Abstract
Age-related Epstein-Barr virus–associated B-cell lymphoproliferative disorder (aEBVLPD) is a disease group characterized by EBV-associated large B-cell lymphoma in elderly without predisposing immunodeficiency. In nearly one- third of cases, aEBVLPD occurs as a polymorphous subtype with reactive cell-rich components, bearing a morphologic similarity to classic Hodgkin lymphoma (cHL). The aim of this study was to clarify clinicopathologic differences between the polymorphic subtype of aEBVLPD (n = 34) and EBV+ cHL (n = 108) in patients aged 50 years or older. Results showed that aEBVLPD was more closely associated with aggressive clinical parameters than cHL, with a higher age at onset (71 vs 63 years); lower male predominance (male-female ratio, 1.4 vs 3.3); and a higher rate of involvement of the skin (18% vs 2%), gastrointestinal tract (15% vs 4%), and lung (12% vs 2%). aEBVLPD was histopathologically characterized by a higher ratio of geographic necrosis, greater increase (> 30%) in cytotoxic T cells among background lymphocytes, higher positivity for CD20 and EBNA2, and absence of CD15 expression. As predicted by the clinical profile, aEBVLPD had a significantly poorer prognosis than EBV+ cHL (P < .001). The polymorphous subtype of aEBVLPD constitutes an aggressive group with an immune response distinct from EBV+ cHL, and requires the development of innovative therapeutic strategies.
Introduction
Epstein-Barr virus (EBV), a member of the herpes virus family, infects more than 90% of the worldwide adult population.1,2 In vitro, EBV randomly infects resting B cells and transforms them into lymphoblastoid cell lines. This property makes it a candidate causative agent for many human cancers, including Burkitt lymphoma,3,4 Hodgkin lymphoma,1 immunodeficiency-associated lymphoproliferative disorders (LPDs),5 and some diffuse large B-cell lymphomas.6 Although the mechanism of this effect has not been precisely identified, 2 events that cause the lymphoblastoid cell to survive and evolve into a lymphoma are assumed: first, the EBV-infected cell must be unable to exit the cell cycle to become a resting memory B cell; and second, the cytotoxic T-cell response must be impeded so that the lymphoblast is not killed. It is thus widely accepted that T cells play a prominent role in controlling EBV-associated oncogenesis.1 In support of this, administration of immunosuppressive agents such as tacrolimus or cyclosporin A to prevent the rejection of organ transplants sometimes causes EBV-positive B-cell LPDs.7,8 On these bases, the tumor microenvironment is thought to play an important role in the pathogenesis and tumor progression of EBV-associated B-cell neoplasms.
The World Health Organization (WHO) classification for hematopoietic neoplasm currently categorizes immunodeficiency-associated LPDs into 4 main groups9 : (1) LPDs associated with primary immunodeficiency syndromes and other primary immune disorders10-12 ; (2) lymphomas associated with infection with human immunodeficiency virus (HIV)13-15 ; (3) posttransplantation LPDs (PTLDs) in patients who have received a solid organ or bone marrow allograft16-19 ; and (4) methotrexate-associated LPDs, most commonly seen in patients with autoimmune disease.20,21 In 2003, we reported 22 elderly patients with EBV-positive B-cell LPD, which in many respects appeared analogous to immunodeficiency-associated B-cell LPDs. Of particular note, we have found no evidence of underlying immunodeficiency among these patients to date,22 leading us to propose senile or age-related EBV+ LPD (aEBVLPD) as a nosologic term based on the hypothesis that the pathogenesis of these 2 conditions may be closely associated with an immunologic deterioration or senescence derived from the aging process.
We and others subsequently provided additional evidence that aEBVLPD constitutes an aggressive clinicopathologic group that is distinct from EBV-negative diffuse large B-cell lymphoma (DLBCL) and relatively resistant to conventional chemotherapies.23 This disease has just been listed in the fourth version of the WHO classification.24 aEBVLPD, particularly a polymorphous subtype, also sometimes features the rich infiltration of reactive cells in the background. This variant accounts for approximately one-third of cases, and represents a differential diagnostic issue from classic Hodgkin lymphoma (cHL) with an EBV association. However, little is known about the clinicopathologic differences between these 2 EBV-positive diseases.
Here, we conducted a retrospective clinicopathologic comparison of 34 cases of age-related polymorphous subtype-EBV+ LPD and 108 cases of EBV+ cHL in patients aged 50 years or older.
Methods
Patient samples
We diagnosed aEBVLPD as previously described23 based on the expression of one or more pan-B-cell antigens and/or light-chain restrictions, together with the harboring of EBV in proliferating, often neoplastic-appearing cells. In the present study, the polymorphous subtype of aEBVLPD was defined by the proliferation of CD20+ large cells, including Hodgkin and Reed-Sternberg (HRS)–like cells; namely, more than 50% of large tumor cells were positive for CD20 and/or CD79a, admixed with relatively abundant reactive components. Differential diagnosis of some cases from cHL in hematoxylin and eosin–stained sections alone was therefore difficult. However, the presence of CD20+ large cells was more suggestive of DLBCL. We excluded cases showing morphologic characteristics of mature T-cell lymphomas and immunodeficiency conditions such as HIV infection or a history of use of immunosuppressive agents.22 In addition, we excluded pyothorax-associated lymphoma and EBV-associated lymphomas of the T- or natural killer (NK)–cell phenotype because these were considered to constitute distinct clinicopathologic groups.25,26 In particular, we paid careful attention to the differential diagnosis of age-related LPD from peripheral T-cell lymphoma with Reed-Sternberg–like B cells or from angioimmunoblastic T-cell lymphoma with proliferation of large B cells.26,27 Patients were limited to those aged 50 years or older in accordance with the disease definition of aEBVLPD, and to well-documented cases for which paraffin sections for immunohistochemical analysis were available. Among 149 consecutive cases with a diagnosis of aEBVLPD, 34 with available clinical datasets diagnosed between 1982 and 2005 were enrolled as the polymorphous type, including the 13 cases of senile EBV+ B-cell LPD we previously reported.22 For the control group, 108 patients with EBV+ cHL (age ≥ 50 years) diagnosed between 1982 and 2005 at Aichi Cancer Center were selected. All EBV+ cHL cases had been examined in our previous study.28 All cases were independently reviewed by 6 pathologists (authors N.A., J.-I.T., K.O., T.Y., N.N., and S.N.) to confirm the diagnosis and immunophenotype, with any disagreements resolved by consensus. This study was conducted with the approval of the Institutional Review Board of Aichi Cancer Center, and informed consent was obtained in accordance with the Declaration of Helsinki.
Histologic and immunohistochemical staining
Tissue samples were fixed in 10% formalin and embedded in paraffin, then sectioned at 5 μm and stained with hematoxylin and eosin. Immunoperoxidase studies were performed on formalin-fixed paraffin sections. Monoclonal antibodies used were anti-CD3, UCHL-1/CD45RO, L26/CD20, Ber-H2/CD30, CD79a, latent membrane protein-1 (LMP-1), and EBV-encoded nuclear antigen-2 (EBNA2; DAKO, Glostrup, Denmark); LeuM1/CD15 (Becton Dickinson, Mountain View, CA); TIA-1 (Coulter Immunology, Hialeah, FL); and granzyme B (Monosan, Uden, The Netherlands). All antibodies were used after antigen retrieval after microwave oven heating.
To assess the percentages of cytotoxic T lymphocytes positive for TIA1 and/or granzyme B, whole-tissue sections of all cases were blindly reviewed twice by 2 of the authors (N.A. and S.N.). Discrepancies were reviewed at a multiheaded microscope until consensus was achieved.
In situ hybridization study
The presence of EBV small ribonucleic acids was examined by in situ hybridization using EBV-encoded small nuclear early region (EBER) oligonucleotides on formalin-fixed, paraffin-embedded sections as described previously.29
Statistical analysis
Differences in characteristics between the 2 groups were examined by the χ2 test, Fisher exact test, Student t test, or Mann-Whitney U test as appropriate. Patient survival data were analyzed by the Kaplan-Meier method. Differences in survival were tested by the log-rank test. Disease-specific survival (DSS) was measured from the date of diagnosis to the date of death due to a lymphoma-related cause. Patients who died from a nonlymphoma-related cause were censored at the time of death. Deaths from treatment-related causes were classified as death from lymphoma. All data were analyzed with the aid of STATA software (version 9.0; STATA, College Station, TX).
Results
Clinical characteristics
Patient characteristics are listed in Table 1. The 142 patients with aEBVLPD (n = 34) or EBV+ cHL (n = 108) consisted of 103 men and 39 women with a median age of 67 years, ranging from 50 to 92 years. Compared with those with EBV+ cHL, patients with aEBVLPD showed a higher ratio of elderly patients (median, 71 vs 63 years; P = .001) and lower male predominance (female-male ratio, 14:20 vs 25:83; P = .04). Of particular note, patients with aEBVLPD had a significantly higher frequency of patients older than 60 years (88% vs 59%, P = .002) as well as a higher ratio of involvement of the skin (18% vs 2%, P = .002), gastrointestinal tract (15% vs 4%, P = .052), and lungs (12% vs 2%, P = .028). As predicted by these data, aEBVLPD patients were more frequently categorized into a high International Prognostic Index (IPI) score group than EBV+ cHL patients, with the difference being statistically significant.
Patient characteristics at diagnosis of age-related EBV-positive B-cell LPDs of polymorphous type and EBV-positive classic Hodgkin lymphoma
| Variable . | Age-related EBV+ B-cell LPDs polymorphous type, n = 34 . | EBV+cHL age 50 or older, n = 108 . | P . |
|---|---|---|---|
| Median age, y (range) | 71 (50-88) | 63 (45-89) | .001 |
| Older than 60 y (%) | 30 (88) | 63 (59) | .002 |
| Sex, male/female | 20/14 (1.4) | 83/25 (3.3) | .04 |
| Performance status, 2-4 (%) | 22 (67) | 43 (48) | .071 |
| Ann Arbor stage, III/IV (%) | 21 (62) | 53 (59) | .77 |
| Extranodal involvement, more than 1 site (%) | 11 (32) | 15 (17) | .06 |
| Extranodal sites (%) | |||
| Skin | 6 (18) | 2 (2) | .002 |
| GI tract | 5 (15) | 4 (4) | .052 |
| Lung | 4 (12) | 2 (2) | .028 |
| Pleural effusion | 3 (9) | 3 (3) | .21 |
| Bone | 2 (6) | 1 (1) | .13 |
| BM/PB | 3 (9) | 9 (10) | .83 |
| Spleen | 2 (6) | 18 (20) | .054 |
| Liver | 2 (6) | 15 (17) | .12 |
| LDH level, high (%) | 19 (56) | 26 (43) | .24 |
| B symptoms, presence (%) | 18 (56) | 44 (49) | .51 |
| Therapy (%) | .018 | ||
| None | 7 (21) | 4 (6) | |
| Without anthracycline | 3 (9) | 18 (29) | |
| With anthracycline | 23 (70) | 40 (65) | |
| IPI (%) | .010 | ||
| L/LI | 15 (45) | 73 (70) | |
| HI/H | 18 (55) | 31 (30) | |
| Age-related score (%) | .192 | ||
| 0 | 7 (21) | 28 (32) | |
| 1 | 16 (47) | 43 (50) | |
| 2 | 11 (32) | 16 (18) | |
| Variable . | Age-related EBV+ B-cell LPDs polymorphous type, n = 34 . | EBV+cHL age 50 or older, n = 108 . | P . |
|---|---|---|---|
| Median age, y (range) | 71 (50-88) | 63 (45-89) | .001 |
| Older than 60 y (%) | 30 (88) | 63 (59) | .002 |
| Sex, male/female | 20/14 (1.4) | 83/25 (3.3) | .04 |
| Performance status, 2-4 (%) | 22 (67) | 43 (48) | .071 |
| Ann Arbor stage, III/IV (%) | 21 (62) | 53 (59) | .77 |
| Extranodal involvement, more than 1 site (%) | 11 (32) | 15 (17) | .06 |
| Extranodal sites (%) | |||
| Skin | 6 (18) | 2 (2) | .002 |
| GI tract | 5 (15) | 4 (4) | .052 |
| Lung | 4 (12) | 2 (2) | .028 |
| Pleural effusion | 3 (9) | 3 (3) | .21 |
| Bone | 2 (6) | 1 (1) | .13 |
| BM/PB | 3 (9) | 9 (10) | .83 |
| Spleen | 2 (6) | 18 (20) | .054 |
| Liver | 2 (6) | 15 (17) | .12 |
| LDH level, high (%) | 19 (56) | 26 (43) | .24 |
| B symptoms, presence (%) | 18 (56) | 44 (49) | .51 |
| Therapy (%) | .018 | ||
| None | 7 (21) | 4 (6) | |
| Without anthracycline | 3 (9) | 18 (29) | |
| With anthracycline | 23 (70) | 40 (65) | |
| IPI (%) | .010 | ||
| L/LI | 15 (45) | 73 (70) | |
| HI/H | 18 (55) | 31 (30) | |
| Age-related score (%) | .192 | ||
| 0 | 7 (21) | 28 (32) | |
| 1 | 16 (47) | 43 (50) | |
| 2 | 11 (32) | 16 (18) | |
EBV indicates Epstein-Barr virus; LPDs, lymphoproliferative disorders; cHL, classic Hodgkin lymphomas; BM/PB, bone marrow or peripheral blood; and LDH, lactate dehydrogenase.
Histologic characteristics of age-related EBV+ LPD and EBV+ cHL
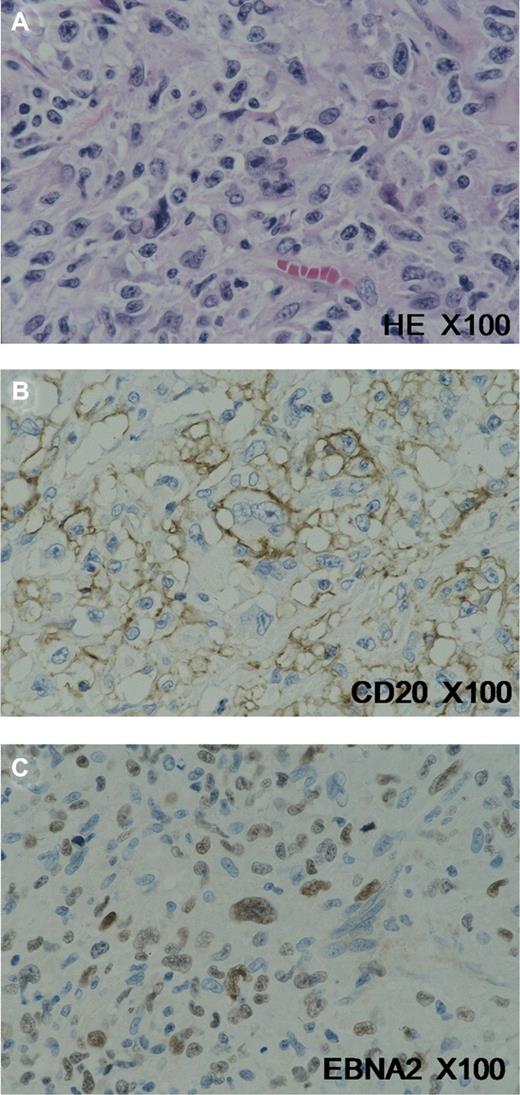
Biopsy specimens of aEBVLPD were obtained from lymph nodes in 27 cases and from extranodal sites in 22. In accordance with the study definition, the polymorphous subtype of aEBVLPD was generally accompanied by a rich cellular infiltration of reactive components such as small lymphocytes, plasma cells, histiocytes, and epithelioid cells, which effaced the overall architecture of the involved lymph node or other tissue. Many large transformed cells/immunoblasts and Hodgkin and Reed-Sternberg (HRS)–like giant cells were frequently observed scattered among the reactive cells (Figure 1A). On the other hand, EBV+ cHL cases were consistent with the established WHO morphologic boundaries9 of mixed cellularity in 96 cases and nodular sclerosis in 12 cases.
Histopathologic profile of age-related EBVLPD. (A) Hodgkin and Reed-Sternberg (HRS)–like giant cells were frequently observed scattered among reactive cells. These cells showed CD20 expression (B), in addition to positive signals for EBNA2 (C). Images were acquired using an Olympus AX80 microscope (Olympus, Tokyo, Japan) with a 100×/1.35 oil Iris lens (Olympus). Staining was with L26/CD20 and EBNA2 (Dako, Glostrup, Denmark). Images were captured with an Olympus DP70 camera and processed with Adobe Photoshop Elements, version 7 (Adobe Systems, San Jose, CA).
Histopathologic profile of age-related EBVLPD. (A) Hodgkin and Reed-Sternberg (HRS)–like giant cells were frequently observed scattered among reactive cells. These cells showed CD20 expression (B), in addition to positive signals for EBNA2 (C). Images were acquired using an Olympus AX80 microscope (Olympus, Tokyo, Japan) with a 100×/1.35 oil Iris lens (Olympus). Staining was with L26/CD20 and EBNA2 (Dako, Glostrup, Denmark). Images were captured with an Olympus DP70 camera and processed with Adobe Photoshop Elements, version 7 (Adobe Systems, San Jose, CA).
Table 2 shows immunophenotypic data of all aEBVLPD cases in the present study. In 4 cases, the morphologic features on hematoxylin and eosin–stained sections more closely resembled cHL, whereas the immunophenotype was more suggestive of DLBCL. CD20 was positive and CD15 was negative in all aEBVLPD cases examined.
Clinical and immunophenotypic features of age-related EBV LPD tumors
| No. . | Age, y . | Sex . | Biopsy specimen . | CD15 . | CD20 . | CD30 . | CD45 . | CD79a . | EBER . | LMP1 . | EBNA2 . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 79 | M | Skin | 0 | 2+ | 2+ | 1+ | 2+ | 2+ | 1+ | 2+ |
| 2 | 82 | M | Cervical LN | 0 | 2+ | 2+ | 0 | 2+ | 2+ | 2+ | 2+ |
| 3 | 88 | M | Cervical LN | 0 | 2+ | 0 | 0 | 2+ | 2+ | 2+ | 0 |
| 4 | 79 | F | Skin | 0 | 2+ | 1+ | 1+ | 2+ | 2+ | 2+ | 0 |
| 5 | 63 | F | Lung, stomach | 0 | 2+ | 0 | 0 | 2+ | 2+ | 2+ | 2+ |
| 6 | 73 | F | Skin, LN | 0 | 2+ | 2+ | 0 | 2+ | 2+ | 2+ | 0 |
| 7 | 83 | M | Pharynx | 0 | 2+ | 2+ | 2+ | 2+ | 0 | ||
| 8 | 68 | M | LN | 0 | 2+ | 0 | 0 | 2+ | 2+ | 2+ | 0 |
| 9 | 85 | M | LN | 0 | 2+ | 0 | 0 | 2+ | 2+ | 2+ | 0 |
| 10 | 78 | M | Spleen | 0 | 2+ | 0 | 1+ | 2+ | 2+ | 2+ | 2+ |
| 11 | 78 | F | LN | 0 | NS | 0 | 2+ | 2+ | 2+ | 2+ | 2+ |
| 12 | 71 | F | LN | 0 | 2+ | 0 | 0 | 2+ | 2+ | 2+ | 0 |
| 13 | 70 | F | Stomach | 2+ | 2+ | 2+ | 0 | ||||
| 14 | 64 | F | LN | 0 | 2+ | 1+ | 2+ | 2+ | 0 | ||
| 15 | 67 | F | LN | 0 | 2+ | 1+ | 2+ | 2+ | 0 | ||
| 16 | 63 | F | Tonsil | 0 | 2+ | 1+ | 2+ | 2+ | 2+ | 0 | |
| 17 | 50 | M | Stomach | 0 | 2+ | 2+ | 2+ | ||||
| 18 | 68 | M | LN | 0 | 2+ | 1+ | 2+ | 2+ | 0 | ||
| 19 | 65 | F | LN | 0 | 2+ | 2+ | 2+ | 2+ | 0 | ||
| 20 | 81 | F | LN | 0 | 2+ | 1+ | 0 | 2+ | 2+ | ||
| 21 | 71 | F | LN | 0 | 2+ | 1+ | 2+ | 1+ | 0 | ||
| 22 | 86 | M | Nasal cavity | 0 | 2+ | 0 | 2+ | 2+ | 1+ | 2+ | |
| 23 | 78 | M | LN | 2+ | 2+ | 2+ | 2+ | 0 | |||
| 24 | 63 | M | LN | 0 | 2+ | 2+ | 2+ | 2+ | 0 | ||
| 25 | 54 | M | LN | 0 | 2+ | 2+ | 2+ | 2+ | 0 | ||
| 26 | 55 | F | Tonsil | 0 | 2+ | 2+ | 2+ | 2+ | 2+ | 2+ | |
| 27 | 73 | M | LN | 2+ | 2+ | ||||||
| 28 | 61 | F | Skin | 0 | 2+ | 1+ | 2+ | 2+ | 0 | ||
| 29 | 55 | M | LN | 2+ | 2+ | ||||||
| 30 | 71 | M | LN | 2+ | 2+ | 2+ | 2+ | NS | |||
| 31 | 79 | M | LN | 2+ | 2+ | ||||||
| 32 | 74 | M | Media-LN | 0 | 2+ | 2+ | 0 | 2+ | 2+ | NS | |
| 33 | 66 | M | LN | 2+ | 2+ | 2+ | 2+ | ||||
| 34 | 82 | M | LN | 0 | NS | 2+ | 2+ | 2+ | 2+ | 0 |
| No. . | Age, y . | Sex . | Biopsy specimen . | CD15 . | CD20 . | CD30 . | CD45 . | CD79a . | EBER . | LMP1 . | EBNA2 . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 79 | M | Skin | 0 | 2+ | 2+ | 1+ | 2+ | 2+ | 1+ | 2+ |
| 2 | 82 | M | Cervical LN | 0 | 2+ | 2+ | 0 | 2+ | 2+ | 2+ | 2+ |
| 3 | 88 | M | Cervical LN | 0 | 2+ | 0 | 0 | 2+ | 2+ | 2+ | 0 |
| 4 | 79 | F | Skin | 0 | 2+ | 1+ | 1+ | 2+ | 2+ | 2+ | 0 |
| 5 | 63 | F | Lung, stomach | 0 | 2+ | 0 | 0 | 2+ | 2+ | 2+ | 2+ |
| 6 | 73 | F | Skin, LN | 0 | 2+ | 2+ | 0 | 2+ | 2+ | 2+ | 0 |
| 7 | 83 | M | Pharynx | 0 | 2+ | 2+ | 2+ | 2+ | 0 | ||
| 8 | 68 | M | LN | 0 | 2+ | 0 | 0 | 2+ | 2+ | 2+ | 0 |
| 9 | 85 | M | LN | 0 | 2+ | 0 | 0 | 2+ | 2+ | 2+ | 0 |
| 10 | 78 | M | Spleen | 0 | 2+ | 0 | 1+ | 2+ | 2+ | 2+ | 2+ |
| 11 | 78 | F | LN | 0 | NS | 0 | 2+ | 2+ | 2+ | 2+ | 2+ |
| 12 | 71 | F | LN | 0 | 2+ | 0 | 0 | 2+ | 2+ | 2+ | 0 |
| 13 | 70 | F | Stomach | 2+ | 2+ | 2+ | 0 | ||||
| 14 | 64 | F | LN | 0 | 2+ | 1+ | 2+ | 2+ | 0 | ||
| 15 | 67 | F | LN | 0 | 2+ | 1+ | 2+ | 2+ | 0 | ||
| 16 | 63 | F | Tonsil | 0 | 2+ | 1+ | 2+ | 2+ | 2+ | 0 | |
| 17 | 50 | M | Stomach | 0 | 2+ | 2+ | 2+ | ||||
| 18 | 68 | M | LN | 0 | 2+ | 1+ | 2+ | 2+ | 0 | ||
| 19 | 65 | F | LN | 0 | 2+ | 2+ | 2+ | 2+ | 0 | ||
| 20 | 81 | F | LN | 0 | 2+ | 1+ | 0 | 2+ | 2+ | ||
| 21 | 71 | F | LN | 0 | 2+ | 1+ | 2+ | 1+ | 0 | ||
| 22 | 86 | M | Nasal cavity | 0 | 2+ | 0 | 2+ | 2+ | 1+ | 2+ | |
| 23 | 78 | M | LN | 2+ | 2+ | 2+ | 2+ | 0 | |||
| 24 | 63 | M | LN | 0 | 2+ | 2+ | 2+ | 2+ | 0 | ||
| 25 | 54 | M | LN | 0 | 2+ | 2+ | 2+ | 2+ | 0 | ||
| 26 | 55 | F | Tonsil | 0 | 2+ | 2+ | 2+ | 2+ | 2+ | 2+ | |
| 27 | 73 | M | LN | 2+ | 2+ | ||||||
| 28 | 61 | F | Skin | 0 | 2+ | 1+ | 2+ | 2+ | 0 | ||
| 29 | 55 | M | LN | 2+ | 2+ | ||||||
| 30 | 71 | M | LN | 2+ | 2+ | 2+ | 2+ | NS | |||
| 31 | 79 | M | LN | 2+ | 2+ | ||||||
| 32 | 74 | M | Media-LN | 0 | 2+ | 2+ | 0 | 2+ | 2+ | NS | |
| 33 | 66 | M | LN | 2+ | 2+ | 2+ | 2+ | ||||
| 34 | 82 | M | LN | 0 | NS | 2+ | 2+ | 2+ | 2+ | 0 |
Scoring for the expression of immunophenotypic markers: 0 indicates a negative stain; 1+, positive on less than 50% of tumor cells; and 2+, positive on more than 50%.
LPD indicates lymphoproliferative disorder; LN, lymph node; and NS, not satisfactory.
Table 3 shows histologic differences between those aEBVLPD and EBV+ cHL cases for which lymph node specimens were available for evaluation. Twelve (55%) of 22 aEBVLPD cases included broad areas of geographic necrosis, a percentage significantly higher than that (8%) in EBV+ cHL cases (P < .001). Small lymphocytes positive for TIA1 and/or granzyme B were observed in all cases, but numbers varied from case to case. In 19 (26%) of the 73 EBV+ cHL cases, these cytotoxic lymphocytes accounted for more than 30% of background reactive lymphoid cells, but for less than 10% in the other cHL cases. In contrast, aEBVLPD was characterized by an increased number of cytotoxic T lymphocytes, accounting for more than 30% of background cells in 11 (65%) of the 17 cases examined (P = .002).
Histologic characteristics of age-related EBV-positive B-cell LPDs and EBV-positive cHL
| . | Age-related EBV+ LPDs polymorphous type, n = 34 . | EBV+ cHL age 50 y older, n = 108 . | P . |
|---|---|---|---|
| Necrosis (%) | 12/22 (55) | 6/80 (8) | < .001 |
| Increasing of cytotoxic T cells (%) | 11/17 (65) | 19/73 (26) | < .001 |
| Immunophenotype (%) | |||
| CD20 | 32/32 (100) | 19/99 (19) | < .001 |
| CD15 | 0/27 (0) | 64/106 (60) | < .001 |
| CD30 | 19/27 (70) | 99/107 (93) | .002 |
| CD45RO | 4/12 (33) | 1/77 (1) | .001 |
| CD56 | 0/13 (0) | 0/21 (0) | > .99 |
| EBER | 34/34 (100) | 108/108 (100) | > .99 |
| LMP1 | 29/29 (100) | 45/52 (87) | .039 |
| EBNA2 | 7/28 (25) | 0/24 (0) | .008 |
| . | Age-related EBV+ LPDs polymorphous type, n = 34 . | EBV+ cHL age 50 y older, n = 108 . | P . |
|---|---|---|---|
| Necrosis (%) | 12/22 (55) | 6/80 (8) | < .001 |
| Increasing of cytotoxic T cells (%) | 11/17 (65) | 19/73 (26) | < .001 |
| Immunophenotype (%) | |||
| CD20 | 32/32 (100) | 19/99 (19) | < .001 |
| CD15 | 0/27 (0) | 64/106 (60) | < .001 |
| CD30 | 19/27 (70) | 99/107 (93) | .002 |
| CD45RO | 4/12 (33) | 1/77 (1) | .001 |
| CD56 | 0/13 (0) | 0/21 (0) | > .99 |
| EBER | 34/34 (100) | 108/108 (100) | > .99 |
| LMP1 | 29/29 (100) | 45/52 (87) | .039 |
| EBNA2 | 7/28 (25) | 0/24 (0) | .008 |
Values are indicated as number of positive/ tested cases.
EBV indicates Epstein-Barr virus; LPDs, lymphoproliferative disorders; and cHL, classic Hodgkin lymphoma.
Several immunophenotypic differences between aEBVLPD and EBV+ cHL were also recognized. According to the definition adopted for this study, all patients with age-related EBV-positive LPDs were positive for EBV and B-cell markers (CD20 and/or CD79a; Figure 1B) on more than 50% of the large tumor cells, in addition to harboring EBV. In 19 of the 99 EBV+ cHL cases examined, a small proportion of HRS and related large cells were also positive for CD20, but less commonly than that for CD79a. Positivity for CD20 remained at less than 10% of large tumor cells in 11 of the 19 cases, from 10% to 30% in 4, and from 30% to 50% in 4. aEBVLPD cases were further characterized by a higher positivity (25% of cases) for EBNA2 (Figure 1C), indicative of type III latency, and an absence (0%) of CD15 expression (Table 3). No significant difference in clinical features between EBNA-2–positive and –negative EBVLPD cases was noted (Table S1, available on the Blood website; see the Supplemental Materials link at the top of the online article).
Therapy and survival
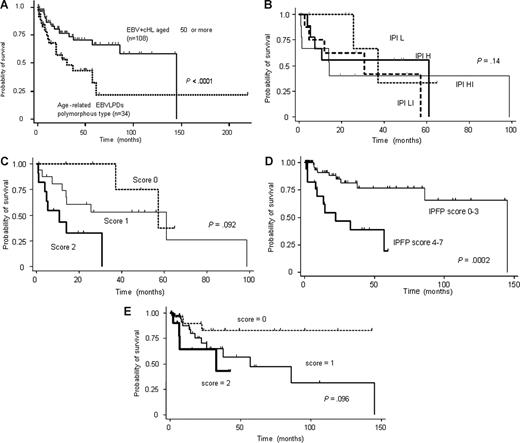
Chemotherapeutic regimens for aEBVLPD included anthracycline in 23 patients and no anthracycline in 3 patients. Seven patients who had not received any therapy because of a poor PS died of the disease (Table 1). Complete remission was achieved in 17 patients (68%) with aEBVLPD and partial remission in 5 (20%), whereas 3 (12%) showed no response. One patient received rituximab and experienced a complete response (CR), but suffered a relapse at 6 months after the CR and died within 1 year. In contrast, 50 (94%) of 53 patients with EBV+ cHL attained remission with the initial therapy, namely an ABVD (doxorubicin, bleomycin, vinblastine, and dacarbazine) regimen in 44 patients and a CMOPP (cyclophosphamide, vincristine, prednisone, and procarbazine) regimen in 9. No significant difference in therapeutic response between aEBVLPD and EBV+ cHL patients was seen, notwithstanding these differences in therapeutic regimen. Disease-specific survival curves in Figure 2A reveal strikingly inferior survival in age-related EBV+ LPD to EBV+ cHL (median survival time, 26 months vs not reached, respectively; P < .001).
Survival curves. (A) Survival curves for age-related EBVLPD and EBV-positive cHL patients. Age-related EBVLPD showed a poorer prognosis than EBV-positive cHL. (B) Survival curves of patients with age-related EBVLPD stratified according to IPI score. (C) Survival curves of patients with age-related EBVLPD stratified according to the prognostic score of age-related EBVLPD. (D) Survival curves of patients with EBV-positive cHL stratified according to the IPFP score. (E) Survival curves of patients with EBV-positive cHL stratified according to the prognostic score of age-related EBVLPD.
Survival curves. (A) Survival curves for age-related EBVLPD and EBV-positive cHL patients. Age-related EBVLPD showed a poorer prognosis than EBV-positive cHL. (B) Survival curves of patients with age-related EBVLPD stratified according to IPI score. (C) Survival curves of patients with age-related EBVLPD stratified according to the prognostic score of age-related EBVLPD. (D) Survival curves of patients with EBV-positive cHL stratified according to the IPFP score. (E) Survival curves of patients with EBV-positive cHL stratified according to the prognostic score of age-related EBVLPD.
In this series, IPI score could not distinguish aEBVLPD patients into groups with significantly different survival (P = .14; Figure 2B). In contrast, disease-specific survival curves according to a prognostic model of aEBVLPD using new parameters,23 namely the presence of B symptoms and age older than 70 years, showed a median overall survival time for aEBVLPD patients with scores of 0, 1, and 2 of 49.1, 25.6, and 10.7 months, respectively (Figure 2C). Disease-specific survival curves of EBV+ cHL patients according to the International Prognostic Factor Project (IPFP) score and a prognostic model of aEBVLPD are shown in Figure 2C and E, respectively. For the EBV+ cHL cases, IPFP score efficiently identified 2 groups of patients with different outcomes (Figure 2D). In contrast, the prognostic model of aEBV+ LPD failed to separate EBV+ cHL (P = .096; Figure 2E).
In addition, univariate Cox analysis identified the following prognostic factors for the 144 EBV+ patients: B symptoms (P = .024), age older than 60 years (P = .001), advanced clinical stage (III/IV; P = .002), extranodal involvement at more than one site (P = .003), elevated LDH (P = .015), presence of necrosis (P = .001), and CD20 positivity (P = .015; Table 4).
Univariate analysis for prognostic factors affecting overall survival
| Variable . | Unfavorable factors . | Hazard ratio (95% CI) . | P . |
|---|---|---|---|
| B symptoms | Positive | 2.67 (1.14-6.24) | .024 |
| Age | > 60 y | 2.43 (1.46-4.06) | .001 |
| Clinical stage | III/IV | 2.22 (1.33-3.72) | .002 |
| Extranodal involvement | > 1 site | 2.13 (1.28-3.54) | .003 |
| LDH | > normal | 1.87 (1.13-3.10) | .015 |
| Sex | Female | 1.43 (0.91-2.27) | .12 |
| PS | 2-4 | 0.82 (0.51-1.33) | .43 |
| Necrosis | Presence | 2.98 (1.55-5.73) | .001 |
| CD20 positivity | > 50% | 2.43 (1.19-4.98) | .015 |
| Background CTL | Increase* | 1.86 (0.94-3.68) | .074 |
| Variable . | Unfavorable factors . | Hazard ratio (95% CI) . | P . |
|---|---|---|---|
| B symptoms | Positive | 2.67 (1.14-6.24) | .024 |
| Age | > 60 y | 2.43 (1.46-4.06) | .001 |
| Clinical stage | III/IV | 2.22 (1.33-3.72) | .002 |
| Extranodal involvement | > 1 site | 2.13 (1.28-3.54) | .003 |
| LDH | > normal | 1.87 (1.13-3.10) | .015 |
| Sex | Female | 1.43 (0.91-2.27) | .12 |
| PS | 2-4 | 0.82 (0.51-1.33) | .43 |
| Necrosis | Presence | 2.98 (1.55-5.73) | .001 |
| CD20 positivity | > 50% | 2.43 (1.19-4.98) | .015 |
| Background CTL | Increase* | 1.86 (0.94-3.68) | .074 |
CI indicates confidence interval; LDH, lactate dehydrogenase; PS, performance status; and CTL, cytotoxic T cell.
More than 30% of cytotoxic T cells among background lymphocytes.
Discussion
We compared the clinicopathologic characteristics of 34 patients with the polymorphous subtype of age-related EBVLPD with those of 108 middle-aged and older patients with EBV+ cHL. These 2 diseases shared several histopathlogic findings, including HRS-like giant cells, a rich cellular infiltration of reactive components, and harboring of EBV; however, aEBVLPD patients in our series appeared to constitute an aggressive clinicopathologic group distinct from EBV+ cHL. Given that aEBVLPD and cHL are treated with different drug regimens, their diagnostic differentiation is relevant to the establishment of therapeutic strategies.
Age-related EBV+ LPD (aEBVLPD) is listed under the nosologic term of EBV+ DLBCL in the fourth version of the WHO classification.24 EBV+ DLBCL was initially divided into 2 subgroups on morphologic grounds, namely a polymorphous subtype and a large-cell lymphoma subtype, but this distinction is no longer considered of clinical importance. Nevertheless, these subgroups represent the broad morphologic spectrum from Hodgkin-like lesions to overt large-cell lymphoma. The polymorphous subtype of aEBVLPD often reveals a varying number of HRS-like giant cells with expression of CD30 and an abundant inflammatory background, posing a serious differential diagnostic problem with cHL. This disease might therefore have been confused with EBV+ cHL in the past due to a lack of clear-cut diagnostic criteria.
Recent advances in molecular biologic techniques have provided additional definitive evidence that HRS cells of cHL are derived from germinal center B cells.30,31 The biologic interface or overlap between cHL and diverse subtypes of B-cell lymphoma is thus assumed. For example, mediastinal gray zone lymphoma (MGZL) has been described as the missing link between cHL and mediastinal large B-cell lymphoma.32,33 Given this overlapping range of histologic appearance between EBV+ cHL and aEBVLPD, the difficulty or even impossibility of distinguishing between them is thus unsurprising. The presence of a background of variable numbers of reactive components such as small lymphocytes, eosinophils, histiocytes, and plasma cells is essential to the diagnosis of cHL, but is often shared by some non-Hodgkin lymphomas such as aEBVLPD or T-cell/histiocyte-rich B-cell lymphoma. In the present study, geographic necrosis and an increased percentage (> 30%) of cytotoxic T cells among background lymphocytes were more characteristic of aEBVLPD, suggesting that the interaction between EBV+ large tumor cells and host immune response in these diseases might be distinct. In addition, in accordance with our disease definition, we applied the diagnosis of aEBVLPD to cases in which more than 50% of large tumor cells were positive for CD20 and/or CD79a. This was practically contrasted with the lower CD20 positivity (< 50%) of HRS cells in a minority (19%) of EBV+ cHL cases, indicating that the key to distinction between these EBV-associated diseases is dependent on recognition of the degree of expression of B-cell markers on tumor cells. Immunohistochemical studies for EBNA2 and CD15 were also helpful in distinguishing aEBVLPD from cHL. These issues require further clarification.
A large number of reports have indicated that the clinical outcome of cHL may be predicted by biologic markers such as the expression of EBV34-40 or CD2041 and the increase in background cytotoxic T cells.42 EBV positivity on HRS cells correlated with significantly poorer survival in elderly patients.35-37 The differential impact of EBV HRS status on outcome between young adult and older adult age groups may be a consequence of either biologically distinct diseases, or alternatively a decline in EBV-specific cellular immunity with age.34 More recently, Jarrett et al indicated that, among patients aged 50 years or older with cHL, EBV positivity was associated with a significantly poorer outcome, and suggested that impaired immune status may contribute to the development of EBV+ cHL in older patients.40 These findings suggest that the pathogenesis of aEBVLPD and EBV+ cHL may be related, to some extent at least.
The role of EBV in the induction of T-cell subpopulation balance has attracted much interest. A recent study has described a population of CD4+CCR5+ CTLs as a possible effector mechanism in response to viral infections in humans.43 These cells, which express TIA-1, increase dramatically in response to EBV infection. In the present study, age-related EBVLPD cases showed high numbers of TIA-1+ cells. Moreover, Alvaro et al reported that high infiltration of TIA-1+ cells in cHL may represent a biologic marker predicting an unfavorable outcome, although no relationship was seen between the proportion of TIA-1 and presence of EBV.43 The specific role of TIA-1+ cells in the control of immune surveillance in these EBV-positive tumors requires further experimental investigation.
The existence of lymphomas with transitional features provides no clue to the optimal therapy for aEBVLPD. In our present series, however, conventional combination chemotherapy had only limited efficacy. For recalcitrant cases with aggressive lymphomas such as some cases of DLBCL, the superiority of high-dose chemotherapy with stem cell support over conventional methods is now under confirmation,44-46 but high-dose chemotherapy is in any case unsuitable for elderly patients with aEBVLPD. Rituximab has significantly improved cure rates for elderly patients with DLBCL,47 and we are now conducting a multi-institutional clinical trial to test the efficacy of chemotherapy with this agent in aEBVLPD patients.
In conclusion, we demonstrated that patients with aEBVLPD have a significantly poorer prognosis than those with EBV+ cHL. Histologic characteristics of aEBVLPD are the presence of necrosis, increase in background cytotoxic cells, and high CD20 positivity of tumor cells. aEBVLPD constitutes a distinct clinicopathologic group that contrasts with EBV+ cHL, particularly with regard to its more unfavorable outcome.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank H. Ishida for technical assistance, and collaborators from the following institutions for their provision of clinical data and specimens: National Sapporo Hospital, Sapporo Municipal Hospital, Asahikawa Red Cross Hospital, Akita University School of Medicine, Akita Kumiai General Hospital, National Miyagi Hospital, Tohoku University School of Medicine, Sendai City Hospital, Fukushima Medical College, Ohta Nishinouchi General Hospital, Red Cross Ashikaga Hospital, Gunma University School of Medicine, Kitazato University School of Medicine, Tsukuba University School of Medicine, Saitama Cancer Center, Chiba University School of Medicine, Takeda General Hospital, Tokyo Women's Medical University Daini Hospital, Matsudo Municipal Hospital, Higashi Matsudo Hospital, Niigata University, Nagano Red Cross Hospital, Shinshu University School of Medicine, Iida Municipal Hospital, Toyama Central Hospital, Kanazawa Medical University, Fukui Red Cross Hospital, Seirei Hamamatsu Hospital, Hamamatsu Medical School, Toyohashi Municipal Hospital, Aichi Prefectural Hospital, Toyota Memorial Hospital, Fujita Health University School of Medicine, Okazaki Municipal Hospital, Kariya General Hospital, Tousei Hospital, Tokoname Municipal Hospital, Ichinomiya Municipal Hospital, Kasugai Municipal Hospital, Japanese Red Cross Nagoya First Hospital, Nagoya Memorial Hospital, Nagoya City University School of Medicine, Aichi Cancer Center, Showa Hospital, Yokkaichi Municipal Hospital, Suzuka Chuo General Hospital, Suzuka Kaisei General Hospital, Mie University School of Medicine, Matsusaka Municipal Hospital, Matsusaka Chuo General Hospital, Katsusaka Saiseikai General Hospital, Yamada Red Cross Hospital, Ise City General Hospital, Kyoto University, Kyoto Prefectural University School of Medicine, Okayama University Medical School, Okayama Saiseikai General Hospital, Chugoku Central Hospital, Okayama Red Cross General Hospital, Kawasaki Medical School, Matsue Red Cross Hospital, Japanese Red Cross Takamatsu Hospital, Fukuoka University School of Medicine, Kyushu Cancer Center, Kyushu University, and Kurume University.
This work was supported in part by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (Tokyo, Japan), a Grant-in-Aid for Cancer Research from the Ministry of Education, Culture, Sports, Science and Technology (Tokyo, Japan), and a Grant-in-Aid for “Delineation of the molecular biological profile of refractory lymphoid malignancy and the development of its tumor type-specific management” from the Ministry of Health, Labour and Welfare (Tokyo, Japan).
Authorship
Contribution: N.A. and S.N. designed and performed research, analyzed data, and wrote the paper; and N.A., K.Y., J.-I.T., T.O., F.I., K.O., T.Y., N.N., S.M., O.Y., Y.S., Y.M., T.K., and S.N. contributed vital new reagents or analytical tools.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Naoko Asano, Department of Laboratory Medicine, Shinshu University Hospital 3-1-1 Asahi, Matsumoto 390-8621, Japan; e-mail: naasano@shinshu-u.ac.jp.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal