Abstract
The conditions leading to the activation/differentiation of T-helper (Th) cells dedicated for B-cell antibody production are still poorly characterized. We now demonstrate that interleukin-6 (IL-6) promotes the differentiation of naive T lymphocytes into helper cells able to promote B-cell activation and antibody secretion. IL-6–driven acquisition of B-cell help capacity requires expression of the signal transducer and activator of transcription 3 (STAT3), but not STAT4 or STAT6 transcription factors, suggesting that the ability to provide help to B cells is not restricted to a well-defined Th1 or Th2 effector population. T cell–specific STAT3-deficient mice displayed reduced humoral responses in vivo that could not be related to an altered expansion of CXCR5-expressing helper T cells. IL-6 was shown to promote IL-21 secretion, a cytokine that was similarly found to promote the differentiation of naive T cells into potent B-cell helper cells. Collectively, these data indicate that the ability to provide B-cell help is regulated by IL-6/IL-21 through STAT3 activation, independently of Th1, Th2, Th17, or follicular helper T cell (TFH) differentiation.
Introduction
On activation by antigen-presenting cells (APCs), naive CD4+ T-helper (Th) precursors can differentiate into functionally distinct T-cell lineages, including Th1, Th2, Th17, and regulatory T (Treg) cells. Among the critical signals that direct the induced patterns of gene expression in maturing helper T-cell subsets are cytokine-induced specific transcription factors. Interleukin-12 (IL-12) regulates Th1 differentiation through activation of the transcription factor signal transducer and activator of transcription 4 (STAT4) and T-bet,1–3 whereas IL-4 drives Th2 differentiation through the actions of STAT6 and GATA-3.4,5 Transforming growth factor-β (TGF-β)–induced FoxP-3 is a master regulator of Treg induction,6 and it has been recently demonstrated that development of Th17 is prompted by a combination of IL-6 plus TGF-β and requires expression of STAT3 and the retinoic acid–related orphan receptor γt (RORγt).7
The help that T cells provide to B cells is a fundamental feature of mammalian immune systems that allow the production of memory B cells and long-lived plasma cells secreting high-affinity antigen-specific immunoglobulins. T-cell help to B cells was long thought to be attributable to the Th2 subset, based on the superior ability of Th2 clones to support in vitro antibody (Ab) production, and the well-documented capacity of Th2-derived cytokines (such as IL-4) to sustain B-cell growth, differentiation, and isotype switch.8,9 However, an increasing number of experimental observations cannot be easily reconciled with this simple view. Th1 cells have been indeed shown to support B-cell responses in vitro and in vivo,10–12 and mouse strains in which Th2 differentiation is strongly impaired (such as cMAF, IL-4, and STAT6 KO mice) retain the ability to secrete antibodies in response to T cell–dependent antigens.13–15 More recently, T cells capable of providing help for B cells were identified in human lymphoid tissues through expression of the chemokine receptor CXCR5 and termed follicular helper T cells (TFH) based on their anatomic localization.16–18 Follicular CXCR5-expressing T lymphocytes appear to be particularly apt as B-cell helpers, as determined by T/B collaboration assays in vitro. These cells fail to secrete large amounts of Th1- or Th2-like cytokines, express a distinct set of genes, and can therefore not be easily classified as either Th1 or Th2.19
It is noteworthy, however, that most T cells up-regulate CXCR5 expression on activation20 and that not all CXCR5+ cells display B-cell help capacity,18 leaving open the question of whether Th cells for B-cell antibody isotype switching and secretion belong to a particular T-cell subset. Moreover, the activation pathway leading naive T cells to acquire B-cell help activity and the relationship between B-cell helpers, and the distinct subpopulations of helper T cells identified to date are still poorly defined. We demonstrate herein that differentiation of T cells endowed with B-cell help capacity strongly relies on the activation of the STAT3 transcription factor both in vitro and in vivo. Accordingly, IL-6, a STAT3-activating cytokine produced by a large array of immune and nonimmune cells, promotes the differentiation of naive T cells into efficient B-lymphocyte helper T cells, independently of Th1, Th2, or Th17 functions. IL-6–mediated STAT3 activation leads to IL-21 secretion that further enhances T-cell help activity for B cells, revealing an unconventional role for IL-21 in promoting T-dependent humoral responses.
Methods
Media and reagents
The medium used throughout this study was RPMI 1640 supplemented with 5% fetal calf serum (FCS), penicillin, streptomycin, glutamine, nonessential amino acids, 1 mM sodium pyruvate, and 5 × 10−5 M2-mercaptoethanol. Murine recombinant IL-4 and IL-6 were purchased from eBioscience (San Diego, CA) and IL-21 from PeproTech (Rocky Hill, NJ). JSI-124 was purchased from Sigma-Aldrich (St Louis, MO). Phycoerythrin (PE)–coupled antibodies for flow cytometry were from eBioscience.
Mice and adoptive transfers
Six- to 8-week-old BALB/c and C57BL/6 mice were purchased from Harlan (Indianapolis, IN). DO11.10, IL-4−/−, IL-6−/−, STAT4−/−, STAT6−/−, and Lck-CRE mice were purchased from The Jackson Laboratory (Bar Harbor, ME). STAT3flox/flox mice were kindly provided by Dr Shizuo Akira (Osaka University, Osaka, Japan). RAG−/− mice were a kind gift of Drs A. Lemoine and M. Braun (Institute for Medical Immunology, Gosselies, Belgium). CD4-CRE mice were a gift from Dr Van Loo (University of Gent, Gent, Belgium). IL-21R−/− have been previously described.21 STAT3flox/flox and Lck-CRE mice were bred to generate T-cell compartment-specific STAT3-deficient mice. The deletion of STAT3 gene in CD4+ T cells was tested by intracellular STAT3 staining and found to be complete (data not shown). In some experiments, STAT3flox/flox were bred with CD4-CRE mice. All experiments using animals were done with the approval of the institutional review boards of all participating institutions.
For adoptive transfers, RAG−/− mice were given 2 × 106 purified CD4+ T cells (isolated from naive wild-type [WT] or knockout [KO] mice) together with 107 purified WT B cells, intravenously, 24 hours before immunization.
Immunizations
Mice were immunized by injecting 50 μg nitrophenyl–keyhole limpet hemocyanin (NP-KLH) or trinitrophenyl-conjugated KLH (TNP-KLH) (Biosearch Technologies, Novato, CA) emulsified in complete Freund adjuvant (CFA), intraperitoneally or 3 × 105 KLH-pulsed dendritic cells (DCs) intravenously. Serum levels of antigen-specific antibodies were determined by enzyme-linked immunosorbent assay (ELISA) according to standard procedures using mouse isotype-specific rat monoclonal antibodies (mAbs). Some mice received 3 × 105 KLH-pulsed DC injected in the footpad (f.p.). Draining lymph nodes were harvested 5 days later and tested for KLH-specific response.
Construction of an IL-6–expressing vector and hydrodynamic injections
The vector pHR′-trip-CMV-eGFP-SIN was derived from the pHR′-CMV-eGFP plasmid (a kind gift from Dr L. Naldini, University of Torino, Torino, Italy22 ) as previously described.23 To express IL-6, the pHR′-trip-CMV-IL-6-SIN plasmid was made by digesting pHR′-trip-CMV-eGFP-SIN with BamH1 and SpeI and inserting mouse IL-6 cDNA in place of enhanced GFP (eGFP) cDNA. The murine IL-6 cDNA was amplified from a PUC8-HP1B5 cDNA clone,24 kindly provided by J.-C. Renauld (Université Catholique de Louvain, Louvain, Belgium). Hydrodynamic procedure for in vivo IL-6 expression was as described previously.25 Briefly, 10 μg plasmid was diluted in 2 mL phosphate-buffered saline (PBS) and then injected at a speed of 5 to 7 seconds into the lateral tail vein of mice.
Cell purifications
CD4+ T cells were purified from naive animals by magnetic depletion of B cells, macrophages, DCs, NK cells, granulocytes, erythroid precursors, and CD8+ T cells, as previously described.26 CD62L+CD4+ T cells were further purified by magnetic separation using biotin-coupled anti-CD62L mAbs (eBioscience) and antibiotin-conjugated magnetic beads (Miltenyi Biotec, Auburn, CA). Separation was performed on an autoMACS column using the PosselD program. B cells were isolated by positive selection with anti-CD19–coupled magnetic beads. The percentage of purified cell fractions in all experiments ranged between 90% and 98%, as estimated by flow cytometry using a fluorescence-activated cell sorter (FACS) cytometer (BD Biosciences, San Jose, CA). Splenic DCs were purified as previously described.27
B-cell help
Serial dilutions of CD4+ T-cell populations were cultured for 7 days with purified syngeneic B cells (5 × 105 cells/well) in the presence of anti-CD3 mAbs or TNP–ovalbumin (OVA; Biosearch Technologies). T cells were irradiated (2000 cGy) before the beginning of the coculture with B cells to prevent their outgrowth during the 7-day culture. IgG1 and IgG2a antibodies in the supernatants were determined by ELISA, using rat monoclonal anti–mouse isotype antibodies, as described.26
Priming of naive T cells
Naive CD4+CD62L+ T cells (106 cells/well in 24-well plates) were preactivated for 48 hours with plastic-coated anti-CD3 mAbs (10 μg/mL) and anti-CD28 mAbs (10 μg/mL). In some experiments, wells were supplemented with recombinant IL-4, IL-6, or IL-21 (20 ng/mL). T cells were recovered, extensively washed, and rested 24 hours in medium before being tested for cytokine secretion and B-cell help capacity. Th1 cells were differentiated by the addition of recombinant IL-12 (10 ng/mL) and anti–IL-4 mAb (10 μg/mL), and Th2 polarization was initiated by the addition of IL-4 (10 ng/mL) and anti–interferon-γ (IFN-γ) mAb (10 μg/mL). Th17 cells were differentiated by addition of TGF-β (3 ng/mL) and IL-6 (20 ng/mL) in the presence of anti–IL-4 and anti–IFN-γ mAbs. Efficacy of polarizations was verified by intracellular cytokine staining on secondary stimulations.
Cytokine detection
IL-21 production was measured in the supernatants of T cells by ELISA using specific matched-paired antibodies (R&D Systems, Minneapolis, MN). For intracellular cytokine staining, Th cells were stimulated for 6 hours with plastic-coated anti-CD3 mAbs, in the presence of brefeldin A (2 μg/mL; Sigma-Aldrich) during the final 2 hours. Cells were resuspended in Fixation-Permeabilization solution (BD Cytofix/Cytoperm kit; BD PharMingen, San Diego, CA), and intracellular cytokine staining was done according to the manufacturer's protocol.
RT-PCR
Total cellular RNA was extracted from the cell lysates by the use of TRIzol reagent, and reverse transcription of mRNA was carried out using M-MLV reverse transcriptase (Invitrogen, Carlsbad, CA). Quantitative polymerase chain reaction (PCR) was performed using an ABI Prism 5700 Sequence Detection System (Applied Biosystems, Foster City, CA). Quantification (with RPL32 as endogenous housekeeping gene) was performed using either standard curves or the ΔΔ cycle threshold (CT) method. Primer sequences can be obtained on request.
Statistical analysis
Differences between groups were analyzed with the Mann-Whitney test for 2-tailed data. A P value less than .1 was considered significant.
Results
In vitro stimulation of naive Th cells promotes acquisition of B-cell help function
The capacity of naive CD4+ lymphocytes to differentiate into effector T cells able to provide help for B-cell IgG class switch and secretion (hereafter referred to as ThB function) was evaluated using a 2-step culture system that was adapted from previously established procedures.16–18,26 Naive CD4+CD62L+ T cells were first stimulated in vitro by a combination of anti-CD3 and anti-CD28 antibodies under neutral or polarizing conditions favoring the acquisition of a Th1, Th2, or Th17-like phenotype. After a brief resting period, these cells were irradiated to prevent cell outgrowth and restimulated in the presence of naive B cells to assay their ability to provide B-cell help. This in vitro assay appears to recapitulate the early steps of a cognate, CD40-dependent T-/B-cell interaction leading to B-lymphocyte proliferation and differentiation into antibody-secreting cells as described in detail in Figures S1 and S2 (available on the Blood website; see the Supplemental Materials link at the top of the online article). In most experiments, the ability of T cells to provide B-cell help was evaluated by examining the accumulation of IgG1 and IgG2a secretion in the culture supernatant at the end of the culture period (day 7). In keeping with previous observations,26,28 CD4+ T lymphocytes activated under neutral conditions displayed superior B-cell help capacities compared with freshly purified, naive T cells, suggesting that the ability to induce B-cell help can be acquired during a short-term in vitro Th cell priming assay (Figure 1A). Acquisition of a ThB function appeared as a general feature of activated T cells, as shown by the ability of Th1-, Th2-, and Th17-like cells (Figure S3, intracellular cytokine staining) to provide B-cell help in vitro. Note, however, that Th17 and Th2 cells consistently displayed superior B-cell help compared with Th0 and Th1 cells (Figure 1B).
In vitro TcR triggering enhances B-cell help function. (A) Naive CD62L+CD4+ T cells purified from the spleen of Balb/C mice were stimulated for 48 hours with plastic-coated anti-CD3 and anti-CD28 mAbs and rested 24 hours in fresh medium. Serial dilutions of irradiated recovered Th cells were incubated with purified B cells (5 × 105 cells/well) and anti-CD3 mAbs (500 ng/mL). Fresh control resting CD62L+CD4+ T cells were purified at the time of the T-B coculture test. (B) CD62L+CD4+ T cells were stimulated in neutral culture medium (Th0) or under polarizing conditions, rested, and cocultured with B cells, as in panel A. Culture supernatants were tested on day 7 for IgG1 and IgG2a contents. Results are expressed as mean plus or minus SD of triplicates (SD < 5% did not appear in the figure). Similar results were obtained in 2 additional experiments.
In vitro TcR triggering enhances B-cell help function. (A) Naive CD62L+CD4+ T cells purified from the spleen of Balb/C mice were stimulated for 48 hours with plastic-coated anti-CD3 and anti-CD28 mAbs and rested 24 hours in fresh medium. Serial dilutions of irradiated recovered Th cells were incubated with purified B cells (5 × 105 cells/well) and anti-CD3 mAbs (500 ng/mL). Fresh control resting CD62L+CD4+ T cells were purified at the time of the T-B coculture test. (B) CD62L+CD4+ T cells were stimulated in neutral culture medium (Th0) or under polarizing conditions, rested, and cocultured with B cells, as in panel A. Culture supernatants were tested on day 7 for IgG1 and IgG2a contents. Results are expressed as mean plus or minus SD of triplicates (SD < 5% did not appear in the figure). Similar results were obtained in 2 additional experiments.
IL-6 drives the expression of ThB function independently of Th2 or Th17 differentiation
These observations prompted us to evaluate the role of IL-4 and IL-6, 2 cytokines known to play a major role in Th2 and Th17 differentiation, respectively, in the acquisition of a potent B-cell help function. Endogenous IL-4 did not appear to play a major role in promoting helper T-cell function for antibody responses, as shown by the adequate B-cell help capacity displayed by IL-4–deficient T cells in vitro (Figure 2A). Accordingly, addition of exogenous IL-4 did not affect the acquisition of ThB function (Figure 2B) while promoting adequate Th2 differentiation (Figure 2C). In marked contrast, Th cells from IL-6–deficient mice displayed reduced B-cell help (Figure 2A), whereas exogenous IL-6 was found to promote ThB differentiation when added during the prestimulation step of naive T cells (Figure 2B). To further demonstrate that the ability of IL-6 to promote B-cell help is unrelated to its well-described effect on B-cell growth and differentiation, naive T cells from IL-6–deficient mice were prestimulated in the presence of exogenous IL-6, extensively washed, rested in control media, and assayed for the ability to promote antibody secretion from WT B cells. Results depicted in Figure 2A demonstrate that IL-6–deficient T cells develop into potent B-cell helpers in response to IL-6, clearly indicating that acquisition of a ThB function is unrelated to the capacity of T cells to secrete IL-6 during the T-/B-cell coculture. The ability of IL-6 to promote acquisition of B-cell helper function was further confirmed using a well-defined in vitro model of cognate help.29 DO11.10 naive T cells were stimulated in the presence or absence of IL-6 and tested for their ability to provide B-cell help in an antigen-specific fashion. As previously demonstrated using polyclonal cell populations, IL-6–treated T cells displayed an increased capacity to induce antigen-specific IgG1 and IgG2 secretion in response to cognate antigen (Figure 2D).
IL-6 promotes humoral responses. (A) CD62L+CD4+ T cells purified from WT or KO mice were stimulated for 48 hours with plastic-coated anti-CD3 and anti-CD28 mAbs. Where indicated, IL-6 was added in the primary culture medium. Cells were washed, rested 1 day in fresh media, and restimulated as in Figure 1. (B) CD62L+CD4+ T cells were stimulated in the presence or absence of recombinant IL-4 or IL-6 (20 ng/mL). Recovered cells were rested 1 day in fresh medium and tested for B-cell help capacity. (C) Flow cytometric analyses of IL-4 and IFN-γ production for each T-cell population obtained in B. (D) CD62L+CD4+ DO11.10 T cells were stimulated in the presence or absence of IL-6, rested 1 day in fresh medium, and restimulated in the presence of CD19+ B cells purified from TNP-KLH–immunized mice and OVA-TNP (500 ng/mL). Culture supernatants were tested for TNP-specific IgG1 and IgG2a antibody contents on day 7. (E) Balb/C inoculated with NP-KLH (50 μg in CFA, intraperitoneally) were further inoculated 1 day later with IL-6–encoding or control (eGFP) plasmid DNA by hydrodynamic tail vein injection. Sera were collected on day 14 and tested for NP-specific isotypes. *P < .05; **P < .01. Results in panels A, B, and D are mean plus or minus SD of triplicate T-B cocultures. Similar results were obtained in 2 (D), 3 (A,C,E), and up to 30 (B) independent experiments.
IL-6 promotes humoral responses. (A) CD62L+CD4+ T cells purified from WT or KO mice were stimulated for 48 hours with plastic-coated anti-CD3 and anti-CD28 mAbs. Where indicated, IL-6 was added in the primary culture medium. Cells were washed, rested 1 day in fresh media, and restimulated as in Figure 1. (B) CD62L+CD4+ T cells were stimulated in the presence or absence of recombinant IL-4 or IL-6 (20 ng/mL). Recovered cells were rested 1 day in fresh medium and tested for B-cell help capacity. (C) Flow cytometric analyses of IL-4 and IFN-γ production for each T-cell population obtained in B. (D) CD62L+CD4+ DO11.10 T cells were stimulated in the presence or absence of IL-6, rested 1 day in fresh medium, and restimulated in the presence of CD19+ B cells purified from TNP-KLH–immunized mice and OVA-TNP (500 ng/mL). Culture supernatants were tested for TNP-specific IgG1 and IgG2a antibody contents on day 7. (E) Balb/C inoculated with NP-KLH (50 μg in CFA, intraperitoneally) were further inoculated 1 day later with IL-6–encoding or control (eGFP) plasmid DNA by hydrodynamic tail vein injection. Sera were collected on day 14 and tested for NP-specific isotypes. *P < .05; **P < .01. Results in panels A, B, and D are mean plus or minus SD of triplicate T-B cocultures. Similar results were obtained in 2 (D), 3 (A,C,E), and up to 30 (B) independent experiments.
The ability of IL-6 to promote the ThB function was not associated with the acquisition of Th17 phenotype because IL-6–treated cells failed to secrete detectable levels of IL-17 under these experimental conditions (Figure S3).
In agreement with in vitro studies, in vivo delivery of an IL-6–encoding plasmid led to higher levels of anti-NP antibody titers in the serum of immune animals (Figure 2E), confirming the capacity of exogenous IL-6 to promote antibody secretion in an in vivo setting.
Acquisition of B-cell help function requires optimal STAT3 signaling
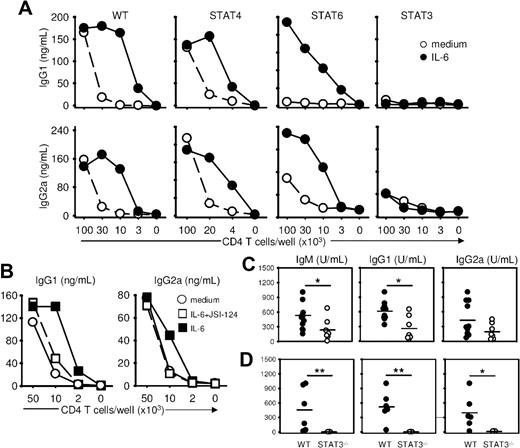
To selectively evaluate the role of transcription factors of the STAT family in regulating the acquisition of the ThB function, we evaluated the ability of naive T cells lacking expression of STAT4, STAT6, or STAT3 to provide help to WT B cells. B-cell help function developed normally in the absence of STAT4 but was impaired in the absence of STAT6. Of interest, both WT STAT4- and STAT6-deficient Th cells developed a strong B-cell help activity in response to exogenous IL-6 (Figure 3A), suggesting that IL-6 could overcome the B-cell help defect observed in the STAT6-negative Th cells. By contrast, STAT3-deficient Th cells failed to provide B-cell help, even when activated in the presence of IL-6 (Figure 3A), establishing an important role of this transcription factor in the acquisition of the ThB function. This conclusion was further supported by experiments in which acquisition of the ThB function by WT helper T cells was shown to be sensitive to a pharmacologic inhibitor of the Jak/STAT3 pathway (Figure 3B). In keeping with published evidence, exogenous IL-6 added during primary stimulation led to reduced levels of IFN-γ secretion and an increase in IL-4 secretion in both WT Balb/c and C57BL/6 congenic STAT3flox/flox mice on secondary TcR triggering (Figure 2C, S5). IL-6 also impaired IFN-γ secretion in STAT6−/− and STAT4−/− T cells but did not affect IFN-γ or IL-4 production in T cells purified from STAT3flox/flox/Lck-CRE mice (Figure S5).
STAT3 is required for the acquisition of a B-cell help function by T cells. (A) Naive T cells from WT, STAT4−/−, STAT6−/−, and T cell–specific STAT3−/− mice were stimulated as in Figure 1, in the presence or absence of IL-6, and tested for B-cell help activity in the presence of B cells (5 × 105 cells/well) and anti-CD3 mAbs (500 ng/mL). Culture supernatants were tested on day 7 for IgG1 and IgG2a/c contents. (B) Naive T cells were activated 48 hours in the presence of medium, IL-6 (20 ng/mL) or IL-6 plus JSI-124 STAT3 inhibitor (100 mM) and tested for ThB function as in panel A. (C) Control and T cell–specific STAT3−/− mice were immunized with NP-KLH (50 μg in CFA, intraperitoneally). Sera were collected on day 14 and tested for NP-specific antibodies. (D) RAG-deficient mice were transferred with CD4+ T cells from control and T cell–specific STAT3−/− mice together with C57BL6 purified B cells and immunized on day 1 as in panel C. Results in panels A and B are expressed as mean plus or minus SD of triplicate T-B coculture. SDs are not visible on the line graph. Similar results were obtained in 3 independent experiments. *P < .05; **P < .01.
STAT3 is required for the acquisition of a B-cell help function by T cells. (A) Naive T cells from WT, STAT4−/−, STAT6−/−, and T cell–specific STAT3−/− mice were stimulated as in Figure 1, in the presence or absence of IL-6, and tested for B-cell help activity in the presence of B cells (5 × 105 cells/well) and anti-CD3 mAbs (500 ng/mL). Culture supernatants were tested on day 7 for IgG1 and IgG2a/c contents. (B) Naive T cells were activated 48 hours in the presence of medium, IL-6 (20 ng/mL) or IL-6 plus JSI-124 STAT3 inhibitor (100 mM) and tested for ThB function as in panel A. (C) Control and T cell–specific STAT3−/− mice were immunized with NP-KLH (50 μg in CFA, intraperitoneally). Sera were collected on day 14 and tested for NP-specific antibodies. (D) RAG-deficient mice were transferred with CD4+ T cells from control and T cell–specific STAT3−/− mice together with C57BL6 purified B cells and immunized on day 1 as in panel C. Results in panels A and B are expressed as mean plus or minus SD of triplicate T-B coculture. SDs are not visible on the line graph. Similar results were obtained in 3 independent experiments. *P < .05; **P < .01.
The role of STAT3 in the generation of B helper T cells was further confirmed in vivo after immunization of conditional STAT3flox/flox/Lck-CRE mice. Mice defective for STAT3 expression in the T-cell compartment showed reduced NP-specific IgG secretion after NP-KLH immunization (Figure 3C). To exclude any potential artifact of CRE expression in non–T-cell lineages, RAG−/− recipient mice were cotransferred with WT syngeneic B cells and CD4+ T cells purified from control or STAT3flox/flox/Lck-CRE mice, and immunized against NP-KLH. As shown in Figure 3D, RAG−/− mice reconstituted with STAT3-deficient CD4+ T cells displayed a severely impaired NP-specific humoral response.
IL-21 secretion, but not CXCR5 expression, correlates with acquisition of B-cell help function
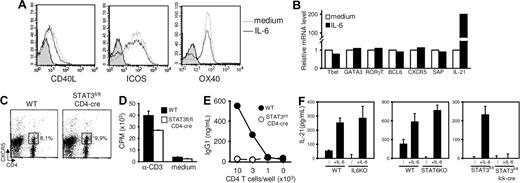
IL-6 treatment during T-cell stimulation did not significantly affect expression of molecules known to be associated with the differentiation and/or function of helper cells for B-cell responses. In particular, IL-6 did not affect CD40L, inducible costimulator (ICOS), OX40, signaling lymphocyte activation molecule–associated protein (SAP), or CXCR5 expression by activated T cells as judged by flow cytometry (Figure 4A; and data not shown) and reverse-transcribed (RT) PCR (Figure 4B). Because CXCR5 is presently thought to identify a T-cell subpopulation particularly apt to provide B-cell help,16–18 we compared the expression of this chemokine receptor induced in vivo after immunization of WT and conditional STAT3flox/flox/CD4-CRE mice. Much to our surprise, the lack of STAT3 expression did not impair the development of CD4+CXCR5+ cells in vivo (Figure 4C). To directly evaluate the ability of CXCR5-expressing T cells to provide B-cell help, CD4+CXCR5+ T cells from NP-KLH–inoculated control and T cell–deficient STAT3 mice were purified by flow cytometry and tested for their ability to activate naive syngeneic B cells in vitro. In agreement with previous observations, cells lacking STAT3 expression failed to provide B-cell help in vitro while retaining the ability to proliferate in response to anti-CD3 mAbs. Both our in vitro and in vivo analyses indicate therefore that acquisition of B-cell help function, but not CXCR5-expression, is regulated by the IL-6/STAT3 axis.
Regulation of IL-21, but not CXCR5, expression by the IL-6/STAT3 axis. (A,B) T cells were stimulated with anti-CD3/anti-CD28 mAbs in the presence or absence of IL-6, rested 1 day, and restimulated for 6 hours in the presence of plastic-coated anti-CD3 mAbs. T cells were tested for FACS profile expression of CD40L, ICOS, and OX40 (A) and mRNA expression of selected genes by quantitative RT-PCR (B). Gray histograms in panel A represent isotype control-stained cells. (C) Control and T cell–specific STAT3−/− mice were immunized in foot pads with KLH emulsified in CFA. Expression of CXCR5+ TFH cells was analyzed on day 7. Numbers in dot-plot quadrants represent the percentages. (D) CXCR5+CD4+ TFH cells were purified from control and T cell–specific STAT3−/− mice and tested for T-cell proliferation in response to anti-CD3 mAbs. (E) Aliquots of cells in panel D were irradiated and tested for B-cell help function in the presence of WT B cells and anti-CD3 mAbs. (F) WT, IL-6–, STAT6-, and STAT3-deficient CD62L+CD4+ T cells were stimulated 48 hours in the presence or absence of recombinant IL-6. IL-21 detection in culture supernatants was performed by ELISA. Results represent mean plus or minus SD of triplicate culture. Similar results were obtained in 2 additional independent experiments.
Regulation of IL-21, but not CXCR5, expression by the IL-6/STAT3 axis. (A,B) T cells were stimulated with anti-CD3/anti-CD28 mAbs in the presence or absence of IL-6, rested 1 day, and restimulated for 6 hours in the presence of plastic-coated anti-CD3 mAbs. T cells were tested for FACS profile expression of CD40L, ICOS, and OX40 (A) and mRNA expression of selected genes by quantitative RT-PCR (B). Gray histograms in panel A represent isotype control-stained cells. (C) Control and T cell–specific STAT3−/− mice were immunized in foot pads with KLH emulsified in CFA. Expression of CXCR5+ TFH cells was analyzed on day 7. Numbers in dot-plot quadrants represent the percentages. (D) CXCR5+CD4+ TFH cells were purified from control and T cell–specific STAT3−/− mice and tested for T-cell proliferation in response to anti-CD3 mAbs. (E) Aliquots of cells in panel D were irradiated and tested for B-cell help function in the presence of WT B cells and anti-CD3 mAbs. (F) WT, IL-6–, STAT6-, and STAT3-deficient CD62L+CD4+ T cells were stimulated 48 hours in the presence or absence of recombinant IL-6. IL-21 detection in culture supernatants was performed by ELISA. Results represent mean plus or minus SD of triplicate culture. Similar results were obtained in 2 additional independent experiments.
IL-6 did not modulate mRNA levels encoding for molecules known to play a role in helper subset differentiation (T-bet, GATA-3, RORγT, or BCL6). In agreement with recent reports,30,31 IL-6 was found to induce high levels IL-21 mRNA in activated T cells (Figure 4B).
We therefore evaluated IL-21 secretion by activated Th cells in culture conditions promoting B-cell help capacity or not. Compared with WT, IL-6-, STAT6-, and STAT3-deficient Th cells displayed reduced IL-21 secretion on anti-CD3/CD28 stimulation. Exogenous IL-6 increased IL-21 production by WT, IL-6-, and STAT6-KO Th cells but not by STAT3-deficient Th cells (Figure 4F), thus establishing a correlation between IL-21 production and the ability of activated T cells to provide B-cell help (Figures 2, 3).
IL-21 has a critical role in regulating humoral responses,32 and IL-21R is expressed by both T and B cells,21,33 thereby allowing complex regulatory mechanism acting at the level of both Th and B-cell populations. We first tested whether IL-21 promoted IgG secretion through IL-21R triggering on B cells by coculturing WT CD4+ T cells with B cells purified from WT or IL-21R–deficient mice. As shown in Figure 5A, the lack of IL-21R did not preclude B-cell differentiation into antibody-secreting cells in the presence of T cells. Similarly, in vitro priming of Th in the presence of IL-6 enhanced B-cell help function toward WT and IL-21R–deficient B cells to similar extent (Figure 5A top right panel), suggesting that T cell–derived IL-21 does not act on B cells to promote antibody secretion. Notably, however, the lack of IL-21R expression by T cells impaired their capacity to promote antibody production in vitro (Figure 5A bottom left panel). Addition of IL-6 to IL-21–KO T cells partially restored their B-cell helper capacities, suggesting that IL-6 might promote ThB function in both an IL-21–/IL-21R–dependent and –independent fashion. To confirm a direct role of IL-21 on T cells, naive T cells were activated in the presence of recombinant IL-21, washed, and rested 24 hours in normal culture medium before secondary stimulation in the presence of WT B cells. As previously shown for IL-6, the addition of IL-21 during T-cell priming promoted optimal ThB function (Figure 5B). Collectively, these results suggested that IL-6–induced IL-21 production during Th cell priming acted at the level of T cell, as a positive loop to induce/maintain optimal ThB function.
IL-21R signaling at the level of Th cells is required to induce optimal B-cell help function. (A) Top panels: WT T cells were activated in the presence (right panel) or absence of IL-6 (left panel) and tested for ThB function in the presence of B cells purified from control or IL-21R−/− mice. Bottom panels: WT or IL-21R–deficient T cells were tested for helping activity toward WT syngeneic B cells. (B) CD62L+CD4+ T cells were stimulated in the presence or absence of recombinant IL-21 or IL-6 cytokines (20 ng/mL). Recovered cells were rested 1 day in fresh medium and tested for B-cell help capacity. Results are expressed of mean plus or minus SD of triplicate cultures and are representative of 2 (A) or 3 (B) independent experiments.
IL-21R signaling at the level of Th cells is required to induce optimal B-cell help function. (A) Top panels: WT T cells were activated in the presence (right panel) or absence of IL-6 (left panel) and tested for ThB function in the presence of B cells purified from control or IL-21R−/− mice. Bottom panels: WT or IL-21R–deficient T cells were tested for helping activity toward WT syngeneic B cells. (B) CD62L+CD4+ T cells were stimulated in the presence or absence of recombinant IL-21 or IL-6 cytokines (20 ng/mL). Recovered cells were rested 1 day in fresh medium and tested for B-cell help capacity. Results are expressed of mean plus or minus SD of triplicate cultures and are representative of 2 (A) or 3 (B) independent experiments.
Vaccination with IL-6–competent DC promotes humoral responses in vivo
In agreement with in vitro studies and previous reports from the literature,34 IL-6–deficient mice were found to produce low titers of antigen-specific IgG1 and IgG2a after immunization with the T-dependent NP-KLH antigen (Figure 6A). Because IL-6 is produced by different cell types in vivo, we next evaluated the role of APC-derived IL-6 production in inducing an in vivo humoral response. WT C57BL6 mice were inoculated with KLH-pulsed mature DCs purified from control or IL-6–deficient mice. Both WT and IL-6–KO DCs elicited Th cell priming in vivo as judged by expression of CXCR5 expression in draining lymph nodes (Figure 6C), cytokine secretion on secondary in vitro restimulation (Figure 6D), and KLH-specific T-cell proliferation in vitro (data not shown). Despite their ability to adequately activate T cells in vivo, IL-6–defective DCs failed to induce an optimal humoral response in vivo, as shown in Figure 6B.
IL-6–derived DC promotes humoral responses in vivo. (A) WT and IL-6–KO mice were inoculated with NP-KLH (50 μg in CFA, intraperitoneally). Sera were collected on day 14 and tested for NP-specific isotypes. (B) KLH-pulsed DCs purified from WT or IL-6−/− mice (3 × 105 cells) were inoculated intravenously into C57BL6 mice. Sera were collected on day 14 and tested for KLH-specific isotypes. (C,D) KLH-pulsed DCs obtained in panel B were inoculated in f.p., and draining lymph nodes were recovered on day 7. Expression of CXCR5+ TFH cells was analyzed by FACS (C), and lymph nodes cells were tested for IL-4 secretion in the presence of graded doses of KLH (D). (E) CD4+ T cells purified from lymph nodes of immunized mice were tested for B-cell help delivery in the presence of CD19+ B from WT naive C57BL6 mice and KLH (10 μg/mL). Culture supernatants were tested on day 7 for IgG1 contents (mean ± SD of triplicate cultures). Numbers in dot-plot quadrants in panel C represent the percentages. Dashed lines in panel A represent normal serum antibody concentrations. **P < .01; ***P < .001. Similar results were obtained in 2 (C,E) or 3 (A,B,D) independent experiments.
IL-6–derived DC promotes humoral responses in vivo. (A) WT and IL-6–KO mice were inoculated with NP-KLH (50 μg in CFA, intraperitoneally). Sera were collected on day 14 and tested for NP-specific isotypes. (B) KLH-pulsed DCs purified from WT or IL-6−/− mice (3 × 105 cells) were inoculated intravenously into C57BL6 mice. Sera were collected on day 14 and tested for KLH-specific isotypes. (C,D) KLH-pulsed DCs obtained in panel B were inoculated in f.p., and draining lymph nodes were recovered on day 7. Expression of CXCR5+ TFH cells was analyzed by FACS (C), and lymph nodes cells were tested for IL-4 secretion in the presence of graded doses of KLH (D). (E) CD4+ T cells purified from lymph nodes of immunized mice were tested for B-cell help delivery in the presence of CD19+ B from WT naive C57BL6 mice and KLH (10 μg/mL). Culture supernatants were tested on day 7 for IgG1 contents (mean ± SD of triplicate cultures). Numbers in dot-plot quadrants in panel C represent the percentages. Dashed lines in panel A represent normal serum antibody concentrations. **P < .01; ***P < .001. Similar results were obtained in 2 (C,E) or 3 (A,B,D) independent experiments.
To further confirm that APC-derived IL-6 regulated development of helper T cells in vivo, draining lymph node CD4+ T cells from mice immunized with WT or IL-6–deficient DCs were restimulated in vitro in the presence of B cells purified from WT naive mice and KLH. Results in Figure 6E indicated that CD4+ T cells purified from mice immunized in an IL-6–deficient environment displayed a reduced capacity to activate B cells in vitro, suggesting an important role for IL-6 in promoting ThB function in vivo.
Discussion
The identification of key cytokines and transcription factors governing T-cell differentiation in response to antigen stimulation has greatly improved our understanding of immune regulation. In particular, it is possible to recapitulate in vitro the differentiation pathways of naive T cells toward predefined effector functions, such as Th1, Th2, or Th17.1–5,7 Surprisingly, however, and despite the major role played by antibodies in immune defense against practically all known pathogens, the signals and molecular pathways enabling a naive T cell to acquire efficient helper capacities for B-cell responses (referred to as ThB function in this study) remain largely unknown.
Acquisition of the B-cell help function by naive T cells is thought to occur in a stepwise fashion, including activation of naive T cells by adequate APCs, migration of activated T cells to B-cell areas, and finally expression of helper function leading to B-cell activation and differentiation into antibody-secreting cells (reviewed by MacLennan et al35 ). Because analysis of this complex differentiation pathway is not easy to perform in an in vivo setting, we adapted from previous studies16–18 an in vitro assay for measuring B-cell helper function of Th cells during both cognate and noncognate interactions. The interest of this model resides in its capacity to evaluate the intrinsic B-cell help function of selected Th cell populations irrespective of their proliferative capacity and pattern of cytokine production.
Two major conclusions can be drawn from the present study: (1) the STAT3 transcription factor is required for the expression of the B-cell help function; and (2) the ability of T lymphocytes to provide B-cell help is not restricted to a specific T-helper cell subset.
The observations reported in this study demonstrate the important role of IL-6, IL-21, and STAT3 in regulating the B-cell help function on activation of naive T cells. Indeed, exogenous IL-6 was shown to induce the differentiation of highly efficient Th cells able to induce B-cell growth and antibody secretion in vitro (Figures 2, 3, 5; and data not shown) and in vivo (Figure 2E), whereas lack of endogenous IL-6 decreased the spontaneous acquisition of the ThB function (Figures 2, 5). Similarly, activation of naive T cells in the presence of exogenous IL-21 increased the ability of T cells to provide B-cell help, whereas only suboptimal help was provided to B cells by in vitro–activated, IL-21R–KO T lymphocytes (Figure 5). Addition of IL-6 to IL-21R–KO T cells partially restored their B-cell helper capacities, suggesting that ability to provide B-cell help is not totally dependent on IL-21 signaling. Finally, these observations were confirmed in an in vivo setting because mice lacking STAT3 expression in the T-cell compartment were strongly impaired in their ability to differentiate into T-helper cells for B lymphocytes (Figure 4).
Although the present study concurs with previous observations supporting important roles for IL-6 and IL-21 in regulating B-cell responses, they differ from the commonly accepted view whereby T cell–derived IL-6 and/or IL-21 represents important effector cytokines regulating antibody production during T cell–dependent responses. It is noteworthy that our culture conditions allowed us to investigate the effect of these cytokines on Th lymphocytes, independent of their potential, often confounding, effect on B cells. In particular, we demonstrated herein that production of IL-6 and IL-21 during the secondary T-/B-cell coculture is not required for enhanced antibody secretion by B cells (Figures 2, 5).
Although both IL-6 and IL-21 display similar functional properties in vitro, the widespread expression of IL-6 by numerous nonimmune and immune cells, including virtually all APCs and B and T lymphocytes, led us to consider IL-6 as the major cytokine able to initiate the B-cell helper program. This hypothesis is supported by our own in vivo data, demonstrating an important role for APC-derived IL-6 in regulating in vivo antibody responses (Figure 6). Irrespective of the cellular source, IL-6 would induce IL-21 secretion by antigen-stimulated T cells, a secretion further sustained through a positive autocrine loop,36 promoting the acquisition of the ThB function. As previously stated, and despite the well-known ability of IL-21 to promote B-cell growth and differentiation,37,38 the role of IL-21 as an effector cytokine sustaining B-cell responses in vivo is still unclear and should be addressed using mouse strains selectively lacking IL-21R expression in the B-cell compartment. At present, secretion of IL-21 may represent a biologic response of T cells activated in a STAT3-dependent fashion and, as such, identify potent B-cell helpers, in keeping with published reports.19,38
The second major issue that was addressed by the present study refers to the relationship between helper function for antibody responses and the established pathways of effector T-cell differentiation. Although this important question cannot be easily addressed and clearly warrants further investigation, observations reported in this study suggest that the ability to provide B-cell help does not define a new T-cell subpopulation but rather represents a functional status compatible with multiple differentiation programs. Indeed, T cells stimulated under neutral, pro-Th1, pro-Th2, or pro-Th17 conditions expressed superior B-cell helper capacities compared with unstimulated cells (Figure 1; and data not shown), although with distinct efficacy. Of interest, the lack of STAT6 and STAT3 led to strongly reduced ThB function, in marked contrast to STAT4-deficient T cells, which differentiate normally into fully competent B-cell helper cells. These data can be easily interpreted in light of the reduced expression of IL-21 in STAT6- and STAT3-deficient Th cells (Figure 4). Note, however, that IL-6 was able to overcome the helper defect in STAT6-negative T cells (Figure 3), further illustrating that acquisition of a B-cell help function and Th2 development can represent both separate and overlapping events. Similarly, although both Th17 development and acquisition of the ThB function are under the control of STAT3, these 2 effector functions are not strictly correlated. Indeed, addition of TGF-β is required for in vitro Th17 development but is dispensable for acquisition of B-cell helper function (Figure S4). Furthermore, and in contrast to Th17 development, IFN-γ/STAT4 and/or IL-4/STAT6 signals do not antagonize helper B-cell differentiation in vitro (Figures 2, 3, S6), further suggesting that Th17 and ThB functions are differentially regulated.
Collectively, our observations concur with most in vivo findings and suggest that expression of ThB function is compatible with multiple T-cell differentiation programs, with Th2 and Th17 pathways appearing as particularly well suited for supporting antibody responses in vivo. Our findings are therefore best explained by assuming that the STAT3-regulated helper function is operative under multiple differentiation programs, a hypothesis that is supported by the observation that IL-21, a STAT3-dependent gene, can be expressed by both Th2 and Th17-like cells39–42 (Figure S7). In support of this conclusion, pharmacologic inhibition of the STAT3 signaling pathway has been found to decrease the B-cell help capacities of T cells activated under pro-Th2 culture conditions (F.E., unpublished observations, 2008).
The molecular mechanism by which IL-6–treated T cells promote B-cell responses in vitro is still ill defined. Although our studies clearly demonstrate that acquisition of the ThB function is under control of STAT3, none of the previously identified molecules thought to play an important role in T-/B-cell collaboration appears as a direct target of the IL-6–initiated signaling pathway. Indeed, IL-6 failed to selectively increase expression of CD40L, ICOSL, CXCR5, OX40, SAP, or BCL6, previously identified as important molecules expressed by T cells and involved in B-cell help. Notably, 2 recent reports published during the review process of the present work concur with our conclusions and confirm the important role of IL-6, IL-21, and STAT3 in the generation of T-helper cells able to promote a humoral response.43,44 In one of these studies,43 STAT3 was shown to regulate the in vivo development of follicular helper cells, a subset of CD4 and CXCR5-expressing cells endowed with superior B-cell help capacities. This was clearly not the case in our own study, as control and conditional T cell–specific STAT3-KO mice displayed similar percentage of CXCR5+ T cells after NP-KLH immunization (Figure 4C). We do not have a clear explanation for these discrepancies because we could not find a significant difference in the mouse strains or immunization protocol used. It is noteworthy that IL-6 also failed to enhance CXCR5 expression in vitro (Figure 4B), suggesting that expression of this chemokine receptor is not under the direct control of STAT3. Furthermore, CXCR5+ cells purified from immunized STAT3flox/flox/CD4-CRE mice were found deficient in B-cell help function (Figure 4E), further suggesting that expression of CXRC5 does not unambiguously identify B-cell helper T lymphocytes, in agreement with previous reports.18 Therefore, although CXCR5-expressing TFH cells and the IL-6–treated cells described in this study display numerous similarities (ability to provide B-cell help in vitro, IL-21 production), their developmental relationship remains to be established.
In conclusion, our study suggests that the acquisition of a B-cell help function is compatible with multiple T-cell differentiation programs, in keeping with numerous in vivo observations that are difficult to reconcile with a classic Th1/Th2/Th17 model of regulation of the humoral immune response. In particular, the concept of T-helper cell subset-independent acquisition of a B-cell help function helps in understanding the widespread observations that both Th1 (such as CFA, CpG, rIL-12) and Th2 (Alum) promoting adjuvants induce high levels of antibody production in vivo. Furthermore, the dual role of IL-6 in regulating both Th17 and ThB differentiation could shed new light on the pathophysiology of autoimmune diseases, such as rheumatoid arthritis or multiple sclerosis inflammatory disorders characterized by both a cellular (Th17) and humoral (presence of autoantibodies) response.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Akira (Osaka University, Osaka, Japan) for providing us the STAT3flox mice; Drs. A Lemoine and M. Braun (Institute for Medical Immunology, Gosselies, Belgium) for giving us the RAG−/− mice; P. Veirman for animal care; Dr L. Naldini (University of Torino, Torino, Italy) and J.-C. Renauld (Université Catholique de Louvain, Louvain, Belgium) for generously providing the vector pHR′-CMV-eGFP and the mouse IL-6–encoding plasmid; Georgette Vansantem-Urbain for her kind help with the FACS-sorting experiments; and A. Marchant and M. Van Mechelen for fruitful discussions all along this work.
This work was supported by the Belgian Program in Interuniversity Poles of Attraction Initiated by the Belgian State, Prime Minister's Office, Science Policy Programming, by a Research Concerted Action of the Communauté française de Belgique (Namur, Belgium) grant from the Fonds Jean Brachet (Gosselies, Belgium), and a collaborative research agreement fee from GSK Biologicals (Rixensart, Belgium), and support from the Division of Intramural Research, National Heart, Lung, and Blood Institute (R.S., W.J.L.). F.A. is a Research Associate at the National Fund for Scientific Research, Fonds National de la Recherche Scientifique (Bruxelles, Belgium). F.E. is supported by a First Post Doc fellow from the DGTRE-Région Wallonne (Namur, Belgium).
National Institutes of Health
Authorship
Contribution: F.E. designed and performed research; S.D. performed research; F.B., R.S., and W.J.L. contributed vital new reagents and analyzed data; and O.L. and F.A. designed research and wrote the paper.
Conflict-of-interest disclosure: R.S. and W.J.L. are inventors on patents and patent applications related to IL-21. All other authors declare no competing financial interests.
Correspondence: Fabienne Andris, Laboratoire de Physiologie animale, Université Libre de Bruxelles–IBMM, 12, rue des Prof Jeener et Brachet, 6041 Gosselies, Belgium; e-mail: fandris@ulb.ac.be.
References
Author notes
*O.L. and F.A. contributed equally to this work.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal