Abstract
Exosomes are secreted cellular vesicles that can be internalized by dendritic cells (DCs), contributing to antigen-specific naive CD4+ T-cell activation. Here, we demonstrate that human immunodeficiency virus type 1 (HIV-1) can exploit this exosome antigen-dissemination pathway intrinsic to mature DCs (mDCs) for mediating trans-infection of T lymphocytes. Capture of HIV-1, HIV-1 Gag-enhanced green fluorescent protein (eGFP) viral-like particles (VLPs), and exosomes by DCs was up-regulated upon maturation, resulting in localization within a CD81+ compartment. Uptake of VLPs or exosomes could be inhibited by a challenge with either particle, suggesting that the expression of common determinant(s) on VLP or exosome surface is necessary for internalization by mDCs. Capture by mDCs was insensitive to proteolysis but blocked when virus, VLPs, or exosomes were produced from cells treated with sphingolipid biosynthesis inhibitors that modulate the lipid composition of the budding particles. Finally, VLPs and exosomes captured by mDCs were transmitted to T lymphocytes in an envelope glycoprotein-independent manner, underscoring a new potential viral dissemination pathway.
Introduction
Dendritic cells (DCs) are specialized antigen-presenting cells that orchestrate innate and adaptive immune responses to invading pathogens. Immature DCs located in the peripheral mucosal tissues recognize and capture microbial pathogens, undergo maturation, and traffic to lymphoid tissues, where they induce adaptive immunity through antigen presentation to naive T cells. Although DCs are required to combat viral infections, viruses, including human immunodeficiency virus type 1 (HIV-1), have evolved strategies to evade their antiviral activity. HIV can gain access into DCs via a nonfusogenic endocytic mechanism, evade classical degradation pathways, and establish productive infection of DC-interacting T cells, a well-studied but poorly understood mechanism of HIV trans-infection of CD4+ T cells.1-3 The efficiency of DC-mediated HIV-1 transmission to T cells can be enhanced by maturing DCs in vitro,2,4,5 although the mechanism underlying this process has not been well defined.6
Previous studies have associated HIV trans-infection with the binding of the viral envelope glycoprotein (gp120) to C-type lectin receptors (CLR) such as DC-SIGN, trypsin-sensitive CLR, and CD4-independent receptors expressed on the DC surface.3,7-11 However, we have recently identified an HIV gp120-independent mechanism of viral binding and endocytosis that is up-regulated upon DC maturation,12 suggesting that HIV-1 might exploit a preexisting cellular pathway of antigen uptake and transmission. Interestingly, previous reports have shown that DCs can endocytose viral-like particles (VLPs) and induce immune responses.13,14 Likewise, small secreted cellular organelles, termed exosomes, are also internalized by DCs and sorted into an endocytic compartment, stimulating antigen-specific naive CD4+ T-cell activation in vivo.15,16
On the basis of similarities in size and composition, it has been previously suggested that retroviruses are, at their most fundamental level, exosomes.17 Indeed, several lines of evidence suggest a link between the biogenesis and release of exosomes and retroviral particles. Both exosomes and HIV can bud from particular cholesterol-enriched microdomains in the T-cell plasma membrane,18-20 sharing glycosphingolipids and tetraspanin proteins that have been used earlier as bona fide lipid raft markers.18,21 Although the endosomal sorting complex machinery requirement during exosome biogenesis has been recently challenged,22 exosome biogenesis can occur in macrophages and DCs by reverse budding into the multivesicular body, an intracellular compartment that can fuse with the plasma membrane, releasing its internal vesicles as exosomes. Interestingly, our previous studies have demonstrated an association of endocytosed HIV-1 particles with intraluminal vesicle-containing compartments within immature DCs.23 Furthermore, HIV-1 particles captured by immature DCs were exocytosed in association with exosomes and could mediate trans-infection of T cells.23
Here, we show that endocytosis of retroviruses and exosomes is up-regulated upon DC maturation, leading internalized particles into the same CD81+ intracellular compartment and subsequent transmission of virus and exosomes to CD4+ T cells. By using HIV-1 Gag-eGFP virus-like particles (VLPHIV-Gag-eGFP), wild-type HIV-1, and Jurkat-derived exosomes labeled with DiI (ExosomesDiI), we have efficiently monitored this novel endocytic pathway. We also found that HIV internalization and transfer is independent of the envelope glycoprotein, allowing viral particles to converge with the exosome dissemination pathway. Interestingly, although virus or exosome capture was insensitive to proteolytic treatments, particle uptake could be efficiently blocked when virus or exosomes were released from producer cells treated with fumonisin B1 or N-butyldeoxynojirimycin hydrochloride, inhibitors that reduce glycosphingolipid levels in the plasma membrane.24,25 The present study suggests that HIV and other retroviruses might be exploiting a preexisting exosome dissemination pathway intrinsic to mature DCs, allowing for HIV-1 transmission to T cells.
Methods
Primary cell cultures
Primary cell isolation procedures were performed as previously described.12 In brief, peripheral blood mononuclear cells (PBMCs) were obtained from HIV-1-seronegative donors and monocyte populations (> 97% CD14+) were isolated with CD14+-positive selection magnetic beads (Miltenyi Biotec, Madrid, Spain). DCs were obtained culturing cells in the presence of 1000 U/mL of granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin-4 (R&D Systems, Minneapolis, MN). mDCs were obtained by culturing iDCs at day 5 for 2 more days in the presence of 100 ng/mL of lipopolysaccharide (LPS; Sigma-Aldrich, Química, Madrid, Spain, or Invitrogen, Carlsbad, CA). The institutional review boards on biomedical research from Hospital Germans Trias i Pujol and Boston University School of Medicine approved this study. Antibodies, cell lines, plasmids, and viral stocks are described in Document S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Exosome generation
Jurkat T cells were cultured in AIM-V serum-free medium (Invitrogen) lacks in contaminating vesicles. Cells were labeled with DiI (Invitrogen–Molecular Probes) following manufacturer's instructions. Supernatants were collected 24 to 48 hours after labeling. Exosome purification was performed as previously described26 by 3 successive centrifugations at 300g (5 minutes), 1200g (20 minutes), and 10 000g (30 minutes) to pellet cells and debris, followed by centrifugation for 1 hour at 100 000g. Exosome stocks were quantified with the use of a Bradford Protein Assay kit (Bio-Rad, Hercules, CA) and a Fluoroskan Ascent FL fluorimeter (Thermo Fisher Scientific, Barcelona, Spain) to perform pulse with equal concentrations of exosomal proteins displaying similar fluorescence intensities.
Exosome and VLP capture assays
A total of 1 × 105 mDCs and iDCs were incubated at 37°C for 4 hours with 2500 pg of VLPHIV-Gag-eGFP p24Gag in a final volume of 0.1 mL. We performed exosome capture by pulsing 105 DCs with 150 μg of ExosomesDiI. Positive DCs were analyzed with a FACSCalibur (BD, Madrid, Spain) and CellQuest and FlowJo software to evaluate collected data.
Competition experiments were performed by preincubating 105 mDCs at a final concentration of 106 cells/mL for 30 minutes with increasing amounts of ExosomeDiI, HIVΔenv-Bru, VLPMLV-Gag, and vesicular stomatitis virus (VSV) particles (previously treated with 400 μg/mL pronase [Roche, Barcelona, Spain] to avoid viral fusion). Effectiveness of pronase treatment was confirmed as detailed Document S1. Alternatively, 200 ng of p24Gag HIVNL43 viral stocks produced in MOLT, MT4, and phytohemagglutinin (PHA)–stimulated PBMCs were preincubated with 105 mDCs at a final concentration of 106 cells/mL for 30 minutes. All mDCs were then pulsed with 625 pg of VLPHIV-Gag-eGFP p24Gag for 1 hour, washed with phosphate-buffered saline (PBS), and fixed to analyze the percentage of eGFP-positive cells by FACS. Of note, after 48 hours of viral pulsing, neither iDCs nor mDCs displayed phenotypic changes, as determined by CD83, CD86, and HLA-DR expression levels. Carboxylated bead assays and kinetic analysis are described in Document S1.
Capture assays after proteolytic treatments
mDCs were left untreated or incubated with 200 μg/mL of pronase for 30 minutes at 4°C. VLPHIV-Gag-eGFP, HIVNL43, and ExosomesDiI were left untreated or were pretreated with 400 μg/mL of pronase for 1 hour at 4°C before they were incubated with mDCs. The effectiveness of pronase treatment was confirmed as detailed in Document S1. Pronase or mock-treated mDCs were exposed to pronase or mock-treated VLPHIV-Gag-eGFP, HIVNL43, or ExosomesDiI during 15 minutes at 37°C to avoid complete recycling of the surface-exposed cellular receptors digested by the proteases. Cells were then washed, fixed, and analyzed by fluorescence-activated cell sorting (FACS) or lysed to measure cell-associated p24Gag with an enzyme-linked immunosorbent assay (ELISA).
VLP, HIV, and exosome capture of particles produced from ceramide-deficient cells
To produce sphingolipid deficient particles, HEK-293T or Jurkat cells were incubated for 2 or 5 days, respectively, in the presence of 50 μmol/L Fumonisin B1 (FB1; Cayman Chemical, Ann Arbor, MI) or 500 μmol/L NB-DNJ (Calbiochem, San Diego, CA). HEK-293T cells were then transfected with HIVLai, HIVNL43, or HIV Gag-eGFP plasmids using FuGENE HD (Roche). Jurkat T cells were labeled with DiI. HIVLai, HIVNL43, VLPHIV-Gag-eGFP, and ExosomesDiI were collected 4 or 7 days after FB1 or NB-DNJ treatment. Mature DCs (105 cells) were exposed to 2500 pg of p24Gag VLPs, 250 μg of ExosomesDiI (displaying equal fluorescent intensity), or 10 ng of p24Gag HIV (produced from either FB1, NB-DNJ, or mock-treated HEK-293T cells or Jurkat cells) for 2 hours at 37°C, washed, fixed, and analyzed with the use of FACS to measure the percentage of positive cells or washed thoroughly to remove unbound particles, lysed, and assayed for cell-associated p24Gag content by an ELISA.
Microscopic analysis
mDCs were pulsed for 12 hours with 150 ng of HIVNL43 p24Gag, 12 500 pg of VLPHIV-Gag-eGFP p24Gag, or 3 mg of ExosomesDiI per 5 × 105 cells, extensively washed in PBS, and fixed in 2.5% glutaraldehyde for 1 hour. Cells were then processed, as described elsewhere,12 for analysis of ultra thin sections with the use of a JEOL JEM 1010 electron microscope (JEOL USA, Peabody, MA). We performed colocalization experiments by pulsing mDCs simultaneously with VLPHIV-Gag-eGFP and ExosomesDiI or with HIVNL43-vpr-eGFP and ExosomesDiI during 6 hours as described in the section “Capture assays after proteolytic treatments.” Colocalization with cellular markers was performed by pulsing mDCs with VLPHIV-Gag-eGFP and ExosomesDiI in parallel for 6 hours as described in “Exosome and VLP capture assays.” Cells were then fixed, permeabilized (Caltag, San Francisco, CA), and stained with 4′,6-diamidino-2-phenylindole (DAPI), CD81, or LAMP-1 (both conjugated with fluorescein isothiocyanate [FITC]; BD) and cytospun into glass slides. Cells were mounted in Dako fluorescent media to analyze them in a confocal microscope (Laser Optic Leica TCS SP2 AOBS; Leica Microsystems, Wetzlar, Germany). Transmission capacity of mDCs was assessed by the use of deconvolution or confocal microscopy, with which we analyzed DAPI-labeled mDCs that were previously pulsed with VLPHIV-Gag-eGFP or ExosomesDiI, extensively washed, and then cocultured for 4 hours with Jurkat T cells labeled with an orange fluorescent or a green fluorescent cell tracker (Invitrogen–Molecular Probes). Cocultures were cytospun, and images were acquired on a Laser Optic Leica TCS SP2 AOBS or an Olympus IX70 microscope equipped for DeltaVision deconvolution (Applied Precision, Issaquah, WA). Z-sections were acquired at 0.2-μm steps using a 60×/1.4 NA Olympus PlanApo objective. The volume viewer function of the Softworx software (Applied Precision) was used to generate 3-dimensional models from the Z stacks.
Results
Maturation of DCs enhances VLP and exosome capture
We have recently identified an HIV envelope glycoprotein-independent mechanism of viral binding and capture that does not rely on CD4 and C-type–lectin receptors expressed on the DC surface.12 To characterize the molecular determinants required for this highly efficient viral capture mechanism that is up-regulated upon DC maturation, we used eGFP-expressing fluorescent virus-like particles (VLPHIV-Gag-eGFP). Expression of HIV Gag protein alone is sufficient for virus-like particle assembly and budding,27-30 and GFP-tagged forms of HIV Gag have been previously used to follow virus particle biogenesis.31,32 We pulsed immature and mature monocyte-derived DCs (iDCs and mDCs, respectively) with VLPHIV-Gag-eGFP for 4 hours at 37°C, fixed the cells, and determined the percentage of eGFP-positive cells by FACS.
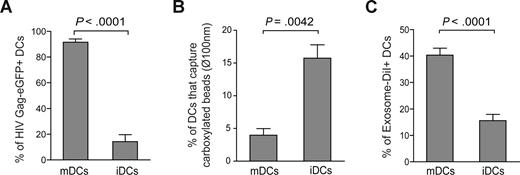
Similar to our previously reported findings with infectious HIV-1 particles,12 VLP capture was enhanced in mDCs compared with iDCs derived from the same donors, suggesting a differential active virus capture process in mDCs (P < .0001, paired t test; Figures 1A and S1A). We also have confirmed equivalent up-regulation of VLP capture with LPS-matured CD1c+ (BDCA-1) blood myeloid DCs (data not shown). Furthermore, DCs matured in the presence of poly I:C (a TLR3 ligand) were comparable with LPS-matured DCs in their ability to capture VLPHIV-Gag-eGFP, suggesting a maturation-signal independent up-regulation of HIV-1 internalization in mDCs (data not shown). To further determine whether mDC-mediated capture was not merely a size-regulated process, DCs were incubated with virus-size carboxylated fluorescent beads of 100 nm diameter. We used a concentration of beads that displayed similar binding at 4°C in iDCs and mDCs (Figure S1B). However, fluorescent bead capture was down-regulated upon maturation, because the percentage of mDCs able to capture these beads at 37°C was lower than in iDCs (P = .0042, paired t test; Figure 1B).
Maturation of DCs enhances VLPHIV-Gag-eGFP and ExosomeDiI capture. (A) Comparative capture of VLPHIV-Gag-eGFP by DCs. A total of 105 DCs were pulsed for 4 hours at 37°C with 2500 pg p24Gag in 0.1 mL, washed with PBS, and fixed to analyze the percentage of eGFP-positive cells by FACS. Data show mean values and SEM from 5 independent experiments, including cells from 6 donors. mDCs captured significantly greater amounts of VLPs compared with iDCs (P < .0001, paired t test). (B) Comparative capture of carboxylated yellow fluorescent beads by DCs. A total of 5 × 105 iDCs and mDCs were incubated at 4°C and 37°C for 2 hours with approximately 1.8 × 1010 beads. Cells were washed, fixed, and analyzed by FACS. Graph displays the percentage of DCs that captured beads at 37°C, after subtracting binding percentages at 4°C. Data show mean values and SEM from 4 independent experiments, including cells from 7 donors. iDCs significantly captured greater amounts of beads compared with mDCs (P = .0042, paired t test). (C) Comparative capture of Jurkat-derived ExosomesDiI by DCs. A total of 105 DCs were pulsed for 8 hours at 37°C with 150 μg exosomes, washed with PBS, and fixed to analyze the percentage of DiI-positive cells by FACS. Data show mean values and SEM from 4 independent experiments, including cells from 11 donors. mDCs capture significantly greater amounts of exosomes compared with iDCs (P < .0001, paired t test).
Maturation of DCs enhances VLPHIV-Gag-eGFP and ExosomeDiI capture. (A) Comparative capture of VLPHIV-Gag-eGFP by DCs. A total of 105 DCs were pulsed for 4 hours at 37°C with 2500 pg p24Gag in 0.1 mL, washed with PBS, and fixed to analyze the percentage of eGFP-positive cells by FACS. Data show mean values and SEM from 5 independent experiments, including cells from 6 donors. mDCs captured significantly greater amounts of VLPs compared with iDCs (P < .0001, paired t test). (B) Comparative capture of carboxylated yellow fluorescent beads by DCs. A total of 5 × 105 iDCs and mDCs were incubated at 4°C and 37°C for 2 hours with approximately 1.8 × 1010 beads. Cells were washed, fixed, and analyzed by FACS. Graph displays the percentage of DCs that captured beads at 37°C, after subtracting binding percentages at 4°C. Data show mean values and SEM from 4 independent experiments, including cells from 7 donors. iDCs significantly captured greater amounts of beads compared with mDCs (P = .0042, paired t test). (C) Comparative capture of Jurkat-derived ExosomesDiI by DCs. A total of 105 DCs were pulsed for 8 hours at 37°C with 150 μg exosomes, washed with PBS, and fixed to analyze the percentage of DiI-positive cells by FACS. Data show mean values and SEM from 4 independent experiments, including cells from 11 donors. mDCs capture significantly greater amounts of exosomes compared with iDCs (P < .0001, paired t test).
In light of this result, we decided to determine whether exosomes that also are derived from cholesterol-enriched membrane microdomains18,19 could incorporate mDC-recognition determinants and be captured by mDCs through a mechanism similar to that exhibited by VLPHIV-Gag-eGFP. Immature or mature DCs were pulsed with DiI-labeled exosomes released from Jurkat T cells (ExosomesDiI) for 4 hours at 37°C, fixed, and analyzed by FACS to determine the percentage of DiI-positive cells. Similar to VLPHIV-Gag-eGFP capture, mDCs were much more efficient than iDCs in capturing exosomes (P < .0001, paired t test; Figure 1C). Overall, these results demonstrate that capture of VLPHIV-Gag-eGFP and ExosomesDiI is up-regulated upon DC maturation and that this uptake mechanism is not merely a size-regulated process but rather requires the specific surface constituents of VLPHIV-Gag-eGFP and ExosomesDiI.
Competition experiments suggest that different particles derived from cholesterol-enriched domains use the same entry pathway into mDCs
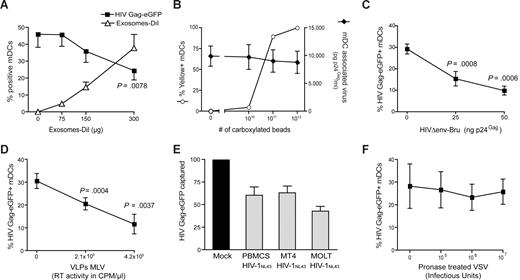
To determine whether VLPHIV-Gag-eGFP and ExosomesDiI share a common entry mechanism, we performed different competition experiments. Mature DCs were preincubated with increasing amounts of ExosomesDiI and subsequently pulsed with a constant amount of VLPHIV-Gag-eGFP for 1 hour at 37°C. Cells were then washed, and the percentage of eGFP- and DiI-positive cells was determined by FACS. Preincubation of mDCs with increasing amounts of ExosomesDiI resulted in a dose-dependent decrease in the percentage of VLPHIV-Gag-eGFP-positive cells (Figure 2A, P = .0078, paired t test). In contrast, preincubating cells with increasing amounts of virus-size fluorescent carboxylated beads resulted in no reduction in the ability of mDCs to capture envelope-glycoprotein deficient HIVΔenv-NL43 (Figure 2B). Hence, the competition observed between ExosomesDiI and VLPHIV-Gag-eGFP suggests that both particles use a common saturable entry mechanism to gain access into mDCs.
Competition experiments suggest that different particles derived from cholesterol-enriched domains use the same entry pathway into mDCs. (A) Capture of VLPHIV-Gag-eGFP by mDCs previously exposed to increasing amounts of Jurkat-derived ExosomesDiI. Cells were preincubated for 30 minutes with increasing amounts of ExosomesDiI and then pulsed with 625 pg of VLPHIV-Gag-eGFP p24Gag for 1 hour at 37°C, washed with PBS, fixed, and analyzed by FACS to determine the percentage of eGFP- and DiI-positive cells. mDCs captured fewer VLPHIV-Gag-eGFP in the presence of increasing amounts of ExosomesDiI (P = .0078, paired t test). (B) Capture of HIVΔenv-NL43 by mDCs previously exposed to increasing amounts of yellow carboxylated 100-nm beads. A total of 5 × 105 mDCs were preincubated for 30 minutes with the beads and then pulsed for 1 hour at 37°C with 130 ng HIVΔenv-NL43 p24Gag in 0.5 mL and extensively washed with PBS. Each sample was then divided and either fixed for analysis by FACS for bead capture or lysed with 0.5% Triton (at a final concentration of 5 × 105 cells per milliliter) to measure p24Gag content in the cell lysate by an ELISA. Results represent the percentage of yellow positive mDCs (○) and the amount of pg of p24Gag bound per mL of cell lysate (♦). (C,D) Capture of VLPHIV-Gag-eGFP by mDCs previously exposed to increasing amounts of HIVΔenv-Bru (C) and VLPMLV-Gag (D). Cells were preincubated for 30 minutes with increasing amounts of HIVΔenv-Bru or VLPMLV-Gag and then pulsed with 625 pg of VLPHIV-Gag-eGFP p24Gag for 1 hour at 37°C, washed with PBS, and fixed to analyze the percentage of eGFP-positive cells by FACS. mDCs capture less VLPHIV-Gag-eGFP in the presence of increasing concentrations of particles derived from cholesterol-enriched membrane microdomains (P values on the graphs, paired t test). (E) The data represent the relative VLPHIV-Gag-eGFP capture by mDCs that had been preincubated with 200 ng of p24Gag of HIVNL43 obtained from either MT4, MOLT, or PHA-stimulated PBMCs and normalized to the level of VLPHIV-Gag-eGFP capture by mock-treated mDCs (set at 100%). mDCs captured less VLPHIV-Gag-eGFP in the presence of these different viral stocks. (F) Capture of VLPHIV-Gag-eGFP by mDCs that had been preincubated with increasing amounts of pronase-treated VSV particles. Cells were preincubated for 30 minutes in the presence of pronase-treated VSV particles and then pulsed with the 625 pg of VLPHIV-Gag-eGFP p24Gag for 1 hour at 37°C, washed with PBS, and fixed to analyze the percentage of eGFP-positive cells by FACS. mDCs captured similar amounts of VLPHIV-Gag-eGFP in the presence of pronase-treated VSV particles. Panels A through F show mean values and SEM from 3 independent experiments, including cells from at least 4 different donors.
Competition experiments suggest that different particles derived from cholesterol-enriched domains use the same entry pathway into mDCs. (A) Capture of VLPHIV-Gag-eGFP by mDCs previously exposed to increasing amounts of Jurkat-derived ExosomesDiI. Cells were preincubated for 30 minutes with increasing amounts of ExosomesDiI and then pulsed with 625 pg of VLPHIV-Gag-eGFP p24Gag for 1 hour at 37°C, washed with PBS, fixed, and analyzed by FACS to determine the percentage of eGFP- and DiI-positive cells. mDCs captured fewer VLPHIV-Gag-eGFP in the presence of increasing amounts of ExosomesDiI (P = .0078, paired t test). (B) Capture of HIVΔenv-NL43 by mDCs previously exposed to increasing amounts of yellow carboxylated 100-nm beads. A total of 5 × 105 mDCs were preincubated for 30 minutes with the beads and then pulsed for 1 hour at 37°C with 130 ng HIVΔenv-NL43 p24Gag in 0.5 mL and extensively washed with PBS. Each sample was then divided and either fixed for analysis by FACS for bead capture or lysed with 0.5% Triton (at a final concentration of 5 × 105 cells per milliliter) to measure p24Gag content in the cell lysate by an ELISA. Results represent the percentage of yellow positive mDCs (○) and the amount of pg of p24Gag bound per mL of cell lysate (♦). (C,D) Capture of VLPHIV-Gag-eGFP by mDCs previously exposed to increasing amounts of HIVΔenv-Bru (C) and VLPMLV-Gag (D). Cells were preincubated for 30 minutes with increasing amounts of HIVΔenv-Bru or VLPMLV-Gag and then pulsed with 625 pg of VLPHIV-Gag-eGFP p24Gag for 1 hour at 37°C, washed with PBS, and fixed to analyze the percentage of eGFP-positive cells by FACS. mDCs capture less VLPHIV-Gag-eGFP in the presence of increasing concentrations of particles derived from cholesterol-enriched membrane microdomains (P values on the graphs, paired t test). (E) The data represent the relative VLPHIV-Gag-eGFP capture by mDCs that had been preincubated with 200 ng of p24Gag of HIVNL43 obtained from either MT4, MOLT, or PHA-stimulated PBMCs and normalized to the level of VLPHIV-Gag-eGFP capture by mock-treated mDCs (set at 100%). mDCs captured less VLPHIV-Gag-eGFP in the presence of these different viral stocks. (F) Capture of VLPHIV-Gag-eGFP by mDCs that had been preincubated with increasing amounts of pronase-treated VSV particles. Cells were preincubated for 30 minutes in the presence of pronase-treated VSV particles and then pulsed with the 625 pg of VLPHIV-Gag-eGFP p24Gag for 1 hour at 37°C, washed with PBS, and fixed to analyze the percentage of eGFP-positive cells by FACS. mDCs captured similar amounts of VLPHIV-Gag-eGFP in the presence of pronase-treated VSV particles. Panels A through F show mean values and SEM from 3 independent experiments, including cells from at least 4 different donors.
Because most retroviruses, including ecotropic murine leukemia virus (MLV), also bud from lipid raft-like plasma membrane domains,33 the binding of VLPHIV-Gag-eGFP to mDCs was competed by incubation with either nonfluorescent HIV-1 particles lacking the envelope glycoprotein (HIVΔenv-Bru) or ecotropic envelope glycoprotein-expressing MLV Gag VLPs (VLPMLV-Gag). Preincubation of mDCs with unlabeled HIVΔenv-Bru or VLPMLV-Gag significantly reduced the percentage of mDCs able to capture VLPHIV-Gag-eGFP in a dose-dependent manner (Figure 2C,D, P values on the graphs, paired t test). Noteworthy, unlabeled HIVNL43 particles derived from different T-cell lines, and stimulated PBMCs also were able to reduce the percentage of mDCs capturing VLPHIV-Gag-eGFP (Figure 2E). In contrast, preincubating mDCs with increasing concentrations of pronase-treated G-protein–deficient VSV particles (known to bud from nonraft membrane microdomains33 ) showed no reduction on mDCs ability to capture VLPHIV-Gag-eGFP (Figure 2F). These results suggest that virus particles or exosomes derived from lipid raft-like plasma membrane domains have a common entry mechanism into mDCs.
Mature DCs retain HIV-1, VLPs, and exosomes within the same CD81+ intracellular compartment
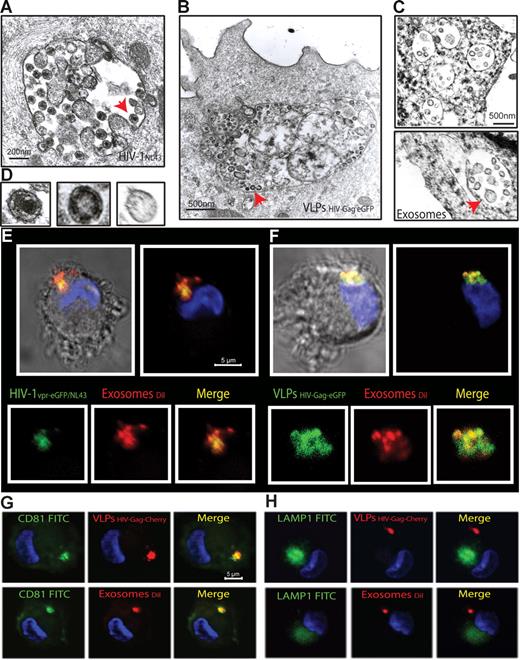
To gain further insights into this mechanism of capture, we monitored mDCs pulsed in parallel with HIVNL43, VLPHIV-Gag-eGFP, and ExosomesDiI by electron microscopy (Figure 3A-D). Most of the mDC-associated particles were found in similar intracellular compartments. Furthermore, when mDCs pulsed simultaneously with HIVNL43 and ExosomesDiI were analyzed, particles displaying viral and exosomal morphology could be found within the same compartment (Figure S2A-C). Confocal microscopy of mDCs pulsed simultaneously with ExosomesDiI and the Vpr-eGFP containing fluorescent wild-type virus HIV-1vpr-eGFP/NL43 or VLPHIV-Gag-eGFP also revealed that both virus and VLPs colocalized with exosomes in the same intracellular compartment (Figure 3E,F, Figure S2D-G, and Videos S1,S2). Similar to HIV-1 trafficking in DCs,12,34 VLPHIV-Gag-Cherry and ExosomesDiI also accumulated in intracellular vesicular compartments, where they colocalized with the CD81 tetraspanin but not with the LAMP-1 lysosomic marker (Figure 3G,H). Previously, we have characterized that β-methyl-cyclodextrin, a cholesterol-sequestering reagent, can block DC viral capture by affecting cellular lipid raft endocytic pathways.10 Interestingly, pretreatment of mDCs with amantadine and chlorpromazine, also blocked VLPHIV-Gag-eGFP uptake (Figure S3). Thus, HIV and exosome trafficking within mDCs relies on active endocytosis that leads captured particles to the same CD81+ compartments.
mDCs retain HIV-1, VLPs and exosomes in the same CD81+ intracellular compartment. (A-C) Electron micrographs of mDCs sections exposed in parallel to HIVNL43, VLPHIV-Gag-eGFP, and ExosomesDiI, showing similar large vesicles containing these particles. Arrows indicate captured particles magnified in panel D, where comparative micrographs show, from left to right: HIVNL43, VLPHIV-Gag-eGFP, and a Jurkat-derived exosome. (E) Confocal images of a section of mDC exposed to HIVvpr-eGFP/NL43 and ExosomesDiI for 4 hours and stained with DAPI. Top images show the mDC, where the red and green fluorescence that merged with DAPI either with or without the bright-field cellular shape are presented. Bottom images show magnification of vesicles containing these particles where individual green and red fluorescence and the combination of both are depicted. (F) Confocal images of a section of a mDC exposed to VLPHIV-Gag-eGFP and ExosomesDiI as described in panel E. (G) Confocal images of a section of a mDC exposed to red fluorescent VLPHIV-Gag-Cherry (top) or ExosomesDiI (bottom) in parallel for 4 hours, fixed, and permeabilized to stain with DAPI and FITC-CD81. Images shown, from left to right, depict individual green and red fluorescence channels and the combination of both merged with DAPI. (H) Confocal images obtained as in panel G, except that cells were stained with DAPI and FITC-LAMP-1. mDCs retain VLPHIV-Gag-eGFP and ExosomesDiI in a CD81+ LAMP1− compartment.
mDCs retain HIV-1, VLPs and exosomes in the same CD81+ intracellular compartment. (A-C) Electron micrographs of mDCs sections exposed in parallel to HIVNL43, VLPHIV-Gag-eGFP, and ExosomesDiI, showing similar large vesicles containing these particles. Arrows indicate captured particles magnified in panel D, where comparative micrographs show, from left to right: HIVNL43, VLPHIV-Gag-eGFP, and a Jurkat-derived exosome. (E) Confocal images of a section of mDC exposed to HIVvpr-eGFP/NL43 and ExosomesDiI for 4 hours and stained with DAPI. Top images show the mDC, where the red and green fluorescence that merged with DAPI either with or without the bright-field cellular shape are presented. Bottom images show magnification of vesicles containing these particles where individual green and red fluorescence and the combination of both are depicted. (F) Confocal images of a section of a mDC exposed to VLPHIV-Gag-eGFP and ExosomesDiI as described in panel E. (G) Confocal images of a section of a mDC exposed to red fluorescent VLPHIV-Gag-Cherry (top) or ExosomesDiI (bottom) in parallel for 4 hours, fixed, and permeabilized to stain with DAPI and FITC-CD81. Images shown, from left to right, depict individual green and red fluorescence channels and the combination of both merged with DAPI. (H) Confocal images obtained as in panel G, except that cells were stained with DAPI and FITC-LAMP-1. mDCs retain VLPHIV-Gag-eGFP and ExosomesDiI in a CD81+ LAMP1− compartment.
Mechanism of VLP and exosome uptake in mDCs is dose dependent and increases over time, allowing efficient transfer to target T cells
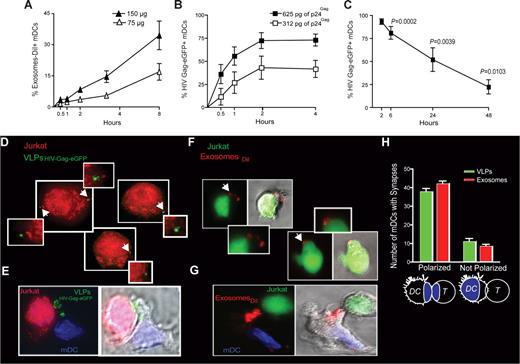
To further characterize the kinetics of this specific exosome, HIV and VLP uptake process that is enhanced upon DC maturation, we monitored mDCs exposed to varying amounts of ExosomesDiI and VLPHIV-Gag-eGFP for 4 to 8 hours at 37°C (Figure 4A,B). Similar to HIVvpr-eGFP/NL4-3 capture by mDCs,12 capture of ExosomesDiI (Figure 4A) and VLPHIV-Gag-eGFP (Figure 4B) by mDCs occurred in a dose-dependent manner and increased over time. Although the percentage of eGFP+ mDCs exposed to VLPHIV-Gag-eGFP reached a plateau during the first 4 hours of culture (Figure 4B), we have previously reported that the mDC-associated virus fraction increases over time, when viral capture of HIVNFN-SX was assayed by a p24Gag ELISA.12 Thus, although particle binding to the mDC-surface is a saturable process that can be outcompeted, active endocytosis continues over time.
VLP and exosome uptake in mDCs is a dose-dependent mechanism that increases over time, allowing efficient transfer to target T cells. (A) Time course of mDCs (n = 4) exposed to 2 different concentrations of ExosomesDiI and fixed at each of the indicated time points and analyzed by FACS. Exosome capture by mDCs increases over time in a dose dependent manner. (B) Time course of mDCs (n = 4) exposed to 2 different concentrations of VLPHIV-Gag-eGFP and fixed at each of the indicated time points and analyzed by FACS. VLPHIV-Gag-eGFP capture by mDCs increases over time in a dose-dependent manner. (C) Fate of VLPHIV-Gag-eGFP captured by mDCs and followed by flow cytometry for 2 days. Graph shows the percentage of Gag-eGFP-positive cells measured by FACS at the indicated time points. P values on the graph reveal that, at 48 hours after pulse with VLPHIV-Gag-eGFP, a significant percentage of mDCs still retained VLPs (one sample t test). Data (mean and SEM from 3 independent experiments) include cells from 4 different donors. (D) Orange cell tracker dye-labeled Jurkat T cells were analyzed by deconvolution microscopy after 4 hours of coculture with mDCs previously pulsed with VLPHIV-Gag-eGFP and extensively washed before coculture. The cells shown in the panels are projections of stack images obtained by merging the red and green fluorescence. Arrows indicate Gag-eGFP dots associated to Jurkat T cells, magnified in the nearby marked boxes (E). Viral synapse could also be observed in these cocultures, where mDCs pulsed with VLPHIV-Gag-eGFP were stained with DAPI. Images shown, from left to right, depict the red and green fluorescence channels merged with DAPI, the bright-field cellular shape and the combination of both. (F) Jurkat T cells labeled with a green cell tracker dye were analyzed by confocal microscopy after 4 hours of coculture with mDCs previously pulsed with ExosomesDiI and extensively washed. Images were obtained by merging the red and green fluorescence. Arrows indicate DiI dots associated with Jurkat T cells, magnified in the nearby marked boxes. Bright-field cellular shape merged with the red and green fluorescence is also shown. (G) Exosome polarization to the site of DC-T cell–contact, where mDCs pulsed with ExosomesDiI were stained with DAPI. Images shown, from left to right, depict the red and green fluorescence channels merged with DAPI, the bright-field cellular shape and the combination of both. (H) Quantification of mDCs forming synapses like those shown in panels E and G. Polarization of particles toward the synapse was considered when VLPsHIV-Gag-eGFP (green) or ExosomesDiI (red) were found within one-third of the cell proximal to the contact zone (as represented in the illustration by the blue colored area). Mean values and SEM of 50 synapses from 2 donors counted by 3 distinct observers.
VLP and exosome uptake in mDCs is a dose-dependent mechanism that increases over time, allowing efficient transfer to target T cells. (A) Time course of mDCs (n = 4) exposed to 2 different concentrations of ExosomesDiI and fixed at each of the indicated time points and analyzed by FACS. Exosome capture by mDCs increases over time in a dose dependent manner. (B) Time course of mDCs (n = 4) exposed to 2 different concentrations of VLPHIV-Gag-eGFP and fixed at each of the indicated time points and analyzed by FACS. VLPHIV-Gag-eGFP capture by mDCs increases over time in a dose-dependent manner. (C) Fate of VLPHIV-Gag-eGFP captured by mDCs and followed by flow cytometry for 2 days. Graph shows the percentage of Gag-eGFP-positive cells measured by FACS at the indicated time points. P values on the graph reveal that, at 48 hours after pulse with VLPHIV-Gag-eGFP, a significant percentage of mDCs still retained VLPs (one sample t test). Data (mean and SEM from 3 independent experiments) include cells from 4 different donors. (D) Orange cell tracker dye-labeled Jurkat T cells were analyzed by deconvolution microscopy after 4 hours of coculture with mDCs previously pulsed with VLPHIV-Gag-eGFP and extensively washed before coculture. The cells shown in the panels are projections of stack images obtained by merging the red and green fluorescence. Arrows indicate Gag-eGFP dots associated to Jurkat T cells, magnified in the nearby marked boxes (E). Viral synapse could also be observed in these cocultures, where mDCs pulsed with VLPHIV-Gag-eGFP were stained with DAPI. Images shown, from left to right, depict the red and green fluorescence channels merged with DAPI, the bright-field cellular shape and the combination of both. (F) Jurkat T cells labeled with a green cell tracker dye were analyzed by confocal microscopy after 4 hours of coculture with mDCs previously pulsed with ExosomesDiI and extensively washed. Images were obtained by merging the red and green fluorescence. Arrows indicate DiI dots associated with Jurkat T cells, magnified in the nearby marked boxes. Bright-field cellular shape merged with the red and green fluorescence is also shown. (G) Exosome polarization to the site of DC-T cell–contact, where mDCs pulsed with ExosomesDiI were stained with DAPI. Images shown, from left to right, depict the red and green fluorescence channels merged with DAPI, the bright-field cellular shape and the combination of both. (H) Quantification of mDCs forming synapses like those shown in panels E and G. Polarization of particles toward the synapse was considered when VLPsHIV-Gag-eGFP (green) or ExosomesDiI (red) were found within one-third of the cell proximal to the contact zone (as represented in the illustration by the blue colored area). Mean values and SEM of 50 synapses from 2 donors counted by 3 distinct observers.
Several studies have argued for long-term retention of HIV-1 infectivity within DCs that can be transmitted to T cells upon contact.35,36 Therefore, we decided to address the ability of mDCs to retain captured particles by taking advantage of the fluorescence intensity of the VLPHIV-Gag-eGFP. Mature DCs were pulsed with VLPHIV-Gag-eGFP, washed, and cultured for 2 days. Cells were periodically harvested and assayed for retention of VLPHIV-Gag-eGFP, as measured by the percentage of eGFP-positive cells left in the culture (Figure 4C; P values on the graph, one sample t test). Although there is a steady decrease in the percentage of eGFP-positive cells over time, results in Figure 4C suggest that a significant percentage of mDCs retain VLPHIV-Gag-eGFP for at least 2 days.
Because mDCs are extremely efficient at transmitting captured HIV-1 particles to T cells,2,4,5 we wanted to determine whether internalized gp120-deficient VLPHIV-Gag-eGFP or ExosomesDiI also could be transferred to T cells. We assessed transmission of VLPHIV-Gag-eGFP and ExosomesDiI by mDCs to T cells by fluorescent microscopy. Mature DCs were pulsed with VLPHIV-Gag-eGFP and then cocultured with Jurkat T cells labeled with an orange cell tracker dye. Fluorescent microscopy analysis of projections of stack images of unconjugated orange Jurkat T cells revealed a few VLPHIV-Gag-eGFP per T cell (Figure 4D). An analysis of conjugated Jurkat T cells with mDCs also revealed VLPHIV-Gag-eGFP polarization to the site of cell-to-cell contact, showing the dramatic rearrangement of VLPs contained in mDC vesicular compartments to the viral synapse upon contact with Jurkat T cells (Figure 4E, Videos S3,S4). Similar results were found when we analyzed mDCs pulsed with ExosomesDiI cocultured with Jurkat T cells, prestained with a green cell tracker dye. We observed unconjugated green Jurkat T cells bearing ExosomesDiI (Figure 4F) and ExosomeDiI polarization to the site of DC-T–cell contact zone (Figure 4G). Most of the VLPs or Exosomes captured by mDCs were recruited to the DC-T–cell contact zone (Figure 4H) together with CD81 (data not shown) as previously reported for HIV-1.4,34 Overall, these results suggest that VLPs and ExosomesDiI captured by mDCs can be transferred to T cells, mimicking HIV-1 transmission to T cells during trans-infection.
VLPs, HIV-1, and exosomes enter mDCs through a mechanism resistant to proteolysis
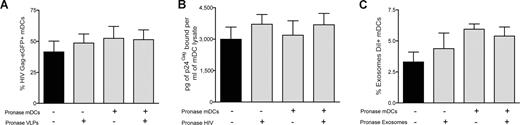
Our data suggest that common molecular determinants expressed on the surface of HIV, VLPs, and exosomes govern entry into mDCs, allowing particle storage and transmission to CD4+ T cells. To gain further insight into the nature of these components, we analyzed the role of host proteins incorporated during viral and exosomal budding and the putative role of cellular protein receptors expressed on mDC surface. We used pronase, a cocktail of proteases that displays high proteolytic activity,37 to treat VLPs, infectious virus particles, exosomes, and cells before particle challenge of mDCs. The efficiency of pronase treatment was confirmed by the lack of expression of gp120 in pronase-treated VLPHIV-Gag-eGFP cotransfected with a viral envelope glycoprotein plasmid (Figure S4A).
We also observed an 18-fold reduction on the infectivity of a wild-type virus, HIVNL43, after equal pronase treatment (Figure S4B). Similarly, pronase treatment of mDCs resulted in an 80%, 100%, 84%, and 85% reduction in cell-surface expression of DC-SIGN, CD4, CD81, and binding of trimeric HIV envelope glycoprotein, respectively (Figure S4C). After pulsing pronase treated or mock-treated mDCs at 37°C (for 15 minutes, to avoid complete recycling of the cellular receptors cut by the proteases) with either pronase-treated or mock-treated VLPHIV-Gag-eGFP, we measured the percentage of eGFP-positive mDCs by FACS (Figure 5A). Surprisingly, protease pretreatment of either mDCs or VLPs did not diminish the percentage of eGFP-positive cells. Similar results were obtained when HIVNL43 or ExosomesDiI were treated with pronase (Figure 5B,C). Furthermore, no differences in intracellular localization of VLPHIV-Gag-eGFP, ExosomesDiI, and HIVNL43-vpr-eGFP were found when we compared mock-treated or pronase-treated mDCs (Figure S4D). Additional experiments performed with pronase-treated mDCs pulsed with VLPs at 16°C to arrest endocytosis also confirmed the lack of effect of pronase treatment on VLP binding to mDCs (Figure S4E,F). Consistently, VLPs, exosomes, and HIV particles captured by pronase-treated mDCs (which were allowed to recover before starting a coculture with Jurkat T cells) also were recruited to the site of DC-T–cell contact (Figure S4G). Thus, broad proteolytic pretreatment of cells or VLPs, HIV-1, or exosomes was not sufficient to prevent particle capture by mDCs.
VLPs, HIV-1 and exosomes enter mDCs through a mechanism resistant to proteolysis. mDC capture of (A) VLPHIV-Gag-eGFP, (B) HIVNL43, or (C) ExosomesDiI. Pronase-treated or -untreated mDCs were pulsed for 15 minutes at 37°C with pronase or mock-treated VLPHIV-Gag-eGFP, HIVNL43, and ExosomesDiI. Protease pretreatments of either the cells or the particles were insufficient to prevent the capture of VLPHIV-Gag-eGFP, ExosomesDiI or HIVNL43 by mDCs. (C-E) Mean values and SEM from 6 independent experiments, including cells from at least 4 different donors.
VLPs, HIV-1 and exosomes enter mDCs through a mechanism resistant to proteolysis. mDC capture of (A) VLPHIV-Gag-eGFP, (B) HIVNL43, or (C) ExosomesDiI. Pronase-treated or -untreated mDCs were pulsed for 15 minutes at 37°C with pronase or mock-treated VLPHIV-Gag-eGFP, HIVNL43, and ExosomesDiI. Protease pretreatments of either the cells or the particles were insufficient to prevent the capture of VLPHIV-Gag-eGFP, ExosomesDiI or HIVNL43 by mDCs. (C-E) Mean values and SEM from 6 independent experiments, including cells from at least 4 different donors.
VLP, HIV-1, and exosome capture can be inhibited when particles are produced from ceramide-deficient cells
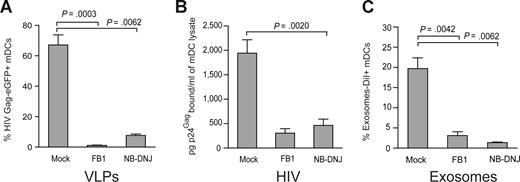
Virus particle budding or exosome biogenesis not only results in selective incorporation of host proteins in the particle membrane but also selects for expression of unusual sphingolipids in the virus particle or exosome membranes.20,22,33,38 Furthermore, it has been previously shown that the lipids of enveloped viruses play a critical role in viral infectivity.38,39 However, the impact of viral lipid composition on mDCs viral capture process has never been evaluated. We decided to address this issue by pretreating HEK-293T producer cells with FB1, an agent that blocks both the de novo synthesis of sphingolipids (by inhibiting the synthesis of dihydroceramide, which serves as precursor to all sphingolipid species, including glycosphingolipids and sphingomyelin) as well as the salvage pathway.24 To inhibit a different step of the metabolism of dihydroceramide-species, we also used NB-DNJ, a reagent that only blocks the synthesis of glycosphingolipids.25 Treatment of HEK-293T cells with FB1 or NB-DNJ had no significant effect on cell viability or VLP release after HIV Gag-eGFP transfection, as measured by p24Gag ELISA or Western blot analysis (Figure S5A,B). Interestingly, challenge of mDCs with VLPHIV-Gag-eGFP released from FB1-treated HEK-293T cells resulted in negligible VLP capture (1.2 ± 0.6% Gag-eGFP+ cells, Figure 6A), compared with mDCs challenged with VLPs generated from mock-treated cells (79.4 ± 14.4% Gag-eGFP+ cells; P = .0003; paired t test; Figure 6A). Analogous results were obtained when VLPs were produced from NB-DNJ–treated HEK-293T cells (P = .0062; paired t test; Figure 6A).
VLP, HIV-1, and exosome capture can be inhibited when particles are produced from ceramide-deficient cells. (A) A total of 105 mDCs were exposed to 2500 pg of p24Gag VLPHIV-Gag-eGFP produced from either FB1, NB-DNJ, or mock-treated HEK-293T cells, fixed, and analyzed by FACS to measure the percentage of eGFP-positive cells. mDCs captured significantly greater amounts of VLPHIV-Gag-eGFP produced from mock treated HEK-293T cells (P = .0003, paired t test). Mean values and SEM from 3 independent experiments, including cells from 5 donors are plotted. (B) mDCs were exposed to 10 ng of HIV-1Lai or HIV-1NL43 p24Gag produced from either FB1, NB-DNJ, or mock-treated HEK-293T cells, washed thoroughly to remove unbound particles, lysed, and assayed to measure the cell-associated p24Gag content by an ELISA. mDCs captured greater amounts of HIV-1 produced from mock-treated HEK-293T cells. Mean values and SEM from 2 independent experiments and cells from 3 donors are plotted. (C) mDCs (105) were exposed to 250 μg of ExosomesDiI produced from either FB1, NB-DNJ, or mock-treated Jurkat cells, fixed, and analyzed by FACS to measure the percentage of DiI-positive cells. mDCs significantly captured greater amounts of ExosomesDiI produced from mock-treated Jurkat cells (P = .0042, paired t test). Mean values and SEM from 3 independent experiments and cells from 6 donors are plotted.
VLP, HIV-1, and exosome capture can be inhibited when particles are produced from ceramide-deficient cells. (A) A total of 105 mDCs were exposed to 2500 pg of p24Gag VLPHIV-Gag-eGFP produced from either FB1, NB-DNJ, or mock-treated HEK-293T cells, fixed, and analyzed by FACS to measure the percentage of eGFP-positive cells. mDCs captured significantly greater amounts of VLPHIV-Gag-eGFP produced from mock treated HEK-293T cells (P = .0003, paired t test). Mean values and SEM from 3 independent experiments, including cells from 5 donors are plotted. (B) mDCs were exposed to 10 ng of HIV-1Lai or HIV-1NL43 p24Gag produced from either FB1, NB-DNJ, or mock-treated HEK-293T cells, washed thoroughly to remove unbound particles, lysed, and assayed to measure the cell-associated p24Gag content by an ELISA. mDCs captured greater amounts of HIV-1 produced from mock-treated HEK-293T cells. Mean values and SEM from 2 independent experiments and cells from 3 donors are plotted. (C) mDCs (105) were exposed to 250 μg of ExosomesDiI produced from either FB1, NB-DNJ, or mock-treated Jurkat cells, fixed, and analyzed by FACS to measure the percentage of DiI-positive cells. mDCs significantly captured greater amounts of ExosomesDiI produced from mock-treated Jurkat cells (P = .0042, paired t test). Mean values and SEM from 3 independent experiments and cells from 6 donors are plotted.
Similarly, wild-type HIV-1 particles derived from FB1- or NB-DNJ–treated HEK-293T cells were deficient in binding to mDCs compared with mDC-capture observed with HIV particles produced from untreated HEK-293T cells (Figure 6B). Although HIV-1 particles derived from HEK-293T cells treated with FB1 had reduced infectivity compared with control viruses obtained from untreated cells,38 we found that NB-DNJ treatment of virus producer cells had no significant impact on viral infectivity compared with untreated stocks (Figure S5C). Furthermore, at the viral dose used to pulse mDCs, infectivity of FB1-treated virus was only 20% (± 10%) less than the wild-type virus (Figure S5D). Analogously, FB1- or NB-DNJ–treated Jurkat T cells released comparable amounts of ExosomesDiI to mock-treated Jurkat T cells, measured both by fluorimetry assays and protein determination (Bradford) assays (Figure S5E,F). Again, after 2 hours at 37°C, mDCs were able to capture greater amounts of exosomes released from mock-treated Jurkat T cells compared with exosomes derived from FB1- or NB-DNJ–treated Jurkat T cells (Figure 6C; P = .0042 and P = .0062; paired t test). Overall, these results suggest that sphingolipids facing the outer layer of VLP, HIV-1, and exosome membranes might be implicated in the initial particle attachment to the surface of mDCs.
Discussion
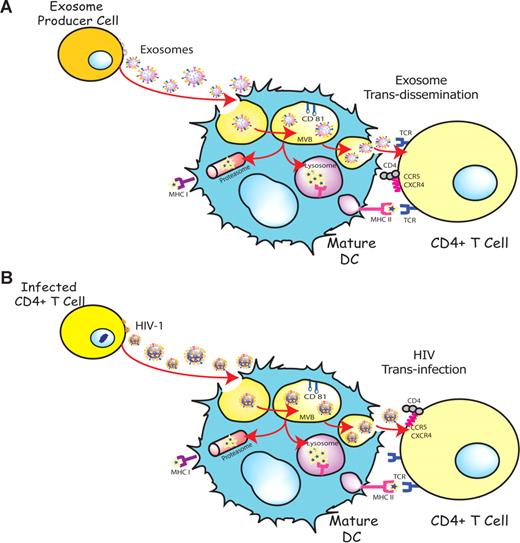
DCs are able to transfer HIV-1 preferentially to antigen-specific CD4+ T cells in the absence of productive DC infection.36,40 This particular viral transmission mechanism is enhanced upon DC maturation and involves trafficking of internalized virus to sites of DC-T cell contact4,34 and its final release, allowing for productive infection of CD4+ T cells.6 Interestingly, exosomes also are internalized and sorted into an endocytic compartment by DCs, stimulating antigen-specific naive CD4+ T-cell responses in vivo.15,16 Our previous work has shown that immature DCs-derived exosomes are constitutively released into the extracellular milieu associated with endocytosed HIV-1 virions.23 The present study suggests that HIV and other retroviruses also might be exploiting an exosome antigen-dissemination pathway intrinsic to mDCs, allowing the final trans-infection of T cells (Figure 7). Indeed, when exosomes are produced by DCs, they can mediate the indirect activation of CD4+ T cells by presenting functional peptide-MHC complexes15,16 through this trans-dissemination mechanism. Our data support the Trojan exosome hypothesis in which retroviruses take advantage of a cell-encoded pathway of intercellular vesicle traffic and exosome exchange, moving between cells in the absence of fusion events.17
HIV can exploit a preexisting exosome trans-dissemination pathway intrinsic to mature DCs, allowing the final trans-infection of CD4+ T cells. (A) Exosomes can transfer antigens from infected, tumoral, or antigen-presenting cells to mDCs, increasing the number of DCs bearing a particular antigen and amplifying the initiation of primary adaptive immune responses through the MHC II pathway, cross-presentation, or the release of intact exosomes, a mechanism described here as trans-dissemination. (B) HIV gains access into mDCs by hijacking this exosome trans-dissemination pathway, thus allowing for the final trans-infection of CD4+ T cells.
HIV can exploit a preexisting exosome trans-dissemination pathway intrinsic to mature DCs, allowing the final trans-infection of CD4+ T cells. (A) Exosomes can transfer antigens from infected, tumoral, or antigen-presenting cells to mDCs, increasing the number of DCs bearing a particular antigen and amplifying the initiation of primary adaptive immune responses through the MHC II pathway, cross-presentation, or the release of intact exosomes, a mechanism described here as trans-dissemination. (B) HIV gains access into mDCs by hijacking this exosome trans-dissemination pathway, thus allowing for the final trans-infection of CD4+ T cells.
Here, we show how, upon maturation, DCs captured greater amounts of VLPHIV-Gag-eGFP and Jurkat-derived ExosomesDiI, accumulating both particles in the same intracellular compartment (Figures 1,3). Direct competition among different particles (exosomes, VLPHIV-Gag-eGFP, HIV-1, and MLVEco) derived from similar cholesterol-enriched membrane microdomains for capture by mDCs further confirmed the specificity of this entry mechanism, which could not be inhibited by 100-nm carboxylated beads or pronase-treated VSV particles budding from nonraft membrane locations (Figure 2).33 Thus, budding from lipid-raft domains is essential to include specific mature DC-recognition determinants that allow viral and exosome capture.
We also found that entry of VLPs and exosomes into mDCs is a dose-dependent mechanism that increases over time and allows efficient transmission of captured particles to target T cells (Figure 4). This scenario might have critical implications for HIV pathogenesis. In vivo, the potential viral dissemination mediated by mDCs is enormous; T cells approach mDCs randomly and make exploratory contacts that last only minutes, enabling each DC to contact thousands of T cells per hour.41 It has been previously shown that circulating LPS, the stimuli used to mature DCs in this study, is significantly increased in chronically infected HIV-positive individuals.42 Furthermore, it also has been reported that in subjects with HIV-1 viremia, blood DCs have increased expression levels of costimulatory molecules (the hallmark of maturation status) that only diminish when the use of highly active antiretroviral therapy (HAART) suppresses viral load.43 Thus, elucidating the mechanisms underlying mDC-enhanced HIV-1 transmission is crucial for the design of effective strategies for therapeutic intervention aimed at blocking viral spread.
Our data also revealed that internalization of HIV-1, VLPs, or exosomes could not be abrogated with an effective protease pretreatment of neither these particles nor mDCs (Figure 5). Nevertheless, this observation does not exclude the potential role of proteins during VLP or exosome capture and might just reflect that the molecular determinants involved in capture were not fully processed by the proteases used. However, treatment of virus, VLP, or exosome-producing cells with inhibitors of sphingolipid biosynthesis extensively reduced particle entry into mDCs (Figure 6) without interfering with net release from producer cells. Although it has been previously shown that FB1 treatment of virus producer cells diminishes infectivity of released HIV-1 particles38 and blocks HCV replication in vitro,39 at the viral input used in this study, differences in infectivity were moderate. Moreover, treatment with NB-DNJ did not affect viral infectivity at all. Therefore, our findings establish a critical role for sphingolipids during mDC binding and endocytosis of particles derived from cholesterol-enriched domains.
In conclusion, capture of retroviruses and exosomes is up-regulated upon DC maturation, leading internalized particles into the same CD81+ intracellular compartment and allowing efficient transmission to T cells. This novel capture pathway in which retroviruses and exosomes converge is insensitive to proteolytic treatment and efficiently blocked by sphingolipid biosynthesis inhibitors. Underscoring the molecular determinants of this highly efficient viral capture process in which retroviruses mimic exosomes to evade the host immune system might lead to new therapeutic strategies for infectious diseases caused by agents such as HIV-1, HTLV-1, or other retroviruses. Furthermore, this knowledge can help in the design of safer candidates to use in a DC-based therapeutic vaccine.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank P. Parrales from the Pathology Department of Hospital Germans Trias i Pujol, P. Bastos from F.E.B.'s laboratory, M.T. Rodriguez from J.M.-P.'s laboratory, and M. Roldán, A. Carvalho, and H. Monton from the UAB Microscopy Service. Some of the FACS data were acquired with equipment maintained by the Boston University Medical Campus Flow Cytometry Core Facility.
Work in the laboratory of S.G. is supported by grants from the National Institutes of Health (NIH; RO1-AI064099). Work in the laboratory of J.M-P. is supported by the Spanish Ministry of Education and Science through grants SAF2004-06991 and SAF2007-64696, the Spanish AIDS network Red Temática Cooperativa de Investigación en SIDA (RD06/0006), the Catalan HIV Vaccine Development Program (HIVACAT), and the Fundación para la Investigación y Prevención del Sida en España (FIPSE) through grants 36356/05, 36523/05, and 36621/06. N.I.-U. is supported by Departament d'Innovació, Universitat i Empresa (DURSI) from the Generalitat de Catalunya and the Spanish AIDS Network RD006/0006. M.N.-G. is supported by Fondo de Investigación Sanitaria (FIS) from the Spanish Health Department through grant 03/0142 to the laboratory of F.E.B. J.B. is supported by the Institute for Research on Health Sciences Germans Trias i Pujol in collaboration with the Catalan Health Department. J.H.C. is supported by a grant from NIH (AI064606). The HIV-1 JRFL gp140 expression plasmid was a gracious gift of Dr Sodroski (Dana-Farber Cancer Institute, Boston, MA). The following reagents were obtained through the NIH AIDS Research and Reference Reagent Program (Division of AIDS, National Institute of Allergy and Infectious Diseases): pGAG-eGFP (cat. no. 11 468) from Dr Resh; HIV-IG (cat. no. 3957) from North American Biological Inc (NABI) and National Heart, Lung, and Blood Institute (Dr Barbosa); anti-HIV-1 p24Gag monoclonal antibodies and Clone AG3.0 (cat. no. 4121) from Dr Allan; Clone 183-H12-5C (cat. no. 3537) from Dr Chesebro and Dr Wehrly; and TZM-BL and Ghost X4/R5 indicator cell line (cat. nos. 8129 and 3942) from Dr Kappes, Dr Kewal Ramani, and Dr Littman.
National Institutes of Health
Authorship
Contribution: N.I.-U. and M.N.-G. designed and performed research, analyzed data, and wrote the paper; J.A., S.C.H., I.E., and M.C.P. designed and performed research and analyzed data; J.B. and F.E.B. designed research and analyzed data; B.C., J.H.C., M.T.F.-F., and L.M. contributed vital analytical tools; and S.G. and J.M.-P. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Javier Martinez-Picado, IrsiCaixa Foundation, Hospital Germans Trias i Pujol, Ctra de Canyet s/n, 08916 Badalona, Spain; e-mail: jmpicado@irsicaixa.es; or Suryaram Gummurulu, Department of Microbiology, R-518, Boston University School of Medicine, 73 E Concord St, Boston, MA 02118; e-mail: rgummulu@bu.edu.
References
Author notes
*N.I.-U. and M.N.-G. contributed equally to this work.
†S.G. and J.M.-P. contributed equally to this work as senior authors.