In this issue of Blood, Zhou and colleagues describe preclinical results in vitro and in vivo, showing constitutive TGF-β activation in MDS and enhanced hematopoiesis following inhibition of TBRI kinase in MDS.
These investigators have previously shown that p38 MAP kinase regulates stem cell apoptosis in human hematopoietic failure.1 Moreover, a small-molecule inhibitor, SCIO-463 of the p38 MAP kinase pathway, improves hematopoiesis in myelodysplastic syndrome (MDS) progenitors in vitro.1 The elegant experiments by Zhou et al extend their study of TGF-β signaling in MDS and show the constitutive downstream activation of smad2 in MDS bone marrow precursors and its overexpression in MDS-derived CD34+ cells. Suppression of TGF-β activity by shRNA down-regulation of TGF-β receptor I kinase (TBRI) as well as pharmacologic inhibition of TBRI (alk5) by a small-molecule inhibitor (SD-208) leads to a reversal of this TGF-β–mediated inhibition of hematopoiesis in MDS. Furthermore, SD-208 treatment alleviates anemia and stimulates hematopoiesis in vivo in a novel murine model of bone marrow failure generated by the constitutive hepatic expression of TGF-β1. Finally, the enhancement of hematopoiesis seen in several MDS subtypes exposed in vitro to SD-208 underscores the importance of TBRI as a potential therapeutic target in low-risk MDS.
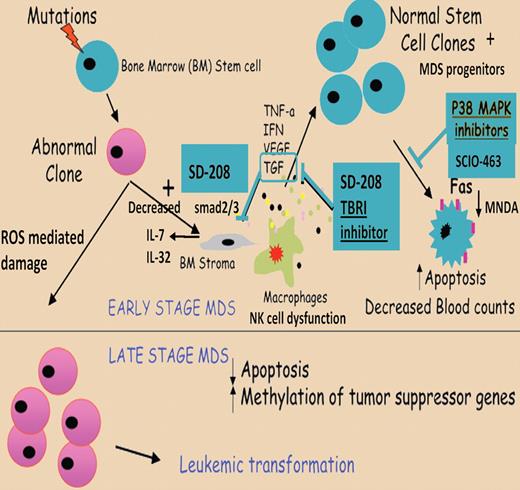
Proposed model of pathogenesis of stem cell apoptosis in MDS. A mutation in a stem cell compartment can give rise to a malignant clone. Interactions of the abnormal stem cell clones with the bone marrow microenvironment can lead to increased proinflammatory cytokine production in the bone marrow. Normal stem cell clones can undergo apoptosis, leading to ineffective hematopoiesis and low blood counts in early stage/low-grade MDS. Elimination of normal stem cells and clonal evolution of abnormal stem cell clones in late stages of MDS lead to development of leukemia. p38 MAPK inhibition can disrupt cytokine-driven apoptosis in low-grade MDS and potentially restore normal hematopoiesis. Apapted from Zhou et al with permission.1
Proposed model of pathogenesis of stem cell apoptosis in MDS. A mutation in a stem cell compartment can give rise to a malignant clone. Interactions of the abnormal stem cell clones with the bone marrow microenvironment can lead to increased proinflammatory cytokine production in the bone marrow. Normal stem cell clones can undergo apoptosis, leading to ineffective hematopoiesis and low blood counts in early stage/low-grade MDS. Elimination of normal stem cells and clonal evolution of abnormal stem cell clones in late stages of MDS lead to development of leukemia. p38 MAPK inhibition can disrupt cytokine-driven apoptosis in low-grade MDS and potentially restore normal hematopoiesis. Apapted from Zhou et al with permission.1
Stem cell apoptosis in MDS, illustrated in the figure, is a facet of the heterogeneity of this disease and the interplay of various mechanisms affecting the marrow microenvironment as well as progenitor proliferation and apoptosis.1 Ineffective hematopoiesis in MDS may be intrinsic to dysregulated gene expression as well as resulting from dysfunctional cell-to-cell contacts within the stromal microenvironment.2,3 TGF-β overactivation in MDS leads to altered stromal cytokine expression with decreased IL-7 and decreased B-cell proliferation4 and enhanced IL-1β and TNFα associated with increased stromal IL-6, IL-8, and IL-32 expression.5 These proinflammatory cytokines are associated with natural killer cell dysfunction5 and may lead to programmed cell death of all hematopoietic cell lineages via autophagy6 or apoptosis.3,7
Our understanding of the molecular pathobiology of MDS and its progression to acute myeloid leukemia (AML) has been made possible by advances in unraveling the molecular underpinnings of acute and chronic leukemias and myeloproliferative syndromes.3 Intrinsic stem and progenitor cell abnormalities in MDS may be attributed to altered DNA methylation and gene silencing. This affects specific hematopoietic lineages, such as Survivin in erythropoiesis and WT-1 and CHK2 during granulopoiesis8 , as well as structural genetic alterations.3 While this report does not address the importance of gene silencing and altered differentiation programs in MDS, it nevertheless offers a clear rationale to test compounds such as SD-208 in all phases of MDS. Correlative studies will be needed to determine whether inhibition of TBRI can alter cytokine, chemokine, and oncogene expression profiles in this disease.
Currently approved treatments in MDS are now directed at intermediate and high-risk patients and include immunomodulators, such as lenalinomide and hypomethylating agents 5-azacytidine and decitabine. Patients with low-grade MDS in the future can look forward to these novel hematopoietic enhancing treatment modalities, which provide an alternative to supportive care, transfusions, and growth factors.3 Finally, since reactive oxygen species can damage stem cell DNA and may accelerate clonal evolution from MDS to AML, it remains to be seen if the early use of SD-208 and SCIO-463 small-molecule inhibitors of TBRI and p38 MAPK will result in delayed disease progression and improved survival in MDS.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal