Protein kinase C (PKC) isoforms have been implicated in several platelet functional responses, but the contribution of individual isoforms has not been thoroughly evaluated. Novel PKC isoform PKC-θ is activated by glycoprotein VI (GPVI) and protease-activated receptor (PAR) agonists, but not by adenosine diphosphate. In human platelets, PKC-θ–selective antagonistic (RACK; receptor for activated C kinase) peptide significantly inhibited GPVI and PAR-induced aggregation, dense and α-granule secretion at low agonist concentrations. Consistently, in murine platelets lacking PKC-θ, platelet aggregation and secretion were also impaired. PKC-mediated phosphorylation of tSNARE protein syntaxin-4 was strongly reduced in human platelets pretreated with PKC-θ RACK peptide, which may contribute to the lower levels of granule secretion when PKC-θ function is lost. Furthermore, the level of JON/A binding to activated αIIbβ3 receptor was also significantly decreased in PKC-θ−/− mice compared with wild-type littermates. PKC-θ−/− murine platelets showed significantly lower agonist-induced thromboxane A2 (TXA2) release through reduced extracellular signal–regulated kinase phosphorylation. Finally, PKC-θ−/− mice displayed unstable thrombus formation and prolonged arterial occlusion in the FeCl3 in vivo thrombosis model compared with wild-type mice. In conclusion, PKC-θ isoform plays a significant role in platelet functional responses downstream of PAR and GPVI receptors.
Introduction
Platelet activation plays an important role in hemostasis, and the abnormal activation of platelets leads to thrombosis.1 After circulating platelets are exposed to collagen-rich subendothelium at the site of vascular injury, platelets become activated, release granule contents, and generate thrombin and the lipid mediator thromboxane A2 (TXA2).2,3 Secreted adenosine diphosphate (ADP), serotonin, and TXA2 amplify the initial stimulus in a positive feedback activation of platelets.2,3 In addition, α-granule proteins, such as P-selectin, mediating adhesive interactions between platelets, leukocytes, and endothelial cells, play a pivotal role in the pathogenesis of thrombosis and inflammation.4 Glycoprotein VI (GPVI) and G-protein–coupled protease-activated receptors (PARs) are 2 dominant signaling receptors that mediate many of the important functional responses in platelets.1,–3 There are significant similarities in GPVI and PAR signaling, as phospholipase C (PLC) is activated by both pathways, which results in the generation of inositol 1,4,5-triphosphate (IP3) and diacylglycerol (DAG). IP3 mediates the release of Ca2+ from intracellular stores, whereas DAG causes direct protein kinase C (PKC) activation.3,5 Platelet aggregation requires the αIIbβ3 receptor to undergo a conformational change from a low- to a high-affinity state to bind ligands, such as fibrinogen, which is considered inside-out signaling. On the other hand, the pathway of outside-in signaling is induced by ligand binding to αIIbβ3.6,7
Human platelets express several PKC isoforms: α, β, η, ϵ, δ, ζ, and θ.8,9 Many functional responses, including platelet secretion, aggregation, and actin reorganization, have been shown to be positively regulated by PKC isoforms.10 PKC-θ, as a member of PKC novel subfamily, is Ca2+-insensitive but DAG-sensitive.11 This isoform contains a carboxyl-terminal catalytic domain with 2 conserved regions, C3 and C4, which are essential for catalytic activity and substrate binding, but lacks the calcium-binding C2 region.12,13 After activation, PKC-θ is phosphorylated at threonine, serine (autophosphorylation site), and tyrosine residues. Among these, phosphorylation of threonine 538 (Thr538) residues in the activation loop is an important event in the activation of PKC-θ and critical to its kinase activity.14,15 This event has been used as a marker for activation of this PKC isoform in other cell system such as muscle resistance artery cells.16 In platelets, PKC-θ has been reported to be tyrosine phosphorylated during outside-in and GPVI signaling at Tyr-90.17 PKC-θ was found to contribute to receptor-mediated outside-in αIIbβ3 signaling and actin reorganization, but it was excluded to be a regulator in agonist-induced inside-out signaling and fibrinogen binding to αIIbβ3.17
In the present study, we show for the first time that GPVI and PAR activation, but not P2Y receptor activation, causes Thr538 phosphorylation on PKC-θ, and this isoform has a significant role in platelet aggregation and activation of αIIbβ3 receptors. Furthermore, this PKC isoform also mediates the agonist-induced ATP release, P-selectin expression, and TXA2 generation downstream of GPVI and PAR signaling. More significantly, we also demonstrated unstable thrombus formation and prolonged arterial occlusion in PKC-θ−/− mice compared with WT littermates in the FeCl3 in vivo thrombosis model. From these results, we conclude that PKC-θ plays an important role in GPVI- and PAR-mediated platelet activation.
Methods
Materials
2MeSADP, apyrase (grade VII), human fibrinogen (type I), acetylsalicylic acid, α-thrombin, and bovine serum albumin (BSA, fraction V) were obtained from Sigma-Aldrich (St Louis, MO). Hexapeptides, AYPGKF and SFLLRN, were custom synthesized at Invitrogen (Carlsbad, CA). Collagen-related peptide (CRP) was purchased from Centerchem (Norwalk, CT). PKC-θ antagonistic RACK peptide and its control peptide were from Drs Daria Mochley-Rosen and Grant Budas (Stanford, CA). PKC-θ isoform selective antibodies, anti-PKC-θ and antiphospho(Thr538)-PKC-θ, were obtained from BD Biosciences PharMingen (San Jose, CA). The antisyntaxin-4 antibody was from BD Biosciences Transduction Laboratories (Lexington, KY). Antiphospho-threonine, antiphospho(Thr202/Tyr204)-extracellular-signal regulated kinase (ERK) and anti-ERK antibodies were purchased from Cell Signaling Technology (Danvers, MA). Luciferin-luciferase reagent was purchased from Chrono-Log (Havertown, PA). Normal mouse IgG and protein A/G Sepharose beads were from Santa Cruz Biotechnology (Santa Cruz, CA). Oligonucleotides to the PKC-θ gene were obtained from Integrated DNA Technologies (Coralville, IA). The antihuman CD62-fluorescein isothiocyanate (FITC) and CD61-PE and IgG isotype control antibodies were purchased from BD Biosciences PharMingen. The antimouse JON/A-PE and CD62-FITC antibodies were obtained from Emfret Analytics (Wuerzburg, Germany). PKC-θ−/− mice were obtained from The Jackson Laboratory (Bar Harbor, ME) and bred in the central animal facility of Temple University Medical School. All other reagents were of reagent grade, and deionized water was used throughout.
This study was approved by the Institutional Review Board of Temple University.
Isolation of human platelets
All experiments using human subjects were performed in accordance with the Declaration of Helsinki. Whole blood was drawn from healthy, consenting human volunteers selected from students, staff, or workers at the Temple University. Donated blood was collected into tubes containing one-sixth volume of acid citrate dextrose (2.5 g sodium citrate, 1.5 g citric acid, and 2 g glucose in 100 mL deionized water). Citrated blood was centrifuged at 230g for 20 minutes at room temperature to obtain platelet-rich plasma (PRP). The PRP was incubated with 1 mM acetylsalicylic acid (aspirin) for 30 minutes at 37°C, and then allowed to remain at room temperature for 15 minutes. The PRP was then centrifuged for 10 minutes at 980g at room temperature to pellet the platelets. Platelets were resuspended in Tyrode's buffer (138 mM NaCl, 2.7 mM KCl, 2 mM MgCl2, 3 mM NaH2PO4, 5 mM glucose, 10 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, and 0.2% bovine serum albumin adjusted to pH 7.4) containing 0.1 U/mL apyrase. Cells were counted, and concentration of cells was adjusted to 2 × 108 platelets/mL.
Isolation of mouse platelets
Studies using mice were performed under protocols that were approved by the Animal Use and Care Committee of Temple University. Blood was collected from vena cava of anesthetized mice into syringes containing one-tenth blood volume of 3.8% sodium citrate as anticoagulant. Red blood cells were removed by centrifugation at 100g for 10 minutes. PRP was removed, and platelets were pelleted at 400g for 10 minutes. The platelet pellet was resuspended in Tyrode buffer (pH 7.4) containing 0.1 U/mL apyrase. The washed platelets were subsequently used for experiments.
Aggregometry
Aggregation of 500 μL of washed human platelets or 250 μL of mouse platelets was analyzed using a lumiaggregometer (Chrono-Log). Aggregation was measured using light transmission under stirring conditions (900 rpm) at 37°C. Agonists were added for platelet stimulation. However, platelets were preincubated with PKC-θ antagonistic RACK peptide or control peptide (vehicle) for 15 minutes at 37°C to study the role of PKC-θ. Each sample was allowed to aggregate for at least 3 minutes. Aggregation tracings are representative of results obtained from at least 3 separate experiments using platelets from 3 different donors.
Measurement of platelet dense granule secretion
Platelet secretion was determined by measuring ATP secretion using the Lumi-chrome reagent during the aggregation, and the corresponding luminescence was measured. The data are represented in the form of actual secretion tracings.
Immunoprecipitation
Washed human platelets were activated with agonists in the presence of PKC-θ antagonistic RACK peptide or control peptide, and the reaction was stopped by the addition of ice-cold 2× lysis buffer. The lysates were incubated for 30 minutes in ice to complete the lysis. The samples were then centrifuged at 10 000g for 10 minutes at 4°C to separate the cytoskeletal elements. The supernatant was isolated, and the antisyntaxin-4 immunoprecipitating antibody was added in a 1:100 dilution and incubated for 1 hour at 4°C. Protein A/G Sepharose beads were then added, and the samples were incubated for overnight with the antibody and the beads at 4°C. The beads were washed 3 times with 1× lysis buffer and the proteins were eluted after addition of 3× sodium dodecyl sulfate (SDS) sample buffer with dithiothreitol (100 mM). Sample were boiled for 10 minutes and processed as described in “Western blot analysis.”
Western blot analysis
After platelet samples were prepared, proteins were separated on 10% SDS–polyacrylamide gel electrophoresis gel and then transferred onto polyvinylidene difluoride membrane. Nonspecific binding sites were blocked by incubation in Tris-buffered saline/Tween (TBST; 20 mM Tris, 140 mM NaCl, 0.1% [vol/vol] Tween 20) containing 5% (wt/vol) BSA for 60 minutes at room temperature, followed by incubating it overnight at 4°C with gentle agitation with primary antibody (in TBST with 5% BSA). After 3 washes for 5 minutes each with TBST, the membranes were probed with the alkaline phosphatase-labeled secondary antibody (1:10 000 dilution in TBST with 5% BSA) for 1 hour at room temperature. After additional washing steps, membranes were then incubated with CDP-Star chemiluminescent substrates (Tropix, Bedford, MA) for 10 minutes at room temperature, and immunoreactivity was detected using Luminescent Image Analyzer model LAS-3000 CH (Fujifilm, Tokyo, Japan).
Measurement of TXA2 generation
Isolated mouse platelets (250 μL) were stimulated by agonists in an aggregometer. The stimulation was performed for 3.5 minutes, and the reaction was stopped by quickly freezing the sample in a dry ice-methanol bath. Before performing the measurement, samples were thawed at room temperature and centrifuged at 15 000g for 3 minutes at room temperature to remove lysed platelets. The supernatant was diluted 1:50 with the standard diluent (assay buffer). Levels of TXB2 were determined in duplicate according to the manufacturer's instructions using a Correlate-EIA Thromboxane B2 Enzyme Immonoassay kit (Assay Designs, Ann Arbor, MI). The data present the average of 3 days of data plus or minus SD.
Flow cytometry
All determinations were performed on FACSCalibur flow cytometer (BD Biosciences, San Jose, CA). Washed human and murine platelets were analyzed to measure surface expression of P-selectin receptor by CD62-FITC antibody, and the level of activated αIIbβ3 receptors on murine platelets by JON/A-PE antibody. Aliquots (0.1 mL) of suspension of washed human platelets (2 × 106/mL) in Tyrode's buffer (pH 7.4) were preincubated with 1 μM PKC-θ RACK peptide or control peptide (vehicle) for 15 minutes at 37°C. Saturating concentrations of CD62-FITC and CD61-PE (BD Biosciences) antibodies were added after the respective agonist, gently mixed, and incubated for 15 minutes at 37°C in the dark in nonstirring condition. Platelets were identified according to their CD61 (against GPIIIb) positivity or forward and side scatter signal during dual-color analysis. As a control for immunolabeling, platelets were incubated with nonimmune IgG isotype control antibody. To fix the platelets, 1% paraformaldehyde dissolved in phosphate-buffered saline was added. A total of 10 000 platelet events were acquired per sample, and the mean fluorescence intensity (MFI) of positive platelets was analyzed.
Genotyping
Tails of the mice were cut 0.3 cm from the tip. DNA from the tail was extracted and amplified using polymerase chain reaction (PCR) kit with the sense primers 5′-TTGGTTCTCTTGAACTCTGC-3′ (wild-type [WT]), or 5′-ACTGCATCTGCGTGTTCGAA-3′ (knockout), and a common antisense primer 5′TAAGAGTAATCTTCCAGAGC-3′. After PCR, the DNA products were run on a 0.8% agarose gel for 30 minutes and then detected using a Fujifilm Luminescent Image Analyzer. The WT and knockout resulted in a 426-bp and 600-bp PCR products, respectively.
In vivo thrombosis model
Adult mice (6-8 weeks old, weight ∼25 g) were anesthetized by intraperitoneal injection of pentobarbital (40 mg/kg). Experimental groups consisted of PKC-θ−/− mice and WT littermates. The right carotid artery was exposed surgically, and a miniature Doppler flow probe (model 0.7, phosphate-buffered saline; Transonic Systems, Ithaca, NY) was placed on the surface of the artery. Normal saline was placed on the surgical wound to allow Doppler monitoring, and the baseline blood flow was recorded using a Transonic T402 flow meter. Thereafter, Whatman filter paper (Maidstone, United Kingdom; 1 × 1 mm) saturated with 10% FeCl3 was applied to the adventitial surface of the carotid artery, immediately proximal to the flow probe. After 2 minutes, the filter paper was removed, saline solution was placed again in the wound, and the carotid blood flow was monitored for 30 minutes. Time to thrombotic occlusion after initiation of arterial injury was defined as the time required for blood flow to decline to 0 mL/min. The operator was blinded to mouse genotype while performing all experiments.
Statistical analysis
Data were analyzed by unpaired Student t test. P values less than .05 were considered significant.
Results
Agonist-induced Thr538 phosphorylation of PKC-θ in platelets
To investigate the activation of PKC-θ in platelets, we examined the activation-dependent Thr538 phosphorylation in the activation loop of PKC-θ using antiphospho(Thr538)-specific PKC-θ antibodies after stimulation by different platelet agonists. Activated PKC-θ is threonine phosphorylated on 538 residues, which is critical for its in vivo kinase activity.14 Previous reports demonstrated that alboaggregin A (GPVI and GPIb-V-IX receptor agonist),18 collagen and fibrinogen binding to platelets induced tyrosine phosphorylation of PKC-θ,17 but have not examined the phosphorylation of Thr538 on this PKC isoform.
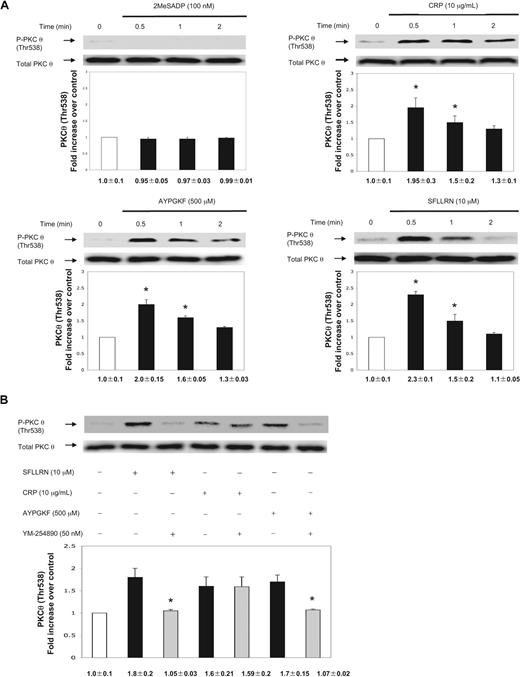
We used aspirin-treated washed human platelets to eliminate positive feedback contribution of TXA2 in PKC-θ activation. 2MeSADP (100 nm) failed to cause PKC-θ threonine phosphorylation (Figure 1A). We next investigated the effect of stronger platelet agonists, including CRP as a GPVI agonist, SFLLRN (PAR1 agonist), and AYPGKF (PAR4 agonist). Thr538 phosphorylation of PKC-θ occurred in a time-dependent manner starting at 30 seconds (Figure 1A). The phosphorylation was still present after CRP and AYPGKF stimulation until 2 minutes, whereas SFLLRN-induced PKC-θ threonine phosphorylation significantly decreased by 2 minutes of activation (Figure 1A). Blots were analyzed by densitometry, and phosphorylation data were expressed in fold increase over control. The difference in the time-dependent phosphorylation of PKC-θ could be a result of the different kinetics of receptor activation.19 These data also show that agonists that cause dense granule secretion also cause activation of PKC-θ, suggesting the functional role of this PKC isoform in the process of dense granule secretion.
Activation of PKC-θ by different platelet agonists. (A) Washed and aspirin-treated human platelets were stimulated with 2MeSADP, collagen-related peptide (CRP), and PAR agonists SFLLRN (PAR1) and AYPGKF (PAR4) peptides with various times as indicated at 37°C. (B) Aspirin-treated, washed human platelets were stimulated with SFLLRN (10 μM) and AYPGKF (500 μM) peptides and CRP (10 μg/mL) in the presence or absence of Gq selective inhibitor YM-254890 (50 nm) at 37°C. The stimulation times for all agonists were 60 seconds. The samples were analyzed for threonine phosphorylation of PKC-θ by Western blotting using monoclonal phospho(Thr538)-specific PKC-θ antibody. Equal lane loading was assured by probing the samples with total PKC-θ in the same blot. The Western blot shown is representative of 3 experiments done from 3 different donors. Data were quantified by densitometry and analyzed the fold increase over control. *P < .05.
Activation of PKC-θ by different platelet agonists. (A) Washed and aspirin-treated human platelets were stimulated with 2MeSADP, collagen-related peptide (CRP), and PAR agonists SFLLRN (PAR1) and AYPGKF (PAR4) peptides with various times as indicated at 37°C. (B) Aspirin-treated, washed human platelets were stimulated with SFLLRN (10 μM) and AYPGKF (500 μM) peptides and CRP (10 μg/mL) in the presence or absence of Gq selective inhibitor YM-254890 (50 nm) at 37°C. The stimulation times for all agonists were 60 seconds. The samples were analyzed for threonine phosphorylation of PKC-θ by Western blotting using monoclonal phospho(Thr538)-specific PKC-θ antibody. Equal lane loading was assured by probing the samples with total PKC-θ in the same blot. The Western blot shown is representative of 3 experiments done from 3 different donors. Data were quantified by densitometry and analyzed the fold increase over control. *P < .05.
PKC-θ is activated via Gq in PAR-mediated platelet activation
PARs activate Gq and G12/13 pathways to cause platelet activation, although PLC stimulation occurs via Gq pathway causing Ca2+ release and PKC activation.3,20,21 To evaluate whether PKC-θ activation is dependent of Gq stimulation downstream of PARs, aspirin-treated washed human platelets were pretreated for 15 minutes with a selective inhibitor of Gq protein, YM-254890 (50 nm).22,23 On stimulation by both SFLLRN and AYPGKF, Thr538 phosphorylation was completely abolished in the presence of the inhibitor (Figure 1B). However, in the CRP-activated control, there was no difference in the level of PKC-θ phosphorylation, as Gq signaling is not linked to GPVI pathway. Thus, we conclude that G12/13 pathways are not sufficient to cause PKC-θ activation. Blots were analyzed by densitometry, and phosphorylation data are expressed in fold increase over control.
Role of PKC-θ in platelet functional responses downstream of GPVI and PARs
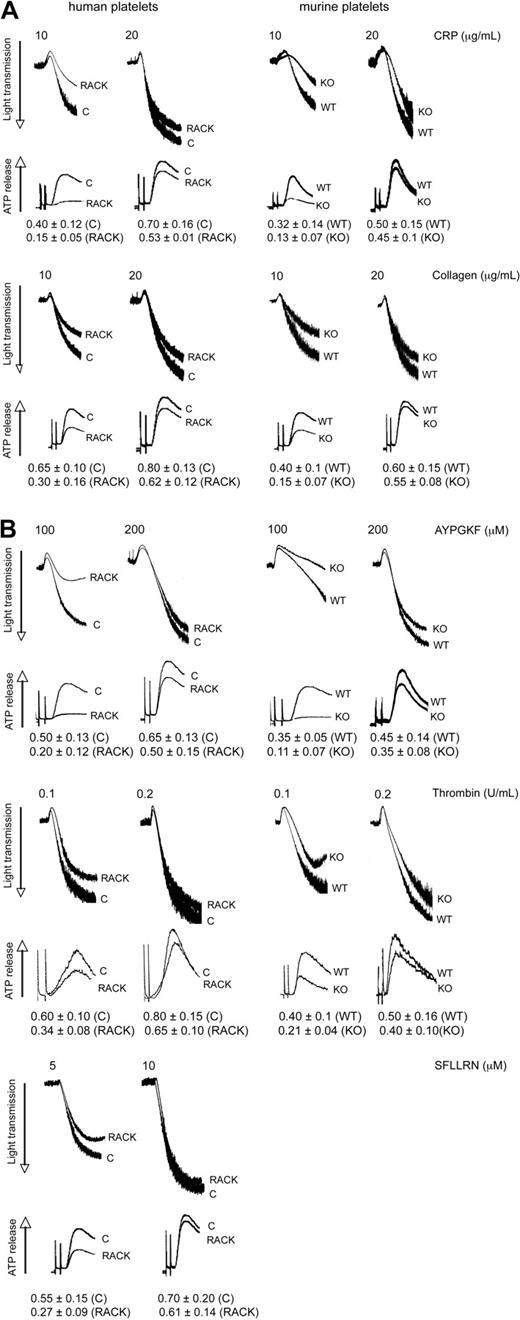
Because PKC-θ is activated downstream of GPVI and PARs, we sought to evaluate the functional role of this PKC isoform in platelet aggregation as well as ATP release and α-granule secretion. These studies were carried out using pharmacologic and gene knockout approaches. Toward pharmacologic approach, we used isoform-specific RACK peptides. Recently, antagonistic RACK peptides for individual PKC isoforms, which selectively inhibit the translocation of the activated PKC isoform to its substrate during platelet activation, have become available to investigate the role of each PKC isoform in terms of different cell functions.24 In the gene knockout approach, we used murine platelets deficient in PKC-θ and WT littermates. In this study, aspirin-treated washed human platelets pretreated with either PKC-θ antagonistic RACK peptide or inactive control peptide (vehicle), and PKC-θ−/− mice with WT littermates, were stimulated with GPVI agonists CRP and collagen (Figure 2A) and PAR agonists AYPGKF, thrombin, and SFLLRN (only in case of human platelets; Figure 2B), and then platelet aggregation and ATP release were examined. As shown in Figure 2A,B, loss of PKC-θ function significantly inhibited the aggregation of platelets and dense granule secretion induced by lower concentrations of these agonists. However, at maximal concentrations of agonists, AYPGKF (500 μM), thrombin (0.5 U/mL), and CRP (50 μg/mL), there was no difference in the aggregation and secretion between WT and PKC-θ−/− murine platelets, or in the presence of the RACK or control peptide in human platelets (data not shown). These results demonstrate that PKC-θ positively regulates aggregation and dense granule secretion on GPVI- and PAR-mediated platelet activation in platelets.
Role of PKC-θ in GPVI- and PAR-mediated platelet aggregation and dense granule secretion. Aspirin-treated washed human platelets in the presence or absence of PKC-θ antagonistic RACK peptide and isolated mouse platelets (as indicated) from PKC-θ−/− mice and WT littermates were stimulated with GPVI agonists (A) CRP (10 and 20 μg/mL) and collagen (10 and 20 μg/mL); and (B) PAR4 agonist AYPGKF (100 and 200 μM), PAR1 and PAR4 agonist thrombin (0.1 and 0.2 U/mL), and PAR1 agonist SFLLRN (5 and 10 μM) for 3.5 minutes at 37°C in stirring condition and their aggregation and simultaneous dense granule secretion were measured and compared. Dense-granule secretion is expressed as ATP released (nmol/108 platelets). The tracings are representative of results from at least 3 different donors.
Role of PKC-θ in GPVI- and PAR-mediated platelet aggregation and dense granule secretion. Aspirin-treated washed human platelets in the presence or absence of PKC-θ antagonistic RACK peptide and isolated mouse platelets (as indicated) from PKC-θ−/− mice and WT littermates were stimulated with GPVI agonists (A) CRP (10 and 20 μg/mL) and collagen (10 and 20 μg/mL); and (B) PAR4 agonist AYPGKF (100 and 200 μM), PAR1 and PAR4 agonist thrombin (0.1 and 0.2 U/mL), and PAR1 agonist SFLLRN (5 and 10 μM) for 3.5 minutes at 37°C in stirring condition and their aggregation and simultaneous dense granule secretion were measured and compared. Dense-granule secretion is expressed as ATP released (nmol/108 platelets). The tracings are representative of results from at least 3 different donors.
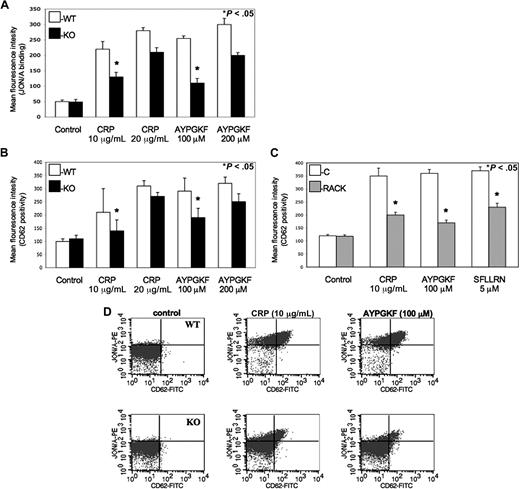
Cifuni et al have recently identified the CalDAG-GEFI and PKC-dependent alternative pathways leading to αIIbβ3 receptor activation via Rap1 in PAR4-stimulated murine platelets.25 To evaluate whether this PKC isoform also directly affects the activation αIIbβ3 receptors to facilitate platelet activation, monoclonal JON/A antibody was used against activated αIIbβ3 receptors when murine platelets were stimulated by agonists and examined by flow cytometry. We found that PKC-θ−/− murine platelets showed a significantly lower level of activated αIIbβ3 receptors compared with WT littermates at lower GPVI and PAR agonist concentrations (Figure 3A). Thus, we demonstrated that PKC-θ has a role in inside-out signaling resulting in activation of αIIbβ3 integrin. Data were presented in MFI values of JON/A antibody binding on platelets.
Role of PKC-θ in αIIbβ3 activation and α-granule secretion downstream of GPVI and PARs. (A) Isolated platelets from PKC-θ−/− mice (■) and WT littermates (□) were stimulated with GPVI agonist CRP (10 and 20 μg/mL) and PAR4 agonsit AYPGKF (100 and 200 μM) to test the JON/A binding to activated αIIbβ3 receptors. (B) WT and PKC-θ−/− murine platelets and (C) washed human platelets with or without PKC-θ RACK peptide were stimulated with CRP (10 and 20 μg/mL) as well as AYPGKF (100 and 200 μM) and PAR1 agonist SFLLRN (5 μM; only in human platelets) for 15 minutes at 37°C in nonstirring condition in the presence of FITC-labeled anti–P-selectin (CD62)-antibody. Reactions were terminated by fixing the platelets with PFA and then analyzed by flow cytometry. Each bar is the average of 3 experiments plus or minus SD from 3 different donors. (D) Dot plots of dual-color labeling for JON/A binding and CD62 expression on murine platelets are representative data of panels A and B.
Role of PKC-θ in αIIbβ3 activation and α-granule secretion downstream of GPVI and PARs. (A) Isolated platelets from PKC-θ−/− mice (■) and WT littermates (□) were stimulated with GPVI agonist CRP (10 and 20 μg/mL) and PAR4 agonsit AYPGKF (100 and 200 μM) to test the JON/A binding to activated αIIbβ3 receptors. (B) WT and PKC-θ−/− murine platelets and (C) washed human platelets with or without PKC-θ RACK peptide were stimulated with CRP (10 and 20 μg/mL) as well as AYPGKF (100 and 200 μM) and PAR1 agonist SFLLRN (5 μM; only in human platelets) for 15 minutes at 37°C in nonstirring condition in the presence of FITC-labeled anti–P-selectin (CD62)-antibody. Reactions were terminated by fixing the platelets with PFA and then analyzed by flow cytometry. Each bar is the average of 3 experiments plus or minus SD from 3 different donors. (D) Dot plots of dual-color labeling for JON/A binding and CD62 expression on murine platelets are representative data of panels A and B.
α-Granule secretion also propagates platelet activation, which is a much slower process than dense granule release.26 The expression of P-selectin (CD62) receptor is a sensitive marker of α-granules release as well as being that of general platelet activation.4 Previous studies demonstrated some data about the potential function of different PKC isoforms in the regulation of α-granule secretion.27,–29 Hence, we evaluated the role of PKC-θ in α-granule release in murine platelets isolated from PKC-θ−/− and WT mice (Figure 3B) and washed human platelets in the presence or absence of PKC-θ RACK peptide (Figure 3C) by measuring P-selectin expression on flow cytometer observed with FITC-labeled CD62 antibody. Agonist-induced α-granule secretion was significantly impaired in platelets lacking PKC-θ (Figure 3B,C). These results show that PKC-θ, similarly as observed in dense granule release, positively regulates α-granule secretion during GPVI and PAR-mediated platelet activation. Data were presented in MFI values of CD62 surface expression on platelet surface. Moreover, murine platelets were further analyzed in dual-color labeling with JON/A and CD62 antibodies to measure the coexpression of the 2 important activation markers. Dot plots are representative data of this analysis at low concentration of agonists (Figure 3D).
PKC-θ mediates syntaxin-4 phosphorylation
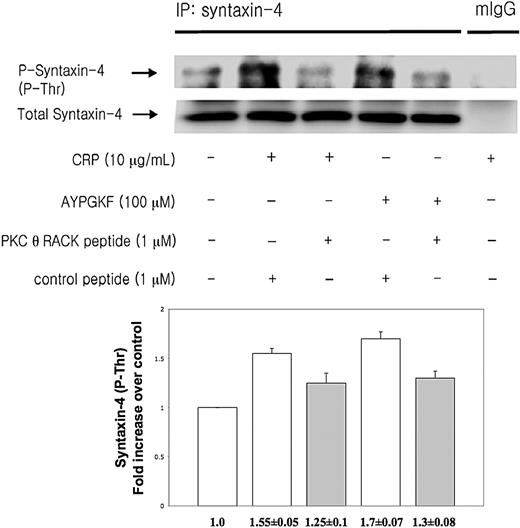
Syntaxin-4 has been shown to be an essential member of tSNARE complex in the regulation of secretion in human platelets.30 PKCs directly phosphorylate syntaxin-4 resulting in inhibition of binding of syntaxin-4 to SNAP-23 proceeding platelet secretion.30 To investigate whether PKC-θ affects platelet secretion via mediating syntaxin-4 phosphorylation, washed human platelets preincubated with either PKC-θ RACK peptide or control peptide were activated by low concentration of CRP and AYPGKF and then immunoprecipitated for syntaxin-4, and then immunoblotted for phosphorylation on threonine residues of syntaxin-4. Normal mouse IgG served as a negative control. We found that human platelets in the presence of PKC-θ RACK peptide showed a strongly reduced threonine phosphorylation of syntaxin-4 after GPVI and PAR4 activation compared with stimulated control samples (Figure 4). Blots were analyzed by densitometry, and phosphorylation data were expressed in fold increase over control. These results indicate that PKC-θ regulates granule secretion through syntaxin-4 phosphorylation.
PKC-θ-mediated syntaxin-4 phosphorylation. Washed human platelets were stimulated with CRP (10 μg/mL) and AYPGKF (100 μM) at 37°C in the presence of PKC-θ RACK peptide or control peptide and then immunoprecipitated for syntaxin-4, and immunoblotted for phosphorylation on threonine residues of syntaxin-4. Normal mouse IgG served as a negative control. Phosphorylation data were quantified and analyzed in fold increase over control.
PKC-θ-mediated syntaxin-4 phosphorylation. Washed human platelets were stimulated with CRP (10 μg/mL) and AYPGKF (100 μM) at 37°C in the presence of PKC-θ RACK peptide or control peptide and then immunoprecipitated for syntaxin-4, and immunoblotted for phosphorylation on threonine residues of syntaxin-4. Normal mouse IgG served as a negative control. Phosphorylation data were quantified and analyzed in fold increase over control.
Role of PKC-θ in GPVI and PAR-mediated thromboxane generation and ERK phosphorylation
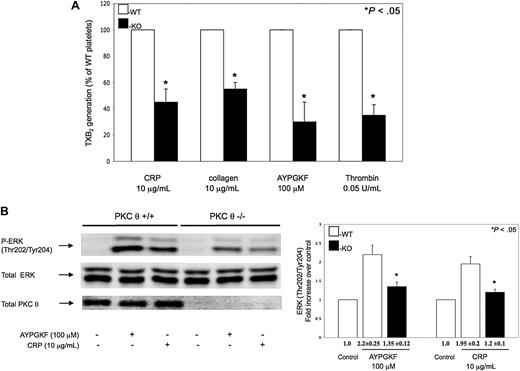
It has been shown that GPVI- and PAR-stimulated platelets generate TXA2 de novo, which is crucial for maximal platelet activation and for the maintenance of normal hemostasis.31,–33 We measured the TXA2 released in PKC-θ−/− murine platelets on stimulation with CRP, collagen, AYPGKF, and thrombin, to evaluate whether PKC-θ mediates TXA2 generation via GPVI and PARs. As seen in Figure 5A, the levels of TXA2 release measured in GPVI and PAR agonist-stimulated PKC-θ−/− murine platelets were significantly lower compared with platelets from WT littermates.
Role of PKC-θ on thromboxane generation and ERK phosphorylation. (A) Non–aspirin-treated mouse platelets separated from PKC-θ−/− mice (■) and WT littermates (□) were stimulated with collagen (10 μg/mL) and CRP (10 μg/mL) as well as AYPGKF (100 μM) and thrombin (0.05 U/mL) for 3.5 minutes at 37°C in stirring condition. Reactions were terminated and the generated TXB2 levels were measured. Data are presented as maximal percentage of TXB2 generated in the WT controls. Each bar is the average ( ± SD) of 3 experiments from 3 different donors. (B) Stimulated washed mouse samples were also analyzed by Western blotting using antibodies against antiphospho-ERK and total ERK as lane loading control. The phenotype of the mice was assured by probing the mice samples with total PKC-θ antibody. The Western blot shown is representative of results from 3 different donors. Phosphorylation data were quantified and analyzed in fold increase over control.
Role of PKC-θ on thromboxane generation and ERK phosphorylation. (A) Non–aspirin-treated mouse platelets separated from PKC-θ−/− mice (■) and WT littermates (□) were stimulated with collagen (10 μg/mL) and CRP (10 μg/mL) as well as AYPGKF (100 μM) and thrombin (0.05 U/mL) for 3.5 minutes at 37°C in stirring condition. Reactions were terminated and the generated TXB2 levels were measured. Data are presented as maximal percentage of TXB2 generated in the WT controls. Each bar is the average ( ± SD) of 3 experiments from 3 different donors. (B) Stimulated washed mouse samples were also analyzed by Western blotting using antibodies against antiphospho-ERK and total ERK as lane loading control. The phenotype of the mice was assured by probing the mice samples with total PKC-θ antibody. The Western blot shown is representative of results from 3 different donors. Phosphorylation data were quantified and analyzed in fold increase over control.
TXA2, as a positive feedback, triggers Gq-mediated responses and a second wave of platelet secretion.34 To evaluate whether platelet secretion is affected by PKC-θ function only because of diminished TXA2 generation, PKC-θ−/− murine platelets were activated by the agonists in the presence or absence of 10 μM indomethacin after preincubation for 10 minutes. Indomethacin as a nonselective cyclo-oxygenase inhibitor that blocks the TXA2 generation. We observed that PKC-θ positively regulates (primary) platelet secretion even in the presence of indomethacin when TXA2 generation was completely abolished (data not shown). Hence, we concluded that the role of PKC-θ in platelet secretion is not the result of its effect on TXA2 generation.
It has been revealed that ERK, which is activated on PAR- and GPVI-stimulated platelets, is an important mediator of activation of cytosolic phospholipase A2.35,–37 TXA2 is produced endogenously from arachidonic acid that is released from the platelet membrane phospholipids via the activation of cytosolic phospholipase A2. Agonist-induced ERK phosphorylation was significantly decreased in PKC-θ−/− murine platelets, which may contribute to lower TXA2 levels (Figure 5B). However, less difference was found between PKC-θ−/− and WT platelets, when murine platelets were activated by increasing concentrations of agonists (data not shown). Blots were analyzed by densitometry, and phosphorylation data are expressed in fold increase over control. These data suggest that PKC-θ has a direct functional role in TXA2 generation through regulating ERK phosphorylation on GPVI and PAR platelet activation.
PKC-θ deficiency impairs in vivo platelet function
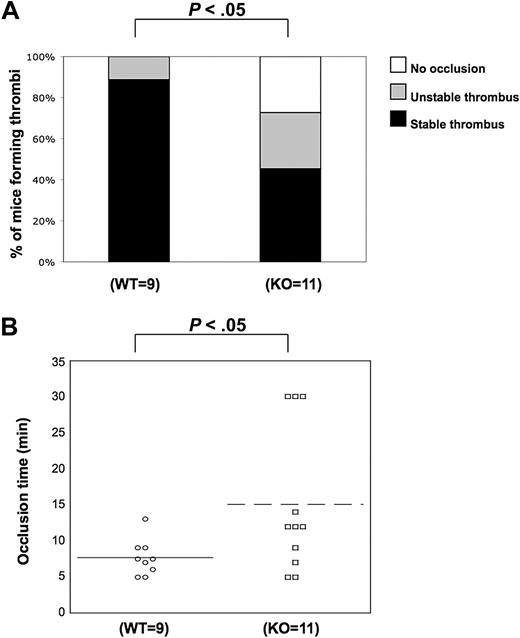
We observed the positive functional role of PKC-θ in several functional responses downstream of GPVI and PARs. These results led us to examine and confirm whether PKC-θ participates in the thrombus formation in vivo. Using the FeCl3 carotid artery-injury model of in vivo thrombosis, we analyzed the time to 100% occlusion in WT (n = 9) and PKC-θ−/− (n = 11) mice. WT and P2Y1-deficient mice were used previously for standardization in our laboratory (data not shown) because the in vivo thrombosis data have been well characterized in P2Y1−/− mice.38 Stable thrombus formation was scored when blood flow remained at 0 mL/min for 30 minutes. Thrombi were considered as unstable when blood flow stopped, and then resumed before 30 minutes of period. In PKC-θ−/− mice, an average prolonged time of occlusion of 15.7 plus or minus 3.3 minutes (mean ± SEM) was observed, and 55% of PKC-θ−/− mice failed to form a stable thrombus in response to injury. However, 95% of WT mice formed a stable thrombus in 7.6 (± 1.1) minutes of occlusion time (Figure 6). The percentage of mice formed thrombi and the mean values of occlusion time calculated between PKC-θ−/− and WT mice were found statistically significant (P < .05). These findings show that PKC-θ is critical for stabilizing thrombus formation after arterial injury.
Role of PKC-θ in thrombus formation in vivo. Using the FeCl3 carotid artery injury model of in vivo thrombosis (2 minutes of exposure to 10% FeCl3), we analyzed the percentage of mice forming stable thrombi (A) and the average time of occlusion (B) in PKC-θ−/− and WT mice. PKC-θ−/− mice demonstrated prolonged average occlusion time (mean ± SEM; 15.7 ± 3.3 minutes) and a failure to form a stable thrombus versus littermates (7.6 ± 1.1 minutes of occlusion time; P < .05 by unpaired Student t test). Fisher exact probability test was used for analyzing data shown in panel A.
Role of PKC-θ in thrombus formation in vivo. Using the FeCl3 carotid artery injury model of in vivo thrombosis (2 minutes of exposure to 10% FeCl3), we analyzed the percentage of mice forming stable thrombi (A) and the average time of occlusion (B) in PKC-θ−/− and WT mice. PKC-θ−/− mice demonstrated prolonged average occlusion time (mean ± SEM; 15.7 ± 3.3 minutes) and a failure to form a stable thrombus versus littermates (7.6 ± 1.1 minutes of occlusion time; P < .05 by unpaired Student t test). Fisher exact probability test was used for analyzing data shown in panel A.
Discussion
PKC isoforms have been appreciated to be essential in full platelet activation as playing mostly a positive role in different platelet functional responses.10 Although PKCs have a high degree of homology, it is presumed that each PKC isoform has distinct important function in platelet aggregation, secretion, αIIbβ3 activation, and the regulation of cytoskeleton.10 This current study was designed to clarify the entire functional role of PKC-θ downstream of PAR and GPVI receptors. Here, we investigated the activation mechanism and the functional role with the molecular mechanism of this PKC isoform in platelet functional responses using pharmacologic and gene knockout approaches using different platelet agonists.
Activated PKC-θ is phosphorylated at threonine, serine (autophosphorylation), and tyrosine residues. We observed the Thr538 phosphorylation site to examine which platelet agonists induce PKC-θ activation. GPVI and PAR agonists through PLC signaling activated PKC-θ in a time-dependent manner, but not by ADP (Figure 1A). Similarly, the activation of another novel PKC isoform, PKC-δ, occurs only with stimulation by GPVI and PAR agonists, but not by ADP.8,9 After activation by CRP and AYPGKF, the threonine phosphorylation of PKC-θ was still present until 2 minutes, whereas SFLLRN-induced PKC-θ phosphorylation significantly decreased by 2 minutes of activation. Our group detected similar threonine-505 phosphorylation manner of PKC-δ on GPVI and PAR stimulation.8 This is not very surprising as the intracellular calcium mobilization by SFLLRN versus AYPGKF is known to be different.19 The SFLLRN-induced increase in intracellular calcium is transient, whereas AYPGKF causes a sustained increase in intracellular calcium levels.19 However, both signaling cascades occur via Gq-coupled pathway (Figure 1B); we have no further evidence that PKC-θ phosphorylation is affected by other different pathways.
To evaluate the role of PKC isoforms in platelet functional responses, different pharmacologic and genetic tools are now available. Here, we used PKC-θ antagonistic RACK peptide in human platelets to selectively inhibit the translocation of PKC-θ to its substrate. These RACK peptides were developed and extensively used by Chen et al, for example, to study the role of PKC-δ and PKC-ϵ in isolated cardiomyocytes.24 In our study, PKC-θ RACK peptide significantly inhibited the aggregation and dense granule secretion of aspirin-treated washed human platelets induced by GPVI and PAR agonists compared with control. These results demonstrate that PKC-θ positively regulates platelet aggregation and dense granule release on GPVI- and PAR-mediated activation in human platelets. Consistent results were obtained from PKC-θ−/− mice platelets in terms of aggregation and secretion caused by GPVI agonists CRP and collagen and PAR agonists AYPGKF and thrombin compared with platelets from WT mice (Figure 2A,B). Furthermore, PKC-θ positively regulated (primary) platelet secretion in PKC-θ−/− mice even in the presence of cyclo-oxygenase inhibitor indomethacin, when TXA2 generation was completely abolished (data not shown). Hence, we conclude that the role of PKC-θ in platelet secretion is not the result of its effect on TXA2 generation. These data also show that only those agonists that cause dense granule secretion activate PKC-θ, emphasizing the functional role of this PKC isoform in the process of dense granule release downstream of both pathways tested. In contrast, PKC-δ plays a positive role in PAR-mediated platelet dense granule secretion but negatively regulates GPVI-induced dense granule release.8 PKC-α/β isoforms positively regulate GPVI-mediated dense granule secretion but do not play a significant role in PAR-mediated dense granule release.8 Others also identified PKC-α to be an essential signaling molecule in Ca2+-induced platelet secretion.27
The expression of P-selectin (CD62) receptor is considered to be one of the most sensitive markers of platelet activation and, by mediating adhesive interactions between platelets, leukocytes, and endothelial cells, plays a pivotal role in the pathogenesis of thrombosis and inflammation.4 Previous studies have demonstrated the role of different PKC isoforms in the regulation of α-granule secretion.27,–29 Here, we found that GPVI and PAR-induced α-granule secretion is positively regulated by PKC-θ signaling (Figure 3B,C). Libersan and Merhi29 showed that thrombin-induced P-selectin expression in platelets requires for novel PKC-ϵ and PKC-η isoforms and atypical PKC-ζ, isoform, but not protein tyrosine kinase or phosphoinositide 3-kinase. However, these studies relied on the nonselective inhibition of these isoforms by rottlerin and hence cannot be conclusive. PKC-α is also involved in α-granule secretion, but the role of PKC-β and PKC-δ was excluded in this process.27 Other investigators found that P-selectin expression was slightly reduced with the use of rottlerin, a PKC-δ selective inhibitor in thrombin-induced but not in collagen-induced platelet activation.28
To provide more evidence that PKC-θ signaling positively modulates granule secretion on GPVI and PAR-induced platelet activation, washed human platelets preincubated with either PKC-θ RACK peptide or control peptide were activated by low concentration of CRP and AYPGKF and then immunoprecipitated for syntaxin-4, and immunoblotted for phosphorylation on threonine residues of syntaxin-4. It has been formerly observed that one of tSNARE protein syntaxin-4 has a very important role in granule release in Ca2+- and thrombin-activated human platelets30,39,40 and appears to be phosphorylated by PKCs on thrombin-stimulated platelet activation.30 PKC phosphorylation of syntaxin-4 was shown to be functionally important because it decreased the binding of phosphorylated syntaxin-4 to SNAP-23, leading to vesicle-associated membrane SNARE-complex interactions during membrane trafficking and fusion.30,39,40 Here, we found that human platelets in the presence of PKC-θ RACK peptide showed a strongly reduced threonine phosphorylation of syntaxin-4 after GPVI and PAR4 activation compared with stimulated control samples (Figure 4). These results give an insight into the molecular mechanism of involvement of PKC-θ in the regulation of granule release. Chung et al suggested that, besides Ca2+-dependent PKC isoforms, Ca2+-independent isoforms are also important in granule secretion, as specific inhibitors of classic PKC isoforms (Go-6983 and Go-6976) had only minimal effect on syntaxin-4 phosphorylation, which promotes the release of granule release.30
The activation of αIIbβ3 receptors requires Ca2+- and PKC-dependent signaling pathways downstream of PARs and GPVI.41,42 Furthermore, Cifuni et al25 recently described the CalDAG-GEFI and PKC-dependent alternative pathways leading to αIIbβ3 receptor activation via Rap1 in PAR4-stimulated murine platelets, emphasizing the essential role of PKCs in this functional response. Among classic isoforms, PKC-β is required for platelet spreading on fibrinogen.43 PKC-θ was shown to be constitutively associated with αIIbβ3 but was excluded as a regulator enzyme in agonist-induced inside-out signaling and fibrinogen binding to αIIbβ3.17 Their conclusions appear not to agree with our observations that PKC-θ has a significant role in platelet aggregation through inside-out signaling. Our data demonstrated (1) the inhibition of aggregation in human platelets treated with PKC-θ RACK peptide as well as in PKC-θ−/− mice compared with vehicle or WT mice; (2) the contribution of PKC-θ to PKC-dependent αIIbβ3 receptor activation when significantly lower levels of activated αIIbβ3 receptors were found in PKC-θ−/− mice compared with WT littermates; and (3) unstable thrombosis formation in PKC-θ−/− mice in vivo. Soriani et al17 examined inside-out signaling in PKC-θ−/− mice vs WT littermates stimulated with ADP, epinephrine, and high concentrations of AYPGKF. We also observed that the role of PKC-θ is not evident when high concentrations of agonists were used, suggesting that platelet stimulation with high concentrations of strong agonists is not appropriate for studying the possible role of PKC isoforms in platelet functional responses.
Our conclusions regarding the role of PKC-θ in platelet function were confirmed when PKC-θ−/− mice displayed an average prolonged time of arterial occlusion in vivo and a failure to form a stable thrombus. Although tail-bleeding times in PKC-θ−/− mice were normal relative to WT mice,17 such discrepancy in the bleeding times and in vivo thrombosis was observed in the mice lacking Akt isoforms.44 Impaired receptor-mediated activation of αIIbβ3 with decreased PKC-θ activity in the association of core-binding factor A2 mutation was also reported in human platelets.45 These findings support our hypothesis that PKC-θ through inside-out signaling is critical for stabilizing thrombus formation after arterial injury. However, as observed by Soriani et al, PKC-θ might also be playing a role downstream of outside-in signaling.17
Secondary mediator TXA2 has been reported to be involved in GPVI and PAR-induced platelet aggregation.35,–37 We showed that both GPVI and PAR-mediated TXA2 release from platelets strongly depends on PKC-θ signaling. In addition, the phosphorylation of downstream effector ERK was significantly decreased in PKC-θ−/− mice platelets, which may contribute to lower TXA2 levels.46,47
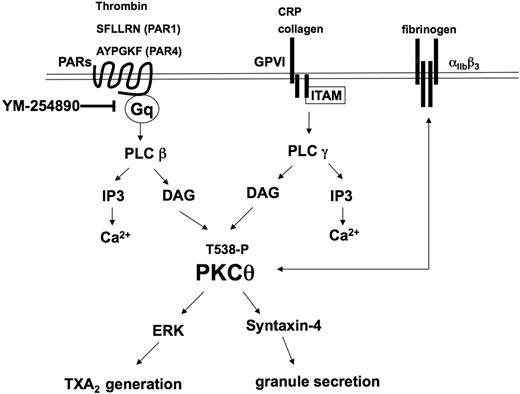
In conclusion, the present study adds new insights into the activation, functional role, and molecular mechanistic involvement of PKC-θ in agonist-induced platelet functional responses (Figure 7). After binding of collagen or CRP to GPVI and PAR agonists (SFLLRN, AYPGKF, and thrombin) to thrombin receptors, PKC-θ is threonine phosphorylated in 30 seconds through the activation of one of PLC isozymes and positively mediates platelet aggregation. Moreover, secretion also requires PKC-θ activation via mediating syntaxin-4 phosphorylation. This PKC isoform triggers the phosphorylation of ERK, which ultimately results in the generation and release of TXA2. Secretion of TXA2 is well known to cause recruitment and activation of platelets at the site of injury. Consequently, PKC-θ is a functionally important signaling mole-cule that is involved in multiple pathways in the activation of platelets and in vivo thrombus formation.
Model depicting the functional role of PKC-θ downstream of GPVI and PAR activation in platelets. Collagen and CRP act through GPVI, whereas thrombin, SFLLRN (PAR1), and AYPGKF (PAR4) act via PARs and cause activation of the Gq/PLC pathways. PLC activation leads to generation of IP3, which mobilizes calcium from intracellular stores. Increased DAG leads to the translocation to the membrane and subsequent phosphorylation of the threonine 538 residues on PKC-θ. Activated PKC-θ has a significant role in granule secretion via mediating syntaxin-4 phosphorylation and thromboxane generation via regulating ERK phosphorylation. Furthermore, PKC-θ is involved in both inside-out and outside-in αIIbβ3 signaling pathways.
Model depicting the functional role of PKC-θ downstream of GPVI and PAR activation in platelets. Collagen and CRP act through GPVI, whereas thrombin, SFLLRN (PAR1), and AYPGKF (PAR4) act via PARs and cause activation of the Gq/PLC pathways. PLC activation leads to generation of IP3, which mobilizes calcium from intracellular stores. Increased DAG leads to the translocation to the membrane and subsequent phosphorylation of the threonine 538 residues on PKC-θ. Activated PKC-θ has a significant role in granule secretion via mediating syntaxin-4 phosphorylation and thromboxane generation via regulating ERK phosphorylation. Furthermore, PKC-θ is involved in both inside-out and outside-in αIIbβ3 signaling pathways.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr G. L. Prasad (Department of Physiology, Temple University School of Medicine) for helpful suggestions and Drs Daria Mochley-Rosen and Grant Budas (Department of Chemical and Systems Biology, Stanford University, Stanford, CA) for providing RACK peptides and suggestions on their use.
This study was supported by the National Institutes for Health (HL80444, HL 81322, HL93231, and HL60683).
National Institutes of Health
Authorship
Contribution: B.N. designed and performed experiments, analyzed data, and wrote the paper; K.B., T.G., Y.S.B., and S.K. performed experiments; and S.P.K. provided overall direction, designed experiments, and analyzed data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Satya P. Kunapuli, Department of Physiology, Temple University School of Medicine, 3420 N Broad Street, Philadelphia, PA 19140; e-mail: spk@temple.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal