Friedman and colleagues use spectratyping to identify donor T cells that respond to host minor histocompatibility antigens in vitro and in patients after allogeneic HSCT.
The use and efficacy of allogeneic hematopoietic stem cell transplantation (HSCT) has been severely hampered by the occurrence of graft-versus-host disease (GVHD), in which donor T cells attack the genetically disparate host. Separating the T cells that mediate GVHD from those that produce beneficial graft-versus-tumor (GVT) effects and/or provide resistance to opportunistic infections has remained the Holy Grail of allogeneic HSCT. In this issue of Blood, Friedman et al suggest that a new era may be coming whereby T-cell receptor (TCR) profiling using spectratyping would allow for analysis of different T-cell populations in the donor that can ultimately respond to host minor histocompatibility antigens (miHA) and mediate GVHD. Spectratyping may allow for better donor selection as well as a means to identify and then remove deleterious T-cell populations from donor grafts.
Allogeneic HSCT offers tremendous potential benefits for cancer therapy due to the existence of GVT responses by donor T cells that can help eradicate the tumor. Unfortunately, some of these donor T cells may also mediate deleterious GVHD, which significantly limits the efficacy and application of this procedure. Early attempts at graft engineering had nonselectively removed the donor T cells from the graft, which reduced GVHD occurrence, but also obviated the GVT effects and led to increased graft rejection as well as increased susceptibility to opportunistic infections. There has been tremendous interest in finding a means to specifically remove the GVHD-inducing donor T cells from the graft but allow other T cells to remain and perform their useful functions. The question is how to identify the “bad” T cells? Recent studies have focused on a number of different ways to transfer “safer” T cells via separation based on differences including CD4+ T-cell subsets (Th2, Tregs), naive phenotype, or activation marker expression after mixed lymphocyte culture (MLC) assays as reviewed by Welniak et al.1 The primary problem is that all these methods are inefficient, GVHD-mediating cells may be missed, and GVT-producing cells may be adversely targeted. Adding to the complexity, numerous investigators tried to correlate in vitro MLC proliferation assays or cytotoxic T-cell precursor frequencies with GVHD to help choose and determine optimal donors.2-4 While in principle these attempts could be sound, thus far these assays have proven unreliable as predictors of GVHD.
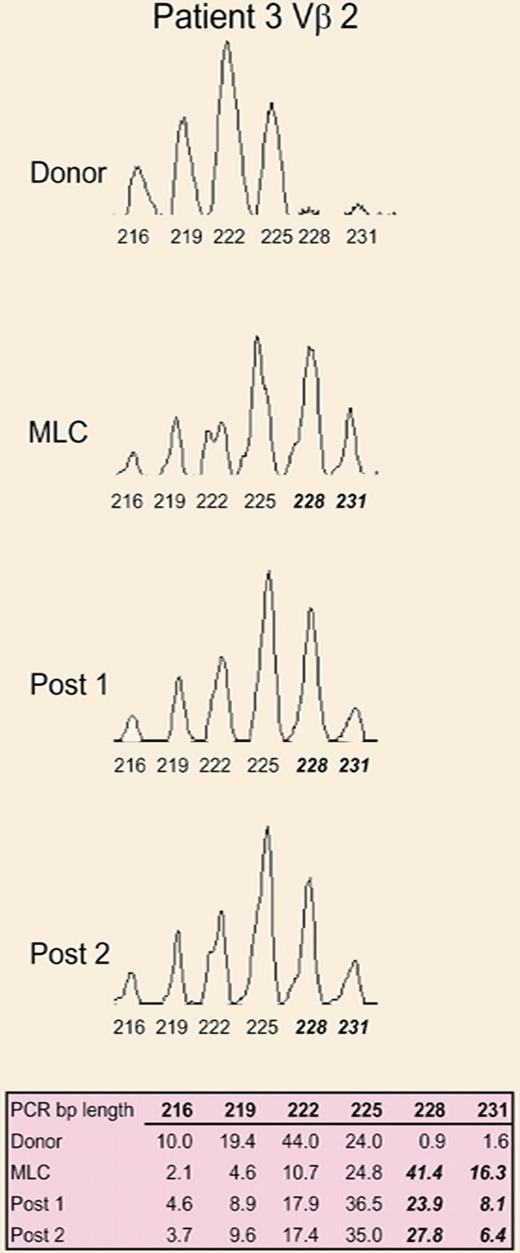
A representative CDR3 size spectratype analysis of the Vβ2 family from the donor, donor/patient mixed lymphocyte culture, and at 2 time points after allogeneic HSCT. See the complete figure in the article beginning on page 3517.
A representative CDR3 size spectratype analysis of the Vβ2 family from the donor, donor/patient mixed lymphocyte culture, and at 2 time points after allogeneic HSCT. See the complete figure in the article beginning on page 3517.
Another means to analyze donor T cells would be by characterizing TCRs, which are miHA-specific. TCR Vβ usage analysis has been shown to separate GVHD and GVT as determined by T- cell clone sequencing in patients.5 However, the sequencing of the third complementarity-determining region (CDR3) of TCR Vβ in T-cell clones after multiple MLCs proved highly variable.3 CDR3 size analysis or “spectratyping” provides a means to examine TCR diversity among the 24 Vβ families of the whole T-cell population. It involves RT-PCR analysis of CDR3 distribution sizes of TCR Vβ families which, statistically, should fall into a Gaussian pattern unless skewing occurs as evidence of oligoclonal expansion. Spectratyping has been used in the past to assess T-cell repertoire recovery after HSCT by several groups.6,7
The present study by Friedman et al is notable for 2 reasons: First, using spectratyping, they show significant skewing of multiple Vβ families after MLC, and second, they observed the same pattern of skewing in patients after bone marrow transplantation and this correlated with GVHD occurrence.
This study opens up some intriguing questions, and clearly more data are needed with larger sample sizes to see if this approach is predictive and robust. The issue of GVT, if these same families affect it or if it is indeed separate as suggested by Michalek et al5 all need to be addressed. Additionally, if these populations are removed, do other miHA-reactive T-cell clones arise due to immunodominance? The data showed that some TCR Vβ families exhibited extensive skewing but also that numerous families are affected to some degree. It will be of interest to determine the influence of the numerous families on alloresponsiveness and clinical outcome.
What do these findings mean? Perhaps in the near future it may be possible to remove the detected dominant Vβ families associated with expansion after MLC. However, to minimize depletion of entire Vβ families, identification and depletion of specific clones within these families may be the preferred scenario. As stated in part of their title, “designer allogeneic blood and marrow transplantation,” using this technique may indeed allow for safer and more efficacious allogeneic HSCT.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■
REFERENCES
National Institutes of Health

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal