Abstract
Macrophages have the capacity to proliferate in response to specific growth factors, such as macrophage-colony stimulating factor (M-CSF). In the presence of several cytokines and activating factors, macrophages undergo growth arrest, become activated, and participate in the development of an immune response. We have previously observed that activation of extracellularly regulated kinase 1/2 (ERK-1/2) is required for macrophage proliferation in response to growth factors. A short and early pattern of ERK activity correlated with the proliferative response. In contrast, slightly prolonged patterns of activity of these kinases were induced by signals that lead to macrophage activation and growth arrest. IFN-γ is the main endogenous Th1-type macrophage activator. Here we report that stimulation with IFN-γ prolongs the pattern of ERK activity induced by M-CSF in macrophages. These effects correlate with IFN-γ–mediated inhibition of the expression of several members of the MAPK phosphatase family, namely MKP-1, -2, and -4. Moreover, inhibition of MKP-1 expression using siRNA technology or synthetic inhibitors also led to elongated ERK activity and significant blockage of M-CSF–dependent proliferation. These data suggest that subtle changes in the time course of activity of members of the MAPK family contribute to the antiproliferative effects of IFN-γ in macrophages.
Introduction
Macrophages perform essential functions in homeostasis, infection, tissue repair, and resolution of inflammation.1 Resident macrophages can be maintained by local proliferation in healthy tissues, including the skin, the lungs, the central nervous system, and the spleen.2,3 In contrast, in response to inflammatory signals, these phagocytic cells stop proliferating and become activated.2 Macrophage-colony stimulating factor (M-CSF) is the major and the only specific growth factor for macrophages.4 M-CSF regulates macrophage differentiation, proliferation, and survival by stimulating a complex array of signal transduction pathways, including mitogen-activated protein kinases (MAPKs).5 Three MAPK modules, namely extracellular regulated kinases 1 and 2 (ERK-1/2), c-Jun-NH2 terminal kinase 1 (JNK-1), and p38, are activated in response to M-CSF.6-9 MAPKs are evolutionarily conserved serine/threonine kinases involved in the transduction of externally derived signals that regulate cell growth, differentiation, and apoptosis.10 These kinases directly modulate downstream targets by phosphorylation, including additional protein kinases, components of the cytoskeleton, phospholipase A2, and transcriptional regulators, such as Ets-1, Elk/TCF, and AP-1, which in turn promote immediate early gene expression. In a previous study, we observed that activation of the ERK cascade is specifically required for macrophages to proliferate in response to M-CSF.11 A growing body of evidence indicates that the time and space properties of MAPK activation are major determinants of their function. This has been mostly established for the ERK-1/2 pathway. Depending on the cell type, subtle differences in the time course of ERK activation change the final biologic outcomes.12-15 Our previous studies have shown that certain macrophage-activating signals, such as lipopolysaccharide, lead to a more prolonged pattern of ERK activity than that induced by proliferative signals such as M-CSF.7,16 In agreement with this concept, recent work on human leukemia TF-1-FMS cells (cells that constitutively express the receptor for M-CSF) by Suzu et al17 demonstrated that a short and defined time course of ERK activity correlated with the capacity of these cells to proliferate in response to M-CSF. In contrast, differentiation toward a macrophage phenotype in response to 12-O-tetradecanoylphorbol coincided with increased and prolonged activation of these kinases.
Macrophage activation is characterized by a series of biochemical and morphologic modifications that allow these cells to perform their functions.18 The T helper 1 cytokine interferon-γ (IFN-γ) signals through Janus kinases and signal transducer and activator of transcription 1 (STAT-1) to trigger classical activation of macrophages. Target genes for IFN-γ in these cells include major histocompatibility complex (MHC) class I and II, which are involved in antigen presentation, immunomodulatory cytokines such as tumor necrosis factor α (TNF-α), chemokines, and antiviral proteins. We have shown that activation by IFN-γ also results in the blockage of macrophage proliferation.19 During the physiologic immune response, it is very likely that macrophages are exposed to proliferating signals and activating factors. The cross-talk between signaling cascades involved in these opposite responses (macrophage activation versus proliferation) has not been extensively explored. Here we demonstrate that IFN-γ inhibits the expression of several phosphatases involved in MAPK dephosphorylation, leading to extended activity times and/or increased activity of those MAPKs initially stimulated by M-CSF. Interestingly, small interfering RNA (siRNA) against the phosphatase MKP-1 partially inhibited the proliferative response to M-CSF. Our studies indicate that, in accordance with results obtained with other cellular systems,12-15 an extended pattern of ERK-1/2 activity in macrophages correlates with a low proliferative response.
Methods
Reagents
Recombinant M-CSF and IFN-γ were purchased from R&D Systems (Minneapolis, MN). In some experiments, L-cell conditioned medium was used as a source of M-CSF. PD98059 was purchased from Calbiochem (San Diego, CA). Actinomycin D and 5,6-dichlorobenzimidazole 1-β-d-ribofuranoside (DBR) were obtained from Sigma-Aldrich (St Louis, MO).
Cell culture and animal models
Bone marrow–derived macrophages were obtained from 7-week-old Balb/c mice (Charles River Laboratories, Wilmington, MA) as described.20 Bone marrow precursors were cultured in DMEM (Sigma-Aldrich), supplemented with 20% fetal calf serum (FCS; Sigma-Aldrich) and 30% L-cell conditioned medium as a source of M-CSF. All experiments were performed with differentiated macrophages at 80% confluence, normally after 6 days of culture. At this point, cells were deprived of M-CSF for 18 hours and then treated with M-CSF in the presence or absence of IFN-γ. Stat1 knockout mice have been described previously.21 Bone marrow from these mice was kindly provided by Dr Robert D. Schreiber (Washington University School of Medicine, St Louis, MO). p21Waf1 knockout mice22 were kindly donated by Dr Philip Leder (Harvard Medical School, HHMI, Boston, MA). Mkp-1 knockout mice were donated by Dr Rodrigo Bravo (Bristol-Myers Squibb Pharmaceutical Research Institute, Princeton, NJ). Jnk-1–deficient mice23 were kindly donated by Dr Richard A. Flavell (Yale University School of Medicine, New Haven, CT). Macrophages deficient in p38α were isolated from mice generated by crossing p38α flox/flox24 with LysM-Cre strains.25 The use of animals was approved by the Animal Research Committee of the University of Barcelona.
RNA extraction and Northern blot analysis
Cells were washed twice in cold PBS and total RNA was extracted as described.11 Total RNA samples (15-20 μg) were separated on 1.2% agarose gels containing formaldehyde and transferred to nylon membranes (Genescreen; NEN Life Science Products, Boston, MA). Specific probes were obtained by labeling full-length cDNAs with α-32P-dCTP (ICN Pharmaceuticals, Costa Mesa, CA). After hybridization at 65°C, membranes were exposed to Kodak X-AR films (Rochester, NY). The bands of interest were quantified with a Molecular Analyst System (Bio-Rad, Hercules, CA).
Quantitative real-time polymerase chain reaction analysis
Cells were washed twice with cold PBS and total RNA was extracted with the EZ-RNA system following the manufacturer's recommendations (Biological Industries, Kibbutz Beit Haemek, Israel). RNA was treated with DNase (Roche, Basel, Switzerland). For cDNA synthesis, 1 μg RNA was subjected to reverse transcription using Moloney murine leukemia virus (M-MLV) Reverse transcriptase RNase H Minus, Point Mutant, oligo(dT)15 primer, and polymerase chain reaction (PCR) Nucleotide mix (Promega, Madison, WI). Real-time (rt)–PCR was performed using the Power SYBR Green Reagent Kit (Applied Biosystems, Foster City, CA) following the manufacturer's recommendations, with the exception that the final volume was 12.5 μL SYBR Green Reaction Mix. The sequence of primers used throughout this study are shown in Table S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article). Annealing for all primers was performed at 60°C for 30 seconds. Real-time monitoring of PCR amplification was performed using the ABI Prism 7900 Sequence Detection System (Applied Biosystems). Data were expressed as relative mRNA levels normalized to L14 expression levels in each sample. A control sample without RNA was included in each reaction.
Protein extraction and Western blot analysis
Cells were washed twice in cold PBS and lysed on ice with lysis solution (1% Triton X-100, 10% glycerol, 50 mM Hepes (pH 7.5), 250 mM NaCl, 1 μg/mL aprotinin, 1 μg/mL leupeptin, 1 μg/mL iodoacetamide, 1 mM PMSF, 1 mM sodium orthovanadate). Insoluble material was removed by centrifugation at 13 000g for 8 minutes at 4°C. Cell lysates (50-100 μg) were boiled at 95°C in Laemmli sodium dodecyl sulfate (SDS)–loading buffer and separated by 10% SDS–polyacrylamide gel electrophoresis (PAGE), unless otherwise stated, and electrophoretically transferred to nitrocellulose membranes (Hybond-ECL; GE Healthcare Europe, Munich, Germany). Membranes were blocked in 5% milk in TBS-0.1% Tween 20 (TBS-T) overnight at 4°C and then incubated with primary antibody for 2 hours at room temperature. We used the following antibodies: rabbit IgG anti–mouse MKP-1 (Santa Cruz Biotechnology, Santa Cruz, CA), monoclonal antidiphosphorylated ERK-1/2 (clone MAPK-YT; Sigma-Aldrich), rabbit polyclonal antibodies against total ERK (obtained after immunization of a rabbit with the peptide IFQETARFQPGAPEAP, corresponding to the C-terminal region of ERK-1 as described26 ), goat polyclonal antibodies against histone H1 (N-19; Santa Cruz Biotechnology), and monoclonal anti–mouse β-actin (Sigma-Aldrich). In general, membranes were washed 3 times in TBS-T, then incubated for 1 hour with peroxidase-conjugated secondary antibodies (Jackson ImmunoResearch Europe [Suffolk, United Kingdom] and Sigma-Aldrich). After 3 washes of 15 minutes with TBS-T, enhanced chemiluminescence detection was performed (GE Healthcare Europe) and the membranes were exposed to X-ray films (GE Healthcare Europe).
Determination of ERK activity by in-gel kinase assay
The analysis of ERK activity was performed as described.27 Total protein (50 μg), obtained as described in “Protein extraction and Western blot analysis,” was separated by 12.5% SDS-PAGE containing 0.1 mg/mL myelin basic protein (MBP; Sigma-Aldrich) copolymerized in the gel. After electrophoresis, SDS was removed by washing the gel with 2 changes of 20% 2-propanol in 50 mM Tris-HCl (pH 8.0) for 1 hour at room temperature. The gel was then incubated with 50 mM Tris-HCl (pH 8.0) containing 5 mM β-mercaptoethanol (buffer A) for 1 hour at room temperature. The proteins were denatured by incubating the gel with 2 changes of 6 M guanidine-HCl for 1 hour at room temperature and then renatured in buffer A containing 0.04% Tween-20 for 16 hours (5 changes) at 4°C. To perform the phosphorylation assay, the gel was first equilibrated in 40 mM Hepes-NaOH (pH 7.4) containing 2 mM DTT, 0.1 mM EGTA, 15 mM MgCl2, and 300 μM sodium orthovanadate for 30 minutes at 25°C and then incubated in the same solution containing 50 μM ATP and 100 μCi (3.7 MBq) γ-32P-ATP (ICN Pharmaceuticals). The reaction was terminated by washing the gel with 5% TCA containing 10 mM sodium pyrophosphate to inhibit phosphatase activity. The gel was dried, exposed to X-ray films (Kodak), and quantified with a Bio-Rad Molecular Analyst.
JNK activity assay
JNK activity was measured as described.28 Briefly, cells were lysed with nuclear extract protocols and immunoprecipitated with protein A–sepharose and anti–JNK-1 Ab. After several washes, the reaction was performed with 1 μg GST-c-jun (1-169; MBL, Woburn, MA) as JNK substrate, 20 μM ATP, and 1 μCi (0.037 MBq) γ32P-ATP. SDS-PAGE electrophoresis was performed and the gel was exposed to Agfa X-ray films (Mortsel, Belgium).
Isolation of cytosolic and nuclear fractions
The cells were washed in cold PBS and resuspended in cold buffer A (10 mM Hepes, pH 7.5, 1 mM EDTA, 0.1 mM EGTA, 10 mM Na4P2O7, 100 mM NaF, 10 mM KCl, 1 mM DTT, 2 mM sodium orthovanadate, and a cocktail of protease inhibitors). Triton (0.3%; final concentration) was added to the samples, which were immediately vortexed for 30 seconds and centrifuged for 30 seconds. The supernatants were collected (cytosolic fractions). The nuclei were lysed by incubation for 30 minutes at 4°C in buffer B (buffer A supplemented with 400 mM NaCl and 1 mM EGTA), and the samples were centrifuged for 15 minutes at 16 000g (4°C). The supernatants were collected (nuclear fractions). Both cytosolic and nuclear fractions were quantitated using Bradford assay and used for Western blotting.
Proliferation assay
Cell proliferation was measured as described20 with minor modifications. Quiescent cells (105 cells/well in 24-well plates) were stimulated with M-CSF for 24 hours. The media were aspirated and replaced with 0.5 mL media containing 3H-thymidine (1 μCi [0.037 MBq]/mL; ICN Pharmaceuticals). After 4 to 6 hours of incubation at 37°C, the media were removed and cells were fixed in ice-cold 70% methanol. After 3 washes in ice-cold 10% trichloroacetic acid, cells were solubilized in 1% SDS and 0.3 M NaOH at room temperature. Radioactivity was counted by liquid scintillation using a 1400 Tri-Carb Packard scintillation counter (Hewlett Packard, Palo Alto, CA). Each point was performed in triplicate and the results were expressed as the mean plus or minus SD.
siRNA
MKP expression was blocked using siRNA technology. The following target sequence was used to develop specific siRNAs against MKP-1: 5′AATCCTCCTGAGTTCCACTG3′ (purchased from Dharmacon, Lafayette, CO). Control siRNAs were designed against Luciferase (5′CGTACGCGGAATACTTCGA3′) and enhanced green fluorescent protein (EGFP; 5′AAGACGTAAACGGCCACAAGTTC3′), purchased from Dharmacon. Validated siRNAs against MKP-2 (sc-38999) and MKP-4 (sc-39003) were purchased from Santa Cruz Biotechnology. siRNAs were transfected into macrophages by electroporation. Briefly, the cells were collected and washed twice with DMEM and then resuspended at a final concentration of 107 cells/mL. Macrophages (4 × 106; 400 μL) were mixed with 1.5 μM siRNA in 4-mm gap cuvettes (BTX no. 640; GENO-tronics, Landgraaf, The Netherlands) and placed on ice for 5 minutes. Electroporation was carried out at 2300 μF, 300 V, 13 Ω (∼27 ms) with an ECM 600 electroporator (BTX). The samples were placed on ice for 10 minutes before reconstitution in DMEM supplemented with 20% FCS, 30% L-cell conditioned medium. Subsequent assays were carried out 24 hours after transfection. In pilot experiments, high transfection efficiencies (> 80%) were observed using a Cy5-labeled unspecific siRNA (detection by cytometry).
Immunofluorescence microscopy
Macrophages were seeded at 50 × 103 cells/well onto poly-l-lysine–treated cover glasses (12 mm diameter; Marienfeld, Lauda-Königshofen, Germany). After treatment, the cells were washed twice with PBS, fixed, and lysed in ice-cold methanol at −20°C for 15 minutes. The cells were then washed twice in PBS and blocked in PBS containing 1% BSA for 20 minutes at room temperature. Primary antibodies against total ERK were obtained after immunization of a rabbit with the peptide IFQETARFQPGAPEAP, corresponding to the C-terminal region of ERK-1 as described.26 The peptide was coupled to KLH and synthesized by the Peptide Synthesis Unit (Facultat de Química, University of Barcelona). Rabbits were immunized with 250 μg/μL peptide at 3-week intervals for 3 months. The primary antibody was diluted (1:300) into 1% BSA in PBS and incubated with the slides for 90 minutes at 37°C in a humidified chamber. The slides were washed 3 times with PBS and incubated with anti–rabbit IgG coupled to Alexa 488 (1:1000) in 1% BSA in PBS-glycine at 37°C for 90 minutes. The nuclei were stained with bisbenzimide Hoescht 33342 (Sigma-Aldrich; 50 μM in PBS) for 5 minutes at room temperature. The slides were then washed 5 times with PBS and then mounted in Mowiol (Calbiochem). The slides were viewed on a laser scanning confocal microscope (Leica SP2 AOBS; Mannheim, Germany) using a 40× oil objective (1.25 lens aperture). Micrograph acquisition was taken using the Leica confocal software.
Results
We previously characterized the activation time course of members of the MAPK family and their upstream regulators in macrophages stimulated with M-CSF.7,29 Activation of the ERK pathway was required for macrophages to proliferate in response to M-CSF.11 Strong ERK activation is normally detected 5 to 10 minutes after the start of M-CSF stimulation and activation drops significantly after 30 minutes of treatment (Figure 1A). In the present study, we examined whether classical activation by IFN-γ influenced M-CSF–induced ERK activation. For this purpose, bone marrow–derived macrophages were incubated with IFN-γ for 5 minutes before addition of M-CSF. In these conditions, ERK activity still peaked at 5 to 10 minutes after stimulation with M-CSF, but significantly higher levels of active ERK were detected throughout the time course experiment up to 3 hours (Figure 1A). Equivalent results were obtained when IFN-γ and M-CSF were added simultaneously (data not shown). The activation of p38 (Figure 1C) was also substantially prolonged, whereas activation of JNK-1 (Figure 1B) was only weakly affected. As a control of these experiments, we also analyzed the effects of IFN-γ treatment alone. Weak activation of ERKs and JNK-1 was detected after 2 hours of treatment with IFN-γ (Figure 1D and E, respectively), but not earlier. This observation suggests that the prolongation of ERK activity within the first 2 hours of treatment with IFN-γ and M-CSF is not necessarily mediated by direct activation by IFN-γ. JNK-2 activity was almost undetectable in our conditions (data not shown). In contrast, IFN-γ signaling did activate p38 (Figure 1F), consistent with the possibility that prolonged p38 activation in macrophages treated with M-CSF and IFN-γ is the consequence of additive direct activation of these signals.
Effects of IFN-γ on MAPK activation. Bone marrow–derived macrophages were obtained after 7 days of culture in the presence of M-CSF. The cells were rendered quiescent by incubating them in medium supplemented with 10% FCS (without M-CSF) for 15 hours before the start of the experiment. In panels A through C, G and H, quiescent macrophages were first treated with IFN-γ (10 ng/mL) or vehicle for 5 minutes and then stimulated with M-CSF (30 ng/mL) for the indicated periods of time. In panels D through F, quiescent macrophages were treated with IFN-γ (10 ng/mL) for the indicated periods of time. (A) The activity of ERK-1 and -2 was analyzed by in-gel kinase assay. As a control for sample quality and quantity, 20 μg whole-cell extract was used to measure β-actin expression by Western blotting. (B) JNK-1 activation was determined by in vitro kinase assay using c-Jun as a substrate. As a control for sample quality and quantity, 20 μg whole-cell extract was used to measure β-actin expression by Western blotting. (C) p38 activation was measured by Western blotting using an anti–phospho-p38 antibody. The expression of β-actin was measured as a control. (D) The phosphorylation of ERK-1 and -2 was measured by Western blotting, using anti–phospho-ERK antibodies. The expression of β-actin was measured as a control. (E) JNK-1 activation was determined by in vitro kinase assay. The loading control for section E is the same than in section D. (F) p38 activation was measured by Western blotting using anti–phospho-p38 antibodies. (G) Confocal microscopy was used to visualize translocation of total ERK to the nucleus. (H) Cytosolic and nuclear fractions were obtained from cells incubated with M-CSF or IFN-γ + M-CSF for the indicated periods of time. The samples were immunoblotted using antibodies specific against diphosphorylated ERK and total ERK. As a control, the expression of nuclear histone H1 was also analyzed. The experiments in panels A and G have been performed 5 times. The rest of the sections have been reproduced 3 times.
Effects of IFN-γ on MAPK activation. Bone marrow–derived macrophages were obtained after 7 days of culture in the presence of M-CSF. The cells were rendered quiescent by incubating them in medium supplemented with 10% FCS (without M-CSF) for 15 hours before the start of the experiment. In panels A through C, G and H, quiescent macrophages were first treated with IFN-γ (10 ng/mL) or vehicle for 5 minutes and then stimulated with M-CSF (30 ng/mL) for the indicated periods of time. In panels D through F, quiescent macrophages were treated with IFN-γ (10 ng/mL) for the indicated periods of time. (A) The activity of ERK-1 and -2 was analyzed by in-gel kinase assay. As a control for sample quality and quantity, 20 μg whole-cell extract was used to measure β-actin expression by Western blotting. (B) JNK-1 activation was determined by in vitro kinase assay using c-Jun as a substrate. As a control for sample quality and quantity, 20 μg whole-cell extract was used to measure β-actin expression by Western blotting. (C) p38 activation was measured by Western blotting using an anti–phospho-p38 antibody. The expression of β-actin was measured as a control. (D) The phosphorylation of ERK-1 and -2 was measured by Western blotting, using anti–phospho-ERK antibodies. The expression of β-actin was measured as a control. (E) JNK-1 activation was determined by in vitro kinase assay. The loading control for section E is the same than in section D. (F) p38 activation was measured by Western blotting using anti–phospho-p38 antibodies. (G) Confocal microscopy was used to visualize translocation of total ERK to the nucleus. (H) Cytosolic and nuclear fractions were obtained from cells incubated with M-CSF or IFN-γ + M-CSF for the indicated periods of time. The samples were immunoblotted using antibodies specific against diphosphorylated ERK and total ERK. As a control, the expression of nuclear histone H1 was also analyzed. The experiments in panels A and G have been performed 5 times. The rest of the sections have been reproduced 3 times.
We next determined whether IFN-γ affected the entry of ERK to the nucleus in response to M-CSF. Confocal microscopy using antibodies against total ERK (Figure 1G), as well as Western blotting of cytoplasmic and nuclear extracts using antibodies against total and phosphorylated ERK (Figure 1H), demonstrated that ERK was capable of entering the nucleus in response to M-CSF and that IFN-γ did not block this process. We also observed that a high percentage of total ERK still remained in the cytoplasm after stimulation of macrophages with M-CSF, which suggests that active ERK could have important extranuclear and/or intranuclear actions during the macrophage response to M-CSF.
To unravel the mechanism that accounts for extended ERK activity, we studied the expression of several phosphatases that are known negative MAPK regulators. These include MKP-1, -2, -3, -4, -5, and -7, PAC-1, and CPG21.30 We first measured the pattern of expression of each of these phosphatases during the response to M-CSF. Real-time PCR experiments demonstrated the capacity of M-CSF to induce the expression of MKP-1, -2, -4, and -7, PAC-1, and CPG21 (Figure 2A). Low levels of expression of MKP-3 and -5 were also observed in macrophages, but we did not detect any induction by M-CSF (data not shown). MKP-1, MKP-2, PAC-1, and CPG21 were induced relatively early during M-CSF signaling. However, several differences in their patterns of induction were observed. The expression levels of MKP-1 peaked at 45 minutes and dropped drastically thereafter, whereas induction of PAC-1 and CPG21 was more sustained. Expression of MKP-2 peaked at 2 hours and remained high until at least 3 hours. In contrast, MKP-4 was induced by M-CSF in a more delayed manner, with highest expression levels at 3 to 6 hours.
Effects of M-CSF and IFN-γ on members of the MKP family. The expression of several MKPs was analyzed by rt-PCR. (A) Macrophages were stimulated with M-CSF for the indicated periods of time. Control cells were left untreated (0). (B) Macrophages were treated with M-CSF, IFN-γ, or both agents together for 30 minutes (for MKP-1), 2 hours (for MKP-2), or 2 to 6 hours (for MKP-4). (C) Macrophages were stimulated with IFN-γ for the indicated times and the expression of MKP-5 and -7 was analyzed. In all rt-PCR experiments, the expression values were normalized to the expression levels of the ribosomal gene L14. Each experiment was performed 3 times and a representative experiment from each one is shown here. Error bars indicate the variation between experiments.
Effects of M-CSF and IFN-γ on members of the MKP family. The expression of several MKPs was analyzed by rt-PCR. (A) Macrophages were stimulated with M-CSF for the indicated periods of time. Control cells were left untreated (0). (B) Macrophages were treated with M-CSF, IFN-γ, or both agents together for 30 minutes (for MKP-1), 2 hours (for MKP-2), or 2 to 6 hours (for MKP-4). (C) Macrophages were stimulated with IFN-γ for the indicated times and the expression of MKP-5 and -7 was analyzed. In all rt-PCR experiments, the expression values were normalized to the expression levels of the ribosomal gene L14. Each experiment was performed 3 times and a representative experiment from each one is shown here. Error bars indicate the variation between experiments.
We next explored the effect of IFN-γ on the expression of each of these phosphatases. IFN-γ did not alter the expression levels of MKP-3 or the induction of PAC-1 and CPG21 by M-CSF (data not shown). However, this cytokine strongly inhibited the expression of MKP-1, -2, and -4 (Figure 2B). This observation suggested that the extension of MAPK activity in the presence of IFN-γ could be mediated by reduced expression of MKP-1, -2, or -4. Interestingly, MKP-5 and -7 were up-regulated late during the response to IFN-γ (Figure 2C), which might help to explain why the activity of MAPKs cannot be prolonged for a very long time after IFN-γ stimulation.
To further understand the nature of the inhibitory effects of IFN-γ on the mRNA expression of MKP-1, -2, and -4 we studied whether the stability of these transcripts was decreased in response to IFN-γ signaling. For this purpose, MKP gene expression was induced upon treatment with M-CSF for 30 minutes (MKP-1) or 2 hours (MKP-2 and MKP-4). After this time, IFN-γ was added and 5 minutes later gene transcription was stopped using a cocktail of actinomycin D and 5,6-dichlorobenzimidazole 1-β-d-ribofuranoside.31 The remaining amount of mRNA for each gene of interest was analyzed during a time course with interval times of 10 minutes. The transcripts for MKP-1, -2, and -4 have relatively short half-lives after induction by M-CSF (approximately 18 minutes for MKP-1, 37 minutes for MKP-2, and 33 minutes for MKP-4). The degradation rates of these transcripts were not increased by IFN-γ (data not shown). In summary, IFN-γ did not exert posttranscriptional effects that resulted in decreased stability of the transcripts for MKP-1, -2, and -4, which suggests that the inhibitory effects of this cytokine are exerted mainly at the transcriptional level. We are currently trying to determine the molecular mechanism underlying the transcriptional regulation of MKPs by IFN-γ.
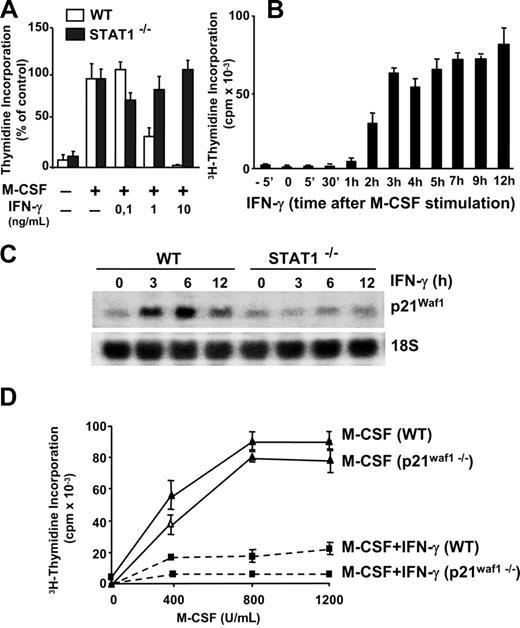
In contrast to other cells of the immune system, such as lymphocytes, macrophage activation is associated with cell growth arrest.19 Treatment with IFN-γ inhibited M-CSF–dependent proliferation as determined by thymidine incorporation (Figure 3A). Functional Stat-1 was required for this process, since the inhibition of proliferation is lost in Stat-1–deficient macrophages (Figure 3A). We extended these studies by stimulating macrophages with saturating concentrations of IFN-γ (10 ng/mL)32 at a range of times before and after the addition of M-CSF (Figure 3B). In these experiments, IFN-γ fully inhibited DNA synthesis only when added before M-CSF or during the first hour of M-CSF signaling. However, the capacity to abolish macrophage growth was gradually lost when IFN-γ was added 2 or more hours after the stimulation with M-CSF. Therefore, efficient blockage of macrophage proliferation requires IFN-γ–mediated regulation of early events that take place within the first hour of M-CSF signaling.
IFN-γ inhibits macrophage proliferation in a Stat-1–dependent, p21Waf1-independent manner. (A) Macrophages were obtained from wild-type (WT) and Stat-1–deficient mice. Quiescent macrophages (105) were incubated for 24 hours with M-CSF either alone or together with the indicated concentrations of IFN-γ. Proliferation was determined by 3H-thymidine incorporation. Each point was represented as the percentage of 3H-thymidine incorporated in the absence of IFN-γ (M-CSF control). Mean values in wild-type and Stat-1–deficient macrophages were 83 000 and 71 000 cpm, respectively. (B) Quiescent cells (105) were incubated with IFN-γ (10 ng/mL) for a range of time periods before and after stimulation with M-CSF. Proliferation was determined by 3H-thymidine incorporation. (C) The expression of p21Waf1 was analyzed by Northern blot in WT and Stat-1–deficient macrophages treated with IFN-γ. Expression of 18S rRNA was used as a control for RNA loading and transfer. (D) Macrophages were obtained from WT and p21Waf1 knockout mice. Macrophages (105) were incubated for 24 hours with the indicated amounts of M-CSF either alone or in combination with IFN-γ. Proliferation was determined by 3H-thymidine incorporation. The experiments in panels A through C were performed 3 times with equivalent results. The data in panel D were reproduced twice. Error bars represent SDs from triplicates in 1 representative experiment.
IFN-γ inhibits macrophage proliferation in a Stat-1–dependent, p21Waf1-independent manner. (A) Macrophages were obtained from wild-type (WT) and Stat-1–deficient mice. Quiescent macrophages (105) were incubated for 24 hours with M-CSF either alone or together with the indicated concentrations of IFN-γ. Proliferation was determined by 3H-thymidine incorporation. Each point was represented as the percentage of 3H-thymidine incorporated in the absence of IFN-γ (M-CSF control). Mean values in wild-type and Stat-1–deficient macrophages were 83 000 and 71 000 cpm, respectively. (B) Quiescent cells (105) were incubated with IFN-γ (10 ng/mL) for a range of time periods before and after stimulation with M-CSF. Proliferation was determined by 3H-thymidine incorporation. (C) The expression of p21Waf1 was analyzed by Northern blot in WT and Stat-1–deficient macrophages treated with IFN-γ. Expression of 18S rRNA was used as a control for RNA loading and transfer. (D) Macrophages were obtained from WT and p21Waf1 knockout mice. Macrophages (105) were incubated for 24 hours with the indicated amounts of M-CSF either alone or in combination with IFN-γ. Proliferation was determined by 3H-thymidine incorporation. The experiments in panels A through C were performed 3 times with equivalent results. The data in panel D were reproduced twice. Error bars represent SDs from triplicates in 1 representative experiment.
IFN-γ induces the expression of the cyclin-dependent inhibitor p21Waf1 in primary macrophages.19 Those studies suggested an essential role for p21Waf1 in the protection of macrophages from apoptosis induced by several stimuli. Time course studies by Northern blotting show that p21Waf1 mRNA expression is induced after 1 hour of treatment with IFN-γ.19 Here we demonstrate that induction of p21Waf1 by IFN-γ is also dependent on Stat-1 signaling (Figure 3C). However, whereas p21Waf1 plays an essential role in growth arrest in several other cell types, inhibition of macrophage proliferation by IFN-γ seems to be independent of p21Waf1 activity, as observed in macrophages derived from p21Waf1 knockout mice (Figure 3D). Taken together, the antiproliferative action of IFN-γ in macrophages is a Stat-1–dependent, p21Waf1-independent process.
In previous studies, we observed a correlation between the type of time course of ERK activity and specific macrophage responses. More precisely, macrophage-activating signals, such as lipopolysaccharide, lead to a more prolonged pattern of ERK activity than that induced by proliferative signals.7,16 Moreover, treatments that block the expression of the phosphatase MKP-1 and result in prolonged ERK activation, also lead to inhibition of macrophage proliferation, including incubation with the PKC inhibitor GF109203X (data not shown), and exposure to the extracellular matrix components decorin and fibronectin.33 Recent work with human leukemia TF-1-FMS cells has provided an independent line of evidence that subtle changes in ERK activity correlate with distinct cellular responses.17 In that study, M-CSF–induced proliferation correlated with a short time course of ERK activity, whereas increased and prolonged activation of these kinases in response to 12-O-tetradecanoylphorbol correlated with differentiation toward a macrophage phenotype.
The observation that IFN-γ stimulation alters the activation pattern of ERK-1/2 made us hypothesize that this effect contributes to the antiproliferative actions of IFN-γ. To test this theory and based on the finding that IFN-γ–mediated inhibition of macrophage proliferation requires functional Stat-1, we first examined whether the prolongation of ERK activity and the induction of MKP-1 expression depend on Stat-1 activation (Figure 4A). Early induction of ERK activity by M-CSF occurred independently of Stat-1. In wild-type (WT) macrophages, ERK activity was relatively sustained upon stimulation with IFN-γ and M-CSF, whereas this treatment did not lead to prolonged ERK activity in macrophages deficient for Stat-1. In correlation with this, IFN-γ did not inhibit MKP-1 expression in Stat-1 knockout macrophages (Figure 4B). In concert, inhibition of MKP-1 expression, extension of ERK activity, and growth arrest in macrophages activated by IFN-γ required intact signaling through Stat-1. The pattern of p38 activity was also substantially sustained in macrophages stimulated with M-CSF and IFN-γ (Figure 1). However, IFN-γ inhibited macrophage proliferation induced by M-CSF in p38α-deficient macrophages (data not shown), which suggests that extended p38α activity does not mediate the antiproliferative effects of IFN-γ. However, we cannot exclude the involvement of other p38 isoforms. Likewise, the contribution of JNK-1 to this process was ruled out, as the IFN-γ–mediated inhibition of proliferation was not impaired in JNK-1–deficient macrophages (data not shown).
IFN-γ inhibits MKP-1 expression and elongates ERK activity through a Stat-1–dependent mechanism. (A) Quiescent macrophages from wild-type (WT) and Stat-1 knockout mice were treated with M-CSF for the indicated periods of times in the presence or absence of IFN-γ. Activation of ERK-1 and -2 was analyzed by in-gel kinase assay. (B). MKP-1 expression was determined by Northern blotting after 30 minutes of treatment with M-CSF in the presence or absence of IFN-γ. These experiments were done 3 times with equivalent results.
IFN-γ inhibits MKP-1 expression and elongates ERK activity through a Stat-1–dependent mechanism. (A) Quiescent macrophages from wild-type (WT) and Stat-1 knockout mice were treated with M-CSF for the indicated periods of times in the presence or absence of IFN-γ. Activation of ERK-1 and -2 was analyzed by in-gel kinase assay. (B). MKP-1 expression was determined by Northern blotting after 30 minutes of treatment with M-CSF in the presence or absence of IFN-γ. These experiments were done 3 times with equivalent results.
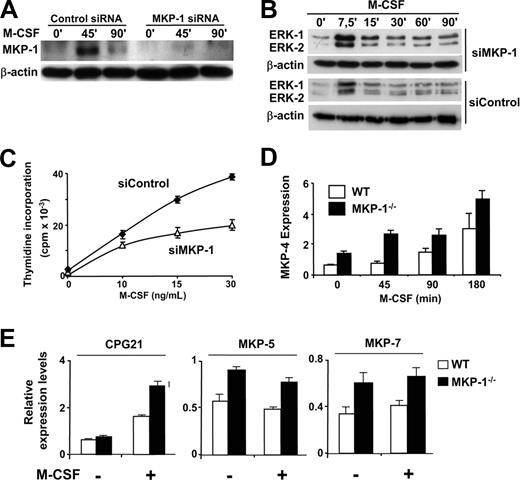
Among the phosphatases tested, MKP-1 was expressed in tight correlation with the pattern of deactivation ERK-1/2 during the response to M-CSF (Figure 2A) and was repressed by IFN-γ. Therefore we used siRNA technology to explore the possibility that inhibition of MKP-1 expression contributes to growth arrest. Transfection of macrophages with siRNA against MKP-1 resulted in reduced induction of the phosphatase in response to M-CSF (Figure 5A), increased and more prolonged ERK activity (Figure 5B), and partial inhibition of the macrophage proliferative response (Figure 5C). The use of siRNA against MKP-2 or -4 did not result in reduced proliferation in response to M-CSF, and the combined use of siRNA against MKP-2 or -4 with siRNA against MKP-1 did not enhance the capability of the latter to inhibit macrophage proliferation (data not shown). Although we cannot discard that inhibition of MKP-2 and -4 may contribute to prolongation of MAPK activity by IFN-γ, the experiments using siRNAs suggest an important role for MKP-1 in the regulation of macrophage proliferation. Moreover, inhibition of macrophage proliferation was also observed when these cells were incubated with antisense oligonucleotides against MKP-1 (data not shown). The use of MKP-1 siRNA did not inhibit c-myc expression in macrophages (data not shown), which suggests that the antiproliferative effects of prolonged ERK activity are not related to changes in c-myc expression. Taken together, blockage of MKP-1 expression in vitro seems to correlate with decreased macrophage proliferation. However, these results were not confirmed in macrophages derived from mice deficient for MKP-1; in these cells, we did not detect significant changes in macrophage proliferation or ERK activity in response to M-CSF (data not shown) compared with the wild-type macrophages, which suggests the existence of compensatory mechanisms perhaps provided by other members of the MKP family. Moreover, treatment with IFN-γ resulted in prolonged ERK activation and growth arrest in both wild-type and Mkp-1 knockout macrophages (data not shown), which suggested that the compensatory mechanisms could be sensitive to down-regulation by IFN-γ. Interestingly, mRNA expression analysis revealed increased and more anticipated levels of MKP-4 during the response to M-CSF in MKP-1–deficient macrophages (Figure 5D) in comparison with the wild-type cells. We extended our studies in the MKP-1–deficient macrophages by performing a systematic analysis of the expression levels of other members of the MKP family. We observed increased basal expression of MKP-5 and MKP-7 and increased induction of CPG21 early during the response to M-CSF (Figure 5E). No significant changes were observed in the pattern of expression of other phosphatases tested such as PAC-1 and MKP-2. The use of siRNA against MKP-4 was not sufficient to inhibit proliferation in Mkp-1 knockout macrophages (data not shown), which may suggest that the compensatory mechanisms that operate in the absence of MKP-1 in the in vivo model are more complex than we initially expected. We are currently developing experimental work to determine the nature of these compensatory effects.
Blockage of MKP-1 expression results in decreased macrophage proliferation. (A-C) Macrophages were transfected with either an siRNA directed against MKP-1 or a control siRNA against luciferase. Twenty-four hours after transfection, the cells were stimulated with M-CSF for the indicated periods of time. (A) MKP-1 expression was analyzed by Western blotting, using β-actin expression as a control. (B) Total ERK activity was analyzed by Western blotting. Diphospho-ERK detection was carried out with specific antibodies. β-actin expression was measured as a control. (C) Thymidine incorporation was determined in triplicate as a measurement of macrophage proliferation. Error bars represent SD from triplicates in 1 representative experiment. (D) Macrophages from wild-type or MKP-1–deficient mice were stimulated with M-CSF for the indicated periods of time. Expression of MKP-4 was determined by rt-PCR using the expression values of L14 for normalization. (E) Macrophages from wild-type or MKP-1–deficient mice were either left untreated or stimulated with M-CSF for 45 minutes. Expression of CPG21, MKP-5, and -7 was determined by rt-PCR using the expression values of L14 for normalization. In all the sections, similar results were obtained in at least 3 independent experiments. Error bars in panels D and E indicate the variation between experiments.
Blockage of MKP-1 expression results in decreased macrophage proliferation. (A-C) Macrophages were transfected with either an siRNA directed against MKP-1 or a control siRNA against luciferase. Twenty-four hours after transfection, the cells were stimulated with M-CSF for the indicated periods of time. (A) MKP-1 expression was analyzed by Western blotting, using β-actin expression as a control. (B) Total ERK activity was analyzed by Western blotting. Diphospho-ERK detection was carried out with specific antibodies. β-actin expression was measured as a control. (C) Thymidine incorporation was determined in triplicate as a measurement of macrophage proliferation. Error bars represent SD from triplicates in 1 representative experiment. (D) Macrophages from wild-type or MKP-1–deficient mice were stimulated with M-CSF for the indicated periods of time. Expression of MKP-4 was determined by rt-PCR using the expression values of L14 for normalization. (E) Macrophages from wild-type or MKP-1–deficient mice were either left untreated or stimulated with M-CSF for 45 minutes. Expression of CPG21, MKP-5, and -7 was determined by rt-PCR using the expression values of L14 for normalization. In all the sections, similar results were obtained in at least 3 independent experiments. Error bars in panels D and E indicate the variation between experiments.
Discussion
Here we have shown that classical activation of macrophages with IFN-γ resulted in stronger and more prolonged activity of members of the MAPK family in response to M-CSF treatment. The mechanism that accounts for prolonged activation of these MAPKs seems to be a combination of their direct activation by M-CSF and IFN-γ, together with inhibition of the expression of several phosphatases. Members of the MKP family mediate dephosphorylation of threonine/serine and tyrosine residues within the MAPK activation motif, which is sufficient for total enzymatic inactivation.34 M-CSF has the capacity to induce the expression of an array of MKPs, which probably act in concert to ensure adequate dephosphorylation and inactivation of MAPKs in a time- and compartment-dependent fashion. Of these phosphatases, IFN-γ selectively inhibited MKP-1, -2, and -4, leading to prolonged MAPK activity. Other authors have also reported repressive effects of IFN-γ on the induction of MKP-1 upon TLR2 engagement in human macrophages.35 Work in progress in our laboratory seeks to define the mechanism that accounts for IFN-γ–mediated repression of MKP expression. Stat-1 has previously been reported to inhibit gene expression mediated by other transcription factors such as AP-1 by sequestering coactivators required for both pathways.36 Another potential mechanism could involve Gsk3 activation.35 It should be noted that most of the cellular JNK-1 activity initially triggered by M-CSF was shut down even in the presence of IFN-γ, which indicates that MKPs other than MKP-1, -2, and -4 are probably responsible for the initial dephosphorylation of JNK-1 in macrophages. Moreover, the prolongation of the activity of ERK and p38 is not indefinite. This finding could be attributed to the action of other MKPs that are not down-regulated by IFN-γ. Indeed, our studies demonstrate that IFN-γ has the capacity to induce the expression of MKP-5 and -7 by itself later during the macrophage response to this cytokine.
In our previous studies, activation of p38α appeared to be essential for the expression of several IFN-γ–induced genes in macrophages, and these effects were exerted, at least in part, through mechanisms involving mRNA stabilization.37,38 However, in the present study, we cannot conclude that the extended activity of p38α participates in the antiproliferative action of IFN-γ, as this cytokine inhibited macrophage proliferation in p38α-deficient macrophages.
In contrast, macrophage proliferation in response to M-CSF might be susceptible to the changes in ERK activity reported here. In several cellular models, changes in ERK activity have been associated with loss of proliferation.39-42 For example, the expression of the adapter MONA in macrophages leads to sustained ERK activation, which has an antiproliferative effect and promotes cell differentiation.43 In the neuroepithelial PC12 cells, transient activation of ERK occurs in response to mitogenic stimuli, whereas sustained activation precedes differentiation into sympathetic-like neurons.44,45 Furthermore, we have long observed that the time course of ERK phosphorylation correlates with specific macrophage responses. Signals that act as growth factors for macrophages, such as M-CSF, granulocyte and macrophage-colony stimulating factor (GM-CSF), or interleukin 3 (IL3), induce rapid and transient activation of ERK-1 and -2. In contrast, activating signals, such as lipopolysaccharide or treatment with exogenous phosphatidylcholine-phospholipase C, which in turn induce macrophage growth arrest, lead to a more delayed pattern of ERK activation.7,16 We observed similar results when macrophages were treated with the matrix extracellular proteins fibronectin and decorin,33 with the PKC inhibitor GF109203X (data not shown), or with cyclosporine A or FK506.46 In all these cases, macrophages underwent growth arrest in correlation with extended ERK activity and MKP-1 inhibition.
Because inhibition of ERK activity blocks macrophage proliferation by itself, we could not use PD98059 to study whether direct inhibition of ERK reverts the antiproliferative effects of IFN-γ. The time course of MKP-1 induction coincided tightly with the pattern of ERK deactivation during the response to M-CSF. The use of siRNA technology to inhibit MKP-1 expression confirmed a relevant role for MKP-1 in the control of the duration of ERK activity, although we cannot discard additional effects of other phosphatases on macrophage ERK dephosphorylation. In agreement with our results, other authors have reported sustained ERK1/2 activation after stimulating MKP-1 degradation via the ubiquitin-proteasome pathway.47
In correlation with augmented ERK activation, substantial inhibition of proliferation was observed in macrophages transfected with siRNA against MKP-1. Unfortunately, equivalent experiments performed in Mkp-1 knockout macrophages did not render the same results. Cells deficient in MKP-1 showed no extended ERK activity and cycled normally in response to M-CSF. This discrepancy could be explained by the existence of compensatory mechanisms to supplement the permanent absence of MKP-1. Indeed, higher levels of expression of other phosphatases were observed in Mkp-1 knockout macrophages at early time points of IFN-γ stimulation, which may help to compensate for the lack of MKP-1.
MAPK-mediated growth arrest has been associated with induction of the cyclin-dependent kinase inhibitor p21Waf1.48,49 In our system, IFN-γ–mediated expression of p21Waf1 plays an important role in the protection against apoptotic stimuli.19 However, the results presented here do not support a role for this cyclin-dependent kinase inhibitor in macrophage growth arrest, which resembles the scenario in other cellular systems.50 Moreover, prolonged ERK activation in the macrophage cell line Raw264.7 does not result in induction of p21Waf1,41 which reflects, once again, that the signaling events that follow sustained ERK activity are specific to each cellular system. Several other studies have related ERK signal duration to stabilization of c-fos, which contains a docking site termed FXFP domain.51,52
Inhibition of the expression of c-myc by IFN-γ occurs through Stat-1–dependent and –independent pathways.53 A γ-activated sequence element in the c-myc promoter is necessary but not sufficient to suppress c-myc expression. It has also been described that IFN-γ regulates the expression of the small protein Mad154 or the protein p20255 that modulates the binding capacity of c-Myc to the promoter of its target genes. Inhibition of c-myc expression and the extension of MAPK phosphorylation may act coordinately to mediate the antiproliferative actions of IFN-γ in macrophages. Our siRNA experiments suggest that extension of MAPK activity does not mimic the inhibitory effects of IFN-γ on c-myc expression (data not shown), thereby indicating that these 2 events are independent.
Whether the inhibition of proliferation depends on the acquisition of a more differentiated phenotype in bone marrow–derived macrophages stimulated with IFN-γ is not clear. We definitely know that during their activation, macrophages lose their capability to proliferate. Although it may be logical to think that this is a consequence of an increased state of differentiation, as it is the case in other cell types, we have to also keep in mind the possibility that growth arrest may take place well before the acquisition of the differentiated phenotype. Indeed, in our hands, the stop of macrophage DNA synthesis by IFN-γ is observed before the cells really acquire significant levels of their markers of activation (as measured by increased levels of expression of surface molecules that participate in antigen presentation and phagocytosis, and by release of proinflammatory mediators). The fact that prolongation of the activity of the ERK pathway correlates with inhibited proliferation, but the ERK pathway per se does not participate in the regulation of many of the genes that configure an activated phenotype in response to IFN-γ,38 makes us think that the inhibition of macrophage proliferation is a direct effect of prolongation of ERK activity rather than an indirect consequence of the acquisition of a more differentiated phenotype.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Philip Leder (Harvard Medical School, HHMI) and Dr Gabriel Gil (Institut Municipal d'Investigacions Biomèdiques, Barcelona, Spain) for the p21Waf1 knockout mice; Dr Robert D. Schreiber and Dr Maria Pilar Gil (Washington University School of Medicine) for providing bone marrow from Stat-1 knockout mice; Dr Rodrigo Bravo (Bristol-Myers Squibb Pharmaceutical Research Institute) for plasmids that harbor full-length cDNAs for Mkp-1 and for the Mkp-1 knockout mice; Dr Joan Massague (Sloan-Kettering Institute, New York, NY) for the plasmid containing full-length p21Waf1; Dr R. A. Flavell (Yale University School of Medicine) for the Jnk-1 knockout mice; Dr Kinya Otsu (Osaka University Graduate School of Medicine, Osaka, Japan) for the p38α flox/flox mice used in generating myeloid-specific p38α knockout mice; and Dr Jin Mo Park (Cutaneous Biology Research Center, Massachusetts General Hospital, Charlestown, MA). We also thank Cristina Vila for support with the Mkp-1 knockout mice and Tanya Yates for editorial assistance.
This work was supported by grants from the Comision Interministerial de Ciencia y Tecnologia (BFU2004-05725/BMC) to A.C. and from the European Commission (International Reintegration Grant) to A.F.V. (IRG-31137). A.F.V. was a Ramon y Cajal investigator. L.A. was supported by a predoctoral fellowship (Formacio Personal Investigador, Generalitat de Catalunya).
Authorship
Contribution: A.F.V. designed research, performed research, collected data, analyzed data, and wrote the paper; L.A., E.S.-T., M.C., C. Casals, and J.X. performed research and analyzed data; C. Caelles provided vital reagents; J.L. designed research and wrote the paper; and A.C. supervised research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Antonio Celada, Macrophage Biology Group, Institute for Research in Biomedicine, Barcelona Science Park, Josep Samitier 1-5, E-08028 Barcelona, Spain; e-mail: acelada@ub.edu.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal