cytoplasmic domain of the platelet and leukocyte low-affinity receptor for IgG, FcγRIIa.10 When ITAM-bearing receptors become engaged or cross-linked, tyrosine residues within the ITAM undergo Src family kinase–dependent phosphorylation, creating docking sites for tandem Src homology 2 (SH2) domains of the protein tyrosine kinase, Syk,11,12 or its T-cell analog, Zap-70.13 The ensuing recruitment and activation of Syk results in the assembly of a multiprotein signaling complex containing the cytosolic adaptor molecule SLP-7614 and one or more members of the Bruton tyrosine kinase (Btk) family,15,16 ultimately resulting in activation of phospholipase Cγ2 (PLCγ2) and/or PLCγ1, which via their lipase activity generate lipid products that support a multitude of cellular activation responses.17-19
Integrins are a family of bidirectional cell adhesion and signaling receptors that mediate both cell-cell and cell-matrix interactions.20 In response to a variety of intracellular activation signals, integrins can undergo changes in their conformation that increase their affinity for ligand—a process known as “inside-out” signal transduction. They also transmit into the cell ligand binding–dependent signals that initiate a wide variety of cellular processes, ranging from activation of protein tyrosine and lipid kinases, to gross morphologic changes in cell shape and migration—a process often referred to as “outside-in” integrin signaling. Although the exact mechanism by which integrins are able to promote and amplify cellular activation is not well understood, recent studies in platelets, neutrophils, and osteoclasts have identified several cytosolic proteins, previously thought to be used only for immunoreceptor signaling, that appear to play an unexpected role in outside-in integrin signaling. For example, platelets deficient in PLCγ2,18,19 or that express a mutant form of Syk unable to associate with phosphorylated ITAMs,21 spread poorly on immobilized fibrinogen. Evidence that ITAM-bearing adaptor proteins themselves might be required to initiate or amplify integrin-mediated cellular activation responses was recently provided from findings made in genetically modified mice deficient in the ITAM-bearing subunits FcRγ and DAP12, whose neutrophils and macrophages were found to be markedly defective in integrin-mediated responses.22,23 Whether ITAM-bearing proteins are widely used for integrin signaling in other cell types, however, is not known, nor has the identity of other clinically important ITAM/integrin pairs been established.
Human platelets express 3 known ITAM-bearing molecules: the FcRγ chain—a 12-kDa homodimer that exists in the plasma membrane noncovalently associated with the major platelet receptor for collagen, GPVI7,8 ; CLEC-2—a recently identified C-type lectin receptor for podoplanin that harbors a single ITAM tyrosine residue within its cytoplasmic domain24,25 ; and FcγRIIa—a low-affinity receptor for IgG immune complexes expressed on platelets and leukocytes.26 The purpose of the present investigation was to determine which among these, if any, might couple integrins to ITAM signaling in human platelets. Our findings may have important implications for understanding the molecular mechanisms of platelet activation and adhesion, and may pave the way for development of a new class of novel antiplatelet agents and antithrombotic therapeutics.
Methods
Patients
The cell, molecular, and biochemical characteristics of platelets from a patient with autoantibody-induced shedding of platelet membrane GPVI have been described previously.27 For some studies, small blood samples were also obtained from a child diagnosed with variant Glanzmann thrombasthenia whose platelets expressed 100% normal levels of both the αIIbβ3 integrin complex and platelet FcγRIIa (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). All blood samples were donated as approved by the Institutional Review Board of the BloodCenter of Wisconsin and informed consent was obtained in accordance with the Declaration of Helsinki.
Antibodies and reagents
The hybridoma cell line for the anti-FcγRIIa monoclonal antibody (mAb), IV.3,28 was purchased from the ATCC (Manassas, VA). Antibodies specific for Syk and PLCγ2 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies specific for tyrosine-phosphorylated Syk tyrosine residues 525 to 526 and tyrosine-phosphorylated PLCγ2 were purchased from Cell Signaling Technology (Danvers, MA). The antiphosphotyrosine mAb, 4G10, was purchased from Millipore (Billerica, MA), and the antiphosphotyrosine PY20 was purchased from Invitrogen (Carlsbad, CA). Protease and phosphatase inhibitor cocktails were purchased from EMD Chemicals (San Diego, CA). Monovalent Fab fragments were prepared from mAb IV.3 and anti–PECAM-1 mAb 235.1 using a Fab preparation kit from Pierce Biotechnology (Rockford, IL). RGDW, collagen-related peptide (CRP)29 and thrombin receptor activating peptide (TRAP = SFLLRN) were synthesized by the Blood Research Institute's Protein Chemistry Core laboratory.
Preparation of washed platelets
Whole blood drawn into acid citrate dextrose was diluted 1:1 with modified Tyrode-HEPES (Tyrode–N-2-hydroxyethylpiperazine-N-2-ethanesulfonic acid) buffer (10 mM HEPES [pH 7.4], 12 mM NaHCO3, 137 mM NaCl, 2.7 mM KCl, 5 mM glucose, 0.25% bovine serum albumin [BSA]). Diluted whole blood was supplemented with 50 ng/mL prostaglandin E1 (PGE1) and spun at 200g for 10 minutes. Platelet-rich plasma was collected, and after the addition of 50 ng/mL PGE1, platelets were pelleted at 750g for 10 minutes. Platelets were washed once in Tyrode-HEPES buffer containing 50 ng/mL PGE1 and 1 mM EDTA, pH 7.4, and finally resuspended in Tyrode-HEPES to a final concentration of 2.5 × 108/mL.
Platelet spreading on immobilized fibrinogen
Washed platelets (50 μL) at a concentration of 5 × 106/mL were preincubated with Fab fragments derived from the anti-FcRγIIa mAb IV.3 or an isotype control Fab derived from a 235.1, a mAb directed against an epitope in the cytoplasmic domain of PECAM-1. For spreading assays, samples were incubated at 37°C for 30, 45, or 60 minutes on 8-chamber glass tissue-culture slides (Becton Dickinson, Franklin Lakes, NJ) that had been coated overnight at 4°C with human fibrinogen (100 μg/mL), rinsed with PBS, and blocked with 1% BSA for 2 hours at room temperature. After gently rinsing 3 times with Tyrode-HEPES buffer, remaining adherent platelets were fixed with 1% paraformaldehyde for 20 minutes and permeabilized for 30 minutes at room temperature with 0.1% Triton X-100 in 100 mM Tris-Cl (pH 7.4), 150 mM NaCl, 10 mM EGTA, 5 mM MgCl2, containing 1× protease inhibitor cocktail, and stained with phalloidin tetramethylrhodamine isothiocyanate (phalloidin-TRITC, 1 μg/mL) for 60 minutes at room temperature in the dark. Samples were mounted in Vectashield mounting medium (Vector Laboratories, Burlingame, CA), and images were acquired with a Photometrics SenSys camera (Photometrics, Tucson, AZ) from 3 consecutive fields using a Zeiss Axioscop microscope (Carl Zeiss, Oberkochen, Germany) with a Zeiss 100× oil-immersion lens (1.3 numeric aperture) and analyzed using Metamorph software (Universal Imaging, Downingtown, PA). Statistical analysis was performed using a 2-tailed Student t test for unpaired samples.
For biochemical analysis, platelets were incubated at 37°C for 45 minutes on 10-cm tissue-culture dishes and lysed directly with 2× lysis buffer (30 mM HEPES [pH 7.4], 300 mM NaCl, 20 mM EGTA [ethylene glycol tetra-acetic acid], 0.2 mM MgCl2, 2% Triton X-100) containing 2× protease and phosphatase inhibitor cocktails, and subjected to SDS–polyacrylamide gel electrophoresis (PAGE) immunoblot analysis.
Platelet aggregation assays
Samples prepared to examine the effects of soluble fibrinogen binding on platelet activation were performed using a whole-blood lumi-ionized calcium aggregation system (Chrono-log, Havertown, PA). Washed platelets (500 μL) in Tyrode-HEPES containing 1 mM CaCl2 were placed in a siliconized glass cuvette at 37°C with constant stirring at 1000 rpm. Platelet activation and secretion were initiated by addition of 10 μM or 0.5 μM TRAP for select RGD and mAb IV.3 inhibition experiments, respectively. Platelets were allowed to aggregate for 4 minutes before addition of 2× lysis buffer for subsequent biochemical analysis.
Results
Outside-in signaling initiated by platelet binding to immobilized fibrinogen uses components of the FcγRIIa→Syk→PLCγ2 signal transduction pathway
Human platelets are known to express 3 ITAM-bearing subunits: CLEC-2,24 the FcRγ chain homodimer,30 and the platelet Fc receptor, FcγRIIa.26 Although CLEC-2 does not become tyrosine phosphorylated during agonist-induced platelet activation (B.B., C.G., and P. J. Newman, unpublished observations, October 2007), 2 preliminary reports31,32 have shown that the GPVI/FcRγ chain complex, in addition to functioning as a major platelet collagen receptor,7,30 becomes tyrosine phosphorylated in response to agonists that bind G protein–coupled receptors (GPCRs) in a manner that, at least in one study,31 required ligand binding to the major platelet integrin, αIIbβ3. ITAM tyrosines within the cytoplasmic domain of FcγRIIa have also been shown to become tyrosine phosphorylated after platelet activation,33 although a relationship between FcγRIIa activation and integrin engagement has not been explored.
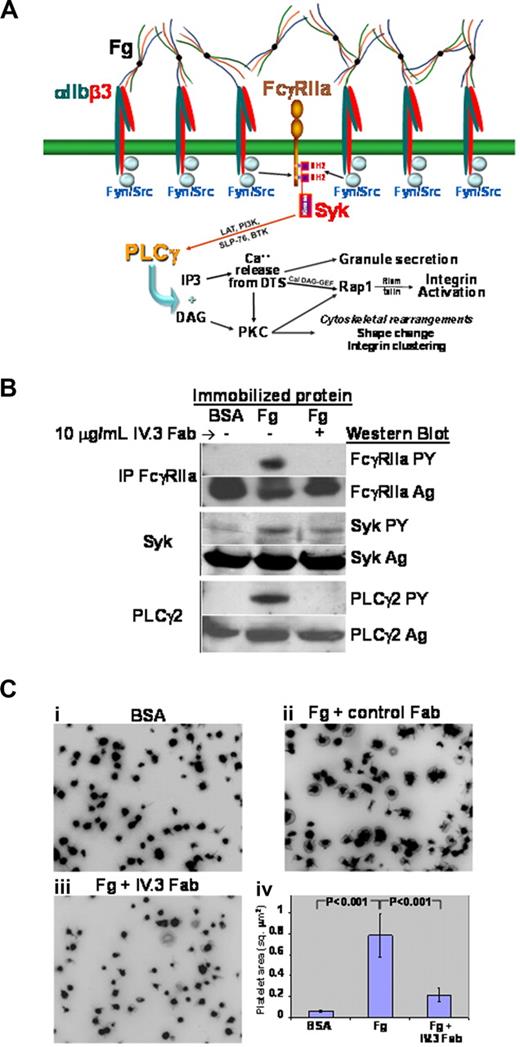
Previous studies have shown that adhesion of resting platelets to immobilized ligands for αIIbβ3 directly initiates outside-in signal transduction, resulting in tyrosine phosphorylation of multiple proteins, cytoskeletal reorganization, and changes in cell shape.34 To determine whether FcγRIIa functions as a ligand binding–dependent amplifier of integrin signaling in human platelets, resting platelets were allowed to settle and spread on immobilized fibrinogen, and the tyrosine phosphorylation state of FcγRIIa, Syk, and PLCγ2 was interrogated by immunoblot analysis. Platelet binding to immobilized fibrinogen (illustrated schematically in Figure 1A) resulted in robust phosphorylation of all 3 signaling components (Figure 1B). Evidence that FcγRIIa is not only coupled to, but required for, integrin signaling downstream of ligand binding was provided by incubating platelets with small Fab fragments derived from the anti-FcγRIIa–specific mAb, IV.3. As shown in Figure 1B, precoating platelets with IV.3 Fab nearly eliminated tyrosine phosphorylation of all 3 signaling components. The physiologic importance of FcγRIIa in transmitting αIIbβ3-mediated outside in signals was revealed by experiments in which IV.3 Fabs were found to markedly inhibit platelet spreading on immobilized fibrinogen (Figure 1C). In addition, IV.3 had a marked inhibitory effect on clot retraction (Figure S1). Taken together, these data strongly suggest that the FcγRIIa→Syk→PLCγ2 pathway is required for both the biochemical and cell biologic events that occur downstream of αIIbβ3 binding to its ligand.
Outside-in signaling initiated by platelet interaction with immobilized fibrinogen activates the FcγRIIa signal transduction pathway. (A) Schematic showing use of the FcγRIIa signaling pathway downstream of αIIbβ3 engagement. Note the importance of physical approximation of the integrin with FcγRIIa, allowing integrin-associated Src family members to function as ITAM kinases, thus initiating the outside-in signaling cascade. (B,C) Washed platelets were plated onto 8-chamber glass tissue-culture slides coated with either BSA or fibrinogen (Fg), and allowed to spread for 45 minutes in the presence or absence of 10 μg/mL IV.3 Fab Immunoprecipitation and Western blot analysis (B) reveals strong activation of FcγRIIa, Syk, and PLCγ2 after platelet binding to immobilized fibrinogen, and inhibition of this outside-in signaling pathway by Fab fragments of mAb IV.3. (C) Effect of IV.3 on platelet spreading. Preincubation of platelets with IV.3 Fab markedly inhibits platelet spreading on immobilized fibrinogen, shown in panels A through C, with quantitative analysis of the pixel area of spread platelets in panel. Data shown are the mean plus or minus SEM from one of 4 representative experiments using 3 different platelet donors.
Outside-in signaling initiated by platelet interaction with immobilized fibrinogen activates the FcγRIIa signal transduction pathway. (A) Schematic showing use of the FcγRIIa signaling pathway downstream of αIIbβ3 engagement. Note the importance of physical approximation of the integrin with FcγRIIa, allowing integrin-associated Src family members to function as ITAM kinases, thus initiating the outside-in signaling cascade. (B,C) Washed platelets were plated onto 8-chamber glass tissue-culture slides coated with either BSA or fibrinogen (Fg), and allowed to spread for 45 minutes in the presence or absence of 10 μg/mL IV.3 Fab Immunoprecipitation and Western blot analysis (B) reveals strong activation of FcγRIIa, Syk, and PLCγ2 after platelet binding to immobilized fibrinogen, and inhibition of this outside-in signaling pathway by Fab fragments of mAb IV.3. (C) Effect of IV.3 on platelet spreading. Preincubation of platelets with IV.3 Fab markedly inhibits platelet spreading on immobilized fibrinogen, shown in panels A through C, with quantitative analysis of the pixel area of spread platelets in panel. Data shown are the mean plus or minus SEM from one of 4 representative experiments using 3 different platelet donors.
The αIIbβ3→FcγRIIa→Syk→PLCγ2 signaling pathway also serves as a weak amplifier of platelet activation after agonist-induced binding of soluble fibrinogen to platelets
To complement the studies shown in Figure 1 in which platelets were allowed to interact with immobilized fibrinogen or fibrin, we next examined the potential for FcγRIIa to amplify platelet responses downstream of already strong agonists such as thrombin or its peptide surrogate, TRAP (thrombin receptor activating peptide, which activates platelets and stimulates granule secretion via the G protein–coupled receptor [GPCR] PAR1).35 As shown in Figure 2, platelet stimulation with TRAP resulted in tyrosine phosphorylation of FcγRIIa, as well as its downstream effectors, Syk and PLCγ2. Notably, phosphorylation of all 3 of these signaling molecules after TRAP-induced platelet activation was strongly inhibited by RGD peptide—which blocks ligand binding to αIIbβ3—consistent with the notion that the binding of soluble fibrinogen to its integrin receptor initiates the FcγRIIa ITAM→Syk→PLCγ2 pathway. Preincubation of platelets with IV.3 Fabs also suppressed tyrosine phosphorylation of these 3 signaling components, consistent with the findings in Figure 1 that this ITAM-bearing signaling receptor is enlisted to amplify platelet responses downstream of fibrinogen binding to the αIIbβ3 integrin complex.
Agonist-induced activation of human platelets results in tyrosine phosphorylation of FcγRIIa, Syk, and PLCγ2 in a ligand binding–dependent manner. Washed human platelets stirring in an aggregometer cuvette were stimulated with TRAP in the presence or absence of 2 mM RGD peptide or 10 μg/mL IV.3 Fab, as indicated. After detergent lysis, proteins were immunoprecipitated using antibodies specific for FcγRIIa, Syk, or PLCγ2, and immunoblots containing immunoprecipitated proteins stained either for antigen (Ag) or phosphotyrosine (PY). Note that FcγRIIa, Syk, and PLCγ2 each become tyrosine phosphorylated after platelet activation, and that preventing ligand binding with RGD peptide (left half of the figure) largely inhibits tyrosine phosphorylation of each of these signaling components, demonstrating that ligand binding to the integrin αIIbβ3 activates the FcγRIIa→Syk→PLCγ2 signaling pathway. Also note that preincubation of platelets with IV.3 (right half of the figure) prevents FcγRIIa, Syk, and PLCγ2 activation in response to TRAP. Data shown are representative of 3 separate experiments performed using 2 different platelet donors.
Agonist-induced activation of human platelets results in tyrosine phosphorylation of FcγRIIa, Syk, and PLCγ2 in a ligand binding–dependent manner. Washed human platelets stirring in an aggregometer cuvette were stimulated with TRAP in the presence or absence of 2 mM RGD peptide or 10 μg/mL IV.3 Fab, as indicated. After detergent lysis, proteins were immunoprecipitated using antibodies specific for FcγRIIa, Syk, or PLCγ2, and immunoblots containing immunoprecipitated proteins stained either for antigen (Ag) or phosphotyrosine (PY). Note that FcγRIIa, Syk, and PLCγ2 each become tyrosine phosphorylated after platelet activation, and that preventing ligand binding with RGD peptide (left half of the figure) largely inhibits tyrosine phosphorylation of each of these signaling components, demonstrating that ligand binding to the integrin αIIbβ3 activates the FcγRIIa→Syk→PLCγ2 signaling pathway. Also note that preincubation of platelets with IV.3 (right half of the figure) prevents FcγRIIa, Syk, and PLCγ2 activation in response to TRAP. Data shown are representative of 3 separate experiments performed using 2 different platelet donors.
Human platelets expressing decreased FcγRIIa fail to spread on immobilized fibrinogen
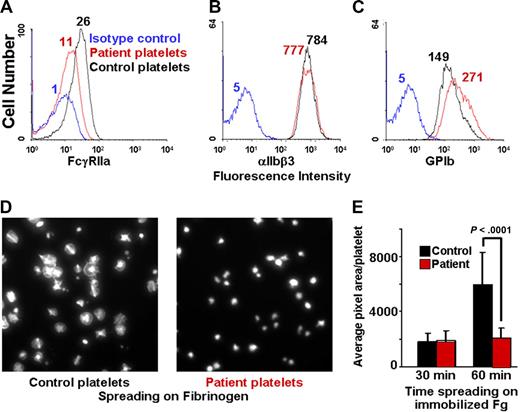
We have previously described a patient with autoantibody-mediated shedding of the extracellular domain of GPVI,27 and recent studies have shown that ectodomain shedding of GPVI is accompanied by calpain-mediated cleavage of the cytoplasmic domain of FcγRIIa at sites amino-terminal to its cytoplasmic ITAM.36 To determine the functional consequences of FcγRIIa inactivation on the ability of human platelets to undergo αIIbβ3-mediated outside-in signal transduction, we examined the ability of platelets derived from this patient to spread on immobilized fibrinogen. As shown in Figure 3A, patient platelets express only approximately 40% of the normal levels of FcγRIIa, and exhibited a correspondingly marked reduction in the ability to spread on immobilized fibrinogen (Figure 3D), consistent with a requirement for ITAM-bearing FcγRIIa in integrin αIIbβ3-mediated outside-in signal transmission.
Human platelets expressing decreased FcγRIIa fail to spread on immobilized fibrinogen. (A-C) Flow cytometric analysis of washed platelets from a patient with an autoantibody specific for GPVI, demonstrating approximately 60% reduced expression of FcγRIIa (as reported by the binding if mAb IV.3), but normal expression of αIIbβ3 and the GPIb complex. (D,E) Washed patient platelets or control platelets from a healthy donor were allowed to spread on glass slides that had been coated with 100 μg/mL fibrinogen. The degree of platelet spreading was analyzed at both 30 and 60 minutes. Note that the patient's platelets failed to spread. Data are the mean plus or minus SEM.
Human platelets expressing decreased FcγRIIa fail to spread on immobilized fibrinogen. (A-C) Flow cytometric analysis of washed platelets from a patient with an autoantibody specific for GPVI, demonstrating approximately 60% reduced expression of FcγRIIa (as reported by the binding if mAb IV.3), but normal expression of αIIbβ3 and the GPIb complex. (D,E) Washed patient platelets or control platelets from a healthy donor were allowed to spread on glass slides that had been coated with 100 μg/mL fibrinogen. The degree of platelet spreading was analyzed at both 30 and 60 minutes. Note that the patient's platelets failed to spread. Data are the mean plus or minus SEM.
The cytoplasmic domain of integrin β3 is required for activation of FcγRIIa-supported platelet spreading on immobilized fibrinogen
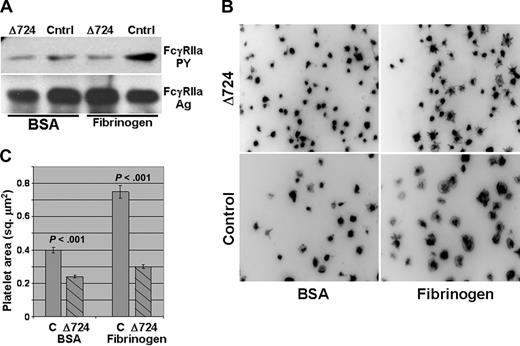
To further define the molecular requirements for functional coupling between ligand-engaged integrin αIIbβ3 and FcγRIIa-mediated platelet amplification, we obtained platelets from a patient harboring a homozygous nonsense mutation encoding an Arg724Stop within the integrin β3 gene, resulting in the expression of a β3 integrin subunit with a truncated cytoplasmic domain that lacks most of the cytoplasmic domain, including the C-terminal RGT752 sequence that has been shown to bind Src.37 Although platelets from this variant Glanzmann thrombasthenic patient constitutively express on their surface normal levels of both integrin αIIbβ3 and FcγRIIa (Figure S2), FcγRIIa failed to become tyrosine phosphorylated when these platelets were incubated in microtiter wells coated with immobilized fibrinogen (Figure 4A). Consequently, their ability to spread on immobilized fibrinogen was significantly compromised compared with platelets from a healthy donor (Figure 4B,C). These data demonstrate that initiation of signal transduction after engagement of integrin αIIbβ3 requires the β3 cytoplasmic domain, and further implicates FcγRIIa as the ITAM-bearing receptor required for outside-in signaling in human platelets.
The cytoplasmic domain of the integrin β3 subunit is required for ligand binding–induced phosphorylation of FcγRIIa. Washed platelets from a patient expressing a homozygous mutant form of a Δ724 β3 integrin subunit were plated onto immobilized fibrinogen or BSA and allowed to spread for 45 minutes at 37°C. Note the failure of platelets harboring a truncated β3 cytoplasmic tail to support tyrosine phosphorylation of FcγRIIa (A) or to spread on immobilized fibrinogen (B,C). Representative pictures are shown in panel B, with the average pixel area of 148 to 350 platelets per condition quantitated and shown in panel C. Error bars show mean plus or minus SEM.
The cytoplasmic domain of the integrin β3 subunit is required for ligand binding–induced phosphorylation of FcγRIIa. Washed platelets from a patient expressing a homozygous mutant form of a Δ724 β3 integrin subunit were plated onto immobilized fibrinogen or BSA and allowed to spread for 45 minutes at 37°C. Note the failure of platelets harboring a truncated β3 cytoplasmic tail to support tyrosine phosphorylation of FcγRIIa (A) or to spread on immobilized fibrinogen (B,C). Representative pictures are shown in panel B, with the average pixel area of 148 to 350 platelets per condition quantitated and shown in panel C. Error bars show mean plus or minus SEM.
Discussion
FcγRIIa (CD32) is a member of the immunoglobulin (Ig) gene superfamily and is composed of 2 extracellular Ig homology domains—the C-terminal of which contains binding sites for the Fc region of IgG, a single-pass transmembrane domain, and a 76–amino acid cytoplasmic tail that contains 2 YxxL sequences separated by a somewhat longer than usual 12–amino acid spacer region that together constitute its single ITAM.10 FcγRIIa is the predominant FcγRII receptor on neutrophils and macrophages,38 and its cross-linking triggers such diverse activation events as calcium mobilization,39 superoxide production,39 and phagocytosis.40,41
Although FcγRIIa was identified on human platelets almost 25 years ago,26 cloned nearly 20 years ago,38,42 and solved at the structural level nearly a decade ago,43 the reason that platelets should express 2000 to 3000 copies44,45 of a low-affinity receptor for monomeric IgG on their surface has never been satisfactorily explained. FcγRIIa is known to be responsible for receptor-mediated endocytosis and packaging into platelet α-granules of plasma IgG,46 and when transfected into nucleated cells mediates internalization of opsonized particles.41,47 The relevance, however, of these functions for human platelet biology is uncertain, and although the importance of FcγRIIa in pathophysiologic disorders such as heparin-induced thrombocytopenia is well understood,48 its role in normal platelet physiology has remained a mystery.
The major finding of the current investigation is that FcγRIIa and its downstream effector molecules represent key components of outside-in signal amplification mediated by the major human platelet integrin, αIIbβ3. We found that FcγRIIa, Syk, and PLCγ2 each become activated after the binding of either soluble or immobilized fibrinogen to αIIbβ3 (Figures 1,2), and used 3 complementary approaches to show that the functional coupling between this integrin and what has previously been thought to be simply a receptor for IgG immune complexes is required for αIIbβ3-mediated outside-in signaling. Thus, the ability of platelets to become activated and spread on fibrinogen was significantly inhibited by preincubating platelets with the blocking anti-FcγRIIa–specific mAb, IV.3 (Figure 1), in platelets that expressed reduced levels of a crippled FcγRIIa receptor (Figure 3), and in platelets missing most of the cytoplasmic domain of the integrin β3 subunit (Figure 4). Taken together, these results establish FcγRIIa as the ITAM-bearing receptor mediating αIIbβ3 outside-in integrin signaling in human platelets.
FcγRIIa is thus the latest of a growing number of proteins, previously thought to be used exclusively for immunoreceptor signaling, to be shown to play a prominent role in integrin-mediated signal transmission. Mócsai et al recently reported that a variety of β2 integrin–mediated signaling responses in neutrophils and macrophages require tyrosine phosphorylation of the ITAM-bearing proteins DAP12 and FcRγ, along with subsequent recruitment of Syk.22 Although previous studies have shown that Syk,21 the adaptor protein SLP-76,21 and PLCγ218,19 are required for platelet spreading on immobilized fibrinogen, the membrane protein responsible for recruiting Syk to the inner face of the plasma membrane had not been identified. Earlier studies in Chinese hamster ovary (CHO) cells with enforced overexpression of Syk suggested that this protein tyrosine kinase might be associated with the β3 cytoplasmic domain in an SH2 domain/ITAM-independent manner,49,50 however more recent studies using platelets that express mutant isoforms of Syk harboring single amino acid substitutions within their SH2 domains that prevent association with phosphorylated ITAMs clearly demonstrated a requirement for the SH2 domains of Syk in integrin-mediated outside-in signaling21 —thereby implicating a yet-to-be-identified ITAM-containing molecule upstream of Syk in integrin-mediated cellular activation. Our identification of FcγRIIa as that ITAM-bearing receptor adds another important, albeit somewhat unexpected, signaling component to the outside-in integrin signaling circuit.
Although many of the molecular details underlying integrin-mediated signal transmission remain to be uncovered, growing evidence in several cellular systems suggests that integrin-associated Src family kinases play a critical role in relaying signals downstream of ligand binding.37,51-56 Wang et al observed more than 10 years ago that a truncated integrin β3 subunit cytoplasmic domain in a variant form of Glanzmann thrombasthenia was unable to support the spreading of human platelets on immobilized fibrinogen,57 thereby underscoring the importance of outside-in signaling to human platelet pathophysiology. Given the observation that human platelets lacking most of the β3 cytoplasmic domain also fail to tyrosine phosphorylate the ITAM tyrosines of FcγRIIa (Figure 4A), it is tempting to speculate that, upon ligand binding, one of the αIIbβ3-associated Src family members37,58 phosphorylates FcγRIIa, creating the docking site for Syk that initiates a signaling pathway leading to activation of PLCγ2, which, via production of the second messengers IP3 and diacylglycerol, amplifies a multitude of platelet activation responses.
The fibrinogen→αIIbβ3→Src→FcγRIIa ITAM→Syk→PLCγ2 outside-in signaling pathway defined in the present work reveals a bit of symmetry in nature relative to the manner by which adhesive ligands appear to activate platelets. Thus, collagen binding to the integrin α2β1 uses both the ITAM-bearing GPVI/FcRγ chain59-61 as well as PLCγ217 to transmit activation signals, as does the integrin α6β1 when platelets encounter the extracellular matrix protein laminin.62,63 Similarly, interaction of von Willebrand factor with the platelet GPIb complex results in activation of Src family kinases, Syk, and PLCγ2,64-67 and we and others have shown a critical role for PLCγ2 in supporting platelet spreading66,68 and thrombus formation68 on immobilized VWF under conditions of flow. Unlike collagen, laminin, or fibrinogen binding–induced activation, however, VWF-induced platelet activation does not appear to require FcγRIIa or the FcRγ chain to transmit adhesion-dependent signals,66,69 although their ability to serve as costimulatory signal amplification molecules, when present, has not been ruled out.65,69 Perhaps a still-to-be-identified ITAM-bearing protein in platelets serves as the primary mediator of GPIb signaling. In this regard, identification of potential candidate ITAM-containing proteins downstream of the interaction of human platelets with fibronectin (via α5β1) or osteopontin (via αvβ3), or of murine platelets with fibrinogen (murine platelets do not express FcγRIIa38,70,71 ), remains an important area for future investigation.
Although the physical nature underlying the functional relationship between integrins and ITAM-bearing receptors in general, and between αIIbβ3 and FcγRIIa in particular, is not understood, several possibilities to explain their stimulus-response coupling come to mind. First, although neither Mócsai et al22 nor we (B.B. and P. J. Newman, unpublished observations, January 2008) have been able to coimmunoprecipitate an integrin with an ITAM-bearing receptor, evidence that they may be nevertheless topographically associated, at least in platelets, derives from the observations that (1) preincubation of certain αIIbβ3-specific antibodies with platelets inhibits the binding of both the anti-FcγRIIa mAb, IV.3, and human aggregated IgG,72 whereas (2) certain other anti-αIIb-β3 mAbs bind in such a way as to present their Fc regions to FcγRIIa, resulting in robust platelet activation73-76 —an event that can be totally blocked by preincubation with IV.3. Our observation that IV.3 Fabs prevent ligand binding–induced, αIIbβ3-mediated activation of FcγRIIa, Syk, and PLCγ2, as well as platelet spreading on immobilized fibrinogen (Figure 1), strongly supports this concept. Whether IV.3 Fabs inhibit integrin-mediated signal transmission by sterically blocking activation-induced approximation of an integrin-associated kinase with the ITAMs of FcγRIIa, or by interfering with a still-to-be-identified intermediary docking protein that mediates integrin-ITAM coupling, remains to be determined.
Platelets in the circulation patrol the bloodstream looking for sites of vascular injury, and rapidly respond to a variety of external stimuli to maintain hemostasis. Ligands for GPCRs, immunoreceptor family members, and integrins trigger signaling pathways that increasingly appear to share common components, and converge to accomplish a common goal, namely granule release, integrin activation, and eventual thrombus formation. Findings from the current study add a key piece of information to our current understanding of the global picture of signaling in platelets by providing a link between GPCRs, immunoreceptors, and integrins. Identification of FcγRIIa as the likely target of integrin-associated Src family kinases, and the docking site for Syk leading to activation of PLCγ2, provides a new piece of the platelet signaling circuit that links outside-in integrin-mediated signal transmission to shared components used in immunoreceptor signaling. These findings may additionally provide a foundation for the development of novel strategies to combat a wide range of clinical disorders associated with bleeding and clotting.
The online version of this article contains a data supplement.
Portions of this work were presented in abstract form at the 49th Annual Meeting of the American Society of Hematology, December 11, 2007, Atlanta, GA.77
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank James Crockett for assistance in performing platelet spreading assays, Nick Cartwright for molecular analysis of the mutation in the patient with variant Glanzmann thrombasthenia, and Dr Sanford Shattil (University of California San Diego) for valuable discussions before submission of this paper. We are indebted to the 2 patients and their families for the generous contribution of platelets deficient in FcγRIIa and αIIbβ3 function.
This work was supported by grant HL-44612 (P.J.N. and D.K.N.) from the National Heart, Lung, and Blood Institute of the National Institutes of Health (Bethesda, MD).
National Institutes of Health
Authorship
Contribution: B.B. designed and performed research, analyzed results, and wrote the paper; C.G. designed and performed research and analyzed results; V.R. performed research; J.C.G. contributed vital new reagents; D.K.N. analyzed results; and P.J.N. designed research, analyzed results, and wrote the paper.
Conflict-of-interest disclosure: P.J.N. is a consultant for Novo Nordisk and a member of the Scientific Advisory Board of the New York Blood Center. All other authors declare no competing financial interests.
Correspondence: Peter J. Newman, Blood Research Institute, BloodCenter of Wisconsin, PO Box 2178, 638 North 18th Street, Milwaukee, WI 53201; e-mail: peter.newman@bcw.edu.
References
Author notes
*B.B. and C.G. contributed equally to this work.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal