Abstract
Dietary flavonoids have many health-promoting actions, including anticancer activity via proteasome inhibition. Bor-tezomib is a dipeptide boronate proteasome inhibitor that has activity in the treatment of multiple myeloma but is not effective in chronic lymphocytic leukemia (CLL). Although CLL cells are sensitive in vitro to bortezomib-induced apoptosis when cultured in medium, the killing activity was blocked when cultured in 50% fresh autologous plasma. Dietary flavonoids, quercetin and myricetin, which are abundant in plasma, inhibited bortezomib-induced apoptosis of primary CLL and malignant B-cell lines in a dose-dependent manner. This inhibitory effect was associated with chemical reactions between quercetin and the boronic acid group, -RB(OH)2, in bortezomib. The addition of boric acid diminished the inhibitory effect of both quercetin and plasma on bortezomib-induced apoptosis. The protective effect was also reduced when myeloma cell lines, but not B-cell lines, were preincubated with quercetin, indicating a direct effect of quercetin on myeloma cells. At high doses, quercetin itself induced tumor cell death. These data indicate that dietary flavonoids limit the efficacy of bortezomib, whereas supplemental inorganic boric acid is able to reverse this. The complex interactions between quercetin, tumor cells, and bortezomib mean caution is required when giving dietary advice to patients.
Introduction
Bortezomib (Velcade) is a first-in-class proteasome inhibitor developed specifically for use as an antineoplastic agent. It is the most potent antineoplastic agent for the treatment of relapsed, refractory multiple myeloma.1 A total of 73% of patients with myeloma responded to treatment with bortezomib combined with pegylated liposomal doxorubicin.2 However, only 4 of 15 patients with acute leukemia showed a decrease in blast count.3
The efficacy of bortezomib in chronic lymphocytic leukemia (CLL) appears to be related to IgV(H) and BCL-6 mutational status,4 down-regulation of CD23, and inactivation of Notch 2.5 However, we have previously shown that all primary CLL cells were sensitive to bortezomib in vitro. Proteasome inhibition increased Bax protein accumulation and Bax activation in CLL cells. Bortezomib also increased the sensitivity of CLL cells to TNF-related apoptosis-inducing ligand-induced apoptosis.6 Although the effectiveness of bortezomib killing of CLL cells in vitro has also been reported by other groups,4,5 it did not display substantial antitumor activity in patients with CLL.7,8 The basis of this differential activity of bortezomib on CLL cells in vivo and in vitro is unknown. Autologous plasma is capable of maintaining survival of CLL cells in vitro and conferring resistance to chemo-radiotherapy,9 and albumin in plasma plays an important role in this survival mechanism.10 We sought to identify factors in the blood, which could prevent bortezomib-mediated killing of leukemic cells in the circulation.
Quercetin is one of the most abundant flavonoids in the human diet and is a potent antioxidant.11 Quercetin, abundant in human plasma, noncovalently binds to serum albumin.12,13 It contributes to the prevention of human diseases by promoting relaxation of cardiovascular smooth muscle and protects low-density lipoprotein from oxidation.14 Recent studies found that quercetin can induce apoptosis by inhibiting proteasome activation,15 causing G2/M-phase arrest16 and increasing p21 expression.17 Other dietary flavonoids, such as myricetin, kaempferol, and apigenin, also have similar functions to quercetin with respect to proteasome inhibition and apoptosis induction.15
In this study, we demonstrate that human plasma affects killing by bortezomib and that the dietary flavonoids, especially quercetin, inhibit bortezomib-induced apoptosis in malignant B-cell lines and primary CLL cells. This inhibitory activity of quercetin was associated with complex formation with bortezomib. However, in myeloma cell lines, quercetin also had an additional direct effect on the cells to increase their sensitivity to bortezomib. Overall, dietary flavonoids show competing interactions with tumor cells and bortezomib, which are dose- and tumor type–dependent.
Methods
Materials
Bortezomib was provided by Millennium Pharmaceuticals (Cambridge, MA) as the boronate ester with D-mannitol. Human albumin solution 20% was from Baxter (Norfolk, United Kingdom). MG-132 and Ac-IETD-AFC were obtained from Biomol International (Exeter, United Kingdom). Quercetin, myricetin, apigenin, kaempferol, lactacystin, propidium iodide (PI), 4,6 diamidino-2-phenylindole (DAPI), the monoclonal anti–β-actin antibody, Ac-LEHD-AFC, 7-amino-4-trifluoromethylcoumarin (AFC), N-acetyl-L-cysteine (NAC), boric acid, and all other chemicals were purchased from Sigma Chemical (Poole, United Kingdom). Ac-DEVD-AFC and MG-262 were purchased from Calbiochem (Nottingham, United Kingdom). Annexin V–fluorescein isothiocyanate (FITC) kit, anticytochrome c clone 6H2.B4 antibody, anti-Bax (6A7) monoclonal antibody, and anti-Bax antibody clone 3 were purchased from BD Biosciences (San Jose, CA). The monoclonal anti-Bax 2D2 antibody (clone YTH-2D2) was purchased from R&D Systems Europe (Abingdon, United Kingdom). MitoTracker red CMXRos, tetramethylrhodamine, methyl ester (TMRM), dihydroethidium (HE), 2′,7′-dichlorodihydrofluorescein diacetate (H2-DCFDA), and M-450 rat anti–mouse IgG1 Dynabeads were from Invitrogen (Carlsbad, CA).
Cell culture
The Epstein-Barr virus (EBV)–transformed human B-cell line HRC57 (provided by Cancer Research UK Cancer Cell Services), the human diffuse large B-cell lymphoma (DLBCL) cell line DoHH2, and the human myeloma cell lines RPMI-8226 and U266, were used in this study. Cells were cultured in RPMI 1640 medium (Sigma Chemical) supplemented with 10% heat-inactivated fetal calf serum, 25 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, 2.0 mM l-glutamine, pH 7.4, penicillin (100 U/mL), streptomycin (100 μg/mL) at 37°C in a 5% CO2 humidified incubator. Peripheral blood was collected after written informed consent was obtained in accordance with the Declaration of Helsinki from patients with CLL and institutional review board approval was received from the East London & City Research Ethics Committee. The mononuclear cells were isolated by density centrifugation over Ficoll.
Apoptosis assays
Apoptosis was determined by both annexin V and DNA content assays. The annexin V assay was performed according to the protocol of the annexin V–FITC kit. Whole cells in the binding buffer suspension were stained with 5 μL annexin V–FITC and 10 μL PI for 15 minutes at the room temperature in the dark. Annexin V–FITC and PI fluorescence were measured by flow cytometry (FACScan; BD Biosciences) on the FL1-H and FL3-H channels, respectively. Annexin V–positive cells (both PI-negative and -positive) were defined as apoptotic. DNA content was measured by flow cytometry. Briefly, cells were permeabilized in 70% ethanol for 40 minutes at 4°C. After washing twice with phosphate-buffered saline (PBS), cells were incubated at 37°C with 25 μg/mL RNase A and 50 μg/mL PI for 1 hour. PI fluorescence of nuclei was measured by flow cytometry. Data analysis was carried out on cells gated on an FL2-area channel versus FL2-width channel display to exclude cell debris and clumped cells. DNA content distribution (PI fluorescence) was analyzed on the FL2-area histogram, and cells with a DNA content less than G0/G1 (hypodiploid) were defined as apoptotic cells.18
Fluorogenic assay for quercetin-albumin binding
The fluorescence elicited by albumin/quercetin interactions was determined in black microtiter plates (Nalge Nunc International, Rochester, NY) at a wavelength of 528 plus or minus 20 nm with an excitation wavelength at 485 plus or minus 20 nm using a Bio-Tek Synergy HT Multi-Detection Microplate Reader (Bio-Tek Instruments, Winooski, VT). The human serum albumin (Baxter) concentration was fixed at 25 μM (0.167%, wt/vol) and quercetin concentration varied between 2 and 20 μM.19 Quercetin and albumin were added to PBS up to 100 μL total volume and incubated for 10 minutes at room temperature.
Caspase activity assay
Cells were lysed and proteins (50 μg) were diluted with fluorogenic assay buffer (20 mM PIPES-KOH, pH 7.4, 10 mM dithiothreitol, 10% sucrose, 1.0 mM ethylenediaminetetraacetic acid, and 0.1% 3-cholamidopropyl)-dimethylammonio-1-propanesulfonate [CHAPS]) to 90 μL. The reaction was initiated by the addition of 5 μL 400 μM (final concentration, 20 μM) fluorescent substrate, Ac-DEVD-AFC for caspase-3, Ac-IETD-AFC for caspase-8, or Ac-LEHD-AFC for caspase-9. After incubation at 37°C for 15 minutes, the reaction was stopped by the addition of 50 μL 1% sodium acetate trihydrate in 175 mM acetic acid and cooling on ice.18 The fluorescence at 400/505 nm for AFC release by the caspase cleavage was measured using a Bio-Tek Synergy HT Multi-Detection Microplate Reader (Bio-Tek Instruments). Measurements were calibrated against a standard linear regression curve of AFC. Caspase activity was defined as μM AFC release per milligram protein per minute (μM/hr per mg of protein).
Measurement of mitochondrial membrane potential (ΔΨm) and reactive oxidative species by flow cytometry
To measure ΔΨm, cells were stained with 20 nM TMRM for 30 minutes at 37°C. The fluorescent intensities were measured in the FL3-H channel with flow cytometer. The intracellular accumulation of reactive oxidative species (ROS) was determined using the fluorescent probe HE to measure O2·̄ at FL3-H and H2-DCFDA for H2O2 at FL1-H. After the treatment, cells (105/mL) were incubated with 40 μM HE and 5 μM H2-DCFDA for 15 minutes at 37°C. ROS generation was then assessed by flow cytometry.20
Immunostaining of Bax translocation and cytochrome c release
After treatment with bortezomib with or without quercetin, the intact cells were first labeled with the mitochondrion-specific dye, MitoTracker red CMXRos. Cells in culture medium were incubated with MitoTracker (100 nM) at 37°C for 15 minutes. After washing, cells were fixed/permeabilized on slides. To detect Bax translocation, cells on the slides were incubated with the anti-Bax antibody clone 3 at 1:50 dilution for 1 hour. To determine cytochrome c release from mitochondria, cells on the slides were incubated with the anti-cytochrome c antibody 6H2.B4 (1:400 dilution) for 2 hours. They were then incubated with FITC-conjugated anti–mouse secondary antibody (Sigma Chemical) at a 1:20 dilution. Slides were washed with PBS, air dried at 4°C in the dark, and stained with DAPI before viewing under a Zeiss Axioskop fluorescence microscope (Carl Zeiss, Jena, Germany).18,21,22
Immunoprecipitation and Western blotting
Cells were washed with PBS and lysed with CHAPS buffer (10 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, pH 7.4, 150 mM NaCl, 1% CHAPS, 1 mM dithiothreitol, 0.1 mM phenylmethylsulfonyl fluoride, 3 μg/mL aprotinin, 25 μg/mL leupeptin, and 25 μg/mL pep-stain); 1 μg anti-Bax (6A7) monoclonal antibody was preincubated with 20 μL Dynabeads (M-450 rat anti–mouse IgG1) at 4°C on the rotor for 3 hours. The cell lysates were normalized for protein content, and 1000 μg of total protein in 300 μL CHAPS lysis buffer was then added to the immunoprecipitation tube containing Bax antibody (6A7)–loaded Dynabeads and incubated at 4°C on the rotor overnight. After rinsing 4 times with CHAPS buffer, beads were collected with a Dynal Magnetic Particle Concentrator (Dynal Biotech, Oslo, Norway). Conformationally changed Bax protein was eluted with 25 μL sample buffer for Western blotting by the monoclonal anti-Bax antibody, clone 2D2.6,23
Raman spectroscopy for determining chemical reactions between quercetin and bortezomib, MG-262, boric acid, or MG-132
Raman spectra of the samples were recorded using a Raman spectrometer, Almega (Thermo Electron, Waltham, MA). The laser used in this study is a 785-nm near infrared high power diode laser, with a laser power of 300 mW, multimode continuous wave beam, and power stability of less than 5%. The laser power can be changed from 15% to 100%, according to the requirements of experiments; and in this study, a laser power of 100 mW was used for the analysis. The spectrometer has a CCD Detector of Andor DV420 open electrode, with a working temperature of −50°C. In this experiment, all of the spectra were collected in quartz tubes within the range of 100 to 3430 cm, using a pinhole of 100 μm. A total of 256 exposures with exposure time of 2 seconds for each exposure were used to obtain a spectrum.
Results
Human plasma counteracts the apoptosis-inducing effect of bortezomib in CLL cells
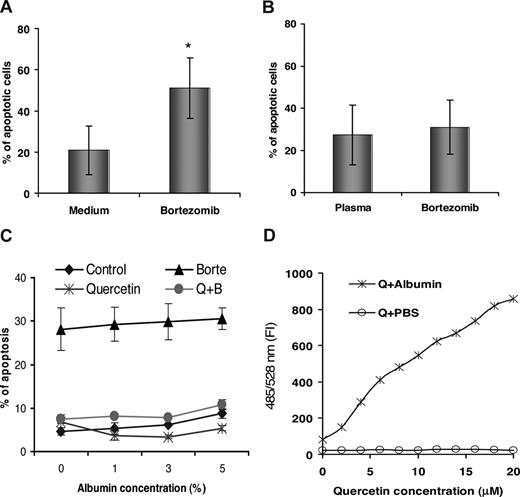
The proapoptotic activity of bortezomib on fresh primary CLL cells was tested in both routine culture medium and 50% autologous plasma. CLL cells in the culture medium were sensitive to 20 nM bortezomib-induced apoptosis (Figure 1A). However, the proapoptotic activity of bortezomib was significantly inhibited when CLL cells were cultured in 50% fresh autologous plasma (Figure 1B). Human serum albumin (HSA) is the most abundant protein in the plasma, with a reference range of 3% to 5% in the blood. However, HSA, at concentrations between 1% and 5%, did not block 20 nM bortezomib-induced apoptosis in the DoHH2 cell line (Figure 1C). Quercetin is the main dietary flavonoid in blood, with antioxidative and proteasome inhibition activities, but can itself induce apoptosis in leukemic Jurkat T cells.15 It shows strong binding to purified albumin19 producing a dose-dependent fluorescence (Figure 1D). The peak concentration of quercetin in the serum is 0.8 μM after the onion meal for 25 hours.24 Therefore, the quercetin concentration in the purified and stored albumin is negligible. Quercetin, at 20 μM, completely inhibited 20 nM bortezomib-induced apoptosis and binding to HSA did not diminish its inhibitory effect (Figure 1C).
A comparison of bortezomib-induced apoptosis in culture medium versus autologous plasma. CLL cells were cultured in the culture medium (A, n = 8) or 50% fresh autologous plasma (B, n = 5) and incubated with or without 20 nM of bortezomib for 12 hours. Apoptotic cell death was measured by the annexin V assay with flow cytometry. *Statistically significant difference (P < .001) between treated and control as analyzed by the t test. (C) Effect of HSA or quercetin on bortezomib-induced apoptosis. DoHH2 cells were preincubated with HSA or HSA plus quercetin for 1 hour at 37°C and then treated with 20 nM bortezomib (Borte) for 16 hours. Control indicates HSA alone; Quercetin, HSA plus quercetin; Borte, HSA plus bortezomib; Q + B, quercetin plus bortezomib. (D) Dose-dependent fluorescence production was determined after quercetin was mixed with HSA. FI indicates fluorescent intensity. Data are mean plus or minus SD from 3 separate experiments. Error bars represent SD.
A comparison of bortezomib-induced apoptosis in culture medium versus autologous plasma. CLL cells were cultured in the culture medium (A, n = 8) or 50% fresh autologous plasma (B, n = 5) and incubated with or without 20 nM of bortezomib for 12 hours. Apoptotic cell death was measured by the annexin V assay with flow cytometry. *Statistically significant difference (P < .001) between treated and control as analyzed by the t test. (C) Effect of HSA or quercetin on bortezomib-induced apoptosis. DoHH2 cells were preincubated with HSA or HSA plus quercetin for 1 hour at 37°C and then treated with 20 nM bortezomib (Borte) for 16 hours. Control indicates HSA alone; Quercetin, HSA plus quercetin; Borte, HSA plus bortezomib; Q + B, quercetin plus bortezomib. (D) Dose-dependent fluorescence production was determined after quercetin was mixed with HSA. FI indicates fluorescent intensity. Data are mean plus or minus SD from 3 separate experiments. Error bars represent SD.
Quercetin blocks bortezomib-induced apoptosis in malignant B cells
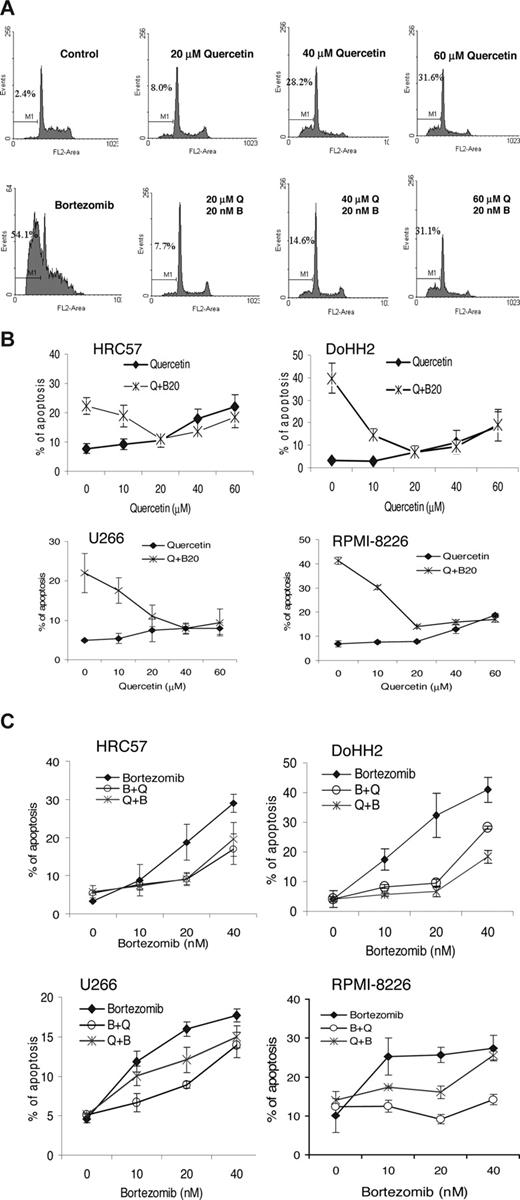
We sought to determine the role of quercetin in bortezomib-induced apoptosis in both CLL and the malignant B-cell lines. Cells were pretreated with 20 to 60 μM quercetin for 1 hour and then incubated with or without 20 nM bortezomib for 16 hours. Apoptotic cells were measured by the DNA content assay using a flow cytometer. Figure 2A is an example showing the dose-dependent inhibitory effect of quercetin on bortezomib-induced apoptosis in primary CLL cells. Quercetin induced apoptosis when the concentration was higher than 20 μM, as shown by the increased sub-G0/G1 cell populations. Interestingly, bortezomib-induced apoptosis was completely inhibited by 20 μM quercetin (Figure 2A). Similar results were seen in both the EBV-transformed HRC57 cell line and the DLBCL DoHH2 cell line (Figure 2B). Quercetin also had the same inhibitory effect on bortezomib-induced apoptosis in 2 myeloma cell lines, U266 and RPMI-8226 (Figure 2B). However, the myeloma cell lines required higher levels of quercetin (40 μM) for maximal inhibition of bortezomib-induced apoptosis, compared with 20 μM for primary CLL, HRC57, and DoHH2 cells. In addition, myeloma cell lines were more susceptible to quercetin-mediated G2/M arrest compared with the B-lymphoma cell lines (data not shown).
Inhibitory effect of quercetin on bortezomib-induced apoptosis. (A) Fresh CLL cells (106) were pretreated with quercetin (20-60 μM) for 1 hour and then incubated with (bottom panel) or without (top panel) bortezomib (20 nM) for 12 hours. Apoptotic cell death was measured by the DNA content assay using flow cytometry. The percentage of cells in the gate M1 indicates apoptotic or sub-G0/G1 cells. The flow cytometric profiles shown are one typical experiment from more than 10 CLL samples. (B) The inhibitory effect of different concentrations of quercetin on bortezomib (20 nM)–induced apoptosis on the EBV transformed HRC57, DLBCL DoHH2, and myeloma cell lines U266 and RPMI-8226. (C) The inhibitory effect of quercetin (20 μM) on different concentrations of bortezomib. Q indicates quercetin; B, bortezomib; Q+B, quercetin added 1 hour before bortezomib; B + Q, quercetin and bortezomib added together. *Statistically significant inhibitory effect of quercetin on bortezomib as analyzed by ANOVA (P < .001). Data are means plus or minus SD from 3 separate experiments.
Inhibitory effect of quercetin on bortezomib-induced apoptosis. (A) Fresh CLL cells (106) were pretreated with quercetin (20-60 μM) for 1 hour and then incubated with (bottom panel) or without (top panel) bortezomib (20 nM) for 12 hours. Apoptotic cell death was measured by the DNA content assay using flow cytometry. The percentage of cells in the gate M1 indicates apoptotic or sub-G0/G1 cells. The flow cytometric profiles shown are one typical experiment from more than 10 CLL samples. (B) The inhibitory effect of different concentrations of quercetin on bortezomib (20 nM)–induced apoptosis on the EBV transformed HRC57, DLBCL DoHH2, and myeloma cell lines U266 and RPMI-8226. (C) The inhibitory effect of quercetin (20 μM) on different concentrations of bortezomib. Q indicates quercetin; B, bortezomib; Q+B, quercetin added 1 hour before bortezomib; B + Q, quercetin and bortezomib added together. *Statistically significant inhibitory effect of quercetin on bortezomib as analyzed by ANOVA (P < .001). Data are means plus or minus SD from 3 separate experiments.
The effect of adding quercetin 20 μM (at which concentration it was not apoptotic to cells) 1 hour before, or simultaneously with, bortezomib is shown in Figure 2C. Bortezomib-induced apoptosis showed a dose-response in all 4 cell lines studied, with significant apoptosis seen at the lowest dose tested (10 nM). Quercetin significantly (P < .001, analysis of variance [ANOVA]) blocked bortezomib-induced apoptosis in all 4 cell lines when both compounds were added simultaneously. When quercetin was added for 1 hour before the addition of bortezomib, the same inhibitory effect on bortezomib-induced killing was seen in HRC57 and DoHH2. However, in both myeloma cells lines, preincubation with quercetin led to less inhibition on bortezomib-induced killing, suggesting that myeloma cells are more active in their uptake and use of quercetin, thereby reducing the chemical binding between quercetin and bortezomib.
Quercetin inhibits bortezomib-induced Bax activation and cytochrome c release
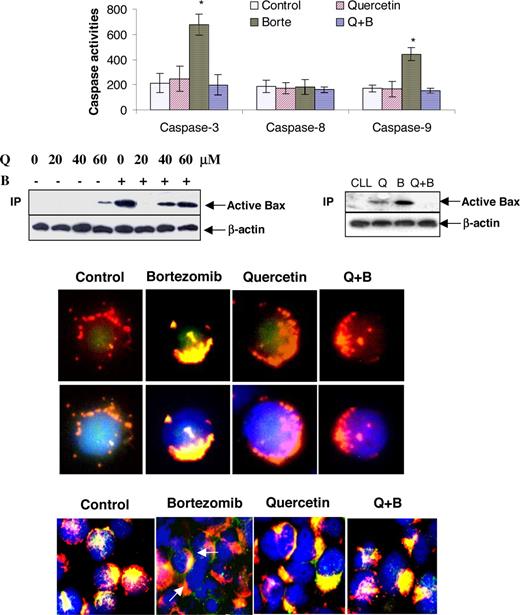
The pathway of bortezomib-induced apoptosis was investigated by looking at caspase activation. Both caspase-9 and caspase-3, but not caspase-8, were activated after DoHH2 cells were treated with bortezomib. The activation of caspases was prevented by quercetin (Figure 3A). This indicates that bortezomib-mediated apoptosis is via the mitochondrial/caspase-9 pathway. Previously, we have shown that bortezomib-induced apoptosis was associated with Bax activation, demonstrated by Bax protein accumulation, translocation to mitochondria, and a conformational change in Bax.6 Quercetin was also found to up-regulate Bax in tumor cells.25 We tested whether the inhibitory effect of quercetin on bortezomib is at the mitochondrial level. DoHH2 cells were pretreated with different concentrations of quercetin (10-60 μM) and then incubated with or without 20 nM bortezomib. At high concentrations, quercetin (60 μM) alone induced a conformational change in Bax. Bortezomib-induced Bax conformational change was completely inhibited by 20 μM quercetin. The inhibitory effect of quercetin on bortezomib-induced Bax conformational change was also confirmed in primary CLL cells (Figure 3B). Bax translocation was tested in primary CLL cells by immunofluorescence staining. The active Bax was only detected in the bortezomib-treated cells, as shown by the yellow-orange color produced by overlapping green-stained active Bax and red-stained mitochondria. This was not seen in the control, quercetin treated, and quercetin/bortezomib-cotreated CLL cells (Figure 3C). These results indicate that bortezomib-induced Bax conformational change and translocation can be blocked by quercetin.
Quercetin blocks bortezomib-induced Bax activation and cytochrome c release. Cells were pretreated with 20 μM quercetin and then incubated with or without 20 nM bortezomib for 12 hours. (A) Caspase activation. DoHH2 cells were used for caspase activity assay, as described in “Caspase activity assay.” *Statistically significantly increased activity of caspase as analyzed by t test (P < .001). Data are means plus or minus SD from 3 separate experiments. (B) Bax conformational change was determined in DoHH2 (left panel) and primary CLL cells (right panel) by immunoprecipitation. DoHH2 cells were treated with different concentrations of quercetin, with or without 20 nM bortezomib. The clone 6A7 anti-Bax antibody was used for the immunoprecipitation, and the clone 2D2 Bax antibody was used for Western blotting. The active Bax indicates the conformationally changed form of Bax. Western blotting for β-actin represents an equal loading of proteins. Bax translocation (C) and cytochrome release from mitochondria (D) were assessed by immunostaining in CLL cells. Mitochondria were stained with Mito-Tracker red CMRos (red). Bax was stained with the conformation-specific clone 3 Bax antibody (C), and cytochrome c was stained with cytochrome c antibody clone 6H2.B4 (D). Slides were then costained with FITC-conjugated antimouse antibody (green). Finally, the nucleus was stained with DAPI (blue). The pictures in the top panel (C) show that cells were stained with Mito-Tracker (red) and Bax, and those in the bottom panel present cells with merged DAPI staining. Arrows in panel D represent released cytochrome c in the apoptotic cells. Q indicates quercetin; B, bortezomib.
Quercetin blocks bortezomib-induced Bax activation and cytochrome c release. Cells were pretreated with 20 μM quercetin and then incubated with or without 20 nM bortezomib for 12 hours. (A) Caspase activation. DoHH2 cells were used for caspase activity assay, as described in “Caspase activity assay.” *Statistically significantly increased activity of caspase as analyzed by t test (P < .001). Data are means plus or minus SD from 3 separate experiments. (B) Bax conformational change was determined in DoHH2 (left panel) and primary CLL cells (right panel) by immunoprecipitation. DoHH2 cells were treated with different concentrations of quercetin, with or without 20 nM bortezomib. The clone 6A7 anti-Bax antibody was used for the immunoprecipitation, and the clone 2D2 Bax antibody was used for Western blotting. The active Bax indicates the conformationally changed form of Bax. Western blotting for β-actin represents an equal loading of proteins. Bax translocation (C) and cytochrome release from mitochondria (D) were assessed by immunostaining in CLL cells. Mitochondria were stained with Mito-Tracker red CMRos (red). Bax was stained with the conformation-specific clone 3 Bax antibody (C), and cytochrome c was stained with cytochrome c antibody clone 6H2.B4 (D). Slides were then costained with FITC-conjugated antimouse antibody (green). Finally, the nucleus was stained with DAPI (blue). The pictures in the top panel (C) show that cells were stained with Mito-Tracker (red) and Bax, and those in the bottom panel present cells with merged DAPI staining. Arrows in panel D represent released cytochrome c in the apoptotic cells. Q indicates quercetin; B, bortezomib.
Cytochrome c release from mitochondria is a crucial event during Bax-dependent apoptosis. Therefore, the effect of bortezomib-induced Bax activation on the localization of cytochrome c was investigated by immunofluorescence staining. In the control, quercetin, or quercetin/bortezomib-treated CLL cells, cytochrome c was detected in the mitochondria, shown by the yellow-orange color produced by overlapping green-stained cytochrome c and red-stained mitochondria. Cytochrome c release from mitochondria was seen in bortezomib-treated cells, as the green cytochrome c was separated from the red mitochondria (Figure 3D). These results confirmed that bortezomib-induced Bax activation was accompanied by cytochrome c release and quercetin can block these apoptotic events.
The inhibitory effect of quercetin on bortezomib is unrelated to its antioxidative property
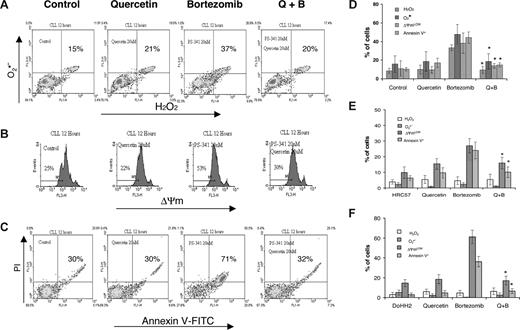
To test whether the inhibitory effect of quercetin on bortezomib is the result of its antioxidative function, ROS, ΔΨm, and apoptosis were measured simultaneously by flow cytometry. Bortezomib induced ROS generation, including H2O2 and O2·̄, ΔΨm reduction, and apoptosis in CLL cells. Quercetin alone (at 20 μM) did not induce apoptotic events, but it completely inhibited bortezomib-induced ROS production, decreases in ΔΨm, and apoptosis (Figure 4A-D). This could suggest that the inhibitory effect of quercetin on bortezomib may be caused by blocking ROS generation. However, bortezomib-induced apoptosis in the transformed HRC57 and the malignant DoHH2 B-cell lines was not associated with ROS production. Bortezomib induced apoptosis and ΔΨm reduction, but not ROS production, in these 2 cell lines. However, quercetin was still able to inhibit bortezomib-induced ΔΨm collapse and apoptosis (Figure 4E,F).
Effect of quercetin on bortezomib-induced oxidative and apoptotic events. Fresh primary CLL cells (106/mL) were pretreated with 20 μM quercetin for 1 hour and then incubated with 20 nM bortezmib for 12 hours. Cells were washed twice with HBSS. For the measurement of ROS (A), cells were stained with both 40 μM HE (O2·̄) and 5 μM H2DCF-DA (H2O2) at 37°C for 30 minutes. ROS production was measured by flow cytometry in the FL1-H and FL3-H channels. For the determination of ΔΨm (B), cells were incubated with 20 nM TMRM at 37°C for 15 minutes, and ΔΨm was measured in the FL3-H channel. For the annexin V assay (C), cells were resuspended in the binding buffer and then stained with annexin V–FITC and PI at room temperature for 15 minutes. Apoptotic cells were analyzed in both the FL1-H and FL3-H channels. Annexin V+/PI− and annexin V+/PI+ cells were counted as apoptotic. The number in each chart indicates the percentage of cells that underwent apoptosis. (D-F) Statistical data from 5 CLL patients (D), the HRC57 cell line (E), and the DoHH2 cell line (F). Statistical significance of the inhibition of quercetin on bortezomib was analyzed by the t test. *P < .001.
Effect of quercetin on bortezomib-induced oxidative and apoptotic events. Fresh primary CLL cells (106/mL) were pretreated with 20 μM quercetin for 1 hour and then incubated with 20 nM bortezmib for 12 hours. Cells were washed twice with HBSS. For the measurement of ROS (A), cells were stained with both 40 μM HE (O2·̄) and 5 μM H2DCF-DA (H2O2) at 37°C for 30 minutes. ROS production was measured by flow cytometry in the FL1-H and FL3-H channels. For the determination of ΔΨm (B), cells were incubated with 20 nM TMRM at 37°C for 15 minutes, and ΔΨm was measured in the FL3-H channel. For the annexin V assay (C), cells were resuspended in the binding buffer and then stained with annexin V–FITC and PI at room temperature for 15 minutes. Apoptotic cells were analyzed in both the FL1-H and FL3-H channels. Annexin V+/PI− and annexin V+/PI+ cells were counted as apoptotic. The number in each chart indicates the percentage of cells that underwent apoptosis. (D-F) Statistical data from 5 CLL patients (D), the HRC57 cell line (E), and the DoHH2 cell line (F). Statistical significance of the inhibition of quercetin on bortezomib was analyzed by the t test. *P < .001.
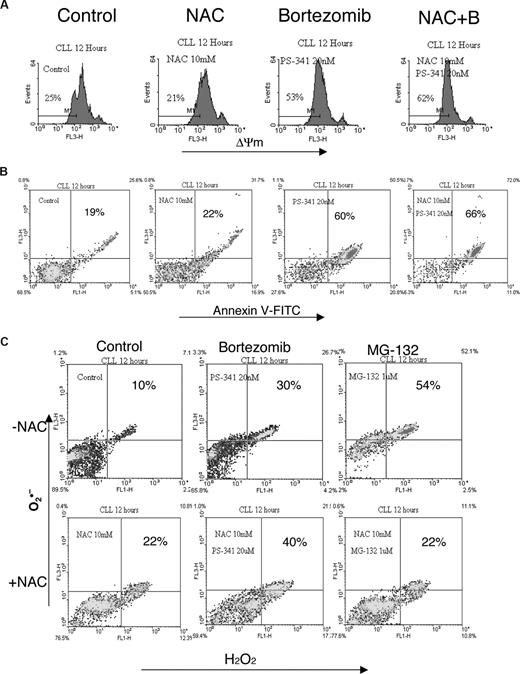
ROS scavengers, but not caspase inhibitors, block all bortezomib-induced apoptosis in mantle cell lymphoma.26 We therefore tested whether the ROS scavenger NAC could inhibit bortezomib-induced apoptosis in the DoHH2 cell line and primary CLL cells. Cells were pretreated with 10 mM NAC26 for 2 hours and then treated with 20 nM bortezomib for 12 hours. NAC did not show any inhibitory effect on bortezomib-induced ROS generation, ΔΨm loss, and apoptosis. Indeed, the hallmarks of bortezomib-mediated apoptosis were enhanced in the primary CLL cells (Figure 5). Further increases in NAC were toxic to CLL cells (data not shown). Nevertheless, MG-132-induced ROS production was inhibited by 10 mM NAC (Figure 5C); and accordingly, apoptosis was partly inhibited by NAC (data not shown). In contrast, quercetin did not show inhibitory effects on MG-132–induced apoptosis in CLL cells (data not shown), suggesting that the inhibitory effect of quercetin on bortezomib may not be at the proteasome level.
Effect of NAC on bortezomib-induced apoptosis. CLL cells were pretreated with 10 mM NAC for 2 hours and then incubated with 20 nM bortezmib or 1 μM MG-132 for 12 hours. ΔΨm (A), annexin V (B), and ROS (C) were determined by flow cytometry. The conditions for the experiment were similar to those described in Figure 4.
Effect of NAC on bortezomib-induced apoptosis. CLL cells were pretreated with 10 mM NAC for 2 hours and then incubated with 20 nM bortezmib or 1 μM MG-132 for 12 hours. ΔΨm (A), annexin V (B), and ROS (C) were determined by flow cytometry. The conditions for the experiment were similar to those described in Figure 4.
The interaction between quercetin and bortezomib, MG-262, or boric acid is associated with their chemical structures
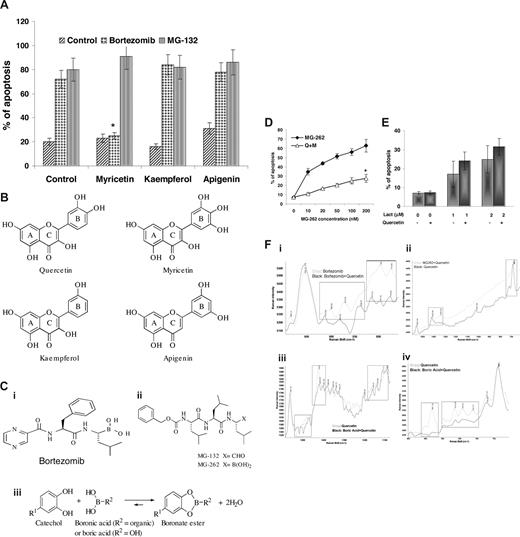
We tested whether other dietary flavonoids also have inhibitory effects on bortezomib. Quercetin, myricetin, apigenin, and kaempferol are all dietary flavonoids that have proteasome inhibitory and antioxidative functions.15,27–29 The inhibitory effect of these flavonoids on bortezomib-induced apoptosis was tested in the DoHH2 cell line. The maximum nonlethal concentrations of these flavonoids were used for these experiments. According to the published data,15 these concentrations were all higher than their IC50 for inhibition of the 26S proteasome. Interestingly, only myricetin showed a significant inhibitory effect on bortezomib-mediated apoptosis. Similar to quercetin, none of these flavonoids had blocking effects on MG-132-induced apoptosis (Figure 6A).
Potential complex formation of bortezomib with catechols. (A) Effect of flavonoids on bortezomib-induced apoptosis. DoHH2 cells were pretreated with 30 μM myricetin, 20 μM kaempferol, or 5 μM apigenin individually for 1 hour and then incubated with or without 20 nM bortezomib or 1 μM MG-132 for 12 hours. Apoptosis was measured by the annexin V assay. Data shown (mean ± SD) were from 3 separate experiments. Inhibition of myricetin on bortezomib-induced apoptosis was statistically analyzed by the t test. *P < .001. (B) Chemical structures of quercetin, myricetin, kaempferol, and apigenin. The B-rings of quercetin and myricetin are substituted catechols. (C) Chemical structure of bortezomib (Ci), MG-262 or MG-132 (Cii), and complex formation of catechol derivatives with boronic acids to form boronate esters (Ciii). (D) Inhibitory effect quercetin on MG-262–induced apoptosis. DoHH2 cells were preincubated with or without 20 μM quercetin for 1 hour and then treated with MG-262 for 12 hours. *Significant inhibition as analyzed by ANOVA (P < .001). (E) Effect of quercetin on lactacystin-induced apoptosis. DoHH2 cells were preincubated with 20 μM quercetin for 1 hour and then treated with lactacystin for 12 hours. Apoptosis was determined by DNA content assay with flow cytometry. Data shown (mean ± SD) were from 3 separate experiments. (F) Detection of chemical reactions by Raman spectroscopy. Chemical reactions between quercetin and bortezomib (Fi), quercetin and MG-262 (Fii), and quercetin and boric acid (Fiii,Fiv). Boxes indicate significantly altered spectra.
Potential complex formation of bortezomib with catechols. (A) Effect of flavonoids on bortezomib-induced apoptosis. DoHH2 cells were pretreated with 30 μM myricetin, 20 μM kaempferol, or 5 μM apigenin individually for 1 hour and then incubated with or without 20 nM bortezomib or 1 μM MG-132 for 12 hours. Apoptosis was measured by the annexin V assay. Data shown (mean ± SD) were from 3 separate experiments. Inhibition of myricetin on bortezomib-induced apoptosis was statistically analyzed by the t test. *P < .001. (B) Chemical structures of quercetin, myricetin, kaempferol, and apigenin. The B-rings of quercetin and myricetin are substituted catechols. (C) Chemical structure of bortezomib (Ci), MG-262 or MG-132 (Cii), and complex formation of catechol derivatives with boronic acids to form boronate esters (Ciii). (D) Inhibitory effect quercetin on MG-262–induced apoptosis. DoHH2 cells were preincubated with or without 20 μM quercetin for 1 hour and then treated with MG-262 for 12 hours. *Significant inhibition as analyzed by ANOVA (P < .001). (E) Effect of quercetin on lactacystin-induced apoptosis. DoHH2 cells were preincubated with 20 μM quercetin for 1 hour and then treated with lactacystin for 12 hours. Apoptosis was determined by DNA content assay with flow cytometry. Data shown (mean ± SD) were from 3 separate experiments. (F) Detection of chemical reactions by Raman spectroscopy. Chemical reactions between quercetin and bortezomib (Fi), quercetin and MG-262 (Fii), and quercetin and boric acid (Fiii,Fiv). Boxes indicate significantly altered spectra.
The chemical structures show that there are similarities between quercetin and myricetin. Quercetin is a catechol or pyrocatechol with 2 hydroxyl groups (−OH) on neighboring carbon atoms of their B rings; and myricetin, a pyrogallol, has 3 −OH groups, whereas both apigenin and kaempferol have only one isolated −OH group on the B ring (Figure 6B). The complex formation in aqueous solution of boron acids with 1,2-dihydroxybenzene (catechol) and its simple derivatives has been well characterized chemically for several decades.30,31 The boronic acid group, −B(OH)2, which is present in bortezomib, can be expected to form cyclic boronate esters with the catechols and pyrogallols groups, but not with flavonoids such as apigenin and kaempferol in which pairs of adjacent hydroxyl groups are absent (Figure 6C). The inhibitory effects of plasma on bortezomib cannot be attributed solely to quercetin as its reported peak serum concentration after a supplemental diet is too low.24 However, there are many dietary flavonoids that have similar structures with quercetin or myricetin (Table 1), so the intake of dietary flavonoids may reduce the killing activity of bortezomib on circulating leukemic cells by the formation of a boronate complex. We also confirmed that several other catechols and pyrogallols, such as epigallocatechin, epigallocatechin gallate, delphinidin, and cyanidin, all have an inhibitory effect on bortezomib-induced apoptosis, but other compounds, such as curcumin and resveratrol, do not (data not shown).
Dietary flavonoids with catechol and pyrogallol structures
| Catechols or pyrocatechols . | Nature source . | Pyrogallols . | Nature source . |
|---|---|---|---|
| Quercetin | Fruits, vegetables, green tea, red wine | Myricetin | Fruits, vegetables and herbs |
| Catechin | Green tea | Gallic acid | Tea |
| Epicatechin | Green tea | Gallocatechin | Green tea |
| Epicatechin gallate | Green tea | Epigallocatechin | Green tea |
| Caffeic acid | Coffee, fruits, vegetables and herbs | Epicatechin gallate | Green tea |
| Rutin | Buckwheat | Epigallocatechin gallate | Green tea |
| Luteolin | Fruits, vegetables | Tannin | Tea, wine, fruits, chocolates |
| Cyanidin | Pigment of fruits | Delphinidin | Pigment of fruits |
| Catechols or pyrocatechols . | Nature source . | Pyrogallols . | Nature source . |
|---|---|---|---|
| Quercetin | Fruits, vegetables, green tea, red wine | Myricetin | Fruits, vegetables and herbs |
| Catechin | Green tea | Gallic acid | Tea |
| Epicatechin | Green tea | Gallocatechin | Green tea |
| Epicatechin gallate | Green tea | Epigallocatechin | Green tea |
| Caffeic acid | Coffee, fruits, vegetables and herbs | Epicatechin gallate | Green tea |
| Rutin | Buckwheat | Epigallocatechin gallate | Green tea |
| Luteolin | Fruits, vegetables | Tannin | Tea, wine, fruits, chocolates |
| Cyanidin | Pigment of fruits | Delphinidin | Pigment of fruits |
The action of quercetin on 2 other proteasome inhibitors was also studied: MG-262, which has a boronic acid moiety,32 and lactacystin, which does not.33 Similar to bortezomib, MG-262, at concentrations between 10 and 200 nM, showed a strong proapoptotic activity on DoHH2 cells. Preincubation with 20 μM quercetin significantly inhibited MG-262-induced apoptosis (Figure 6D). Lactacystin-induced apoptosis in DoHH2 cells was not inhibited by quercetin (Figure 6E).
Raman spectroscopy was used to illustrate the chemical reactions between quercetin and bortezomib, MG-262, or boric acid (Figure 6F). For the chemical reaction between bortezomib and quercetin, the spectral range of 990 to 760 cm showed considerable differences between the spectra of bortezomib and that of bortezomib plus quercetin (Figure 6Fi). Distinct differences are observed in the regions of 950 to 830 cm, which are the result of C-C and C-O stretching, respectively.34–36 The peaks in the area of 820 to 760 cm can be attributed to ring vibrations.34,37 The spectral differences clearly indicate that chemical structure is changing (Figure 6F) and indicate shifts of peak positioning, which are mainly the result of changes in the electronegativity of the molecules and polarizability of the chemical bonds.
The chemical reaction between quercetin and MG-262 was also observed by the differences between the spectra of quercetin and that of MG-262 plus quercetin in the spectral region of 1540 to 670 cm (Figure 6Fii). The differences in the region of approximately 1400 cm can be attributed to C-O cm symmetric stretch.34,38 The shift in the 680-cm peak originated from C-C twisting mode.34,36,39
To further confirm that quercetin reacts with the boronic containing chemicals, the inorganic boric acid was tested. The spectra for quercetin and quercetin plus boric acid show the reaction between the 2 chemicals (Figure 6Fiii,iv). Figure 6Fiii shows the spectra changing in the region of 1580 to 1080 cm, indicating that the chemical structure is altered. The spectral differences were also observed in the area of 1150 to 1080 cm, demonstrating C-C stretching.34,39 In addition, peaks located in the region of 1450 to 1410 cm originate from symmetric stretching vibration of C-O,35 and those in approximately 1500 m can be assigned to C-C stretching in rings.34 Spectra presented in Figure 6Fiv,i, illustrated within the same region, indicate that similar changes take place in the reactions between quercetin with bortezomib and quercetin with boric acid. However, the chemical reaction between quercetin and MG-132 was not detected (Figures S1,S2, available on the Blood website; see the Supplemental Materials link at the top of the online article).
Inorganic boric acid restored the apoptosis-inducing effect of bortezomib in the presence of quercetin
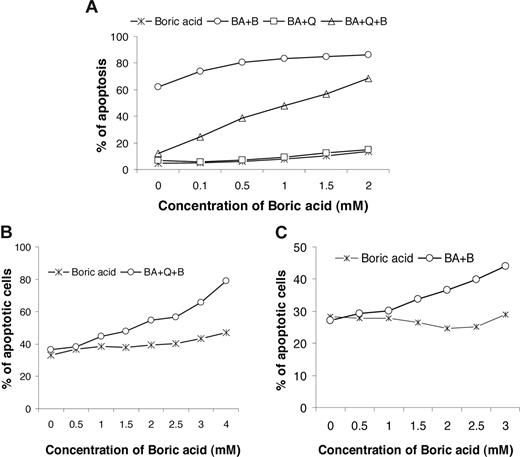
The inorganic boric acid, B(OH)3, was used to abolish quercetin-mediated inhibition of bortezomib by competitively binding to quercetin. Boric acid alone or in combination with quercetin did not induce apoptosis in DoHH2 or CLL cells (Figure 7). Boric acid sensitized DoHH2 cells to killing by bortezomib (Figure 7A). Importantly, boric acid effectively prevented quercetin-mediated inhibition of bortezomib-induced apoptosis in a dose-dependent manner in both DoHH2 and CLL cells (Figure 7A,B). Finally, it was observed that boric acid can restore the apoptotic effect of bortezomib when CLL cells were treated with autologous plasma (Figure 7C).
Effect of boric acid in bortezomib-induced apoptosis. DoHH2 (A) or CLL (B,C) cells were pretreated in various concentrations of boric acid for 2 hours and then treated with 20 nM of bortezomib with or without 20 μM of quercetin for 16 hours. Cells were suspended in the culture medium (A,B) or 50% autologous plasma (C). BA indicates boric acid; Q, quercetin; B, bortezomib. Data shown (mean ± SD) were from 5 separate experiments; error bars have been omitted for clarity.
Effect of boric acid in bortezomib-induced apoptosis. DoHH2 (A) or CLL (B,C) cells were pretreated in various concentrations of boric acid for 2 hours and then treated with 20 nM of bortezomib with or without 20 μM of quercetin for 16 hours. Cells were suspended in the culture medium (A,B) or 50% autologous plasma (C). BA indicates boric acid; Q, quercetin; B, bortezomib. Data shown (mean ± SD) were from 5 separate experiments; error bars have been omitted for clarity.
Discussion
Bortezomib has shown poor efficacy in the treatment of CLL in vivo, despite potent in vitro activity.7,8 We demonstrate that the apoptotic effect of bortezomib on CLL can be blocked by the presence of 50% autologous plasma. This indicates the presence of survival factors or active compounds that can inhibit the killing effect of bortezomib. This inhibitory effect is the result of chemical reactions between quercetin and boronic group containing chemicals, but not related to HSA. We found that a flavonoid, quercetin, blocks bortezomib-induced apoptosis, measured in terms of Bax conformational change and translocation, cytochrome c release, ROS generation, ΔΨm reduction, caspase activation, externalization of phosphatidylserine (annexin V assay), and DNA fragmentation.
Quercetin is one of the abundant flavonol-type flavonoids, commonly found in vegetables and fruits. The average daily intake of flavonoids (quercetin, myricetin, kaempferol) and 2 other flavone-type flavonoids (apigenin and luteolin), was estimated to be 23 mg/day, with quercetin (mean intake, 16 mg/day) as the most consumed of these 5 flavonoids.40 Quercetin is rich in the plasma and is extensively plasma-bound, almost exclusively to HSA.12 The molecular interaction between serum albumin and quercetin involves ionic interactions between the negatively charged quercetin and several ionic and polar amino acids at the putative binding site.41 Binding to albumin, quercetin elicits fluorescence. The plasma concentration of quercetin is tightly associated with its dietary intake.42 Therapeutically, it has been used in antiaging, anticancer, and anti-inflammatory treatment. Flavonoids, such as quercetin, myricetin, kaempferol, and apigenin, also have an inhibitory effect on the chymotrypsin-like activity of 26S and 20S proteasome.15 Using computational modeling, the potential interactions of these flavonoids with the chymotrypsin site (β5 subunit) of the proteasome were detected.15 These flavonoids also have apoptosis-inducing effects on cancer cells.
Quercetin and bortezomib share similarities with respect to proteasome inhibition and the induction of apoptosis (Table 2).1,12,15,26,29,43–46 They both can be used in the treatment of cancer. However, there are 2 differences between these 2 compounds: (1) bortezomib is a boronic dipeptide acid and quercetin is a flavonoid; and (2) bortezomib is a ROS-inducing agent and quercetin is an antioxidant. The question is whether they are synergistic or counteract each other in combination. We observed that quercetin prevented bortezomib-induced ROS generation and apoptosis in primary CLL cells. However, quercetin also blocked bortezomib-induced apoptosis in B-cell lines in which ROS generation was not evoked. Studies from other groups demonstrated that bortezomib-induced apoptosis in mantle cell lymphoma cells was caused by ROS generation and could be prevented by 10 mM NAC, a ROS scavenger.26 In our study, 10 mM NAC blocked bortezomib-induced apoptosis neither in B-cell lines nor in the primary CLL cells. Quercetin induces Bax accumulation and activation at higher doses, which was associated with apoptosis, in good agreement with previous work.15 Quercetin at 20 μM concentration, which was not lethal to B cells, blocked bortezomib-induced Bax activation, cytochrome c release from mitochondria, caspase-3 and caspase-9 activation, and apoptosis. Moreover, neither quercetin nor bortezomib mediated caspase-8 activation, implying that bortezomib-induced apoptosis is via the mitochondrial/caspase-9 pathway.
Comparison of apoptosis-inducing effects of quercetin and bortezomib
| Property . | Quercetin . | Bortezomib . | References . |
|---|---|---|---|
| Compound | Flavonoid | Boronic dipeptide | 1, 12 |
| Proteasome binding site | Inhibition on β5 subunit | Inhibition on β5 subunit | 15 |
| ROS | Inhibition | Generation | 26, 29 |
| NF-κB | Inhibition | Inhibition | 43 |
| Bax | Activation | Activation | 15, 43 |
| Caspase-3 | Activation | Activation | 29, 44 |
| Cell cycle | G2/M arrest | G2/M arrest | 45, 46 |
| Proliferation | Inhibition | Inhibition | 45, 46 |
| Property . | Quercetin . | Bortezomib . | References . |
|---|---|---|---|
| Compound | Flavonoid | Boronic dipeptide | 1, 12 |
| Proteasome binding site | Inhibition on β5 subunit | Inhibition on β5 subunit | 15 |
| ROS | Inhibition | Generation | 26, 29 |
| NF-κB | Inhibition | Inhibition | 43 |
| Bax | Activation | Activation | 15, 43 |
| Caspase-3 | Activation | Activation | 29, 44 |
| Cell cycle | G2/M arrest | G2/M arrest | 45, 46 |
| Proliferation | Inhibition | Inhibition | 45, 46 |
Proteasome activity in human tumor samples has been measured in parallel with its activity in blood. Bortezomib-mediated proteasome inhibition in prostate and lymph node samples was similar to that in blood. Inhibition of activity in the bone marrow was approximately one half that observed in blood.47 The reasons for this differential activity include differential tissue distribution of bortezomib, as well as differential susceptibility of cells at different tissue sites. Interestingly, these observations have not been translated to clinical efficacy in CLL, where the effects of bortezomib used as a single agent are disappointing, but activity was seen at all sites, with a reduction in lymphocytosis, lymphadenopathy, and splenomegaly.8 On the other hand, bortezomib is highly active in myeloma, another B-lymphoid malignancy, suggesting differential sensitivity to bortezomib according to tumor type. We found that myeloma cell lines did not show increased sensitivity to bortezomib-induced apoptosis compared with transformed HRC57 or lymphoma DoHH2 cells. However, whereas quercetin was able to inhibit bortezomib-induced apoptosis of primary CLL, HRC57, and DoHH2 cells, this protective effect was diminished for the myeloma cell lines when they were preincubated with quercetin. These results suggest that quercetin (at 20 μM) has a direct effect on myeloma cells, but not CLL, HRC57 or DoHH2 cells, to increase sensitivity to, and/or synergize with, bortezomib, which is balanced by a protective effect from bortezomib-induced apoptosis, which involves direct interaction with bortezomib and the formation of boronate complexes. Whether dietary intake of quercetin could alter bortezomib-mediated killing of cancer cells in patients with leukemia is unknown, but the lowest concentration tested in our study was 10 μM, which is 12.5 times higher than measured serum levels (0.8 μM) even after supplemental quercetin.24,48 Other dietary flavonoids in both catechol and pyrogallol groups are also able to inhibit bortezomib activity in vivo, as shown for myricetin in this study and listed compounds in Table 1. Given the ability of inorganic boric acid to restore the activity of bortezomib in the presence of quercetin, it would be important to study the effects of both dietary quercetin, other catechols, and supplemental boric acid on the susceptibility of cells to bortezomib-mediated killing in vivo and in vitro. Indeed, ingested inorganic boric acid is nontoxic; it is even safer than table salt. Combined bortezomib and boric acid would have great improvement on the clinical treatment on human leukemia.
Not all dietary flavonoids can inhibit bortezomib. Myricetin showed a similar effect to quercetin on the inhibition of bortezomib, but kaempferol and apigenin did not. Quercetin is catechol and myricetin is pyrogallol. Bortezomib is a boronic acid derivative. A notable feature of the chemistry of both boric and boronic acids is their strong tendency to form complexes with either catechols or pyrogallols.30,31 The efficacy of bortezomib was tested in the presence of inorganic boric acid. Boric acid sensitized CLL cells to bortezomib-induced apoptosis in a dose-dependent manner. It also abolished quercetin or serum-mediated inhibition of bortezomib; this is consistent with competition between boric acid and bortezomib for binding to quercetin in the plasma. Furthermore, quercetin also interferes with MG-262, another boronic acid-based proteasome inhibitor but not MG-132 without boronic acid group. Raman spectroscopy revealed the chemical reactions that can take place in the plasma between quercetin and bortezomib, boric acid, or MG-262.
This study suggests that the inability of bortezomib to induce apoptosis in circulating leukemic cells may be partly the result of the presence of catechols and pyrogallols in the plasma. Quercetin did not inhibit the apoptotic activity of MG-132, a tripeptidyl aldehyde proteasome inhibitor, or lactacystin, a β-lactone–related proteasome inhibitor. Flavopiridol, a novel anticancer drug, is a synthetic flavone but does not contain a catechol structure in the molecule.49 It is a potent chemotherapeutic drug in the treatment of CLL50 and is also able to synergize with bortezomib in inducing apoptosis of CLL in vitro51 ; this combination could also be of interest clinically, with the lack of a catechol group, meaning Flavopiridol would not exert inhibitory binding effects with bortezomib.
In conclusion, quercetin, a dietary flavonoid, can block the hallmarks of bortezomib-induced apoptosis in both cell lines and primary CLL cells. However, not all flavonoids or ROS scavengers can inhibit bortezomib. The inhibitory effect is associated with the catechol and pyrogallol structures of some flavonoids, such as quercetin, cyanidin, myricetin, epigallocatechin, epigallocatechin gallate, and delphinidin, and complex formation with the boric acid group in bortezomib. The differential in vivo activity of bortezomib seen in myeloma and CLL may partly be attributable to the effect of dietary flavonoids: quercetin primes myeloma cells, but not CLL cells, such that they become more sensitive to bortezomib-induced killing. Further work will elucidate the in vivo significance of these findings, which in turn will inform the need for dietary advice on the intake of flavonoids, as well as drug manipulation of flavonoid activity.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Millennium Pharmaceuticals, Inc (Cambridge, MA) for the generous supply of bortezomib; Drs J. Fitzgibbon and M. Streetly for kindly providing the HRC57, DoHH2, U266, and RPMI-8226 cell lines; and Dr M. Yu for involvement in the project discussion.
This work was supported by the Barts and The London Charitable Foundation (London, United Kingdom; L.J., A.C.N.) and the Cancer Research Committee of St Bartholomew's Hospital (London, United Kingdom; L.J.).
Authorship
Contribution: F.-T.L. performed the research; S.G.A. designed and performed the research and wrote part of the paper; P.B.W. designed the research and interpreted the chemical reaction; Z.M. and I.U.R. performed Raman spectroscopy; J.G.G. and A.C.N. designed the research, wrote part of the paper, and raised funds; and L.J. designed and performed the research, wrote the paper, and raised funds.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Li Jia, Centre for Haematology, Institute of Cell and Molecular Sciences, Queen Mary University of London, 4 Newark Street, London E1 2AT, United Kingdom; e-mail: L.jia@qmul.ac.uk.
References
Author notes
*F.-T.L. and S.G.A. contributed equally to this study.