Abstract
We hypothesized that transplacental leukocyte trafficking during pregnancy, which induces long-term, stable, reciprocal microchimerism in mother and child, might influence outcome of patients with acute leukemia given parental donor haploidentical hematopoietic stem cell transplantation (HSCT). We analyzed the outcome of 118 patients who received transplants for acute leukemia in 2 centers. Patients received highly T cell–depleted haploidentical grafts after myelo-ablative conditioning. Five-year event-free survival was better in patients who received transplants from the mother than from the father (50.6% ± 7.6% vs 11.1% ± 4.2%; P < .001). Better survival was the result of both reduced incidence of relapse and transplantation-related mortality. The protective effect was seen in both female and male recipients, in both lymphoid and myeloid diseases; it was more evident in patients receiving transplants in remission than in chemotherapy-resistant relapse. Incidences of rejection and acute graft-versus-host disease were not significantly influenced. Multivariate analysis confirmed donor sex in parental donor transplantation as an independent prognostic factor for survival (hazard ratio, father vs mother = 2.36; P = .003). In contrast, in a control cohort of patients who received transplants from haploidentical siblings, donor sex had no influence on outcome. Although obtained in a retrospective analysis, these data suggest that the mother of the patient should be preferred as donor for haploidentical HSCT.
Introduction
Transplacental trafficking of maternal and fetal cells during pregnancy establishes long-term, reciprocal microchimerism in both mother and child1-4 because of exposure of the 2 immune systems to the non–self-alloantigens. This reciprocal exposure has been hypothesized to lead to tolerance in the context of unmanipulated hematopoietic stem cell transplantation (HSCT) from an HLA-haplotype disparate donor (typically the mother or a sibling mismatched for noninherited maternal antigens).5-7 However, despite potent immunosuppressive therapy administered after transplantation to prevent graft-versus-host disease (GVHD), the majority of patients who received transplants using this approach developed acute and/or chronic GVHD.5
A radically different approach to successful haploidentical HSCT is to combine the infusion of high doses of stem cells with rigorous T-cell depletion; this approach has proved to result in low incidence of GVHD despite the absence of posttransplantation pharmacologic GVHD prophylaxis.8,9 In view of the low number of mature T cells transferred with the graft, the graft-versus-leukemia (GVL) effect in this transplant is mediated mainly by alloreactive natural killer (NK) cells, whose lytic effect on leukemia blasts occurs in the presence of killer-cell immunoglobulin-like receptor (KIR)-ligand mismatches in the graft-versus-host direction.10 However, T lymphocytes have recently been proposed as potential effectors of GVL reactions in T cell–depleted haploidentical HSCT.11 This may occur in the T cell–depleted haploidentical transplantation setting because, in contrast to standard T cell–replete HSCT, the function of the few cotransplanted T cells is not blunted by posttransplantation pharmacologic immunosuppressive prophylaxis. In this study, we reasoned that, in the setting of T cell–depleted haploidentical HSCT, previous exposure of the maternal immune systems to the child alloantigens inherited from the father might influence transplantation outcomes. We therefore conducted a retrospective analysis on the outcome of patients with acute leukemia given T cell–depleted HSCT from an HLA-disparate parent in 2 centers.
Methods
Study design
In a retrospective analysis, we investigated the probability of event-free survival (EFS) and the cumulative incidences of relapse mortality, transplantation-related mortality (TRM), GVHD, and rejection in 118 consecutive patients who received transplants, after T-cell depletion of the graft, from either the father or the mother in 2 centers (Division of Hematology, University of Perugia and Pediatric Hematology/Oncology, University of Pavia) between 1993 and 2006 for acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL). All patients were at high risk for disease relapse, and none had an HLA-matched related or unrelated donor. Ninety patients received transplants in Perugia, whereas 28 patients were treated in Pavia. Outcome of patients who received transplants from a maternal donor was compared with those of patients receiving grafts from the father. A cohort of 79 patients receiving transplants from a haploidentical sibling donor in the same period of time, using the same transplantation protocol, served as controls.
Patients
The study cohort consisted of 118 acute leukemia patients receiving haploidentical HSCT from the mother (n = 47) or the father (n = 71). The transplantation regimen and graft processing have been previously described in detail.9,12 Briefly, patients were conditioned with an 8-Gy single dose or 12-Gy fractionated total body irradiation, thiotepa, fludarabine, and antithymocyte globulin. The first 6 patients received cyclophosphamide instead of fludarabine. Hematopoietic progenitors were collected through peripheral blood apheresis after mobilization with granulocyte colony stimulating factor. T-cell depletion was performed by soybean agglutination and E-rosetting in the first 6 patients and then by positive selection of CD34+ cells in all subsequent patients. No posttransplantation pharmacologic GvHD prophylaxis was given. Patient and graft characteristics are shown in Table 1.
Patient characteristics
| . | Mother donor, n = 47 . | Father donor, n = 71 . | P . | ||
|---|---|---|---|---|---|
| n . | % . | n . | % . | ||
| Transplantation center | |||||
| Perugia | 37 | 78.8 | 53 | 74.6 | .61 |
| Pavia | 10 | 21.2 | 18 | 25.4 | |
| Disease | |||||
| Acute lymphoblastic leukemia | 24 | 51.1 | 43 | 60.6 | .31 |
| Acute myeloid leukemia | 23 | 48.9 | 28 | 39.4 | |
| Disease status at transplantation | |||||
| Remission | 33 | 70.2 | 34 | 47.9 | .02 |
| Relapse | 14 | 29.8 | 37 | 52.1 | |
| Patient sex | |||||
| Male | 25 | 53.2 | 45 | 63.4 | .27 |
| Female | 22 | 46.8 | 26 | 36.6 | |
| KIR ligand mismatch | |||||
| Yes | 21 | 44.7 | 19 | 26.8 | .13 |
| No | 20 | 42.6 | 40 | 56.3 | |
| Not assessed | 6 | 12.8 | 12 | 16.9 | |
| Median patient age at transplantation, range | 19 | 2-52 | 17 | 2-41 | .18 |
| Median donor age, range | 45 | 28-66 | 45 | 21-70 | .92 |
| CMV serology | |||||
| Donor positive/recipient positive | 38 | 80.9 | 54 | 76.1 | .92 |
| Donor positive/recipient negative | 5 | 10.6 | 8 | 11.3 | |
| Donor negative/recipient positive | 2 | 4.3 | 3 | 4.2 | |
| Donor negative/recipient negative | 1 | 2.1 | 4 | 5.6 | |
| Not assessed | 1 | 2.1 | 2 | 2.8 | |
| Graft composition, median, SD | |||||
| CD34 (106/kg) | 12.1 | 7.7 | 14.5 | 9.3 | .22 |
| CD3 (104/kg) | 1.0 | 1.1 | 1.0 | 3.4 | .79 |
| Year of transplantation, median (range) | 2002 (1993-2006) | 1999 (1993-2006) | .22 | ||
| . | Mother donor, n = 47 . | Father donor, n = 71 . | P . | ||
|---|---|---|---|---|---|
| n . | % . | n . | % . | ||
| Transplantation center | |||||
| Perugia | 37 | 78.8 | 53 | 74.6 | .61 |
| Pavia | 10 | 21.2 | 18 | 25.4 | |
| Disease | |||||
| Acute lymphoblastic leukemia | 24 | 51.1 | 43 | 60.6 | .31 |
| Acute myeloid leukemia | 23 | 48.9 | 28 | 39.4 | |
| Disease status at transplantation | |||||
| Remission | 33 | 70.2 | 34 | 47.9 | .02 |
| Relapse | 14 | 29.8 | 37 | 52.1 | |
| Patient sex | |||||
| Male | 25 | 53.2 | 45 | 63.4 | .27 |
| Female | 22 | 46.8 | 26 | 36.6 | |
| KIR ligand mismatch | |||||
| Yes | 21 | 44.7 | 19 | 26.8 | .13 |
| No | 20 | 42.6 | 40 | 56.3 | |
| Not assessed | 6 | 12.8 | 12 | 16.9 | |
| Median patient age at transplantation, range | 19 | 2-52 | 17 | 2-41 | .18 |
| Median donor age, range | 45 | 28-66 | 45 | 21-70 | .92 |
| CMV serology | |||||
| Donor positive/recipient positive | 38 | 80.9 | 54 | 76.1 | .92 |
| Donor positive/recipient negative | 5 | 10.6 | 8 | 11.3 | |
| Donor negative/recipient positive | 2 | 4.3 | 3 | 4.2 | |
| Donor negative/recipient negative | 1 | 2.1 | 4 | 5.6 | |
| Not assessed | 1 | 2.1 | 2 | 2.8 | |
| Graft composition, median, SD | |||||
| CD34 (106/kg) | 12.1 | 7.7 | 14.5 | 9.3 | .22 |
| CD3 (104/kg) | 1.0 | 1.1 | 1.0 | 3.4 | .79 |
| Year of transplantation, median (range) | 2002 (1993-2006) | 1999 (1993-2006) | .22 | ||
Whereas patients who received transplants in Perugia represented a young adult population (median age, 19 years; range, 4-52 years), patients who received transplants in Pavia were predominantly children (median age, 7 years; range, 2-25 years).
Approval was obtained from the Umbria Region Ethics Committee and from the Perugia University Institutional Review Board. Patient informed consent was obtained in accordance with the Declaration of Helsinki.
Endpoints and definitions
Endpoints were EFS (defined as time elapsing between first HSCT to death/relapse, whichever occurred first; patients alive and in remission of leukemia were censored at time of last follow-up), relapse mortality (RM, defined as death occurring after relapse or disease progression after transplantation), and TRM (defined as death in remission). Cumulative incidence of grade II-IV acute and chronic GVHD and of rejection were secondary end points.
Donor versus recipient NK alloreactivity was either determined functionally as previously described (presence of NK clones able to lyse patient hematopoietic targets)10 or derived from high-resolution HLA typing of both donor and patient (patients lacking a KIR ligand present in the donor along with the corresponding KIR).13 Acute and chronic GVHD was diagnosed and graded at each transplantation center in accordance with the Seattle criteria.14,15 Only patients surviving in hematologic remission and with donor chimerism for more than 100 days after transplantation were evaluated for chronic GVHD occurrence.
Statistics
The comparison of the cohorts was performed using Pearson χ2 or Fisher exact test for categorical and Student t test or Mann-Whitney test for continuous variables. EFS was estimated using the Kaplan-Meier method plus or minus SE16 ; comparison between groups was performed by the log-rank test. Cumulative incidences (± SE) of RM and TRM were calculated taking into account the respective competing risks.17 Both death from any cause and graft rejection were competing risks for estimation of acute GvHD cumulative incidence. Univariate competing risk outcomes were compared using the Gray test.18
For multivariate analysis of survival, Cox proportional hazard models were used.19 Donor type (father vs mother) was included in all models; disease, disease stage at transplantation, NK alloreactivity, patient age, donor age, graft composition (CD34+ and CD3+ count), center, conditioning regimen, cytomegalovirus serology in the donor/recipient pair, and year of transplantation were incorporated using forward stepwise inclusion of variables with a threshold level of .05. Variables included in the final models were tested using a time-dependent covariate method to determine whether the proportional hazards assumption was met.
Statistical analyses were carried out in SPSS version 12.0 (SPSS, Chicago IL) and R, version 2.2.1 (http://www.r-project.org).
Results
Table 1 compares characteristics of patients receiving transplantation from a maternal or a paternal donor. Patients receiving the allograft from the mother had a more favorable risk profile, more patients being in remission at time of transplantation (P = .02). The mothers were more frequently NK-alloreactive toward the recipient than the fathers, although the difference was not statistically significant (P = .13). Other factors were evenly distributed. The majority of patient/recipient pairs were cytomegalovirus serology+/+. Cytomegalovirus reactivation after transplantation was frequent and evenly distributed among recipients of mother donor and father donor grafts.
Survival
Forty of the 118 patients (33.9%) are alive (median follow-up, 3.4 years; range, 0.5-13.8 years). Five-year Kaplan-Meier estimate of EFS for the whole cohort is 27.8% plus or minus 4%. The probability of EFS at 5 years of patients who received transplants from maternal donors was 50.6% plus or minus 7% compared with 11.1% plus or minus 4% for patients who received transplants from paternal donors (P < .001, Figure 1A), the unadjusted hazard ratio being 2.56 (95% confidence interval [CI], 1.60-4.00).
Event-free survival of patients receiving parental donor haploidentical HSCT for acute leukemia. Stratified by donor sex (A, mother donors, N = 47; father donors, N = 71) and by both donor sex and NK alloreactivity (B, NK alloreactive mother donor transplantation, N = 21; NK nonalloreactive mother donor transplantation, N = 20; NK alloreactive father donor transplantation, N = 19; NK nonalloreactive father donor transplantation, N = 40).
Event-free survival of patients receiving parental donor haploidentical HSCT for acute leukemia. Stratified by donor sex (A, mother donors, N = 47; father donors, N = 71) and by both donor sex and NK alloreactivity (B, NK alloreactive mother donor transplantation, N = 21; NK nonalloreactive mother donor transplantation, N = 20; NK alloreactive father donor transplantation, N = 19; NK nonalloreactive father donor transplantation, N = 40).
The survival advantage in maternal transplant recipients was observed in both ALL (n = 67, 5-year EFS, 45.8% ± 10% vs 13.1% ± 6%, P = .10) and AML (n = 51, 5-year EFS, 55.3% ± 11% vs 7.5% ± 5%, P < .001, respectively). The benefit of having the mother as donor was more evident for patients who received transplants in remission (n = 67, 5-year EFS, 62.0% ± 9% vs 12.6% ± 7%, P = .004) than for those in chemotherapy-resistant disease (n = 51, 5-year EFS, 24.2% ± 12% vs 9.6% ± 5%, P = .38).
As alloreactivity against minor histocompatibility antigens encoded by Y-chromosome (H-Y-mHAg) occurs when the donor is a female and the recipient is a male, it might have contributed to the better outcome of maternal transplant recipients. Therefore, we analyzed outcomes of male and female patients separately. Better survival after maternal donor transplantation was seen in both male (n = 70, 55.7% ± 10% vs 13.2% ± 5% after paternal donor transplantation, P = .004) and female recipients (n = 48, 43.5% ± 12% vs 7.7% ± 7% after paternal donor transplantation, P = .002). This finding rules out a major role for female donor T cell–mediated recognition of H-Y-mHAg in male recipients.
To investigate whether better survival provided by a maternal donor was due only to donor sex, we compared the outcome of patients given haploidentical transplantation from either a male or a female sibling. In contrast to parent-to-child transplantation, donor sex played no role in haploidentical sibling donor transplantation. The 5-year EFS was 27.0% plus or minus 6% and 25.5% plus or minus 9% for patients who received transplants from an HLA haploidentical sister (n = 30) or a brother (n = 49), respectively (P = .63). Similarly, in multivariate Cox analysis adjusting for disease status at transplantation and NK alloreactivity, the relative risk (RR) of sister donor versus brother donor transplantation was 1.08 (95% CI, 0.67-1.87; P = .79), suggesting that female donor sex as such is not associated with improved outcome in haploidentical HSCT. Finally, in 22 of 79 patients with a complete family HLA typing allowing identification of the origin of shared and mismatched haplotypes in sibling transplantation, no significant difference in survival emerged between patients who received transplants from noninherited paternal antigen-mismatched sibling donors and those who received transplants from noninherited maternal antigen-mismatched sibling donors (46.7% ± 16% vs 66.0% ± 15%; P = .53).
Multivariate analysis
Multivariate analysis using Cox proportional hazards models confirmed the results of the univariate analysis, with a RR of death for paternal versus maternal donor transplantation recipients of 2.36 (95% CI, 1.35-4.15; P = .003). Multivariate analysis also confirmed previously identified prognostic risk factors in haploidentical T cell–depleted HSCT, such as patient age, disease status at transplantation, and NK alloreactivity (ie, KIR-ligand incompatibility in the graft-vs-host direction, Table 2). Whereas the unadjusted RR of NK-nonalloreactive versus NK-alloreactive transplantation was 2.24; time-dependent multivariate analysis showed that the protective effect of NK alloreactivity was statistically significant only in patients surviving more than 6 months after transplantation. An adjusted RR for the whole posttransplantation period could not therefore be calculated. When the posttransplantation period was split into a first period covering the first 6 months after transplantation and a second period including the subsequent observation time, the proportional hazards assumption was confirmed. Within the first period, NK alloreactivity influenced only marginally EFS, whereas it strongly affected the subsequent probability of death of patients surviving the first 6 months after the allograft (Table 2). All other prognostic factors were not time dependent. Prognostic factors without significant impact on survival and therefore not included in the final model were: patient sex, original disease, center, conditioning regimen, CD3+ cell content of the graft, CD34+ cell content of the graft, cytomegalovirus serology in the donor/recipient pair, and year of transplantation. When sibling and parent donor transplantations were combined in a multivariate analysis, an excess mortality for transplantation from donors other than the mother emerged (RR vs mother donor transplantation = 1.82, 95% CI, 1.14-2.90; P = .01). Adjusted hazard ratios of father donor transplantation were comparable with those of sibling donor transplantation (mother donor RR = 1.00; father donor RR = 1.79, brother donor RR = 1.81, sister donor RR = 1.83).
Multivariate analysis for risk of death after transplantation
| Risk factor . | RR . | 95% CI . | P . |
|---|---|---|---|
| Donor type | |||
| Maternal donor | 1.00 | — | — |
| Paternal donor | 2.36 | 1.35-4.15 | .003 |
| Disease status at transplantation | |||
| Remission | 1.00 | — | — |
| Relapse | 1.69 | 1.05-2.71 | .03 |
| NK alloreactivity | |||
| KIR ligand mismatch | 1.00 | — | — |
| No KIR ligand mismatch (up to 6 months) | 1.16 | 0.61-2.22 | .65 |
| No KIR ligand mismatch (after 6 months) | 5.07 | 1.70-15.2 | .004 |
| Patient age per increasing year | 1.033 | 1.007-1.061 | .01 |
| Risk factor . | RR . | 95% CI . | P . |
|---|---|---|---|
| Donor type | |||
| Maternal donor | 1.00 | — | — |
| Paternal donor | 2.36 | 1.35-4.15 | .003 |
| Disease status at transplantation | |||
| Remission | 1.00 | — | — |
| Relapse | 1.69 | 1.05-2.71 | .03 |
| NK alloreactivity | |||
| KIR ligand mismatch | 1.00 | — | — |
| No KIR ligand mismatch (up to 6 months) | 1.16 | 0.61-2.22 | .65 |
| No KIR ligand mismatch (after 6 months) | 5.07 | 1.70-15.2 | .004 |
| Patient age per increasing year | 1.033 | 1.007-1.061 | .01 |
Pretransplant variables without statistically significant impact on survival: patient sex, disease, center, date of transplantation, donor age, CMV serologic status in the donor/recipient pair, and graft CD3 and CD34 count.
— indicates not applicable.
As the benefit of receiving a transplant from the mother was of the same magnitude of that of having an NK-alloreactive donor, we directly compared the impact of these 2 prognostic factors by grouping patients into 4 cohorts according to donor type and KIR-ligand compatibility. The best EFS was found in patients who received transplants from an NK-alloreactive mother (68.2% ± 10.8%, n = 21), whereas the poorest outcome was observed in recipients of a NK-nonalloreactive father HSCT (3.0% ± 3.0%, n = 40). Survival rates were comparable in patients grafted from NK-alloreactive fathers and NK-nonalloreactive mothers (29.6% ± 11.5%, n = 19; and 33.8% ± 10.8%, n = 20, Figure 1B).
Finally, we stratified patients according to their original disease and age. The majority of AML and pediatric cases of ALL are sensitive to NK alloreactivity, whereas adult ALL is typically NK-resistant.20,21 EFS probabilities after stratification into NK-sensitive (all AML and pediatric ALL patients) and NK-resistant diseases (adult ALL patients) are shown in Table 3 and indicate that for all patients an NK-alloreactive mother is the donor of choice, whenever available. In NK-sensitive diseases, the protective effect of NK alloreactivity was superior to that of a maternal donor. In case of an NK-resistant disease, on the other hand, donor sex was more important, as all patients who received transplants from a paternal donor died, irrespective of the presence of NK alloreactivity.
Survival according to donor sex and NK alloreactivity
| Risk factor for donor type . | NK-sensitive diseases,* % . | NK-resistant diseases,† % . |
|---|---|---|
| NK alloreactive mother | 70.8 | 60.0 |
| NK nonalloreactive mother | 34.1 | 33.3 |
| NK alloreactive father | 48.6 | 0.0 |
| NK nonalloreactive father | 6.7 | 0.0 |
| Risk factor for donor type . | NK-sensitive diseases,* % . | NK-resistant diseases,† % . |
|---|---|---|
| NK alloreactive mother | 70.8 | 60.0 |
| NK nonalloreactive mother | 34.1 | 33.3 |
| NK alloreactive father | 48.6 | 0.0 |
| NK nonalloreactive father | 6.7 | 0.0 |
All pediatric acute leukemias and adult acute myeloid leukemia.
Adult acute lymphoblastic leukemia.
Relapse mortality and transplantation-related mortality
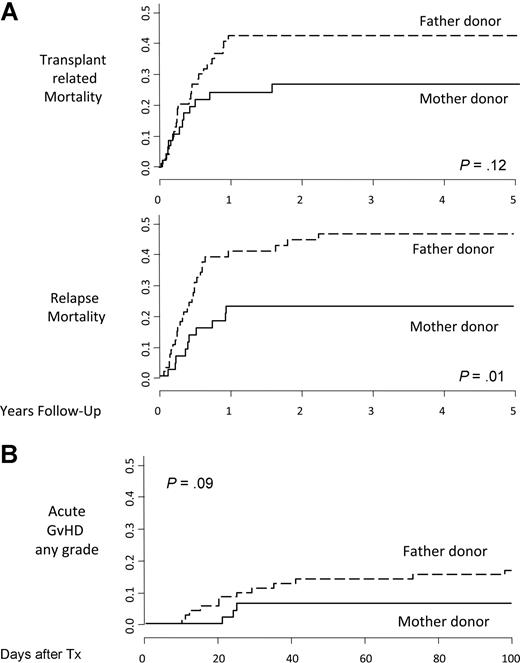
When analyzing causes of treatment failure, the use of the mother as donor was associated with a reduction in both RM (22.7% ± 6% vs 46.5% ± 6%; P = .01) and TRM (26.6% ± 7% vs 42.3% ± 6%; P = .12; Figure 2A).
Cumulative incidences. Relapse mortality and transplantation-related mortality (A) and acute graft-versus-host disease (B) in recipients of parental donor transplants stratified by donor sex.
Cumulative incidences. Relapse mortality and transplantation-related mortality (A) and acute graft-versus-host disease (B) in recipients of parental donor transplants stratified by donor sex.
When stratifying patients for age and disease, adult ALL patients, resistant to NK alloreactivity and therefore at very high risk of disease relapse, were those mostly benefiting from reduction in RM if receiving transplants from maternal donors (22.2% ± 10% vs 62.7% ± 10%; P = .01).
GVHD and rejection
Cumulative incidence of grade II to IV acute GVHD was lower, although not statistically significant, in maternal donor HSCT patients than in those receiving the allograft from the father (6.4% ± 4% vs 16.9% ± 4%, respectively; P = .09; Figure 2B). No difference was seen in the incidence of chronic GvHD between maternal and paternal donor transplantation recipients (12.1% vs 16.2%; P = .37).
Three of the 47 patients (6.4%) who received transplants from the mother rejected their first transplant, whereas 7 of the 71 paternal donor grafts were rejected (9.9%; P = .38). All but one of these patients were eventually successfully received transplants
Discussion
In this retrospective analysis, we found an improved long-term EFS for patients receiving haploidentical HSCT from the mother compared with the father. Although patients who received transplants from mothers had a more favorable risk profile regarding disease state at transplantation and NK alloreactivity, multivariate analysis confirmed donor sex to be an independent prognostic factor in parent-to-child transplantation. Furthermore, analysis of a control cohort of patients receiving sibling donor haploidentical transplants in the same time period showed no difference between patients who received transplants from either a male or female sibling, indicating that donor gender as such probably does not play a role. Transplantation from mothers provided as good a survival advantage as transplantation from NK-alloreactive donors. Improved survival derived mainly from a lower incidence of relapse, suggesting that maternal grafts exerted a more potent alloreactive effect that was active preferentially against leukemia cells, as it was not associated with increased incidence of GVHD. Because the survival advantage was independent of patient gender, alloreactivity was apparently not directed against H-Y-mHAg.
Better outcome of mother-to-child transplantation may be the result of the maternal immune system exposure to fetal antigens during pregnancy. The immune system in the mother, unlike that of the fetus, is mature and therefore capable of being sensitized by paternal histocompatibility antigens. As examples of this sensitization, antibodies directed against paternal HLA-antigens22 and T lymphocytes directed against paternal major and minor histocompatibility antigens23,24 are frequently detected in multiparous women. Under these circumstances, T lymphocytes probably mediated the enhanced GVL effect after maternal donor transplantation. In vitro studies have recently demonstrated the existence of T cells from haploidentical donors reacting against recipient leukemia blasts, but not nonmalignant targets.11 The few T cells transferred with the graft (in this study, a median of 104/kg, ie, a total of ∼0.5-1 million cells), apparently underwent unopposed proliferation after transplantation by virtue of the absence of pharmacologic GVHD prophylaxis. Further immunologic studies aimed at investigating whether differences in frequency of leukemia specific T cells exist between maternal and paternal donors are warranted.
The other main prognostic factor influencing the outcome after highly T cell–depleted haploidentical HSCT is donor versus recipient NK alloreactivity. Its impact and mechanism of action have been studied extensively,20 and its importance is confirmed by our results. Because identification of a donor with the potential for NK alloreactivity constitutes one of the main criteria for donor selection in haploidentical transplantation, we were especially interested in comparing the effects of NK alloreactivity and donor sex. In multivariate analysis and when patients were stratified for NK alloreactivity and maternal donor, the prognostic impact of the 2 factors was similar. When analysis was restricted to diseases sensitive to NK alloreactivity (ie, AML and pediatric ALL), NK alloreactivity had a stronger impact than maternal donors, whereas patients with NK-resistant diseases (ie, adult ALL) benefited more from a maternal donor. In view of these results, it is reasonable to speculate that for an adult patient with ALL an NK-nonalloreactive mother may be a more appropriate donor than an NK-alloreactive father. In NK-sensitive malignancies, an NK-alloreactive donor, whatever the sex and relationship to the patient, should be preferred. Obviously, the NK-alloreactive mother is the donor of choice as this donor combines the 2 good prognosis factors.
A small Japanese registry study comparing T cell–replete maternal to paternal HSCT reported improved survival with maternal donors.25 This study involved patients with a wide variety of diseases (including nonmalignant disorders), treated with several different preparative regimens, with more than half the patients being phenotypically HLA-matched with their parental donor. In contrast, the present study analyzed a homogeneous population of patients because they were all affected by acute leukemia and given highly T cell–depleted grafts after the same preparative regimen. Another study, published in abstract form, compared survival after transplantation of a graft combining T cell–replete bone marrow and T cell–depleted peripheral blood stem cells followed by posttransplantation pharmacologic GVHD prophylaxis.26 Even though in this study the transplantation regimen differed greatly from that used in our cohort and the patients had a wide spectrum of diseases, survival rates were surprisingly similar to those reported in the present study: 55% for maternal donor transplantations and 8% for paternal donor transplantations (P = .02), thus confirming the advantage for mother donor grafts.
In conclusion, we found that, in haplotype-mismatched parent-to-child HSCT, patients grafted from the mother had reduced relapse rate compared with recipients of paternal grafts. Given the absence of increased risk of GVHD, this translated into better EFS. The beneficial effect seems to be a direct consequence of pregnancy, as no similar effect was found for female haplotype-mismatched sibling donors. The effect was most pronounced in patients with adult ALL, an NK-resistant disease with a very high risk of relapse, and was independent of, and additive to, the beneficial effects of NK alloreactivity in alloreactive NK cell–sensitive disease, such as AML and pediatric ALL. Our data strongly suggest further studies are warranted to determine whether donor gender should be incorporated into donor selection criteria in haplotype-mismatched parent-to-child HSCT.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Geraldine Anne Boyd for editorial assistance.
M.S. is supported by research grants from the Swiss Cancer League (grant BIL OCS 01 597-08-2004) and the Swiss National Science Foundation (grant PBBSB-107328). This work was partially supported by grants from Associazione Italiana Ricerca sul Cancro, Consiglio Nazionale delle Ricerche, Ministero dell'Università e della Ricerca Scientifica e Tecnologica, European Union (FP6 program ALLOSTEM), a Translational Research Grant from the Leukemia & Lymphoma Society, the National Institutes for Health (Project No. 1 PO1 CA100265), and Fondazione IRCCS Policlinico San Matteo. L.R. is a Leukemia & Lymphoma Society Special Fellow in Clinical Research.
National Institutes of Health
Authorship
Contribution: M.S. designed research, collected data, performed statistical analysis, and drafted the manuscript; L.R. and A.M. performed research and analyzed and interpreted data; M.E.B. and C.d.A. performed research; C.B. designed research; F.L. and F.A. analyzed and interpreted data and drafted the manuscript; and A.V. designed research, analyzed and interpreted data, and drafted the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Martin Stern, Department of Hematology, University Hospital, Petersgraben 4, 4031 Basel, Switzerland; e-mail: sternm@uhbs.ch.