Abstract
We report 3 cases of a previously uncharacterized form of histiocytosis presenting in early infancy and showing ALK immunoreactivity. The patients presented with pallor, massive hepatosplenomegaly, anemia, and thrombocytopenia. Liver biopsy showed infiltration of the sinusoids by large histiocytes with markedly folded nuclei, fine chromatin, small nucleoli, and voluminous lightly eosinophilic cytoplasm that sometimes was vacuolated or contained phagocytosed blood cells. One patient developed cutaneous infiltrates that morphologically resembled juvenile xanthogranuloma. The histiocytes were immunoreactive for histiocytic markers (CD68, CD163, lysozyme), S100 protein, ALK (membranous and cytoplasmic pattern), and dendritic cell markers (fascin, factor XIIIa), but not CD1a and langerin. One case successfully analyzed by molecular techniques revealed TPM3-ALK fusion. Thus the spectrum of diseases exhibiting ALK translocation should be expanded to include ALK+ histiocytosis. The disease in the 3 patients (2 having been given chemotherapy) resolved slowly over many months.
Introduction
Histiocytosis in childhood is a heterogeneous group of localized or disseminated diseases.1-3 Some forms of histiocytosis result from an underlying genetic defect such as mutation in the perforin gene (familial hemophagocytic lymphohistiocytosis), some are precipitated by an infection such as Epstein-Barr virus–associated hemo-phagocytic syndrome, and some represent monoclonal proliferations such as Langerhans cell histiocytosis, although most do not have an obvious cause.1,4-9 We report 3 cases of a previously undescribed form of histiocytosis in infants, characterized by distinctive morphology and ALK expression.
Methods
The index case was found to express ALK at diagnosis (Royal Children's Hospital), and the other 2 cases (Queen Elizabeth Hospital) were retrospectively identified based on similarities in clinicopathologic features. Available clinical records and pathologic materials were retrieved for review. Immunohistochemical studies were performed in an automated immunostainer (BOND-MAX; Leica Microsystems, Wetzlar, Germany) using a polymer detection system.
RNA was extracted from frozen liver and muscle biopsies of case 1, and paraffin sections of liver biopsy of case 2, using the RNAeasy Midi Kit (Qiagen, Courtaboeuf, France). Synthesis of the first-strand cDNA and polymerase chain reaction (PCR) amplification were performed in a single tube using reverse-transcription (RT)–PCR Access Kit (Promega, Charbonnières, France). The first step consisted of an ALK gene–specific reverse transcription, followed by 30 cycles of amplification using C-TPM1 (positions 97-117 on cDNA TPM3 sequence) and ALK1 (positions 4211-4187 on cDNA ALK sequence) primers. The second round was performed on a 1-μL aliquot from the first amplification, using C-TPM3 (positions 445-466 on cDNA TPM3 sequence) and ALK2 (positions 4171-4148 on cDNA ALK sequence) primers, yielding a 307-bp PCR product.10
Results and discussion
Clinical features
The 3 female infants presented with pallor (Table 1). All had prominent hepatosplenomegaly, but no fever. There was marked anemia and thrombocytopenia, but no leukopenia. Marrow examination showed no obvious abnormal infiltrate or hemophagocytosis. Serologic studies for the common viruses and Toxoplasma were negative. Clinically, storage disease and malignancy were suspected, prompting performance of liver biopsy. Cases 1 and 2 were given dexamethasone and etoposide, whereas case 3 was not given any specific treatment. The hematologic pictures improved slowly over many months, and the hepatosplenomegaly also gradually resolved. The patients were well at 2.5 to 7 years, and showed normal development.
Summary of clinical findings
| Case; sex/age . | Case 1; F/neonate . | Case 2; F/3 mo . | Case 3; F/3 mo . |
|---|---|---|---|
| Presentation | Pallor; failure to thrive; abdominal distension; ascites; lower limb edema | Pallor; mild jaundice | Noted to have persistent pallor on regular checkup |
| Hepatosplenomegaly | Gross (liver 11 cm and spleen 9 cm below costal margin) | Gross (liver 6 cm and spleen 4 cm below costal margin) | Demonstrated on ultrasound examination; a 2-cm hypodense area in dome of liver on CT scan |
| Blood counts | Hb level: 43 g/L; Platelet count: 3 × 109/L | Hb level: 34 g/L; Platelet count: 12 × 109/L | Hb level: 63 g/L; Platelet count: 57 × 109/L |
| Serum albumin level (N: 29-45 g/L) | 15 g/L | 19 g/L | NA |
| Serum ferritin level (N: 8-135 μg/L) | 361 μg/L | 1060 μg/L | NA |
| Serum triglycerides (N: 0.9-2 mM) | 1.7 mM | 1.9 mM | NA |
| Plasma fibrinogen (N: 0.8-3.8 g/L) | 3.3 g/L | 3.93 g/L | NA |
| Treatment and outcome | Mechanical ventilation for increased ascites impairing breathing. Given dexamethasone and etoposide. Gradual improvement, with reduction of ascites but persistence of hepatosplenomegaly. Successfully extubated after 16 days. Then developed multiple brownish to purplish macules, over scalp and trunk, which gradually regressed. At 2.5 years, the child was growing well, with normal blood counts and liver function tests; 3-cm hepatomegaly persisted. | Given dexamethasone and etoposide for about 8 months. Anemia gradually improved, platelet count rose, and biochemical profile normalized. Second biopsy was taken at 1 year (reported then as normal, but abnormal histiocytes were identified retrospectively on ALK immunostaining), and no further treatment was given. Well, with normal growth at 5 years. | Empirically treated with antibiotics. The hemoglobin gradually dropped further, and platelet count remained on the low side. Progressive increase in size of liver (12 cm) and spleen (3 cm). No specific treatment given. Then, condition gradually improved, hepatosplenomegaly regressed, and blood counts normalized. Well at 7 years. |
| Case; sex/age . | Case 1; F/neonate . | Case 2; F/3 mo . | Case 3; F/3 mo . |
|---|---|---|---|
| Presentation | Pallor; failure to thrive; abdominal distension; ascites; lower limb edema | Pallor; mild jaundice | Noted to have persistent pallor on regular checkup |
| Hepatosplenomegaly | Gross (liver 11 cm and spleen 9 cm below costal margin) | Gross (liver 6 cm and spleen 4 cm below costal margin) | Demonstrated on ultrasound examination; a 2-cm hypodense area in dome of liver on CT scan |
| Blood counts | Hb level: 43 g/L; Platelet count: 3 × 109/L | Hb level: 34 g/L; Platelet count: 12 × 109/L | Hb level: 63 g/L; Platelet count: 57 × 109/L |
| Serum albumin level (N: 29-45 g/L) | 15 g/L | 19 g/L | NA |
| Serum ferritin level (N: 8-135 μg/L) | 361 μg/L | 1060 μg/L | NA |
| Serum triglycerides (N: 0.9-2 mM) | 1.7 mM | 1.9 mM | NA |
| Plasma fibrinogen (N: 0.8-3.8 g/L) | 3.3 g/L | 3.93 g/L | NA |
| Treatment and outcome | Mechanical ventilation for increased ascites impairing breathing. Given dexamethasone and etoposide. Gradual improvement, with reduction of ascites but persistence of hepatosplenomegaly. Successfully extubated after 16 days. Then developed multiple brownish to purplish macules, over scalp and trunk, which gradually regressed. At 2.5 years, the child was growing well, with normal blood counts and liver function tests; 3-cm hepatomegaly persisted. | Given dexamethasone and etoposide for about 8 months. Anemia gradually improved, platelet count rose, and biochemical profile normalized. Second biopsy was taken at 1 year (reported then as normal, but abnormal histiocytes were identified retrospectively on ALK immunostaining), and no further treatment was given. Well, with normal growth at 5 years. | Empirically treated with antibiotics. The hemoglobin gradually dropped further, and platelet count remained on the low side. Progressive increase in size of liver (12 cm) and spleen (3 cm). No specific treatment given. Then, condition gradually improved, hepatosplenomegaly regressed, and blood counts normalized. Well at 7 years. |
None of the patients had fever.
N indicates normal range; CT, computed tomography; and NA, not available.
Pathologic findings
The liver biopsies of all 3 cases showed sinusoidal infiltration by single or small aggregates of histiocytes. The histiocytes were very large, with irregularly folded or lobulated nuclei, fine chromatin, and small nucleoli; some contained 2 to 4 nuclei. The voluminous eosinophilic cytoplasm often contained small vacuoles, and occasionally phagocytosed lymphocytes, polymorphs, normoblasts, red cells, or hemosiderin (Figure 1A-C). In case 1, histiocytes also formed aggregates in the portal tracts. Ultrastructural studies were available in cases 1 and 2, and showed cells with short cell processes and abundant cytoplasm containing mitochondria, rough endoplasmic reticulum, ribosomes, lysosomes, and phagolysosomes, but not Birbeck granules. There was no ultrastructural evidence of metabolic disease.
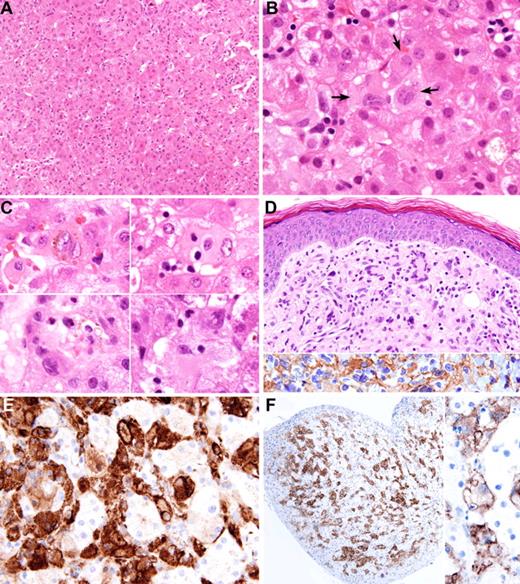
Liver and skin biopsies. (A) Liver biopsy of case 2 shows infiltration of sinusoids by large histiocytes, which on causal examination are difficult to distinguish from the hepatocytes. (B) Liver biopsy of case 1 shows aggregates of histiocytes (arrows) in the sinusoids. The histiocytes have irregularly folded nuclei and abundant lightly eosinophilic cytoplasm. (C) Morphologic spectrum of proliferated histiocytes in the liver sinusoids. The histiocytes have 1 or 2 nuclei, which often show marked irregular foldings and small nucleoli. Some contain phagocytosed blood cells, brown pigment, or fine vacuoles. (D) Skin lesion of case 1 shows dermal infiltrate of mononuclear cells and multinucleated giant cells; the latter showed wreathlike nuclei (upper panel). These cells are immunoreactive for ALK (lower panel). (E) The histiocytes in the sinusoids show positive immunostaining for CD163. (F) Immunostaining for ALK highlights the sinusoidal distribution of the histiocytes (left panel). Higher magnification shows cell membrane and weak cytoplasmic staining (right panel). Images were captured with Olympus DP71 camera mounted on an Olympus microscope model BX61 (Tokyo, Japan). The objectives used for capturing the images were as follow: (A) 20× objective; (B) 100× objective; (C) 100× objective; (D) 40× objective; (E) 60× objective; (F, left) 10× objective; (F, right) 60× objective. Images were acquired using Olympus DP Controller, and whitening of the background and cropping of the images were performed using Adobe Photoshop CS (Adobe Systems, San Jose, CA).
Liver and skin biopsies. (A) Liver biopsy of case 2 shows infiltration of sinusoids by large histiocytes, which on causal examination are difficult to distinguish from the hepatocytes. (B) Liver biopsy of case 1 shows aggregates of histiocytes (arrows) in the sinusoids. The histiocytes have irregularly folded nuclei and abundant lightly eosinophilic cytoplasm. (C) Morphologic spectrum of proliferated histiocytes in the liver sinusoids. The histiocytes have 1 or 2 nuclei, which often show marked irregular foldings and small nucleoli. Some contain phagocytosed blood cells, brown pigment, or fine vacuoles. (D) Skin lesion of case 1 shows dermal infiltrate of mononuclear cells and multinucleated giant cells; the latter showed wreathlike nuclei (upper panel). These cells are immunoreactive for ALK (lower panel). (E) The histiocytes in the sinusoids show positive immunostaining for CD163. (F) Immunostaining for ALK highlights the sinusoidal distribution of the histiocytes (left panel). Higher magnification shows cell membrane and weak cytoplasmic staining (right panel). Images were captured with Olympus DP71 camera mounted on an Olympus microscope model BX61 (Tokyo, Japan). The objectives used for capturing the images were as follow: (A) 20× objective; (B) 100× objective; (C) 100× objective; (D) 40× objective; (E) 60× objective; (F, left) 10× objective; (F, right) 60× objective. Images were acquired using Olympus DP Controller, and whitening of the background and cropping of the images were performed using Adobe Photoshop CS (Adobe Systems, San Jose, CA).
The skin biopsy of case 1 showed a noncircumscribed dermal lesion resembling early juvenile xanthogranuloma predominated by nonlipidized cells. The lesional cells were similar to those seen in the liver, but were admixed with occasional multinucleated giant cells with wreathlike nuclei but that lacked the peripherally located vacuoles characteristic of Touton giant cells (Figure 1D).
In all 3 cases, the lesional cells in the liver showed strong positive staining for CD68 (PGM1; Dako, Glostrup, Denmark), CD163 (10D6; Novocastra, Newcastle upon Tyne, United Kingdom), and lysozyme (antiserum; Dako) (Figure 1E); heterogeneous staining for S100 protein (antiserum; Dako) and factor XIIIa (NCL_FXIIIa; Novocastra); and uniform staining for ALK (ALK1; Dako) and ALKc (from Dr B. Falini, Italy) in a membranous and weak cytoplasmic pattern (Figure 1D,F). Immunostaining with anti–phospho-ALK (antiserum; Cell Signaling Technology, Beverly, MA) showed granular cytoplasmic labeling suggesting ALK fusion protein phosphorylated on tyrosine 664.11 There was focal staining for CD45RB (PD7/26; Dako) and fascin (55K-2; Dako). CD1a (O10; Novocastra), langerin (12D6; Novocastra), CD20 (L26; Dako), CD3 (PS1; Novocastra), and CD30 (BerH2; Dako) were negative. The Ki67 (antiserum; Dako) proliferative index was low (< 2%).
Marrow biopsies were available for ALK immunostaining in cases 2 and 3; these biopsies were originally interpreted on morphologic grounds as negative. Surprisingly, scanty ALK positive cells, solitary or in tiny aggregates, were demonstrated. On retrospective comparison with corresponding fields in the hematoxylin-eosin–stained sections, the abnormal histiocytes could be identified.
RT-PCR demonstrated chimeric TPM3-ALK transcripts in the liver and muscle biopsies of case 1, suggesting presence of t(1;2)(q25;p23).10 The test was unsuccessful in case 2, presumably due to RNA degradation, and there was insufficient material in case 3 for the study.
Occurrence of ALK translocation
Primary systemic anaplastic large cell lymphoma commonly exhibits a unique t(2;5) or variant chromosomal translocation, with ALK gene fused with a housekeeping gene, such as NPM, TPM3, and TFG, resulting in expression of ALK protein.12-15 Currently, only 3 other tumor types are known to exhibit ALK translocation and ALK expression—ALK+ large B-cell lymphomas, inflammatory myofibroblastic tumors, and less than 5% of non–small cell lung carcinomas.16-23 This report expands the spectrum to include ALK+ histiocytosis. In all these proliferations, the formation of X-ALK homodimers/polymers using dimerization sites at the N-terminus of ALK partners mimics ligand binding, and is responsible for activation of the ALK catalytic domain and oncogenic properties of the fusion protein.12
ALK+ histiocytosis as a distinctive disease
We propose the designation “ALK+ histiocytosis” for this distinctive entity presenting in early infancy, characterized by proliferation of morphologically distinctive histiocytes with unique expression of ALK. The presence of liver and subtle bone marrow indicates that the disease is systemic. Features suggesting that the histiocytes are macrophages include the following: presence of phagocytosis; immunoreactivity for CD163, a histiocytic marker not expressed on dendritic cells; and ultrastructural features of phagocytic cells. On the other hand, positive staining for factor XIIIa and fascin suggests a relationship with dendritic cells. Thus, the macrophage versus dendritic cell lineage of ALK+ histiocytosis remains to be clarified.
ALK expression is a unique feature of this form of histiocytosis, since it is absent in other histiocytic proliferations that we have studied, including Langerhans cell histiocytosis (10 cases), juvenile xanthogranuloma (8 cases solitary, 1 case systemic), familial hemophagocytic lymphohistiocytosis (3 cases), and Rosai-Dorfman disease (6 cases). Although presence of ALK gene translocation may suggest that ALK+ histiocytosis is a neoplastic disorder, we are cautious of this interpretation because of the favorable outcome in case 3 even without cytotoxic therapy, and in case 2, even though no further cytotoxic therapy was given for residual disease. Furthermore, the delayed clinical response to cytotoxic therapy in cases 1 and 2 renders it difficult to ascribe the response to chemotherapy versus natural resolution of disease.
In summary, ALK+ histiocytosis is a distinct form of histiocytic proliferative disorder that clinically may suggest a storage disorder. Based on this limited series, the disease tends to resolve slowly, but can be life threatening during the active phase.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: J.K.C.C. and C.W.C. conceived the study, collected and analyzed the data, and drafted the paper; E.A., W.Y.W.T., K.C.L., and K.T. contributed patient materials and analyzed the data; and L.L. and G.D. performed special studies and analyzed the data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: John K. C. Chan, Department of Pathology, Queen Elizabeth Hospital, Wylie Road, Kowloon, Hong Kong, SAR China; e-mail: jkcchan@ha.org.hk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal