The p53 protein plays a key role in securing the apoptotic response of chronic lymphocytic leukemia (CLL) cells to genotoxic agents. Transcriptional induction of proapoptotic proteins including Puma are thought to mediate p53-dependent apoptosis. In contrast, recent studies have identified a novel nontranscriptional mechanism, involving direct binding of p53 to antiapoptotic proteins including Bcl-2 at the mitochondrial surface. Here we show that the major fraction of p53 induced in CLL cells by chlorambucil, fludarabine, or nutlin 3a was stably associated with mitochondria, where it binds to Bcl-2. The Puma protein, which was constitutively expressed in a p53-independent manner, was modestly up-regulated following p53 induction. Pifithrin α, an inhibitor of p53-mediated transcription, blocked the up-regulation of Puma and also of p21CIP1. Surprisingly, pifithrin α dramatically augmented apoptosis induction by p53-elevating agents and also accelerated the proapoptotic conformation change of the Bax protein. These data suggest that direct interaction of p53 with mitochondrial antiapoptotic proteins including Bcl-2 is the major route for apoptosis induction in CLL cells and that p53's transcriptional targets include proteins that impede this nontranscriptional pathway. Therefore, strategies that block up-regulation of p53-mediated transcription may be of value in enhancing apoptosis induction of CLL cells by p53-elevating drugs.
Introduction
Chronic lymphocytic leukemia (CLL) was formerly thought to be an indolent disease but is now known to be characterized by a high rate of turnover, with 0.1% to 1% of the malignant clone being replaced each day.1,2 CLL comprises at least 2 subtypes, characterized by the extent of mutation within the variable region of the immunoglobulin heavy chain (IgVH) gene of the malignant cells. Patients with extensive mutation are associated with a good prognosis, whereas poor prognosis patients harbor IgVH genes with low levels of mutation and typically require early therapeutic intervention.1 Conventional drugs used in CLL therapy include the alkylating agent chlorambucil and the nucleoside analog fludarabine. The importance of the p53 pathway in the response to these drugs is underscored by the poor prognosis and drug resistance of CLL patients with mutations and/or deletions of the p53 genes or the gene encoding ATM protein kinase, the key upstream regulator of p53.3
p53 is up-regulated by a post-translational regulatory mechanism consequent to the induction of DNA damage.4,5 The p53 protein is a transcription factor that induces a G1 phase blockade, thus enabling DNA repair. The G1 blockade is mediated by up-regulation of the p53 target p21CIP1, an inhibitor of cyclin/cyclin-dependent kinase complexes.5 p53 also up-regulates transcription of proapoptotic BH3-only Bcl-2 family members Puma and Noxa.5 Puma and Noxa induce apoptosis by binding to and neutralizing the ability of antiapoptotic proteins, including Bcl-2 and Bcl-XL, to negatively regulate the proapoptotic Bax protein.6 Bax consequently undergoes a conformational change that allows its insertion into the mitochondrial outer membrane, resulting in the efflux of cytochrome c from the mitochondrial intermembrane space to the cytosol. Cytoplasmic cytochrome c binds to the Apaf-1 protein, enabling it to activate caspase 9, thus initiating a cascade of caspase activation events that results in apoptosis.6
The conventional view of p53-mediated apoptosis has emphasized its role as a transcription factor.5 In contrast, recent studies have identified a novel nontranscriptional mechanism that initiates apoptosis by direct binding of p53 to antiapoptotic Bcl-2 family proteins at the mitochondrial surface, resulting in Bax activation and apoptosis.7,8 This binding is dependent on the conformation of the DNA-binding domain of p53 and mutations that abolish p53's DNA binding and transcriptional activation function simultaneously impair its ability to interact with Bcl-2 family members.9,10
The accumulating evidence for transcription-independent apoptosis induction by p53 prompted us to determine its subcellular localization in CLL cells and whether selective pharmacological blockade of p53's transcriptional function blocked apoptotic killing. In the experiments described here, p53 was induced by treatment with chlorambucil, fludarabine, or nutlin 3a, a nongenotoxic agent that elevates p53 by direct inhibition of its interaction with the negative regulator Mdm211 and that induces p53-dependent apoptosis of CLL cells.12,–14 Pifithrin α (PFTα), a selective inhibitor of p53-mediated transcription,15,,,,,,–22 was used to define the relative contributions of transcriptional and nontranscriptional p53 functions to apoptosis induction. The antiapoptotic actions of PFTα have been described in numerous studies that showed that this agent blocked cell death both in vitro16,17,19,–21,23,–25 and in vivo15,18,22,26,27,29 in a wide range of cell types, including lymphoblastoid16 and myeloid17 cells, neurons,23,24,26,28 renal tubular cells,20,25 hepatocytes,27 cardiomyocytes,18 epithelial cells,21 and colon cancer cells.19 We were therefore surprised to observe that PFTα treatment of CLL cells augmented, rather than suppressed, apoptosis induction by p53-elevating agents.
Methods
Materials
Tissue culture media and preformed polyacrylamide gels were from Invitrogen (Paisley, United Kingdom). PFTα was from Calbiochem (Nottingham, United Kingdom). Nutlin 3a and 3b were kindly donated by Hoffmann-La Roche (Nutley, NJ). ZVAD fluoromethylketone was from BioMol (Exeter, United Kingdom). Chlorambucil was from Sigma-Aldrich (Poole, United Kingdom), and fludarabine from Schering Healthcare (Burgess Hill, United Kingdom). All other reagents were of the highest grade available.
Patients and cell isolation
CLL was diagnosed according to established clinical criteria.29 Age, sex, lymphocyte count, Rai staging, and IgVH gene mutation status on individual patients are summarized in Table 1. This study was approved by the Local Research Ethics Committee of the Royal Free Hospital. Written consent was obtained from patients prior to collection of heparinized peripheral blood samples in accordance with the Declaration of Helsinki. Procedures for the isolation of malignant cells and the determination of their purity have been described in detail.30 All isolates used contained more than 95% CD19/CD5-positive cells.
Clinical data on patients studied
| Patient number . | Age, y/sex . | Lymphocyte count, ×109/L . | Rai stage . | IgVH status . |
|---|---|---|---|---|
| 1 | 74/F | 68 | IV | M |
| 2 | 80/F | 19 | IV | U |
| 3 | 44/M | 23 | II | U |
| 4 | 46/F | 65 | II | M |
| 5 | 61/M | 214 | IV | M |
| 6 | 78/M | 47 | III | M |
| 7 | 69/M | 25 | IV | U |
| 8 | 84/F | 44 | III | M |
| 9 | 85/M | 56 | I | M |
| 10 | 60/F | 46 | II | M |
| 11 | 58/M | 55 | II | U |
| 12 | 76/M | 35 | III | M |
| 13 | 53/M | 81 | III | M |
| 14 | 66/F | 68 | 0 | ND |
| 15 | 70/F | 47 | IV | M |
| 16 | 77/F | 96 | 0 | U |
| 17 | 65/M | 84 | III | ND |
| 18 | 84/M | 87 | IV | M |
| 19 | 58/M | 124 | IV | U |
| 20 | 71/M | 22 | 0 | M |
| 21 | 64/M | 36 | 0 | M |
| 22 | 73/F | 47 | IV | M |
| 23 | 40/M | 44 | IV | U |
| 24 | 60/F | 42 | IV | U |
| 25 | 63/M | 56 | II | ND |
| 26 | 75/M | 129 | IV | U |
| 27 | 63/F | 142 | III | U |
| 28 | 60/F | 116 | II | U |
| 29 | 78/F | 42 | III | U |
| 30 | 74/M | 57 | III | U |
| Patient number . | Age, y/sex . | Lymphocyte count, ×109/L . | Rai stage . | IgVH status . |
|---|---|---|---|---|
| 1 | 74/F | 68 | IV | M |
| 2 | 80/F | 19 | IV | U |
| 3 | 44/M | 23 | II | U |
| 4 | 46/F | 65 | II | M |
| 5 | 61/M | 214 | IV | M |
| 6 | 78/M | 47 | III | M |
| 7 | 69/M | 25 | IV | U |
| 8 | 84/F | 44 | III | M |
| 9 | 85/M | 56 | I | M |
| 10 | 60/F | 46 | II | M |
| 11 | 58/M | 55 | II | U |
| 12 | 76/M | 35 | III | M |
| 13 | 53/M | 81 | III | M |
| 14 | 66/F | 68 | 0 | ND |
| 15 | 70/F | 47 | IV | M |
| 16 | 77/F | 96 | 0 | U |
| 17 | 65/M | 84 | III | ND |
| 18 | 84/M | 87 | IV | M |
| 19 | 58/M | 124 | IV | U |
| 20 | 71/M | 22 | 0 | M |
| 21 | 64/M | 36 | 0 | M |
| 22 | 73/F | 47 | IV | M |
| 23 | 40/M | 44 | IV | U |
| 24 | 60/F | 42 | IV | U |
| 25 | 63/M | 56 | II | ND |
| 26 | 75/M | 129 | IV | U |
| 27 | 63/F | 142 | III | U |
| 28 | 60/F | 116 | II | U |
| 29 | 78/F | 42 | III | U |
| 30 | 74/M | 57 | III | U |
ND indicates not determined, M; mutated IgVH; and U, unmutated IgVH.
Normal T lymphocytes were isolated by negative selection using the MACS pan T-cell isolation kit (Miltenyi Biotec, Bisley, United Kingdom).
Cell incubation
CLL cells (107 mL−1) were routinely cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum, 100 U mL−1 penicillin, and 100 μg mL−1 streptomycin. Cultures were incubated for 24 hours at 37°C prior to addition of drugs. Incubations with chlorambucil or nutlin 3a were incubated for 18 hours. Incubations with fludarabine were for 48 hours, because induction of p53 by fludarabine was found in preliminary experiments to be delayed, compared with p53 induction by chlorambucil or nutlin 3a.
In experiments designed to study subcellular distribution of p53 and other proteins, Bax conformation change or the suppression of p53 target induction by cytotoxic agents, 100 μM ZVAD, a pan-caspase inhibitor, was added to media to block protein degradation consequent to caspase activation.
Protein extraction and cell fractionation
Whole cell lysates were prepared by extraction of cell pellets with a buffer containing 2% nonidet P40, 0.5% sodium deoxycholate, and 0.2% sodium dodecyl sulfate as described.30 Subcellular fractionation was carried out by differential detergent fractionation (DDF) essentially as described.31 Fraction 1, consisting of cytosolic material, was obtained by extraction with buffer 1, which contained 0.02% digitonin. Fraction 2 (mitochondria plus membranous organelles) was extracted with buffer 2, which contained 0.5% triton X-100. Fraction 3 (nuclear material) was solubilized directly into gel loading buffer (300 mM sucrose, 250 mM Tris-HCl, pH 8.5, 0.5 mM EDTA, 80 mM dithiothreitol, 2% sodium dodecyl sulfate, and 0.2% Serva Blue G250) with heating at 70°C for 10 minutes. The DDF protocol was found by Waterhouse et al32 to give the most reproducible results in studies of apoptotic mechanisms.
Protein concentrations were determined by a sensitive modification of the Lowry procedure.33
Coimmunoprecipitation of Bcl-2 and p53
Bcl-2 was immunoprecipitated by a modification of a published procedure.34 One hundred microliters of 5 μg mL−1 protein A/G (Calbiochem) in 100 mM sodium carbonate, pH 8.2, were added to the wells of a Nunc Immobilizer Amino Strip (VWR, Lutterworth, United Kingdom) and incubated at room temperature for 4 hours. The strips were then washed with 0.1% Tween 20 in TBS (20 mM Tris-HCl, pH 7.8, 137 mM NaCl). One microgram of hamster anti–Bcl-2 antibody (Clone 3F11; BD Biosciences, Oxford, United Kingdom) was added per well and allowed to bind to the immobilized protein A/G overnight at 4°C. The antibody-protein A/G complexes were crosslinked by addition of 50 μL of 20 mM dimethylpimelidate (Perbio, Cramlington, United Kingdom) in triethanolamine followed by incubation for 30 minutes at room temperature. The crosslinker was quenched by addition of 0.2 M ethanolamine (pH 8.2). The wells were then washed 3 times with 0.1 M sodium citrate (pH 3) and 3 times with 0.2% Tween 20 in TBS.
Cells were lysed in a buffer35 containing 1% [(3-cholamidopropyl) dimethylammonio]-1-propanesulfonate (CHAPS; Perbio, United Kingdom) and the lysates incubated in the prepared wells for 4 hour at 4°C. The supernatants were removed and stored for future analysis. Wells were washed 4 times with 0.2% Tween 20 in TBS. Forty microliters of gel loading buffer were added to each well and the plate heated at 70°C for 10 minutes prior to gel electrophoresis and western transfer. In control experiments we established that an isotype-matched control antibody failed to immunoprecipitate either Bcl-2 or p53.
Quantitation of Bax conformation change
Acquisition of the proapoptotic conformation of Bax was quantified by immunoprecipitation.35 Cells were lysed in 1% CHAPS buffer and immunoprecipitated essentially as described above (“Coimmunoprecipitation of Bcl-2 and p53”) using the conformation-specific Bax 6A7 antibody (Calbiochem). Precipitates were analyzed by Western blotting using a pan-Bax antibody (Cell Signaling Technologies).
Assays for apoptosis
Apoptosis was quantified by Western blot assessment of the cleavage of the caspase 3 substrate poly(ADP ribose) polymerase (PARP).36,37 Late stage apoptosis was quantified morphologically, by counting of cells with or without evidence of nuclear condensation and fragmentation in Giemsa-stained cytospin preparations as described.38 A total of at least 800 cells were counted in 3 randomly selected fields. The percentage of apoptotic cells in each field was computed, and the data are presented as means plus or minus SEM.
Gel electrophoresis and Western blotting
Procedures for gel electrophoresis and western transfer have been described elsewhere.30 Antibodies against the following proteins were used: p53 (DO-1; Santa Cruz Biotechnology, Santa Cruz, CA); Poly (ADP ribose) polymerase, p21CIP1 (BD Biosciences); Puma and Mdm2 (Calbiochem) actin (Sigma-Aldrich); lactate dehydrogenase V (Abcam, Cambridge, United Kingdom); Hsp60 (Cambridge Biosciences, Cambridge, United Kingdom); cytochrome c, cytochrome c oxidase IV, histone 2A and lamin (Cell Signaling Technologies); Bcl-2, and horseradish peroxidase–conjugated secondary antibodies (DAKO, Ely, United Kingdom). When several blots were analyzed in a single experiment, they were simultaneously exposed to the same solution in a single glass cylinder using a rotisserie apparatus (Thermo Fisher Scientific, Loughborough, United Kingdom) in order to ensure uniformity of exposure to both primary and secondary antibodies.
Immunoreactive bands were visualized by Enhanced Chemiluminescence (GE Healthcare, Little Chalfont, United Kingdom). As required by journal policy, backgrounds were not subtracted from the autoradiograms shown in the figures. However, backgrounds were subtracted prior to digital quantification of band intensities using Quantity 1 software (Bio Rad, Hemel Hempstead, United Kingdom).
Results
Subcellular distribution of p53
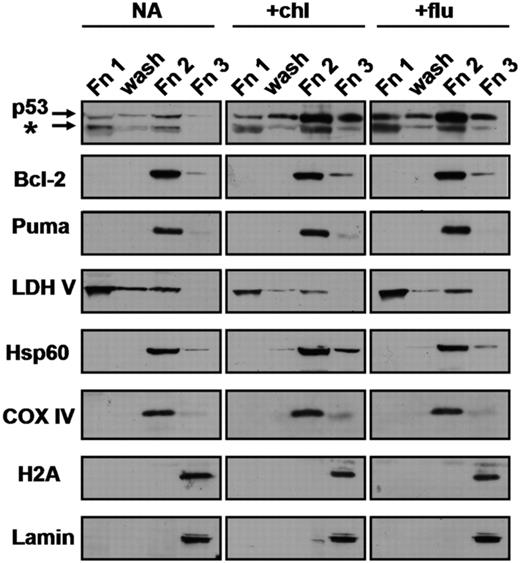
Untreated CLL cells or cells treated with p53-inducing agents were partitioned by DDF (“Methods”) into cytosolic, mitochondria plus organelle and nuclear fractions, designated as fractions 1, 2, and 3, respectively (Figure 1). Although nuclear p53 was clearly detectable, the major fraction of the p53 induced by chlorambucil or by fludarabine treatment (60% and 74%, respectively) was associated with fraction 2. The stability of this association is emphasized by the resistance to washing of the p53 localized to fraction 2 (Figure 1). The proapoptotic BH3-only Puma protein was constitutively expressed and was associated exclusively with fraction 2 in untreated or drug-treated cells. Western blot analysis established that the distribution of the nuclear markers lamin and histone 2A, the mitochondrial markers cytochrome oxidase subunit IV, heat-shock protein 60 (Hsp60) and Bcl-2 and the cytosolic marker lactate dehydrogenase V essentially validated the performance of the DDF procedure (Figure 1). Although we observed a minor fraction of Bcl-2 and Hsp60 in fraction 3, histone 2A and lamin were exclusively recovered in fraction 3, ruling out the possibility that the p53 detected in fractions 1 and 2 were the result of contamination by the nuclear fraction. In addition, the considerably higher ratio of p53 to lactate dehydrogenase band intensity in fraction 2 compared with fraction 1 rules out the possibility that p53 in fraction 2 resulted from contamination by the cytoplasmic fraction.
Subcellular distribution of p53 in CLL cells. Cells from patient 21 were incubated in the presence of 100 μM ZVAD with no further additions (NA), 50 μM chlorambucil (chl) or 10 μM fludarabine (flu). Following 18-hour incubation, cells were fractionated by DDF as described in “Methods,” except that the pellet remaining following extraction with DDF buffer 1 was washed once with buffer 1 prior to extraction with buffer 2. The resulting fractions (Fn1, wash, Fn2 and Fn3) were analyzed by Western blotting. To allow direct visual assessment of p53 distribution, a volume of each fraction equivalent to 20 × 106 cells was loaded on each lane. This resulted in the loading of 50 μg Fn1, 2 μg wash fraction, 20 μg Fn2 and 18 μg Fn3. LDH V, lactate dehydrogenase V; Hsp60, heat shock protein 60; COX IV, cytochrome oxidase IV; H2A, histone 2A. The p53 antibody detected a major band of 53 kDa and a minor band of 48 kDa, indicated by the asterisk. This band may correspond to the alternatively spliced form, p53β, described by Bourdon et al.40 In this and all figures, lanes that were contiguous on autoradiograms are boxed together.
Subcellular distribution of p53 in CLL cells. Cells from patient 21 were incubated in the presence of 100 μM ZVAD with no further additions (NA), 50 μM chlorambucil (chl) or 10 μM fludarabine (flu). Following 18-hour incubation, cells were fractionated by DDF as described in “Methods,” except that the pellet remaining following extraction with DDF buffer 1 was washed once with buffer 1 prior to extraction with buffer 2. The resulting fractions (Fn1, wash, Fn2 and Fn3) were analyzed by Western blotting. To allow direct visual assessment of p53 distribution, a volume of each fraction equivalent to 20 × 106 cells was loaded on each lane. This resulted in the loading of 50 μg Fn1, 2 μg wash fraction, 20 μg Fn2 and 18 μg Fn3. LDH V, lactate dehydrogenase V; Hsp60, heat shock protein 60; COX IV, cytochrome oxidase IV; H2A, histone 2A. The p53 antibody detected a major band of 53 kDa and a minor band of 48 kDa, indicated by the asterisk. This band may correspond to the alternatively spliced form, p53β, described by Bourdon et al.40 In this and all figures, lanes that were contiguous on autoradiograms are boxed together.
More than 85% of the p53 induced by nutlin 3a was also associated with fraction 2. Bcl-2 and Puma were again recovered exclusively in this fraction (Figure S1; available on the Blood website; see the Supplemental Materials link at the top of the online article). Using confocal microscopy, Kojima et al13 have also shown the association of p53 with mitochondria in nutlin-treated CLL cells. Our data confirm these observations by an independent method and extend them to include cells treated with the clinically relevant cytotoxic agents chlorambucil and fludarabine. Preferential association of p53 with fraction 2 of cells treated with p53-elevating agents was demonstrated using cells from 14 additional patients (patients 1, 4-11, 13, 16, 17, 20 and 21; Table 1). The proportion of fraction 2-associated p53 in CLL cells treated with chlorambucil, fludarabine, or nutlin varied between 60% and 90% in different experiments.
As a further control, untreated CLL cells were permeabilized with DDF buffer 1 and then incubated with recombinant p53 before proceeding with the DDF protocol, which included a washing step. Western blot analysis revealed that only a minor fraction of the p53 (< 10% of total) associated with fraction 2, the majority being recovered in fraction 1 (data not shown), the inverse of the distribution seen when intact CLL cells were treated with p53-elevating agents (Figure 1 and Figure S1A). This experiment suggests that the association of p53 with fraction 2 is unlikely to be the result of adventitious association of soluble p53 with this fraction during the DDF procedure.
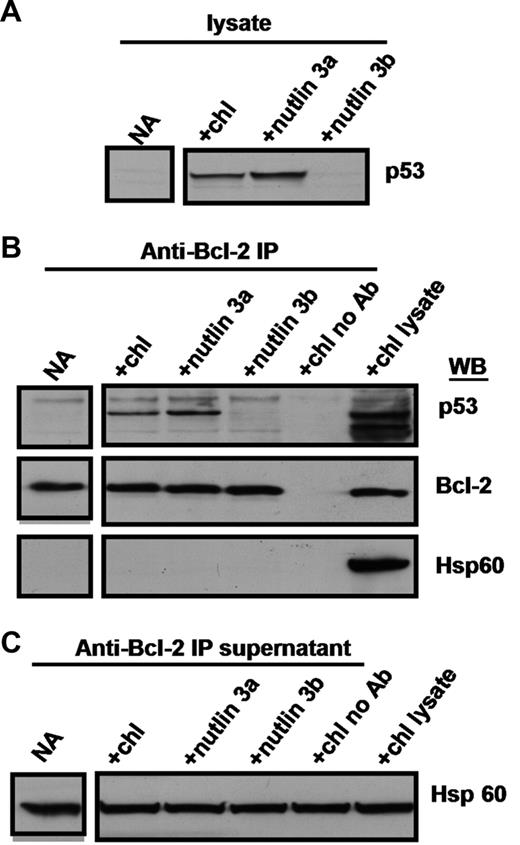
Coimmunoprecipitation of p53 and Bcl-2
The Bcl-2 protein is largely localized to mitochondria.6 A physical association between induced p53 and Bcl-2 was shown by coimmunoprecipitation experiments, emphasizing the potential mechanistic significance of the predominantly mitochondrial localization of p53. Treatment of CLL cells with chlorambucil or nutlin 3a resulted in the induction of p53, whereas the inactive enantiomer nutlin 3b failed to do so (Figure 2A). Immunoprecipitation with an anti–Bcl-2 antibody resulted in co-precipitation of Bcl-2 and p53 from lysates of cells treated with chlorambucil or nutlin 3a, but not from untreated or nutlin 3b–treated lysates (Figure 2B). Omission of the anti–Bcl-2 antibody failed to precipitate either protein (Figure 2B). The selectivity of the Bcl-2-p53 coimmunoprecipitation is emphasized by the observation that the abundant mitochondrial protein Hsp60 was present in the supernatants remaining after immunoprecipitation (Figure 2C), but was entirely undetectable in the immunoprecipitates (Figure 2B). Similar results were obtained using cells from patients 10, 13, 15, 21, 22, 25, 28, and 29.
Coimmunoprecipitation of p53 and Bcl-2. Cells from patient 19 were incubated for 18 hours with no addition, 50 μM chlorambucil, 10 μM nutlin 3a or 10 μM nutlin 3b. CHAPS lysates were prepared and analyzed directly (A), or following immunoprecipitation with anti-Bcl-2 antibodies (B). Immunoprecipitation supernatants were analyzed for Hsp60 to document equal inputs (C).
Coimmunoprecipitation of p53 and Bcl-2. Cells from patient 19 were incubated for 18 hours with no addition, 50 μM chlorambucil, 10 μM nutlin 3a or 10 μM nutlin 3b. CHAPS lysates were prepared and analyzed directly (A), or following immunoprecipitation with anti-Bcl-2 antibodies (B). Immunoprecipitation supernatants were analyzed for Hsp60 to document equal inputs (C).
Some models of apoptosis regulation postulate that Bcl-2 can sequester Bax and that the release of the latter protein initiates pore formation and cytochrome c release.39 However, we were unable to detect Bax in immunoprecipitates of CLL cells (data not shown), thereby effectively ruling out the possibility that displacement of Bax consequent to binding of p53 to Bcl-2 may contribute to the induction of apoptosis.
PFTα blockade of up-regulation of p53 transcriptional targets
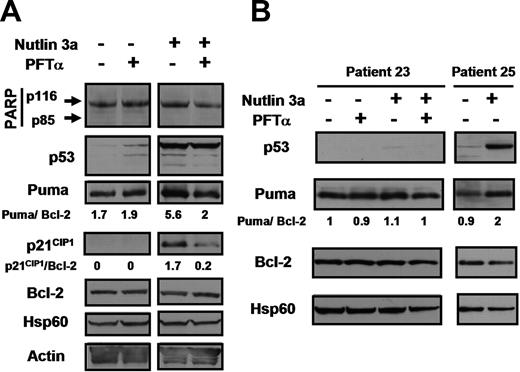
We determined whether PFTα, which has been shown to selectively block p53-mediated transcription in diverse cellular systems,15,,,,,,–22 prevented the up-regulation of the well-characterized p53 target gene p21CIP1 in CLL cells. Treatment of CLL cells with nutlin 3a resulted in up-regulation of p53. p21CIP1 was completely undetectable in untreated cells and was dramatically elevated by nutlin treatment (Figure 3A). PFTα resulted in an approximately 8-fold inhibition of p21CIP1 induction by nutlin without affecting the level of p53. This and similar experiments were carried out in the presence of the pan-caspase inhibitor ZVAD in order to convincingly eliminate the possibility that the observed levels of proteins were affected by caspase cleavage. The complete lack of cleavage of the caspase 3 substrate poly(ADP ribose) polymerase (PARP) documents the effective suppression of caspase activity by ZVAD (Figure 3).
Effect of PFTα on nutlin-induced transcription of p21CIP1 and Puma. Cells from patient 19 (A) or from patient 23 (B) were incubated in media containing 100 μM ZVAD. Nutlin 3a (10 μM) and 25 μM PFTα were added as indicated. Whole cell lysates were prepared (“Methods”) and analyzed by Western blotting. Lysates from patient 25 were analyzed on the same blot with patient 23 lysates to provide a positive control for the p53 antibody. Expression levels of the p53 targets p21CIP1 and PUMA were quantified by densitometry. The band intensities were normalized with respect to Bcl-2 band intensities, since expression of Bcl-2 has been shown to be extremely stable in CLL cells.38
Effect of PFTα on nutlin-induced transcription of p21CIP1 and Puma. Cells from patient 19 (A) or from patient 23 (B) were incubated in media containing 100 μM ZVAD. Nutlin 3a (10 μM) and 25 μM PFTα were added as indicated. Whole cell lysates were prepared (“Methods”) and analyzed by Western blotting. Lysates from patient 25 were analyzed on the same blot with patient 23 lysates to provide a positive control for the p53 antibody. Expression levels of the p53 targets p21CIP1 and PUMA were quantified by densitometry. The band intensities were normalized with respect to Bcl-2 band intensities, since expression of Bcl-2 has been shown to be extremely stable in CLL cells.38
Puma was expressed in untreated cells and this expression was essentially unaffected by PFTα (Figure 3A), suggesting that constitutive Puma expression was independent of p53.
Nutlin treatment resulted in an approximately 3-fold up-regulation of Puma. PFTα resulted in the suppression of Puma expression by nutlin-treated cells to a level close to that seen in untreated cells (Figure 3A). Therefore, Puma expression by CLL cells was only modestly increased following p53 induction and this p53-dependent increment in expression was almost completely blocked by PFTα.
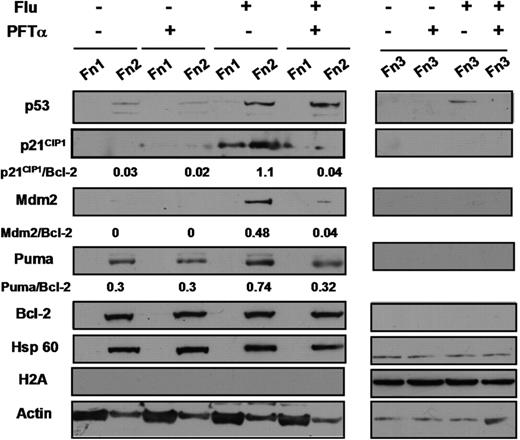
Dramatic up-regulation of p21CIP1 accompanied by a modest induction of Puma in CLL cells treated with fludarabine or chlorambucil and the blockade of these induction events by PFTα are documented in Figure 4 and Figure S2, respectively. The p53 target Mdm2 showed an induction pattern very similar to that of p21CIP1 (Figure 4). Induced p21CIP1 was strikingly absent from the nuclear fraction (Fn 3) and was predominantly localized to the mitochondrial/ organellar fraction (Fn 2; Figure 4). The potential significance of the nuclear exclusion of p21CIP1 is discussed below (see “Discussion”). Similar patterns of expression of p21CIP1 and Puma in response to p53-elevating agents and their suppression by PFTα has been documented in a total of 16 experiments, using cells from patients 1, 2, 6-10, 12, 13, 15-20, and 25.
Effect of PFTα on fludarabine-induced transcription of p21CIP1 and Puma. Cells from patient 4 were incubated in the presence of 100 μM ZVAD. Fludarabine (10 μM) and/or 25 μM PFTα were added as indicated. Cells were fractionated by DDF and analyzed by Western blotting. The nuclear fractions (Fn 3) were run on a separate gel from fractions 1 and 2.
Effect of PFTα on fludarabine-induced transcription of p21CIP1 and Puma. Cells from patient 4 were incubated in the presence of 100 μM ZVAD. Fludarabine (10 μM) and/or 25 μM PFTα were added as indicated. Cells were fractionated by DDF and analyzed by Western blotting. The nuclear fractions (Fn 3) were run on a separate gel from fractions 1 and 2.
To confirm that constitutive expression of Puma by CLL cells was indeed p53 independent, we studied cells isolated from patient 23, a poor prognosis patient with unmutated IgVH and a deletion of the long arm of chromosome 17 (the site of the p53 gene), in more than 99% of the malignant cells. The patient's cells had completely lost inducibility of p53, p21CIP1 and Mdm2 in response to nutlin 3a in vitro (Figure S3), but expressed Puma at a level similar to that seen in a cells from a patient (patient 25) with a functional p53 system (Figure 3B). Puma levels in patient 23's cells were unaffected by nutlin, PFTα or a combination of these agents, whereas the cells from patient 25 showed the modest nutlin-induced increase in expression characteristic of CLL cells with functional p53. Identical results were observed using cells from 2 additional patients with 17p deletion and an undetectable p53 response (patients 24 and 28, not shown), confirming that substantial levels of p53-independent constitutive Puma expression could be maintained by CLL cells.
PFTα augments the ability of p53-elevating agents to induce apoptosis of CLL cells
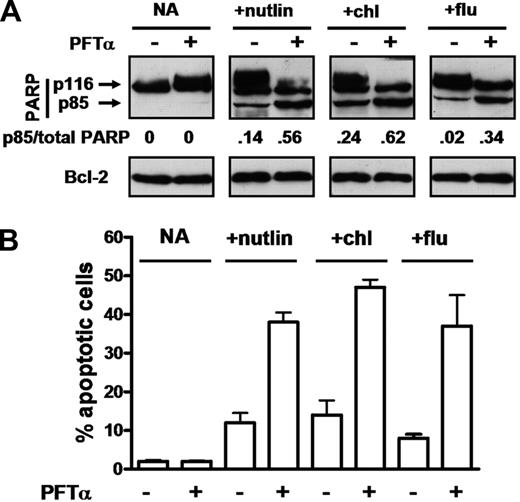
Western blot quantitation of cleavage of the caspase 3 substrate PARP provides a sensitive and accurate measure of an early event in apoptosis induction.36,37 Incubation of cells from patient 19 with PFTα alone resulted in no detectable increase in apoptosis, as judged by this molecular criterion (Figure 5A). Nutlin alone induced a modest level of PARP cleavage, which was enhanced more than 3-fold in a culture incubated with PFTα and nutlin. PFTα also enhanced PARP cleavage induced by chlorambucil and by fludarabine (Figure 5A).
Effect of PFTα on apoptosis induction by p53-elevating agents. Cells from patient 19 were incubated in the absence of ZVAD with 10 μM nutlin 3a, 50 μM chlorambucil or 10 μM fludarabine. PFTα (25 μM) was added as indicated. Apoptosis was assessed by Western blot analysis of PARP cleavage (A) or by morphology (B). The densitometric intensity of the caspase 3–cleaved p85 PARP band is expressed as a ratio relative to the total intensity of the p85 and p116 (intact) bands. Note that the ability of PFTα to block p53-mediated transcription in cells from patient 19 is documented in Figure 3.
Effect of PFTα on apoptosis induction by p53-elevating agents. Cells from patient 19 were incubated in the absence of ZVAD with 10 μM nutlin 3a, 50 μM chlorambucil or 10 μM fludarabine. PFTα (25 μM) was added as indicated. Apoptosis was assessed by Western blot analysis of PARP cleavage (A) or by morphology (B). The densitometric intensity of the caspase 3–cleaved p85 PARP band is expressed as a ratio relative to the total intensity of the p85 and p116 (intact) bands. Note that the ability of PFTα to block p53-mediated transcription in cells from patient 19 is documented in Figure 3.
Photomicrographs of cells treated with fludarabine in the presence or absence of PFTα are shown in Figure S4. Quantitative data, derived by evaluation of the percentage of apoptotic cells, are shown in Figure 5B, confirming the conclusion that PFTα augmented apoptotic killing induced by fludarabine. Apoptosis induced by chlorambucil or nutlin were similarly enhanced by PFTα (Figure 5B).
Clinically achievable concentrations of chlorambucil and of fludarabine are approximately 5 and 2 μM respectively.41,42 Additional experiments were therefore carried out to establish that PFTα augmented apoptosis induction at these concentrations of the cytotoxic drugs (Figure S5). The ability of PFTα to enhance apoptosis induced by p53-elevating agents was demonstrated in a total of 24 experiments (patients 1, 2, 4-13, 15-17, 19-22, and 25-30). Inhibition of apoptosis by PFTα was not seen in any patient studied.
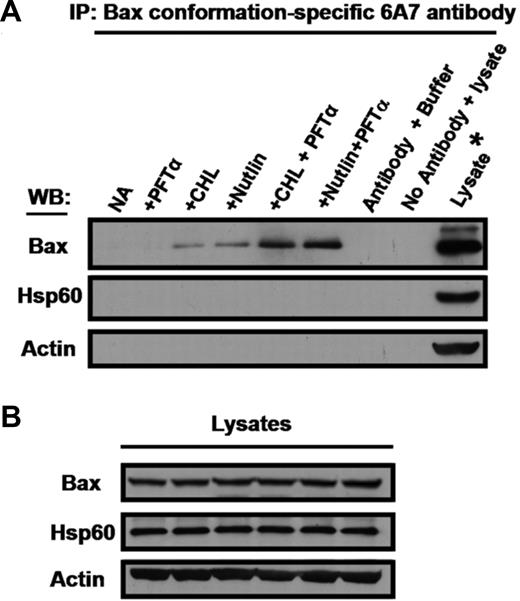
PFTα augments the ability of p53-elevating agents to induce the proapoptotic conformation of Bax
A conformational change which results in exposure of the N-terminus of the proapoptotic Bax protein is a critical rate-determining event which results in cytochrome c release, caspase activation and apoptosis.6 We therefore carried out immunoprecipitation experiments using an antibody specific for the proapoptotic Bax conformation to determine whether the observed augmentation of apoptosis by PFTα was reflected at this level. PFTα alone failed to elicit the conformation change. Modest levels of conformation change were elicited by nutlin or chlorambucil, and these levels were substantially augmented by PFTα (Figure 6). For comparison, cultures identical to those in Figure 6 but omitting ZVAD were incubated in parallel for the quantitation of PARP cleavage and morphological apoptosis (Figure S6). These data complement the observed augmentation of Bax conformation change by PFTα. Therefore, the ability of PFTα to augment apoptosis induction by p53-elevating agents has been confirmed by 3 independent criteria.
Effect of PFTα on Bax conformation change induced by p53-elevating agents. Cells from patient 26 were incubated in the presence of ZVAD. Chlorambucil, nutlin and PFTα were added as indicated. Following 18-hour incubation, cells were lysed in CHAPS buffer. (A) Lysates were analyzed by immunoprecipitation with conformation-specific Bax 6A7 antibody. Unprecipitated lysate from cells incubated with nutlin + PFTα was loaded on the right hand lane to define the migration positions of Bax, Hsp60 and actin. The nutlin + PFTα lysate was also used to generate the negative control in which the 6A7 antibody was omitted from the immunoprecipitation reaction (penultimate lane). (B) Aliquots of the lysates were analyzed directly by Western blotting to document equal inputs into the corresponding immunoprecipitation reactions shown in panel A. Similar results were obtained using cells from 2 additional patients (patients 8 and 9).
Effect of PFTα on Bax conformation change induced by p53-elevating agents. Cells from patient 26 were incubated in the presence of ZVAD. Chlorambucil, nutlin and PFTα were added as indicated. Following 18-hour incubation, cells were lysed in CHAPS buffer. (A) Lysates were analyzed by immunoprecipitation with conformation-specific Bax 6A7 antibody. Unprecipitated lysate from cells incubated with nutlin + PFTα was loaded on the right hand lane to define the migration positions of Bax, Hsp60 and actin. The nutlin + PFTα lysate was also used to generate the negative control in which the 6A7 antibody was omitted from the immunoprecipitation reaction (penultimate lane). (B) Aliquots of the lysates were analyzed directly by Western blotting to document equal inputs into the corresponding immunoprecipitation reactions shown in panel A. Similar results were obtained using cells from 2 additional patients (patients 8 and 9).
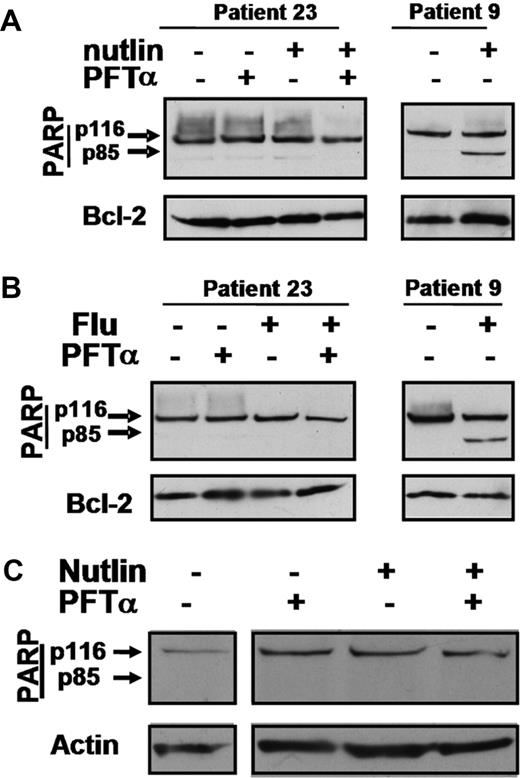
PFTα does not augment killing of CLL isolates lacking functional p53
The complete inability of the malignant cells from patient 23 to activate a p53 response is documented in Figure S3. Nutlin 3a or fludarabine alone or in combination with PFTα failed to induce apoptosis of these cells (Figure 7A,B). Similar results were obtained using cells from an additional extensively treated poor prognosis patient whose cells had also lost p53 inducibility (patient 24, data not shown), suggesting that the interaction between PFTα and cytotoxic agents is strictly dependent on an intact p53 system.
Actions of PFTα in cells from a p53-dysfunctional patient and in normal T lymphocytes. Cells from p53-dysfunctional patient 23 were incubated with 10 μM nutlin 3a (A) or 10 μM fludarabine (B). PFTα (25 μM) was added as shown. Whole cell lysates were analyzed by Western blotting. Cell extracts from patient 9 were included as controls to identify the migration positions of p116 and p85 PARP bands. The complete lack of a p53 response by the cells of this patient are documented in Figure S3. (C) Western blot analysis of PARP in normal T lymphocytes incubated with PFTα and/or nutlin. Similar results were obtained in 2 additional experiments.
Actions of PFTα in cells from a p53-dysfunctional patient and in normal T lymphocytes. Cells from p53-dysfunctional patient 23 were incubated with 10 μM nutlin 3a (A) or 10 μM fludarabine (B). PFTα (25 μM) was added as shown. Whole cell lysates were analyzed by Western blotting. Cell extracts from patient 9 were included as controls to identify the migration positions of p116 and p85 PARP bands. The complete lack of a p53 response by the cells of this patient are documented in Figure S3. (C) Western blot analysis of PARP in normal T lymphocytes incubated with PFTα and/or nutlin. Similar results were obtained in 2 additional experiments.
PFTα does not augment killing of normal T lymphocytes by nutlin 3a
Incubation of purified normal T lymphocytes with nutlin 3a did not result in the induction of apoptosis as quantified by PARP cleavage analysis (Figure 7C), in agreement with earlier observations.12 PFTα failed to augment apoptosis when co-incubated with nutlin. Similar results were obtained in 2 additional experiments using T cells isolated from different normal subjects. Therefore, these cells were substantially resistant to concentrations of nutlin and PFTα which were toxic to CLL cells.
Discussion
The data here show that the major fraction of p53 induced by treatment of CLL cells with genotoxic (chlorambucil, fluarabine) or nongenotoxic (nutlin 3a) agents associated stably with a cellular fraction enriched in mitochondria and other organelles. Immunoprecipitation studies showed that induced p53 bound to Bcl-2, which is itself largely associated with mitochondria.6 The proapoptotic Puma protein was constitutively expressed in a p53-independent manner and was modestly up-regulated following p53 induction. The up-regulation of Puma and also of p21Cip1 was substantially inhibited by PFTα. Having confirmed the ability of PFTα to block p53 target gene expression in CLL cells, we used this agent to assess the relative contributions of transcriptional and non-transcriptional mechanisms to p53-induced apoptosis of CLL cells. The observation that PFTα markedly augmented apoptosis induction by all 3 p53-elevating agents was unexpected. Although PFTα has been shown to block p53-dependent apoptosis in the majority of studies reported to date,15,,,,,,,,,,,,–28 the killing of acute myeloid leukemia cells by cytosine arabinoside43 and of mouse embryo fibroblasts and human glioblastoma cells by topotecan44 is augmented by this agent. It is therefore likely that the impact of PFTα on apoptosis induction in different systems is highly dependent on the relative importance of pro- and antiapoptotic signaling pathways induced by p53 in a particular cell type.
Although the data here do not rule out a role for Puma in apoptosis induction, suppression of Puma elevation by PFTα did not block cell killing. Therefore, p53-mediated up-regulation of Puma is unlikely to be crucial for apoptosis induction in CLL cells, contrasting with the requirement for Puma induction in neuronal cell death.24 The data here are therefore compatible with the interpretation that a nontranscriptional mechanism involving direct binding of p53 to mitochondrial antiapoptotic proteins is the major route to apoptosis induction by p53 in CLL cells. This hypothesis is supported by the growing evidence for transcription-independent mechanisms of p53-mediated cytotoxicity.7,,–10
The apparently paradoxical observation that PFTα augments, rather than supresses, apoptosis induction by different cytotoxic agents suggests that the predominant consequence of p53-induced transcription in CLL cells is to up-regulate expression of a gene or genes whose products block proapoptotic signaling mediated by the predominantly nontranscriptional pathway of p53-dependent cell killing. Therefore, selective blockade of p53-mediated transcription by PFTα would increase rather than suppress cell killing by the nontranscriptional mechanism. This hypothesis is underscored by the observation that PFTα failed to augment apoptosis induction in CLL cells which were completely lacking in functional p53. Because PFTα did not affect p53 induction in cell isolates with wild-type p53, but did augment Bax conformation change, the putative antiapoptotic p53 transcriptional target(s) may impact on the death pathway at the mitochondrial surface, at a point beyond p53 induction but preceding Bax activation.
While p53 has conventionally been considered to regulate apoptosis in a positive manner,5,6 recent studies have emphasized the complex and dualistic nature of p53's transcriptional targets, which can either induce5,6 or block45 apoptosis, depending on the cellular context. The precise impact of p53-mediated transcription therefore depends on the selection of transcriptional targets, which is itself the result of the complex interactions between several variables which are highly dependent on the cellular context. These variables include the nature and extent of genotoxic damage, sites of post-translational modification of p53 (including phosphorylation and acetylation) and the spacing of p53 binding sites in individual promoters.46
Microarray studies have established that hundreds of genes are induced or repressed in a p53-dependent manner following irradiation of mice47 or of CLL cells.48 Furthermore, p53-dependent changes in gene expression were highly cell type-specific.47 Therefore, identification of the key p53 gene targets whose blockade by PFTα results in augmentation of apoptosis in CLL cells presents a formidable challenge. While p21CIP1 has widely been recognized as an inhibitor of the cell cycle, a function which requires its nuclear localization, this protein can act as a blocker of apoptosis in some circumstances. The subcellular localization of p21CIP1 is modulated by a complex network of phosphorylation events and its targeting to extranuclear compartments results in the blockade of the apoptotic pathway at the mitochondrial surface.49 p21CIP1 induced following p53 up-regulation was exclusively localized to the cytosolic and organellar fractions of CLL cells. It is therefore plausible that this protein may be a candidate mediator of p53's putative antiapoptotic actions. Direct proof of this hypothesis will however require application of knockdown strategies. Unfortunately, these strategies are at present ineffective in resting CLL cells.50
While the precise mechanisms involved in mediating p53's antiapoptotic actions require further clarification, the data here show clearly that its proapoptotic actions are augmented by pharmacological agents which block p53-dependent transactivation. The ability of PFTα to prevent the p53-dependent generation of craniofacial abnormalities in a mouse model of Treacher Collins syndrome strikingly demonstrate that pharmacologically active levels of this compound can be maintained for extended periods in vivo without significant deleterious side-effects.28 The LC90 values for both chlorambucil and fludarabine vary widely even in previously untreated patients.51 Therefore, the simultaneous administration of cytotoxic drugs and pharmacological inhibitors of p53's transcriptional function may be of therapeutic value, especially in the treatment of CLL patients who are relatively drug resistant despite the presence of an intact p53 pathway.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Leukaemia Research Fund, United Kingdom.
Authorship
Contribution: A.J.S., A.G.P., and R.G.W. designed studies, analyzed data, and wrote the paper; A.J.S., B.C.Y., S.M.H., E.P.N., J.D.H.-R., V.M.D., and R.G.W. carried out research; A.G.P., A.V.H., P.K., and K.C. recruited and obtained written consent from patients and collated clinical data; and L.T.V. analyzed data and synthesized the nutlins used in this study.
Conflict-of-interest disclosure: L.T.V. is an employee of Hoffman-La Roche Inc. The other authors declare no competing financial interests.
Correspondence: Dr R. Gitendra Wickremasinghe, Department of Hematology, Royal Free and University College Medical School, Rowland Hill Street, London NW3 2PF, United Kingdom; e-mail: r.wickremasinghe@medsch.ucl.ac.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal