The recommended dose (bolus 0.4 mg/kg followed by 0.15 mg/kg per hour) of lepirudin, a direct thrombin inhibitor licensed for treatment of heparin-induced thrombocytopenia (HIT), is too high. Starting in 2001, we omitted the bolus and reduced maintenance dose by at least one-third. Analyzing 53 HIT patients treated between January 2001 and February 2007, we observed that therapeutic anticoagulation intensity already 4 hours after lepirudin start had been reached with the following initial lepirudin doses (median): 0.078 mg/kg per hour [creatinine clearance (CrCl) more than 60 mL/min], 0.040 mg/kg per hour (CrCl 30-60 mL/min), and 0.013 mg/kg per hour (CrCl < 30 mL/min). The efficacy of this treatment was documented by increasing platelets and decreasing D-dimers. Based on this experience, we derived a lepirudin dosing regimen, which was prospectively evaluated treating 15 HIT patients between March 2007 and February 2008. We show that omitting the initial lepirudin bolus and administering 0.08 mg/kg per hour in patients with CrCl more than 60 mL/min, 0.04 mg/kg per hour in patients with CrCl 30-60 mL/min, and 0.01 to 0.02 mg/kg per hour in those with CrCl less than 30 mL/min is efficacious and safe, as documented by increasing platelet counts, decreasing D-dimer levels, and rare thrombotic (1 of 46) and major bleeding (4 of 46) complications.
Introduction
Heparin-induced thrombocytopenia (HIT) is a drug-induced antibody-mediated condition characterized by a highly prothrombotic state.1,2 HIT is caused by IgG antibodies directed against heparin-bound platelet factor 4 (PF4). Macromolecular ternary complexes (HIT-antibody/PF4/heparin) are able to activate platelets, endothelial cells, and monocytes, leading to excessive in vivo thrombin generation.3 The treatment of HIT requires not only the immediate discontinuation of all heparin but also the introduction of alternative nonheparin anticoagulation in therapeutic doses, to counterbalance the strong procoagulant state.1,2 Drugs approved for HIT treatment are the direct thrombin inhibitors lepirudin and argatroban and the heparinoid danaparoid.
Lepirudin (Refludan; Celgene International Sàrl, Boudry, Switzerland), a recombinant hirudin, is an irreversible, specific, direct thrombin inhibitor that is renally excreted and has a half-life of 60 to 90 minutes.4 The problems related to its usage are: the difficulty of its laboratory monitoring,5 the high bleeding risk,6 and fluctuations of its half-life, which can be dramatically increased by renal dysfunction or antibodies delaying its elimination.7 As approved by the European Medical Evaluation Agency (www.emea.europa.eu/humandocs/Humans/EPAR/refludan/refludan.htm) and the US Food and Drug Administration (www.fda.gov/cder/consumerinfo/druginfo/refludan.htm), the manufacturer recommends—in the summary of product characteristics—starting lepirudin treatment with a 0.4 mg/kg bolus dose followed by a continuous intravenous infusion of 0.15 mg/kg per hour, adjusted to maintain an activated partial thromboplastin time (aPTT) 1.5 to 2.5 times the baseline value. Lower dosages are recommended for patients with impaired renal function: 50% of the standard dose for patients with a creatinine clearance (CrCl) 45 to 60 mL/min, 30% for CrCl 30 to 44 mL/min, or 15% for CrCl 15 to 29 mL/min; patients with a CrCl less than 15 mL/min should receive no more than a bolus of 0.1 mg/kg every second day.
Late in 2000, we observed that HIT patients receiving the recommended dose of lepirudin were systematically found to be overanticoagulated at first laboratory monitoring and always required a dose reduction. Starting in 2001, we therefore reduced the dosing regimen of lepirudin as follows: initially by omitting the bolus and by decreasing the recommended maintenance dose by one-third, taking into account the renal function for further adjustments; over time, we realized that this was not yet adequate and successively further reduced starting doses. Three recent publications reporting that lower lepirudin doses than those officially recommended may be sufficient are in agreement with our observation; however, no experimentally established dosing regimen was proposed.6,8,9
The aim of the present 2-phase study (“Patients”) was to define an adequate in-house lepirudin dosing scheme for HIT patients with normal and variably decreased renal function. To the best of our knowledge, this is the first study to propose lepirudin dosing regimens for HIT patients with normal and moderately or severely impaired renal function, thus providing evidence for reduced lepirudin doses, as recently recommended by the American College of Chest Physicians (ACCP) panel.2
Methods
Patients
This was a single-center 2-phase study conducted between January 2001 and February 2008 at the University Hospital Inselspital (Bern, Switzerland). In a first, retrospective investigational study, we collected clinical and laboratory data on 53 HIT patients treated with lepirudin between January 2001 and February 2007. The data allowed us to establish an in-house dosing scheme for lepirudin according to the degree of renal function.10 In a second, prospective study between March 2007 and February 2008, we treated 15 HIT patients with the in-house lepirudin dosage (“Prospective evaluation cohort”) to verify the validity of our approach. The study was conducted in accordance with institutional guidelines for observational studies at our University Hospital and in accordance with Principle 32 of the Declaration of Helsinki. Noteworthy, at our institution, we evaluate all patients with suspected HIT by assessing the pretest clinical probability according to the 4T score11 and by combining it with the antibody titer detected by a rapid particle gel immunoassay, ID-HPF4-PaGIA,12 which has a turnaround time of less than 1 hour. Therefore, lepirudin treatment can be started (after informed patient consent) without delay, usually within a few hours after HIT has been suspected.13
Data collection
Retrospective investigational study.
We reviewed the clinical files of all patients with a laboratory test for HIT, identifying those treated with lepirudin. We collected information about personal data (age, sex, body weight) and workup for suspected HIT (date of evaluation, 4T pretest clinical probability,11 assessed by the consulting hematologist). For HIT patients treated with lepirudin between 2001 and 2003, the 4T score was retrospectively calculated by 2 authors (M.T., L.A.). We also collected information about detection of antiheparin/PF4-antibodies by ID-HPF4-PaGIA and by enzyme-linked immunosorbent assay (ELISA; GTI-PF4), lepirudin treatment (initial and final lepirudin dosage, number of dose adjustments [increments, reductions, and interruptions lasting ≥ 2 hours], duration of therapy), laboratory data (hemoglobin, hematocrit, leukocyte and platelet count, aPTT, thrombin time, D-dimers, serum creatinine), complications occurring during lepirudin treatment (symptomatic thrombotic and bleeding events), and blood products transfused (packed red blood cells [RBCs], fresh frozen plasma, platelet concentrates), confirming that no patient received platelet concentrates during acute HIT. Major bleeding was defined as fatal or life-threatening and/or associated with a 20 g/L or greater decrease of hemoglobin level and/or requiring transfusion of more than 2 units packed RBCs.
Prospective evaluation study.
The aforementioned data were prospectively collected. Day 0 is the day of evaluation for suspected HIT and start of lepirudin treatment.
Laboratory methods
Blood was drawn into 10-mL plastic syringes (Monovette; Sarstedt, Nümbrecht, Germany) containing 1 mL 0.106 M trisodium citrate. Plasma was prepared within 1 hour by twice centrifuging at 1500g for 10 minutes each at room temperature. Aliquots were stored in polypropylene tubes at −70°C. Anti-PF4/heparin antibodies were detected by ELISA (GTI-PF4 Enhanced; Genetic Testing Institute, Waukesha, WI) measured at 492 nm with a microtiter plate reader (Anthos ht III; Hemotec, Gelterkinden, Switzerland) and by a rapid particle-gel immunoassay (ID-HPF4-PaGIA)14 according to the manufacturer's instructions (DiaMed SA, Cressier sur Morat, Switzerland). In case of a positive test result with undiluted plasma, the antibody titer was determined as previously described.12 Briefly, for a 1:2 dilution, 50 μL plasma was mixed with 50 μL diluent II (DiaMed SA), and subsequent dilutions were obtained by mixing 50 μL of the preceding one with 50 μL diluent II. The reported titer is the last positive detection followed by either borderline or negative results. Coagulation assays were performed on a Behring Coagulation System automated analyzer (Dade Behring, Deerfield, IL). aPTT was measured with Pathromtin SL (Dade Behring); the results were the average of duplicate measurements.15 Thrombin time (TT) was measured in duplicate with thrombin reagent (Dade Behring), with final thrombin concentrations of 1.5 U/mL (TT1.5) and 5 U/mL (TT5). D-dimers were measured by an automated quantitative immunoassay, according to the manufacturer's instructions (VIDAS D-dimer New; bioMérieux, Marcy l'Etoile, France).
Lepirudin monitoring
The in-house therapeutic range had been previously defined by measuring both aPTT and TTs in several samples of pooled normal plasma spiked with increasing concentrations of lepirudin (Table 1). The lepirudin summary of product characteristics published in the official Swiss Pharmacopoea16 states that “to define specific and exact aPTT reference values the in-house reagent/coagulometer system can be calibrated by measuring standardized human plasma spiked with 0.15 μg/mL lepirudin (lower limit) and 1.5 μg/mL lepirudin (upper limit).” When treating HIT patients with lepirudin, we aim for an unclottable TT1.5 and a clottable TT5; in case of an unclottable TT5 we allow for a maximum aPTT prolongation of 2.5 times the patient's baseline value (Table 1); in case of TT1.5 and TT5 in target range and a more than 2.5 times prolongation of the aPTT, we dismiss the latter result because in our hands TT assays are robust and many variables besides lepirudin can affect the aPTT. The therapeutic target we aim for is narrower than that stated in the summary of product characteristics and is in line with the lepirudin concentration of 0.6 to 1.0 μg/mL reported by Greinacher and Warkentin.4 Laboratory monitoring is performed at 4-hour intervals after starting lepirudin infusion or any dosage adjustment; after steady state has been reached (defined as 2 consecutive TT/aPTT values in the target range), laboratory monitoring is performed once daily.2
Defining target ranges for lepirudin
| Lepirudin, μg/mL . | TT1.5, seconds . | TT5, seconds . | aPTT, seconds . | aPTT ratio . |
|---|---|---|---|---|
| minimum-maximum . | minimum-maximum . | median (IQR) . | median (IQR) . | |
| 0.00 | 13.2-16.2 | 29.0 (28.8-30.2) | 1 | |
| 0.10 | 21.4-29.6 | 39.0 (38.8-41.0) | 1.35 (1.34-1.38) | |
| 0.15 | 34.4-46.5 | 42.1 (41.2-45.5) | 1.45 (1.42-1.53) | |
| 0.25 | No clot | 8.5-9.4 | 49.4 (46.9-52.0) | 1.71 (1.62-1.75) |
| 0.50 | No clot | 16.1-38.4 | 59.8 (58.4-63.8) | 2.06 (2.02-2.15) |
| 0.75 | No clot | 50.6-No clot | 69.0 (66.2-73.2) | 2.39 (2.33-2.43) |
| 1.00 | No clot | 91.3-No clot | 72.9 (70.8-81.2) | 2.56 (2.46-2.69) |
| 1.25 | No clot | No clot | 79.8 (77.3-86.5) | 2.81 (2.68-2.87) |
| 1.50 | No clot | No clot | 85.1 (82.1-92.3) | 3.01 (2.84-3.06) |
| 1.75 | No clot | No clot | 90.9 (86.3-98.3) | 3.15 (3.03-3.25) |
| 2.00 | No clot | No clot | 95.8 (92.0-102.7) | 3.29 (3.18-3.47) |
| Lepirudin, μg/mL . | TT1.5, seconds . | TT5, seconds . | aPTT, seconds . | aPTT ratio . |
|---|---|---|---|---|
| minimum-maximum . | minimum-maximum . | median (IQR) . | median (IQR) . | |
| 0.00 | 13.2-16.2 | 29.0 (28.8-30.2) | 1 | |
| 0.10 | 21.4-29.6 | 39.0 (38.8-41.0) | 1.35 (1.34-1.38) | |
| 0.15 | 34.4-46.5 | 42.1 (41.2-45.5) | 1.45 (1.42-1.53) | |
| 0.25 | No clot | 8.5-9.4 | 49.4 (46.9-52.0) | 1.71 (1.62-1.75) |
| 0.50 | No clot | 16.1-38.4 | 59.8 (58.4-63.8) | 2.06 (2.02-2.15) |
| 0.75 | No clot | 50.6-No clot | 69.0 (66.2-73.2) | 2.39 (2.33-2.43) |
| 1.00 | No clot | 91.3-No clot | 72.9 (70.8-81.2) | 2.56 (2.46-2.69) |
| 1.25 | No clot | No clot | 79.8 (77.3-86.5) | 2.81 (2.68-2.87) |
| 1.50 | No clot | No clot | 85.1 (82.1-92.3) | 3.01 (2.84-3.06) |
| 1.75 | No clot | No clot | 90.9 (86.3-98.3) | 3.15 (3.03-3.25) |
| 2.00 | No clot | No clot | 95.8 (92.0-102.7) | 3.29 (3.18-3.47) |
Pooled normal plasma was spiked with increasing concentrations of lepirudin. Thrombin time (TT) and activated partial thromboplastin time (aPTT) were measured with different reagent lots (n = 7). See “Lepirudin monitoring” for details.
No clot signifies TT > 120 seconds.
Renal function
CrCl was either measured and/or calculated according to the Cockroft-Gault formula17 : (1.04 for female; 1.23 for male) × (140 − age, years) × body weight (kg)/serum creatinine (μmol/L). Patients were divided into 3 groups, according to the degree of renal function impair-ment: normal (CrCl > 60 mL/min, n = 34), moderately decreased (CrCl 30-60 mL/min, n = 20), and severely decreased (CrCl < 30 mL/min, n = 14).
Statistical analysis
Quantitative data are expressed as median and interquartile range (IQR) or range. Comparison of categorical data was performed by the Fisher exact test or the χ2 test as appropriate. Comparison between 2 nonpaired groups was performed by the Mann-Whitney rank sum test. Comparison between more than 2 groups was performed by nonparametric analysis of variance. Significance was set at the 5% level. Data were analyzed by SigmaStat software (version 3.1; San Jose, CA). Platelet values on Figures 2A and 3A are reported as mean plus or minus 1 SD; D-dimers on Figures 2B and 3B are depicted by box plots visualizing the median value (horizontal line within the box), the 25th and 75th percentiles (lower and upper borders of the box), the 10th and 90th percentiles (lower and upper whiskers), and each outlier outside the 10th and 90th percentiles (black dots).
Results
Patient population
Overall, we treated 68 HIT patients with lepirudin (median age, 69.3 years; range, 26.5-92.2 years). The population of the first, retrospective investigational study (January 2001 to February 2007) consisted of 21 women (median age, 73.0 years; range, 50.6-88.7 years) and 32 men (median age, 69.1 years; range, 26.5-87.0 years). During the second, prospective validation study (March 2007 to February 2008), an additional 15 consecutive HIT patients (6 women and 9 men; median age, 66.5 and 69.2 years; range, 28.4-83.8 and 30.3-92.2 years, respectively) were treated with the in-house lepirudin dosage scheme derived from the results of the first study. Table 2 summarizes relevant personal data, renal function, and results of HIT workup of both patient cohorts.
Patient characteristics
| Characteristics . | . | Investigation cohort (N = 53) . | Evaluation cohort (N = 15) . | ||
|---|---|---|---|---|---|
| Personal data | |||||
| Age, y | Median (IQR) | 69.4 | (58.9-74.0) | 69.2 | (61.2-74.7) |
| Sex, females | n (%) | 21 | (40) | 6 | (40) |
| Body weight, kg | Median (IQR) | 79.7 | (70.7-87.7) | 77.5 | (71.0-80.0) |
| HIT workup | |||||
| Platelet count at day 0, 109/L | Median (IQR) | 53 | (37-88) | 53 | (28-66) |
| Thrombosis at day 0 (HIT-T) | n (%) | 27 | (51) | 7 | (47) |
| D-dimer at day 0, μg/L | Median (IQR) | 5510 | (3401-6129) | 4874 | (3277-10 688) |
| Pretest score (4T) | Median (range) | 4 | (4-7) | 4 | (4-6) |
| Titer (ID-HPF4-PaGIA) | Median (range) | 8 | (2-128) | 4 | (2-256) |
| OD (GTI-PF4) | Median (IQR) | 2.801 | (1.822-3.000) | 2.401 | (1.300-2.868) |
| Renal function | |||||
| Creatinine, μmol/L | Median (IQR) | 114 | (75-150) | 130 | (81-198) |
| CrCl > 60 mL/min | n (%) | 29 | (55) | 5 | (33.3) |
| CrCl 30-60 mL/min | n (%) | 15 | (28) | 5 | (33.3) |
| CrCl < 30 mL/min | n (%) | 9 | (17) | 5 | (33.3) |
| Characteristics . | . | Investigation cohort (N = 53) . | Evaluation cohort (N = 15) . | ||
|---|---|---|---|---|---|
| Personal data | |||||
| Age, y | Median (IQR) | 69.4 | (58.9-74.0) | 69.2 | (61.2-74.7) |
| Sex, females | n (%) | 21 | (40) | 6 | (40) |
| Body weight, kg | Median (IQR) | 79.7 | (70.7-87.7) | 77.5 | (71.0-80.0) |
| HIT workup | |||||
| Platelet count at day 0, 109/L | Median (IQR) | 53 | (37-88) | 53 | (28-66) |
| Thrombosis at day 0 (HIT-T) | n (%) | 27 | (51) | 7 | (47) |
| D-dimer at day 0, μg/L | Median (IQR) | 5510 | (3401-6129) | 4874 | (3277-10 688) |
| Pretest score (4T) | Median (range) | 4 | (4-7) | 4 | (4-6) |
| Titer (ID-HPF4-PaGIA) | Median (range) | 8 | (2-128) | 4 | (2-256) |
| OD (GTI-PF4) | Median (IQR) | 2.801 | (1.822-3.000) | 2.401 | (1.300-2.868) |
| Renal function | |||||
| Creatinine, μmol/L | Median (IQR) | 114 | (75-150) | 130 | (81-198) |
| CrCl > 60 mL/min | n (%) | 29 | (55) | 5 | (33.3) |
| CrCl 30-60 mL/min | n (%) | 15 | (28) | 5 | (33.3) |
| CrCl < 30 mL/min | n (%) | 9 | (17) | 5 | (33.3) |
IQR indicates interquartile range; day 0, day of clinical and laboratory evaluation for suspected HIT; HIT-T, HIT with thrombosis; OD, optical density; and CrCl, creatinine clearance.
Retrospective investigational study
Lepirudin starting dose and anticoagulation intensity at first monitoring.
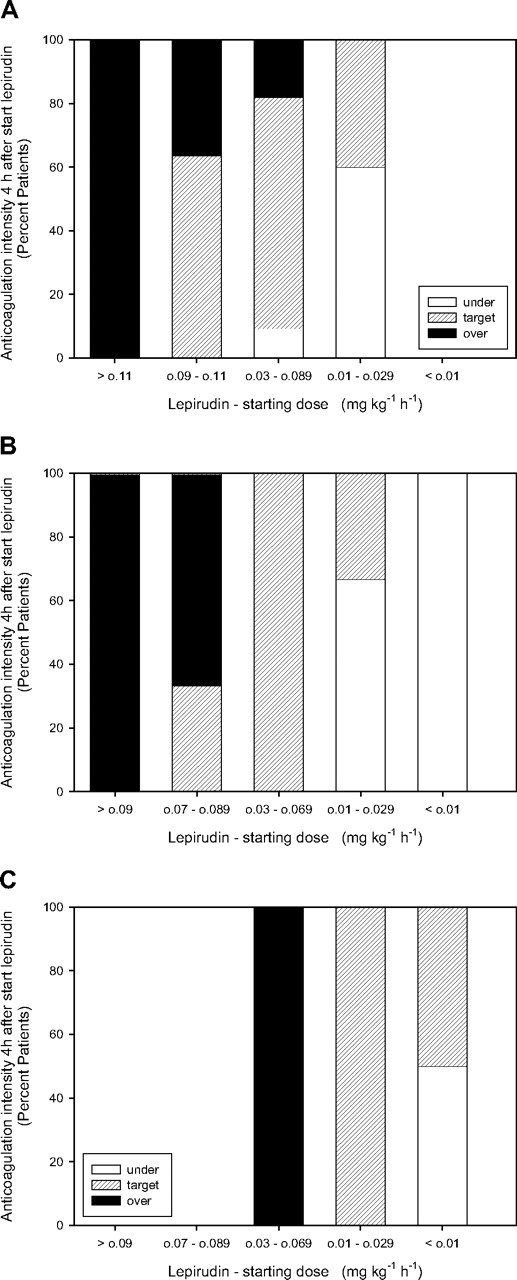
Among the 29 patients with a normal renal function, median lepirudin starting dose was 0.087 mg/kg per hour (IQR, 0.048-0.096 mg/kg per hour). However, a starting dose more than 0.11 mg/kg per hour always led to overtreatment (Figure 1A). Seventeen of 29 patients (58.6%) were within therapeutic range (“Lepirudin monitoring”) already at first laboratory monitoring 4 hours after initiation of lepirudin infusion, showing a median aPTT prolongation of 2.03 (IQR, 1.79-2.83) times the baseline value and a clottable TT5 (median, 21.9 seconds; IQR, 17.9-54.3 seconds) in 15 of 17 instances. For these 17 patients, the median starting dose was 0.078 mg/kg per hour (IQR, 0.049-0.093 mg/kg per hour). Eight patients (27.6%) who were overanticoagulated at first monitoring had received a median starting lepirudin dose of 0.098 mg/kg per hour (IQR, 0.081-0.124 mg/kg per hour). Four patients (13.8%) who were undertreated had received a median dose of 0.022 mg/kg per hour (IQR, 0.014-0.039 mg/kg per hour) (Table 3).
Anticoagulation intensity at first laboratory monitoring 4 hours after starting lepirudin infusion (investigation cohort). (A) Patients with normal renal function (CrCl > 60 mL/min, n = 29) classified according to lepirudin starting dose: more than 0.11 mg/kg per hour (n = 2), 0.09 to 0.11 kg/hr (n = 11), 0.03 to 0.089 kg/hr (n = 11), and 0.01 to 0.029 kg/hr (n = 5). (B) Patients with moderately impaired renal function (CrCl 30-60 mL/min, n = 15) classified according to lepirudin starting dose: more than 0.09 mg/kg per hour (n = 4), 0.07 to 0.089 mg/kg per hour (n = 3), 0.03 to 0.069 mg/kg per hour (n = 4), 0.01 to 0.029 mg/kg per hour (n = 4), and less than 0.01 mg/kg per hour (n = 1). (C) Patients with severely impaired renal function (CrCl < 30 mL/min, n = 9) classified according to lepirudin starting dose: 0.03 to 0.069 mg/kg per hour (n = 1), 0.01 to 0.029 mg/kg per hour (n = 4), and less than 0.01 mg/kg per hour (n = 4). ■ represent patients with clotting times greater than therapeutic ranges; ▨, patients with clotting times within therapeutic ranges; □, patients with clotting times less than therapeutic ranges.
Anticoagulation intensity at first laboratory monitoring 4 hours after starting lepirudin infusion (investigation cohort). (A) Patients with normal renal function (CrCl > 60 mL/min, n = 29) classified according to lepirudin starting dose: more than 0.11 mg/kg per hour (n = 2), 0.09 to 0.11 kg/hr (n = 11), 0.03 to 0.089 kg/hr (n = 11), and 0.01 to 0.029 kg/hr (n = 5). (B) Patients with moderately impaired renal function (CrCl 30-60 mL/min, n = 15) classified according to lepirudin starting dose: more than 0.09 mg/kg per hour (n = 4), 0.07 to 0.089 mg/kg per hour (n = 3), 0.03 to 0.069 mg/kg per hour (n = 4), 0.01 to 0.029 mg/kg per hour (n = 4), and less than 0.01 mg/kg per hour (n = 1). (C) Patients with severely impaired renal function (CrCl < 30 mL/min, n = 9) classified according to lepirudin starting dose: 0.03 to 0.069 mg/kg per hour (n = 1), 0.01 to 0.029 mg/kg per hour (n = 4), and less than 0.01 mg/kg per hour (n = 4). ■ represent patients with clotting times greater than therapeutic ranges; ▨, patients with clotting times within therapeutic ranges; □, patients with clotting times less than therapeutic ranges.
Renal function, anticoagulation intensity at first control, and lepirudin dose (investigation cohort)
| CrCl, mL/minAnticoagulation intensity* . | n . | Lepirudin dose, mg kg−1 hour−1 . | P . | . | |
|---|---|---|---|---|---|
| Median (interquartile range) . | |||||
| Start . | End . | Paired . | |||
| More than 60, n = 29 | |||||
| Over | 8 | 0.098 (0.081-0.124) | 0.033 (0.014-0.055) | 0.007 | |
| Target | 17 | 0.078 (0.049-0.093) | 0.034 (0.027-0.058) | 0.001 | |
| Under | 4 | 0.022 (0.014-0.039) | 0.036 (0.030-0.042) | 0.235 | |
| 0.001 | 0.867 | ANOVA | |||
| 30-60, n = 15 | |||||
| Over | 6 | 0.098 (0.086-0.102) | 0.015 (0.011-0.029) | 0.005 | |
| Target | 7 | 0.040 (0.025-0.050) | 0.037 (0.017-0.047) | 0.294 | |
| Under | 2 | 0.010 (0.009-0.011) | 0.011 (0.007-0.016) | 0.737 | |
| < 0.001 | 0.313 | ANOVA | |||
| Less than 30, n = 9 | |||||
| Over | 0 | ||||
| Target | 7 | 0.013 (0.008-0.026) | 0.007 (0.003-0.018) | 0.182 | |
| Under | 2 | 0.008 (0.006-0.009) | 0.005 | NA | |
| 0.316 | NA | ANOVA | |||
| CrCl, mL/minAnticoagulation intensity* . | n . | Lepirudin dose, mg kg−1 hour−1 . | P . | . | |
|---|---|---|---|---|---|
| Median (interquartile range) . | |||||
| Start . | End . | Paired . | |||
| More than 60, n = 29 | |||||
| Over | 8 | 0.098 (0.081-0.124) | 0.033 (0.014-0.055) | 0.007 | |
| Target | 17 | 0.078 (0.049-0.093) | 0.034 (0.027-0.058) | 0.001 | |
| Under | 4 | 0.022 (0.014-0.039) | 0.036 (0.030-0.042) | 0.235 | |
| 0.001 | 0.867 | ANOVA | |||
| 30-60, n = 15 | |||||
| Over | 6 | 0.098 (0.086-0.102) | 0.015 (0.011-0.029) | 0.005 | |
| Target | 7 | 0.040 (0.025-0.050) | 0.037 (0.017-0.047) | 0.294 | |
| Under | 2 | 0.010 (0.009-0.011) | 0.011 (0.007-0.016) | 0.737 | |
| < 0.001 | 0.313 | ANOVA | |||
| Less than 30, n = 9 | |||||
| Over | 0 | ||||
| Target | 7 | 0.013 (0.008-0.026) | 0.007 (0.003-0.018) | 0.182 | |
| Under | 2 | 0.008 (0.006-0.009) | 0.005 | NA | |
| 0.316 | NA | ANOVA | |||
Over indicates TT/aPTT above therapeutic ranges; Target, TT/aPTT within therapeutic ranges; Under, TT/aPTT below therapeutic ranges 4 hours after lepirudin start; and NA, not applicable.
Among the 15 patients with a moderately decreased renal function (CrCl 30-60 mL/min), median lepirudin starting dose was 0.050 mg/kg per hour (IQR, 0.025-0.091 mg/kg per hour). Starting with lepirudin doses more than 0.090 mg/kg per hour always led to overtreatment and doses less than 0.010 mg/kg per hour to undertreatment (Figure 1B). Seven of 15 patients (46.7%) were within therapeutic range already 4 hours after initiation of lepirudin infusion, showing a median aPTT prolongation of 2.09 (IQR, 1.81-2.75) and a clottable TT5 (median, 28.3 seconds; IQR, 9.4-52.7 seconds) in 7 of 7 cases. These 7 patients had received a median starting dose of 0.040 mg/kg per hour (IQR, 0.025-0.050 mg/kg per hour). Six patients (40%) who were overanticoagulated at first monitoring had received a median starting dose of 0.098 mg/kg per hour (IQR, 0.086-0.102 mg/kg per hour). Two patients (13.3%) who were undertreated had received a starting dose of 0.009 and 0.011 mg/kg per hour, respectively (Table 3).
In 9 patients with severely impaired renal function (CrCl < 30 mL/min), median lepirudin starting dose was 0.012 mg/kg per hour (IQR, 0.007-0.020 mg/kg per hour). Seven of 9 patients (77.8%) were therapeutically anticoagulated at first monitoring, showing a median aPTT prolongation of 2.11 (IQR, 1.98-2.26) and a clottable TT5 (median, 8.4 seconds; IQR, 7.9-9.3 seconds) in 7 of 7 cases. They had received a median starting dose of 0.013 mg/kg per hour (IQR, 0.008-0.026 mg/kg per hour). Two patients (22%) who were undertreated had received a starting dose of 0.005 and 0.012 mg/kg per hour, respectively (Table 3).
The median lepirudin starting doses resulting in therapeutic anticoagulation intensity at first monitoring (ie, 0.078, 0.040 and 0.013 mg/kg per hour) significantly differed among the 3 groups of HIT patients with normal, moderately, or severely impaired renal function (P < .001).
Duration and dose adjustments of lepirudin treatment.
The median duration of all lepirudin treatments was 7 days (IQR, 4-14 days; range, 1-75 days). During the entire treatment period, we registered a median of 4 dose adjustments per patient (IQR, 2-8 dose adjustments per patient; range, 0-16 dose adjustments per patient), 61% of which were dose reductions. Although treatment length was similar among all HIT patients independently from the initial anticoagulation intensity, the number of dose adjustments was lower among patients who were within therapeutic limits already at first monitoring (median, 3, IQR, 1-6, range, 0-11) compared with those who were not (median, 7, IQR, 3-12, range, 0-16; P = .003). Dose adjustments were downwards in 60% of patients within therapeutic range already at first monitoring, in 75% of those who were initially overanticoagulated, and in 35% of those with insufficient anticoagulation 4 hours after starting lepirudin (P < .001). Table 3 displays initial and final lepirudin dosages among the different patient categories.
Efficacy of lepirudin starting dose.
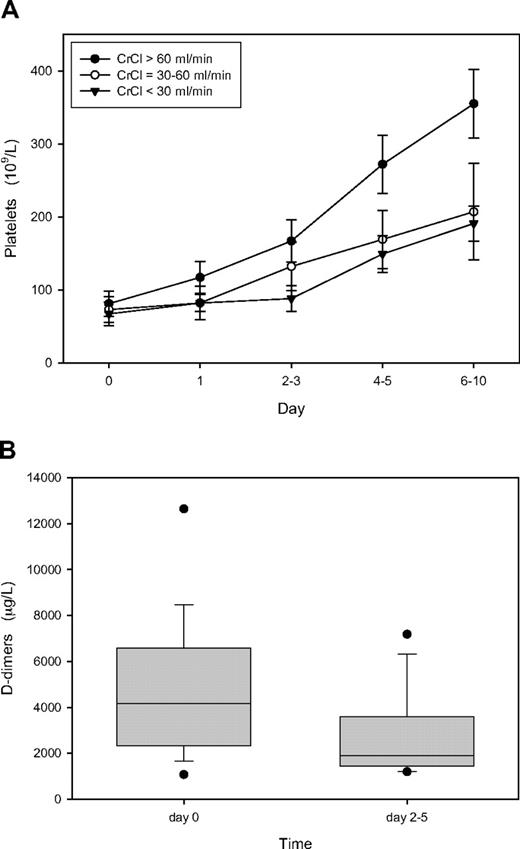
The biologic efficacy of omitting a bolus and using a reduced lepirudin starting dose was assessed by the course of platelet count and D-dimer values after initiation of the alternative anticoagulation. Figure 2A shows that the platelet count increased and normalized among the 31 patients who were within therapeutic range already at first monitoring despite reduced starting lepirudin dose. Moreover, D-dimer levels decreased in all 31 patients from a median of 4170 μg/L at HIT diagnosis to 1892 μg/L at follow-up, between day 2 and day 5 (Figure 2B). For comparison, during the same time frame, D-dimers decreased from median 5668 μg/L to 3322 μg/L in patients initially overanticoagulated but remained stable in those with initial subtherapeutic anticoa-gulation (1888 μg/L vs 1612 μg/L).
Course of platelet count and D-dimer among the 31 patients of the investigation cohort who were within therapeutic ranges already at first monitoring after starting lepirudin. (A) Platelet count; mean values plus or minus 1 SD. ● represent patients with normal renal function (CrCl > 60 mL/min; n = 17); ○, those with moderately impaired renal function (CrCl 30-60 mL/min; n = 7); ▼, those with severely impaired renal function (CrCl < 30 mL/min; n = 7). (B) Box plot of D-dimers in all 31 patients depicting the median value (horizontal line within the box), the 25th and 75th percentiles (bottom and top borders of the box), the 10th and 90th percentiles (bottom and top whiskers), and outliers outside the 10th and 90th percentiles (black dots). Day 0 indicates the day of clinical and laboratory evaluation for suspected HIT and start of lepirudin treatment.
Course of platelet count and D-dimer among the 31 patients of the investigation cohort who were within therapeutic ranges already at first monitoring after starting lepirudin. (A) Platelet count; mean values plus or minus 1 SD. ● represent patients with normal renal function (CrCl > 60 mL/min; n = 17); ○, those with moderately impaired renal function (CrCl 30-60 mL/min; n = 7); ▼, those with severely impaired renal function (CrCl < 30 mL/min; n = 7). (B) Box plot of D-dimers in all 31 patients depicting the median value (horizontal line within the box), the 25th and 75th percentiles (bottom and top borders of the box), the 10th and 90th percentiles (bottom and top whiskers), and outliers outside the 10th and 90th percentiles (black dots). Day 0 indicates the day of clinical and laboratory evaluation for suspected HIT and start of lepirudin treatment.
Of note, among the 31 patients with therapeutic anticoagulation already at first monitoring, only 1 thromboembolic complication occurred during lepirudin treatment (Table 4, patient D). In this patient, therapeutic anticoagulation with lepirudin was interrupted for several hours on 2 occasions: on day 5, for the placement of an intravenous catheter (complication diagnosed after this interruption: distal deep vein thrombosis of the left leg) and on day 7, for a suspected (but eventually not confirmed) intra-abdominal hemorrhage (complication diagnosed after the second interruption: massive pulmonary embolism).
Thrombotic complications during lepirudin treatment
| Patient . | Sex . | Age, y . | HIT-T . | Anti-PF4/H antibodies . | CrCl, mL/min . | Lepirudin treatment . | Anticoagulation at first monitoring . | Dose adjustments . | Event . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dose, mg kg−1 hr−1 . | Length, days . | ↑ . | ↓ . | Stop* . | |||||||||||
| Titer . | OD . | Start . | End . | n . | n . | n . | Localization . | Day . | |||||||
| A | Male | 36.0 | Arterial | 32 | 2.426 | 64 | 0.100 | 0.014 | 7 | Over | 3 | 5 | 2 | Amputation, distal | 7 |
| B | Female | 73.5 | Vein bypass | 16 | 2.129 | 35 | 0.150 | 0.003 | 17 | Over | 3 | 13 | 3 | Amputation, proximal | 6 |
| C | Male | 50.2 | Arterial | 64 | 2.801 | 95 | 0.100 | 0.070 | 5 | Over | 0 | 3 | 0 | Amputation, distal | 4 |
| D | Female | 88.7 | Arterial | 16 | 2.260 | 24 | 0.020 | 0.020 | 7 | Target | 0 | 0 | 2 | DVT; PE | 5; 7 |
| Patient . | Sex . | Age, y . | HIT-T . | Anti-PF4/H antibodies . | CrCl, mL/min . | Lepirudin treatment . | Anticoagulation at first monitoring . | Dose adjustments . | Event . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dose, mg kg−1 hr−1 . | Length, days . | ↑ . | ↓ . | Stop* . | |||||||||||
| Titer . | OD . | Start . | End . | n . | n . | n . | Localization . | Day . | |||||||
| A | Male | 36.0 | Arterial | 32 | 2.426 | 64 | 0.100 | 0.014 | 7 | Over | 3 | 5 | 2 | Amputation, distal | 7 |
| B | Female | 73.5 | Vein bypass | 16 | 2.129 | 35 | 0.150 | 0.003 | 17 | Over | 3 | 13 | 3 | Amputation, proximal | 6 |
| C | Male | 50.2 | Arterial | 64 | 2.801 | 95 | 0.100 | 0.070 | 5 | Over | 0 | 3 | 0 | Amputation, distal | 4 |
| D | Female | 88.7 | Arterial | 16 | 2.260 | 24 | 0.020 | 0.020 | 7 | Target | 0 | 0 | 2 | DVT; PE | 5; 7 |
HIT-T indicates HIT with thrombosis; OD, optical density; CrCl, creatinine clearance; Over, TT/aPTT above therapeutic ranges; Target, TT/aPTT within therapeutic ranges; Vein bypass, occlusion of a greater saphenous vein bypass in the context of chronic peripheral arterial occlusive disease; Amputation, limb amputation (distal, below-knee; proximal, above-knee); DVT, deep vein thrombosis; and PE, pulmonary embolism.
Stop indicates instances in which lepirudin infusion was temporarily interrupted for 2 hours or longer.
We observed 3 additional thromboembolic complications for patients on lepirudin (Table 4): thrombosis of muscle flap of the distal left lower leg after polytrauma leading to below-knee amputation on day 7, occlusion of a great saphenous vein bypass in the context of a chronic peripheral arterial occlusive disease leading to amputation of the right leg on day 6, and rethrombosis of the right superficial femoral artery and truncus tibiofibularis leading to below-knee amputation on day 4. All 3 patients were initially overanticoagulated and experienced frequent dose reductions, including a total of 5 interruptions (each lasting at least 2 hours) among 2 of them; all 3 patients were still on lepirudin at time of amputation. Interestingly, no thromboembolic complications were observed among the 8 patients with an insufficient anticoagulation intensity at first control.
Bleeding during lepirudin treatment.
Overall, no fatal bleedings were observed. Seventeen of 53 patients (32.1%) required transfusion of packed RBCs during lepirudin treatment (Table 5): 6 among the 14 patients with a too high starting dose (43.6%), 10 among the 31 patients with an adequate starting dose (32.6%), and 1 among the 8 patients with insufficient starting dose (12.5%). The incidence of severe bleeding (defined as fatal or life-threatening and/or associated with a hemoglobin decrease of more than or equal to 20 g/L or requirement of > 2 packed RBCs), was higher among those patients who were initially overanticoagulated (28.6%) compared with those with adequate starting dose (9.7%). This is also reflected by a higher median number of packed RBC units administered per transfused patient (Table 5).
Bleeding complications during lepirudin treatment
| Anticoagulation intensity* . | . | Investigation cohort . | Evaluation cohort . | ||
|---|---|---|---|---|---|
| Over (n = 14) . | Target (n = 31) . | Under (n = 8) . | Target (n = 15) . | ||
| Life-threatening bleeding | n | 1 | 0 | 0 | 0 |
| Hb decrease, g/L | Median (IQR) | 11 (9-15) | 10 (3-14) | 10 (6-17) | 10 (3-14) |
| Hb decrease ≥20 g/L | n/N (%) | 3/14 (21.4) | 3/31 (9.7) | 2/8 (25.0) | 1/15 (6.7) |
| Patients requiring PRBC† | n/N (%) | 6/14 (43.6) | 10/31 (32.6) | 1/8 (12.5) | 5/15 (33.3) |
| Patients requiring > 2 PRBCs | n/N (%) | 4/14 (28.6) | 3/31 (9.7) | 1/8 (12.5) | 1/15 (6.7) |
| Units of PRBC/transfused patient | Median | 5 | 2 | 6 | 2 |
| Anticoagulation intensity* . | . | Investigation cohort . | Evaluation cohort . | ||
|---|---|---|---|---|---|
| Over (n = 14) . | Target (n = 31) . | Under (n = 8) . | Target (n = 15) . | ||
| Life-threatening bleeding | n | 1 | 0 | 0 | 0 |
| Hb decrease, g/L | Median (IQR) | 11 (9-15) | 10 (3-14) | 10 (6-17) | 10 (3-14) |
| Hb decrease ≥20 g/L | n/N (%) | 3/14 (21.4) | 3/31 (9.7) | 2/8 (25.0) | 1/15 (6.7) |
| Patients requiring PRBC† | n/N (%) | 6/14 (43.6) | 10/31 (32.6) | 1/8 (12.5) | 5/15 (33.3) |
| Patients requiring > 2 PRBCs | n/N (%) | 4/14 (28.6) | 3/31 (9.7) | 1/8 (12.5) | 1/15 (6.7) |
| Units of PRBC/transfused patient | Median | 5 | 2 | 6 | 2 |
Over, TT/aPTT above therapeutic ranges; Target, TT/aPTT within therapeutic ranges; Under, TT/aPTT below therapeutic ranges at initial monitoring 4 hours after starting lepirudin infusion.
PRBCs indicates packed red blood cells.
Prospective evaluation cohort
Based on the results of the retrospective investigation, in March 2007 we started to treat HIT patients with following dosing schedule: 0.08 mg/kg per hour (without initial bolus) for patients with normal renal function (n = 5), 0.04 mg/kg per hour for those with a CrCl 30 to 60 mL/min (n = 5), and 0.01 to 0.02 mg/kg per hour for those with a severely impaired renal function (0.02 mg/kg per hour for 3 patients with a CrCl close to 30 mL/min and 0.01 mg/kg per hour for 2 patients with a CrCl < 15 mL/min). Patient characteristics are summarized in Table 2.
All 15 patients were within therapeutic ranges already at first control, showing a clottable TT5 (median, 14.9 seconds; IQR, 11.6-26.9 seconds) in 14 of 15 cases and a median aPTT prolongation of 2.02 (IQR, 1.84-2.21) times the baseline value. The median duration of lepirudin treatment was 8 days (IQR, 5-18 days; range, 3-98 days), with a median of 1 dose adjustment (IQR, 0-2 dose adjustments; range, 1-3 dose adjustments) 62% of which were dose reductions. Final lepirudin doses were 0.073 mg/kg per hour (median; range, 0.067-0.080 mg/kg per hour) in patients with normal renal function, 0.030 mg/kg per hour (median; range, 0.023-0.050 mg/kg per hour), and 0.013 mg/kg per hour (median; range, 0.010-0.023 mg/kg per hour) among patients with moderate and severe renal impairment, respectively.
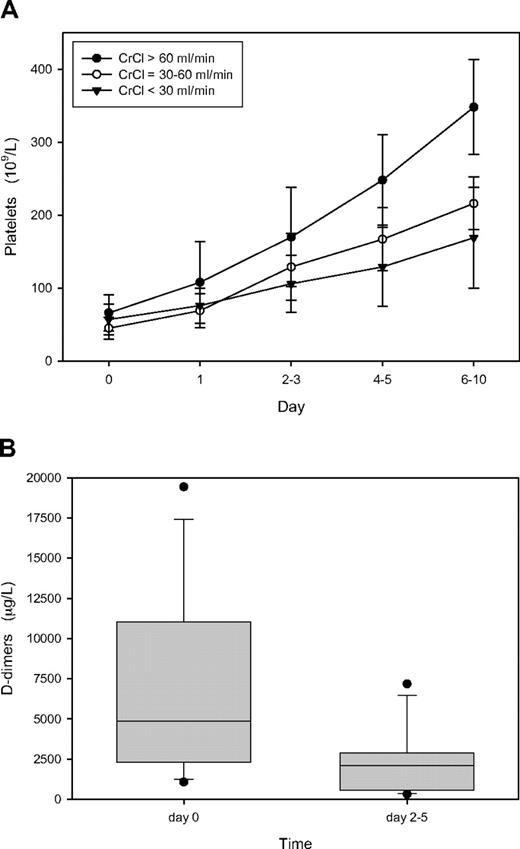
The biologic efficacy of our in-house lepirudin dosage scheme is demonstrated in Figure 3, showing that the platelet count normalized in all 3 patient groups (Figure 3A) and that D-dimer levels decreased in all 15 patients from a median of 4874 μg/L at HIT diagnosis to 2111 μg/L at follow-up, between days 2 and 5 (Figure 3B). No thromboembolic complications occurred during lepirudin treatment.
Course of platelet count and D-dimer among the 15 patients of the evaluation cohort. (A) Platelet count; mean values plus or minus 1 SD. ● represent patients with normal renal function (CrCl > 60 mL/min; n = 5); ○, those with moderately impaired renal function (CrCl 30-60 mL/min; n = 5); ▼, those with severely impaired renal function (CrCl < 30 mL/min; n = 5). (B) Box plot of D-dimers in all 15 patients depicting the median value (horizontal line within the box), the 25th and 75th percentiles (bottom and top borders of the box), the 10th and 90th percentiles (bottom and top whiskers), and outliers outside the 10th and 90th percentiles (●). Day 0 indicates the day of clinical and laboratory evaluation for suspected HIT and start of lepirudin treatment.
Course of platelet count and D-dimer among the 15 patients of the evaluation cohort. (A) Platelet count; mean values plus or minus 1 SD. ● represent patients with normal renal function (CrCl > 60 mL/min; n = 5); ○, those with moderately impaired renal function (CrCl 30-60 mL/min; n = 5); ▼, those with severely impaired renal function (CrCl < 30 mL/min; n = 5). (B) Box plot of D-dimers in all 15 patients depicting the median value (horizontal line within the box), the 25th and 75th percentiles (bottom and top borders of the box), the 10th and 90th percentiles (bottom and top whiskers), and outliers outside the 10th and 90th percentiles (●). Day 0 indicates the day of clinical and laboratory evaluation for suspected HIT and start of lepirudin treatment.
No fatal bleedings were observed. Five of 15 patients required transfusion of packed RBCs during lepirudin treatment, with a median of 2 units packed RBCs administered per transfused patient (Table 5). The incidence of severe bleeding was 6.7%, comparable with that observed among the 31 patients of the investigational cohort with adequate anticoagulation intensity already at the first control after lepirudin start (Table 5).
Discussion
The European Medicines Evaluation Agency- and FDA-approved lepirudin dose for HIT patients (eg, for patients with normal renal function administration of an 0.4 mg/kg bolus followed by a continuous intravenous infusion of 0.15 mg/kg per hour, adjusted to maintain an aPTT 1.5-2.5 times the baseline value) leads to overanticoagulation. Recent publications have reported that lower doses may be sufficient.6,8,9
In the present work, we show that omission of the initial bolus and a reduced lepirudin starting dose (0.08 mg/kg per hour for patients with normal renal function, 0.04 mg/kg per hour for patients with CrCl 30-60 mL/min, and 0.01-0.02 mg/kg per hour for those with CrCl < 30 mL/min) is efficacious and safe. This in-house dosing scheme was derived from the retrospective analysis of 53 HIT patients10 and prospectively evaluated treating additional 15 consecutive HIT patients.
Overall, among the 46 patients (retrospective cohort, n = 31; prospective cohort, n = 15) within therapeutic range already at first monitoring, the aPTT was approximately twice the baseline value (median, 2.08; IQR, 1.82-2.55); the platelet count increased and normalized within 10 days (Figures 2A, 3A); the D-dimer value decreased within 2 to 5 days (Figures 2B, 3B), demonstrating a dampening of the in vivo thrombin generation characteristic of acute HIT.3 The rate of thrombotic complications observed during lepirudin treatment in the whole patient population is 5.9% (4 of 68) and compares very well with the values of 11.2% observed in the HAT-3 study6 and of 13.8% reported by the Groupe d'Etude sur l'Hémostase et la Thrombose (GEHT)–HIT study group.9 Of note, 3 of the 4 thromboembolic complications occurred among patients who were initially overanticoagulated and required frequent dose reductions (Table 4). Among the 31 patients of the retrospective cohort with a therapeutic anticoagulation intensity already at first monitoring, we observed only one thromboembolic complication and none among the 15 patients of the prospective evaluation cohort, giving an overall value of 2.2% (1 of 46) among HIT patients with early adequate anticoagulation intensity. Taken together, these data strongly suggest a good efficacy of our reduced dosing schedule.
The occurrence of progressive thromboses among 3 of the 14 patients who were initially overanticoagulated is noteworthy (Table 4). As suggested by Greinacher and Warkentin,4 premature discontinuation of lepirudin may lead to “rebound hypercoagulability” because of persisting in vivo thrombin generation. The 3 patients reported here experienced many more dose reductions than increases (21 vs 6); among 2 of them (patients A and B in Table 4), lepirudin administration was interrupted a total of 5 times for periods of at least 2 hours each, and final lepirudin doses were much lower than expected for the degree of renal function. These observations suggest that excessive lepirudin dose reductions and, in particular, prolonged interruptions of lepirudin infusion may be responsible for the failure of controlling the severe procoagulant state of acute HIT. This concept is further illustrated by patient D of Table 4: despite therapeutic anticoagulation with lepirudin from the very beginning, 2 interruptions over several hours led to severe and ultimately fatal thromboembolic complications.
Concerning safety, we did not register any fatal bleeding. Overall, 22 of 68 patients (32.4%) required transfusion of packed RBCs (Table 5). We observed major bleedings (defined as fatal or life-threatening and/or associated with a 20 g/L or greater decrease of hemoglobin level and/or requiring transfusion of > 2 units packed RBC) in 10 of 68 patients (14.7%), which is in the range (14.0%-21.7%) reported by a combined analysis of the 3 HAT lepirudin studies.6 In the present study, 4 of the 46 patients within therapeutic range already at first monitoring (8.7%) required more than 2 units packed RBCs compared with 4 of 14 (28.6%) of those who were initially overanticoagulated (P = .077) and median units of packed RBC administered per transfused patient were 2 compared with 5 (P = .072; Table 5). In sum, among the HIT patients reported in this study, those with a therapeutic anticoagulation already at first monitoring appear to have less severe bleeding. This observation is in line with the data reported by the GEHT-HIT study group.9 However, in countries were argatroban is available, this drug may represent a safer treatment option for HIT patients with severe renal failure because its elimination is mainly hepatobiliary.2
Of note, among all 46 patients (retrospective cohort, n = 31; prospective cohort, n = 15) with therapeutic anticoagulation intensity already at first monitoring, we observed a doubling of the baseline aPTT (see third paragraph in “Discussion”) and 93.5% of them (43 of 46) had a clottable TT5 (median, 17.2 seconds; range, 9.4-45.7 seconds). A comparison of these data with the in vitro calibration studies (Table 1) suggests that a plasma lepirudin concentration close to 0.50 μg/mL is sufficient to achieve an adequate anticoagulation in HIT patients. This is approximately one-third of the upper limit of the therapeutic range (0.15-1.50 μg/mL) reported in the lepirudin product information leaflet16 and at the lower limit of the therapeutic concentration (0.6-1.0 μg/mL) reported by Greinacher and Warkentin.4
Among the investigational patient cohort, there was a significant dose reduction from a median starting dose of 0.078 mg/kg per hour to a final one of 0.034 mg/kg per hour in patients with normal renal function who were within therapeutic ranges since first monitoring (Table 3). This might be explained by the development of antibodies decreasing lepirudin clearance7 or by a reduced lepirudin requirement consecutive to the attenuation of the procoagulant state characteristic of acute HIT.3 This observation suggests that even lower lepirudin dosages may be required in the course of HIT treatment and that lepirudin dose should be assessed daily by clotting assays and course of platelets and D-dimers.
In conclusion, we show that omission of the initial bolus and a starting lepirudin dose of 0.08 mg/kg per hour for HIT patients with normal renal function (CrCl > 60 mL/min), 0.04 mg/kg per hour for those with moderately impaired renal function (CrCl 30-60 mL/min), and 0.01 to 0.02 mg/kg per hour for those with CrCl less than 30 mL/min is an efficacious treatment of the prothrombotic state of acute HIT and appears to be associated with a less severe bleeding tendency compared with that induced by the officially recommended lepirudin dosage. Our proposal is in line with the recently published ACCP guideline2 ; however, with some differences: the ACCP panel stratifies renal function by serum creatinine only and for patients with normal and moderately reduced renal function recommends slightly higher lepirudin doses as we report. Full validation of our proposed regimen will require appropriately designed multicenter studies.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank all the physicians of our Department of Hematology who were involved in the care of HIT patients over the years, the technicians of the Central Hematology Laboratory who perform routine diagnostic HIT assays, Irmela Sulzer for performing the GTI-ELISA, and Therese Jost for administrative assistance.
L.A. was supported by a grant from the Swiss National Science Foundation (grant 3200-065337.01).
Authorship
Contribution: M.T. collected and analyzed the data and wrote the first draft of the manuscript; B.L. helped design research and wrote the manuscript; and L.A. conceived the study, collected and analyzed the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Lorenzo Alberio, Department of Hematology and Central Hematology Laboratory, University Hospital Inselspital, Freiburgstrasse 10, CH-3010 Bern, Switzerland; e-mail: lorenzo.alberio@insel.ch.