Abstract
Basophils are effector cells of the innate immune system that are associated with allergic inflammation and infections with helminth parasites. However, their development and in vivo functions are largely unknown. Here, we characterize basophil development, turnover, tissue localization, and effector function during infection with the helminth Nippostrongylus brasiliensis. Our results demonstrate that under homeostatic conditions basophils have a lifespan of about 60 hours. N brasiliensis–induced basophilia is caused by increased de novo production of basophils in the bone marrow. Basophils were found near the marginal zone in the red pulp of the spleen, in the lamina propria of the small intestine, and in the lung parenchyma. Activated basophils promoted systemic eosinophilia, were associated with differentiation of alternatively activated macrophages in the lung, and contributed to efficient worm expulsion, demonstrating that basophils play a crucial role as effector cells in type 2 immune responses.
Introduction
Basophil development and function is poorly understood, although this rare cell type was identified by Paul Ehrlich in 1879.1 Basophils constitute less than 0.3% of leukocytes in the peripheral blood. They are functionally closely related to mast cells, since both cell types express the high-affinity receptor for IgE and produce similar effector molecules, including histamine, lipid mediators (eg, leukotrienes and prostaglandins), serine proteases, and interleukins (eg, IL-4, IL-13, and IL-6).2 However, the developmental and functional relationship of basophils to other cell types is not clearly established. Helminth infections of mice cause a dramatic increase in basophil numbers, which is thought to be mediated by IL-3.3 However, naive IL-3–deficient mice have normal levels of basophils, indicating that IL-3 promotes basophil development and/or survival but is not essential for the basophil lineage.3 Increased numbers of basophils are found in the skin during the late-phase response of contact dermatitis4 or atopic dermatitis.5 Basophils are found in the lungs of patients with allergic asthma, and probably contribute to pathogenesis during fatal asthma.6,7 On the other hand, they might contribute to protective immunity against helminths since basophils are an important source for IL-4 and IL-13.8 Recently, basophils have been shown to be implicated in polarization of T-helper 2 (Th2) cells by allergens with protease activity9 and IgG1-mediated anaphylaxis,10 which further demonstrates their important functions during type 2 immune responses.
The turnover of basophils has not yet been described, and their localization in tissues is essentially unknown. Basophils could enhance Th2-associated immune responses since they release IL-4 within minutes of activation.11,12 Activation can occur by cross-linking of surface-bound IgE molecules or by a large variety of substances in an antigen-unspecific manner (reviewed in Falcone et al2 ). Mast cells and basophils express constitutively low levels of IL-4, which can be visualized by using sensitive IL-4 reporter mice expressing enhanced green fluorescent protein (eGFP) under control of regulatory elements of IL-4 (4get mice13 ). Infection of 4get mice with the helminth Nippostrongylus brasiliensis causes massive type 2 immune responses in the lung and small intestine, providing a valuable model to study basophil function during parasitic infections in vivo.
Here, we analyzed basophil development and turnover by flow cytometry, BrdU incorporation, and adoptive transfer. We determined the localization of basophils in the spleen, lung, and small intestine of N brasiliensis–infected mice by immunofluorescence staining of tissue sections. Basophils were found to promote differentiation of alternatively activated macrophages in the lung, induce systemic eosinophilia, and contribute to worm expulsion in N brasiliensis–infected mice, demonstrating their central role during the effector phase of a type 2 immune response against helminths.
Methods
Mice
IL-4 reporter mice (4get mice; C.129-Il4tm1Lky/J) have been described13 and were kindly provided by R. M. Locksley (Howard Hughes Medical Institute, University of California, San Francisco). In brief, these mice carry an IRES-eGFP construct inserted after the stop codon of the Il4 gene. 4get/rag−/− mice were generated by crossing 4get mice to Rag2-deficient mice (Rag2tm1Fwa; Taconic Farms, Germantown, NY), which had been backcrossed to BALB/c background. Mast cell–deficient c-KitW-sh mice had been backcrossed to a C57BL/6 background and were obtained from D. Lee (Brigham & Women's Hospital, Boston, MA) who obtained them from G. Caughey (University of California, San Francisco), who obtained them from P. Besmer (Memorial Sloan-Kettering Cancer Center, New York, NY). IL-4/IL-13–deficient mice14 were obtained from A. N. McKenzie (Medical Research Council, Cambridge, United Kingdom) and crossed to BALB/c/Thy1.1 mice. Eosinophil-deficient ΔdblGATA mice were obtained from C. Gerard (Children's Hospital, Boston, MA) and crossed to 4get mice.15 BALB/c mice were obtained from The Jackson Laboratory (Bar Harbor, ME). Mice were housed according to institutional guidelines and used at 6 to 8 weeks of age. All animal experiments were approved by the federal government, Regierung von Oberbayern, and by the institutional review board.
N brasiliensis infection
Third-stage larvae (L3) of N brasiliensis were washed extensively in sterile 0.9% saline (37°C) and injected subcutaneously (500 organisms) into mice. Mice were provided antibiotics containing water (2 g/L neomycin sulfate, 100 mg/L polymyxin B sulfate; Sigma-Aldrich, St Louis, MO) for the first 5 days after infection.
Cell culture
The basophil-like cell line IC-216 was cultured in RPMI 1640 (PanBiotech, Aidenbach, Germany) supplemented with 10% fetal calf serum (FCS; Invitrogen, Carlsbad, CA), 2 mM l-glutamine, 1 mM sodium pyruvate, 100 μg/mL penicillin, 100 μg/mL streptomycin (Biochrom AG, Berlin, Germany) and 5 × 10−5 M β-mercaptoethanol containing 1 ng/mL recombinant murine IL-3 (ImmunoTools, Friesoythe, Germany). The bone marrow of 4get mice was cultured in supplemented Dulbecco modified Eagle medium (DMEM; PanBiotech) for 11 days in the presence of recombinant mouse IL-3 and stem cell factor (SCF; ImmunoTools) at the indicated concentrations and either stained for flow cytometry or subjected to negative selection with magnetic beads using biotinylated anti–c-Kit mAb (Invitrogen) and anti-biotin microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany). Magnetic-activated cell sorter (MACS)–purified basophils (IL-4/eGFP+, c-Kit−) contained generally 1% mast cells or less (IL-4/eGFP+, c-Kit+) as assessed by flow cytometry (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). A total of 2.5 to 4 × 106 cells were injected in phosphate-buffered saline (PBS) intravenously into either naive BALB/c mice or into BALB/c mice that had been infected with N brasiliensis 4 days before. Transferred basophils were identified in the blood as IgE+B220− IL-4/eGFP+ in a FSClo/SSClo gate.
Semiquantitative RT-PCR analysis
RNA was extracted from sorted cells or total lung tissue using an RNA isolation kit (Fluka, Buchs, Switzerland) and 2 μg total RNA was used for first-strand cDNA synthesis with reverse transcription (RT) using oligo(dT) primers. Polymerase chain reaction (PCR) was performed with 3-fold serially diluted cDNA templates using the primer pairs listed in Document S1. PCR conditions were the following: 3 minutes initial denaturation at 94°C, 30 to 38 cycles with 30 seconds of denaturation at 94°C, 30 seconds of annealing at 56 to 58°C, and 60 seconds of amplification at 72°C. After 10 minutes of terminal amplification at 72°C, samples were run on a 1% agarose gel.
BrdU staining
For short-term labeling (15 hours), 1 mg 5-bromo-2′-deoxyuridine (BrdU) was injected intraperitoneally in 100 μL PBS. For long-term labeling (36 hours or 84 hours), BrdU was injected at 0, 12, and 24 hours. Single-cell suspensions from total lung tissue, bone marrow, spleen, or blood were stained for surface IgE; counterstained for B220, CD4, and CD8; and washed twice with PBS. Cells were resuspended in 0.15 M NaCl, fixed by adding dropwise −20°C ethanol (final concentration, 70%) and incubated for 30 minutes on ice. After additional washing with PBS, the cells were permeabilized overnight at 4°C in PBS, 1% paraformaldehyde (Roth, Karlsruhe, Germany), and 0.01% Tween-20 (Sigma-Aldrich). Cells were washed in PBS and resuspended in 0.15 M NaCl and 4.2 mM MgCl2 (pH 5.0). Then, genomic DNA was fragmented by adding 50 Kunitz units of DNAse I (Sigma-Aldrich) and incubated for 10 minutes at 20°C, followed by 30 minutes on ice. Finally, cells were stained with FITC-labeled anti-BrdU antibody (BD Pharmingen, Heidelberg, Germany) for 30 minutes at 20°C. Basophils were identified as IgE+B220−CD4−CD8− cells in a FSClo/SSClo gate.
Flow cytometry and cell sorting
Fetal livers of embryos of 4get mice were harvested at day 16.5 of development and passed through a 45-μm cell strainer (BD Falcon, Cowley, United Kingdom). Bone marrow was collected from the tibia and femur of 4get mice and washed once in supplemented RPMI 1640. Lungs were perfused with PBS via the right heart ventricle, cut into small pieces, and passed through a 45-μm cell strainer. Single-cell suspensions were washed once in fluorescence-activated cell sorter (FACS) buffer (PBS/2% FCS/1 mg/mL sodium azide), incubated with anti-CD16/CD32 blocking antibody (2.4G2) for 5 minutes at 25°C and stained with diluted antibody mixtures. The antibodies used are listed in Document S1. For analysis of histamine content, nuclear morphology, and expression of mouse mast cell protease (MMCP) bone marrow cultures were harvested at day 11 and sorted into eGFP+c-Kit− cells (basophils) and eGFP+c-Kit+ cells (immature mast cells) with a purity of 98% or greater using a high-speed cell sorter (FACSAria; BD Immunocytometry Systems, San Jose, CA).
Analysis of nuclear morphology
Highly purified basophils (eGFP+c-Kit−) and mast cells (eGFP+c-Kit+) of day 11 bone marrow cultures were mounted on a slide by cytospin centrifugation and stained with a Hemacolor staining kit for microscopy (Merck, Darmstadt, Germany) according to the manufacturer's instructions.
Immune fluorescence staining
Cryosections of paraformaldehyde-fixed spleens of 4get mice that had been infected with N brasiliensis 10 days before were stained with purified rabbit anti-GFP antibody (Abcam, Cambridge, United Kingdom), followed by biotinylated donkey anti–rabbit F(ab′)2 secondary antibody (Jackson ImmunoResearch Laboratories, Suffolk, United Kingdom), which was detected by streptavidin–horseradish peroxidase (HRP) and tyramide-FITC using the TSA Fluorescein system (Perkin Elmer, Boston, MA). Sections were stained in addition with PE-labeled anti–Siglec-F or biotinylated anti-IgE (R35-118; BD Pharmingen) and Cy3-conjugated streptavidin (Jackson ImmunoResearch Laboratories), respectively. Sections were finally stained with Alexa 647–labeled anti-B220 (Invitrogen). To detect mast cells, sections were stained with biotinylated anti–c-Kit followed by streptavidin-HRP and tyramide-biotin using the TSA biotin system. Then, Cy3-conjugated streptavidin was used for visualization and slides were counterstained with Alexa 647–labeled anti-B220. For detection of basophils in the lung and small intestine of infected BALB/c or c-KitW-sh mice, cryosections were either double-stained with Alexa 647–labeled anti-B220 and biotinylated anti-IgE or single-stained with biotinylated anti-FcϵRI followed by the TSA biotin system and Cy3-conjugated streptavidin. Basophils in lungs of c-KitW-sh were detected by anti-CD200R3 (Ba103; kindly provided by H. Karasuyama, University of Tokyo, Tokyo, Japan), followed by Cy3-conjugated donkey anti–rat (Jackson ImmunoResearch Laboratories). Indicated sections were counterstained with DAPI (Sigma-Aldrich). For detection of eosinophils in lung and small intestine, acetone-fixed cryosections of infected BALB/c mice were stained with purified anti-MBP (Lee Laboratory, Mayo Clinic, Scottsdale, AZ), followed by Cy3-conjugated donkey anti–rat and counterstaining with DAPI. Pictures were acquired with a 10× UPlanSApo objective on a Olympus microscope BX41 equipped with a F-View II camera and cell F software (Olympus, Tokyo, Japan). Original magnification was ×160 for spleen samples and ×80 for lung and small intestine samples.
Basophil depletion and reconstitution of 4get/rag−/− mice
4get/rag−/− mice (Thy1.2) were either used directly or after reconstitution with 7 × 107 spleen and lymph node cells from Thy1.1 congenic IL-4/IL-13−/− mice 8 days before N brasiliensis infection. Basophils were depleted by 2 to 5 intraperitoneal injections of 0.2 to 1 mg anti-Thy1.2 mAb (30H12) starting 2 days before N brasiliensis infection. To sensitize basophils, in vivo mice received 50 μL serum intravenously from N brasiliensis–infected wild-type mice on the day and 4 days after N brasiliensis infection. Mice were analyzed on indicated days after infection.
Basophil stimulation, β-hexosaminidase assay, and ELISA
β-hexosaminidase release was assessed as previously described with minor modifications.17 Briefly, bone marrow–derived basophils of c-KitW-sh mice or the basophil-like cell line IC-2 were preincubated either with TNP-specific mouse IgE (Igelb418 ), kindly provided by H.-M. Jaeck (University of Erlangen, Erlangen, Germany), or with serum of N brasiliensis–infected BALB/c or IL-4/IL-13−/− mice for 1 hour at 20°C. Cells were washed with Tyrode buffer (130 mM NaCl, 5 mM KCl, 1.4 mM CaCl2, 1 mM MgCl2, 5.6 mM glucose, 10 mM HEPES [pH 7.4], and 0.1% bovine serum albumin [BSA]) and incubated in the same buffer with or without NESL3 or NEXL3 for 1 hour at 37°C before supernatant was collected to determine β-hexosaminidase activity or for 6 hours before RNA was isolated for RT-PCR analysis. For cytokine detection in supernatants, bone marrow–derived basophils of c-KitW-sh mice were preincubated with serum of N brasiliensis–infected BALB/c mice and cultured overnight with 1:10 diluted NESL3 or NEXL3. IL-4 and IL-13 concentrations in the supernatant were detected by standard enzyme-linked immunosorbent assay (ELISA) techniques using the anti–IL-4 mAbs 11B11 for coating and BVD6–24G2 (BioLegend, San Diego, CA) for detection or the ELISA development kit for IL-13 (PeproTech, Hamburg, Germany). Histamine content in sorted basophils and mast cells was determined with a histamine EIA (Cayman, Tallinn, Estonia).
Statistics
Statistics were calculated by the Mann-Whitney U test using Prism software (GraphPad, San Diego, CA). P values less than .05 were considered statistically significant.
Results
Differential expression of serine proteases and surface receptors by bone marrow–derived basophils and mast cells
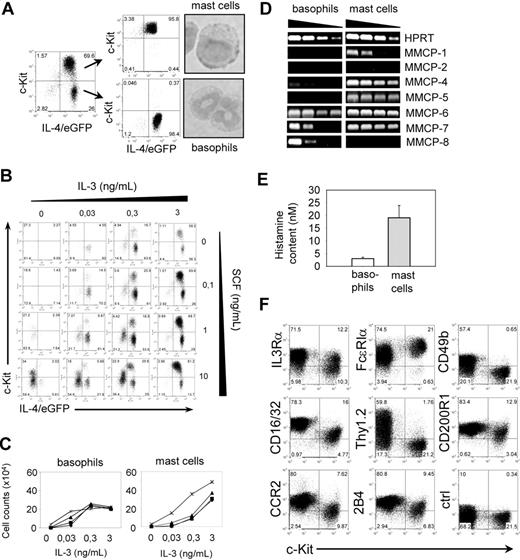
Mast cells and basophils are the only 2 cell types in mice that express the high-affinity receptor for IgE (FcϵRI) and both express constitutively low levels of IL-4 mRNA so that they appear as GFP+ cells in bicistronic IL-4 reporter mice (4get mice), which facilitates detection and isolation of these cells.13,19,20 However, they differ by expression of the SCF receptor c-Kit, which is expressed by mast cells but not by mature basophils.21 Bone marrow cells cultured in the presence of SCF and IL-3 differentiate into basophils (IL-4/eGFP+c-Kit−) and mast cells (IL-4/eGFP+c-Kit+; Figure 1A). Both cell types can further be distinguished by nuclear morphology with segmented nuclei in basophils and round-shaped nuclei in mast cells (Figure 1A).21 To develop optimized culture conditions that lead to preferential differentiation of basophils, bone marrow cells from 4get mice were cultured for 11 days in the presence of varying concentrations of IL-3 and SCF. In general, increasing amounts of IL-3 resulted in increased total numbers of basophils and mast cells, irrespective of the SCF concentration (Figure 1B,C). However, at low IL-3 concentrations (0.03 ng/mL), basophils could only develop in the presence of high concentrations of SCF (Figure 1C). Bone marrow from mast cell–deficient c-KitW-sh mice did not show this effect (data not shown), suggesting that high levels of SCF might induce IL-3 release from mast cells, which then promotes basophil development. The best yield of basophils and the lowest frequency of mast cells were obtained with 0.3 ng/mL IL-3 in the absence of SCF.
Characterization of bone marrow–derived basophils and mast cells. (A) Postsort analysis and nuclear morphology of purified basophils (IL-4/eGFP+c-Kit−) and mast cells (IL-4/eGFP+c-Kit+) after 11 days of culture of bone marrow cells in the presence of IL-3. (B) Titration of IL-3 and SCF to determine optimal culture conditions to generate basophils in vitro. Cultures were analyzed 11 days after setup. The experiment has been repeated with similar results. (C) Total numbers of basophils and mast cells on day 11 of culture in the presence of indicated concentrations of IL-3 and different concentrations of SCF (♦ indicates 0 ng/mL; ■, 0.03 ng/mL; ▴, 0.3 ng/mL; and ×, 3 ng/mL). The experiment has been repeated with similar results. (D) Semiquantitative RT-PCR of purified in vitro–generated basophils and mast cells to determine the expression level of different mast cell proteases. (E) Histamine content in sorted basophils (□) and mast cells (▩). The concentration was determined in 100 μL total cell lysate and normalized to 1000 cells. Bars show the mean plus or minus SD of triplicate wells. (F) Expression of different surface markers on mast cells (c-Kit+ cells) and basophils (c-Kit− cells). Dot plots are gated on autofluorescence−IL-4/eGFP+ cells. For panels A, B, and F, the numbers in the quadrants indicate the frequency of each population.
Characterization of bone marrow–derived basophils and mast cells. (A) Postsort analysis and nuclear morphology of purified basophils (IL-4/eGFP+c-Kit−) and mast cells (IL-4/eGFP+c-Kit+) after 11 days of culture of bone marrow cells in the presence of IL-3. (B) Titration of IL-3 and SCF to determine optimal culture conditions to generate basophils in vitro. Cultures were analyzed 11 days after setup. The experiment has been repeated with similar results. (C) Total numbers of basophils and mast cells on day 11 of culture in the presence of indicated concentrations of IL-3 and different concentrations of SCF (♦ indicates 0 ng/mL; ■, 0.03 ng/mL; ▴, 0.3 ng/mL; and ×, 3 ng/mL). The experiment has been repeated with similar results. (D) Semiquantitative RT-PCR of purified in vitro–generated basophils and mast cells to determine the expression level of different mast cell proteases. (E) Histamine content in sorted basophils (□) and mast cells (▩). The concentration was determined in 100 μL total cell lysate and normalized to 1000 cells. Bars show the mean plus or minus SD of triplicate wells. (F) Expression of different surface markers on mast cells (c-Kit+ cells) and basophils (c-Kit− cells). Dot plots are gated on autofluorescence−IL-4/eGFP+ cells. For panels A, B, and F, the numbers in the quadrants indicate the frequency of each population.
Murine mast cells are commonly classified by the expression pattern of different MMCPs.22-24 Since expression of these genes in basophils has not been described, we determined the expression pattern of MMCPs in highly purified (Figure 1A) bone marrow–derived basophils and mast cells by semiquantitative RT-PCR. MMCP-4, MMCP-6, and MMCP-7 were detectable in both cell types but with higher expression levels in mast cells, whereas expression of MMCP-1 and MMCP-5 was restricted to mast cells. In contrast, MMCP-8 was only expressed in basophils (Figure 1D). Analysis of the total histamine content revealed that mast cells contained about 6 times more histamine compared with basophils (Figure 1E). To further define differences between basophils and mast cells, which could be used to distinguish both cell types by flow cytometry, we compared the expression level of surface markers. IL-3Rα, FcϵRI, FcγRIIb/III (CD16/32), CD200R1, 2B4, and CCR2 were expressed by both cell types, whereas CD49b and Thy1.2 were only expressed on basophils (Figures 1F, S2). Therefore, we could efficiently and selectively deplete basophils in 4get/rag−/− mice with an anti-Thy1.2 mAb while leaving mast cells untouched (see “Basophil depletion reveals their effector functions in vivo”).
Basophil development and turnover in vivo
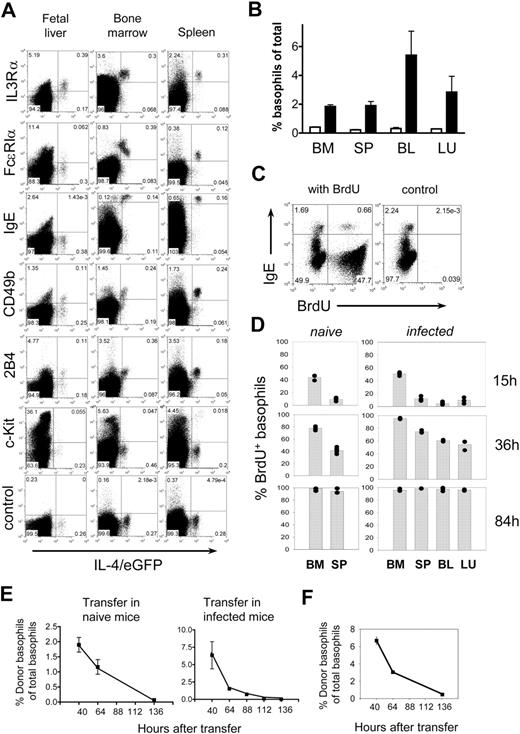
The development of basophils in vivo is largely unknown. Early hematopoiesis in the fetal liver can give rise to many hematopoietic cell lineages, including eosinophils.25,26 To determine whether basophil-like cells are also present in the fetal liver and to characterize their phenotype, we stained embryonic day 16.5 fetal liver cells for basophil-associated surface markers and compared the staining pattern with basophils isolated from the bone marrow or spleen of adult mice. A small population of non-CD4 T cells, noneosinophils that expressed IL-4/eGFP, and IL-3Rα, but not c-Kit, was present in the fetal liver (Figures 2A, S3). Although these cells did not express FcϵRIα and were negative for IgE, which can readily be detected on the surface of basophils in bone marrow and spleen of naive adult mice, they were morphologically similar to basophils isolated from adult mice (Figure S4 and Welle22 ). Mast cell precursors in the fetal liver of 4get mice express c-Kit and are IL-4/eGFP−,19 which distinguishes them from the basophil-like population described here. Two other basophil-associated markers, CD49b and 2B4, were detected only on a fraction of basophil-like cells in the fetal liver, whereas both markers were expressed by all basophils in the bone marrow and spleen (Figure 2A). We conclude that basophil-like cells and immature eosinophils25,26 are present in the fetal liver, where they constitute the first IL-4–expressing cells of the developing hematopoietic system.
Basophil development and turnover. (A) Expression of surface markers on developing basophils. Dot plots of embryonic day 16.5 fetal liver samples from 4get mice were gated on Ter119-−Siglec-F− cells to gate out eosinophils (Siglec-F+) and erythroblasts (Ter119+), whereas bone marrow cells and splenocytes were gated on CD4− and Siglec-F− cells in a FSCloSSClo gate that excludes mast cells (Figure S3). Since Th2 cells, natural killer (NK) T cells, eosinophils, mast cells, and basophils are the only cell types that express IL-4/eGFP in 4get mice, this gating strategy leaves only basophils in the IL-4/eGFP+ population. The experiment has been repeated with comparable results. (B) Frequency of basophils (defined as indicated in Figure S5) in bone marrow (BM), spleen (SP), blood (BL), and lung (LU) of 4get mice before (□) or 9 days after (■) infection with N brasiliensis. (C) Detection of incorporated BrdU in basophils (IgEhi cells) of BALB/c mice by flow cytometry. For panels A and C, the numbers in the quadrants indicate the frequency of each population. (D) BrdU incorporation in basophils (IgE+B220− cells) of naive (left) or N brasiliensis–infected BALB/c mice (right) at indicated time points after BrdU administration with 3 to 5 mice per group. The graphs show pooled results from 2 independent experiments. Each dot represents 1 mouse. (E) Kinetics of disappearance of transferred basophils from 4get mice in naive (left) and N brasiliensis–infected BALB/c mice (right). Graphs show the mean plus or minus SD of 3 individual mice from 2 independent experiments. (F) Decline of transferred basophils in the lung of N brasiliensis–infected BALB/c mice. The graph shows the mean of 2 mice per timepoint.
Basophil development and turnover. (A) Expression of surface markers on developing basophils. Dot plots of embryonic day 16.5 fetal liver samples from 4get mice were gated on Ter119-−Siglec-F− cells to gate out eosinophils (Siglec-F+) and erythroblasts (Ter119+), whereas bone marrow cells and splenocytes were gated on CD4− and Siglec-F− cells in a FSCloSSClo gate that excludes mast cells (Figure S3). Since Th2 cells, natural killer (NK) T cells, eosinophils, mast cells, and basophils are the only cell types that express IL-4/eGFP in 4get mice, this gating strategy leaves only basophils in the IL-4/eGFP+ population. The experiment has been repeated with comparable results. (B) Frequency of basophils (defined as indicated in Figure S5) in bone marrow (BM), spleen (SP), blood (BL), and lung (LU) of 4get mice before (□) or 9 days after (■) infection with N brasiliensis. (C) Detection of incorporated BrdU in basophils (IgEhi cells) of BALB/c mice by flow cytometry. For panels A and C, the numbers in the quadrants indicate the frequency of each population. (D) BrdU incorporation in basophils (IgE+B220− cells) of naive (left) or N brasiliensis–infected BALB/c mice (right) at indicated time points after BrdU administration with 3 to 5 mice per group. The graphs show pooled results from 2 independent experiments. Each dot represents 1 mouse. (E) Kinetics of disappearance of transferred basophils from 4get mice in naive (left) and N brasiliensis–infected BALB/c mice (right). Graphs show the mean plus or minus SD of 3 individual mice from 2 independent experiments. (F) Decline of transferred basophils in the lung of N brasiliensis–infected BALB/c mice. The graph shows the mean of 2 mice per timepoint.
The number of basophils can increase substantially during type 2 immune responses (Figures 2B, S5), which could be caused by increased de novo generation of basophils, prolonged survival of mature basophils, or a combination of both possibilities. Since the lifespan of basophils has not been reported, we sought to determine basophil turnover in naive and N brasiliensis–infected mice by administration of BrdU, a base analog that gets incorporated into the genomic DNA during the S phase of the cell cycle. Incorporated BrdU was determined by flow cytometry at various time points. Staining for surface IgE and intracellular BrdU allowed clear discrimination between BrdU+ and BrdU− basophils (Figure 2C). In naive mice, about 44% of basophils incorporated BrdU in the bone marrow during a 15-hour labeling period, whereas only 9% of basophils in the spleen incorporated BrdU during this time frame (Figure 2D). This difference could be explained by the delay between de novo generation of basophils in the bone marrow and their exit to the periphery. At 36 hours after BrdU administration, the frequency of newly generated basophils increased to 78% in the bone marrow and 41% in the spleen. Therefore, in both organs, an increase of about 33% BrdU+ cells was observed within a 21-hour time window. Given the fact that the total number of basophils is constant under steady-state conditions, we can calculate that it takes less than 60 hours to completely replace the pool of basophils in bone marrow and spleen. Therefore, the lifespan of basophils under steady-state conditions is about 60 hours. When N brasiliensis–infected mice were analyzed, they showed a small but significant increase in BrdU incorporation of basophils in the bone marrow during the 15-hour labeling period (51.1% BrdU+ cells in infected mice [n = 5] vs 43.8% BrdU+ cells in naive mice [n = 3]; P = .036 by Mann-Whitney U test). At 36 hours, the frequency of newly generated basophils in infected mice reached 95% in the bone marrow, 75% in the spleen, 61% in the blood, and 53% in the lung. Thus, the frequency of BrdU+ basophils in the spleen of infected mice almost doubled as compared with naive mice. At 84 hours, essentially all basophils in these organs were labeled with BrdU (Figure 2D). The higher frequency of BrdU+ basophils in infected mice suggests that basophilia is caused at least in part by increased de novo production of basophils in the bone marrow. Alternatively, basophilia could also be the consequence of prolonged survival, as it has been demonstrated for eosinophils.27 Therefore, to determine whether parasite infections can also prolong the survival of basophils, in vitro–generated basophils were transferred to naive or N brasiliensis–infected recipient mice. Transferred basophils rapidly disappeared from the peripheral blood of naive and infected recipient mice (Figure 2E) and also did not accumulate in the lung of infected mice (Figure 2F), which indicates that the inflammatory milieu in infected mice does not prolong the survival of basophils, suggesting that basophilia is indeed caused by increased de novo production during infection.
Localization of basophils, eosinophils, and mast cells in the lung, spleen, and small intestine
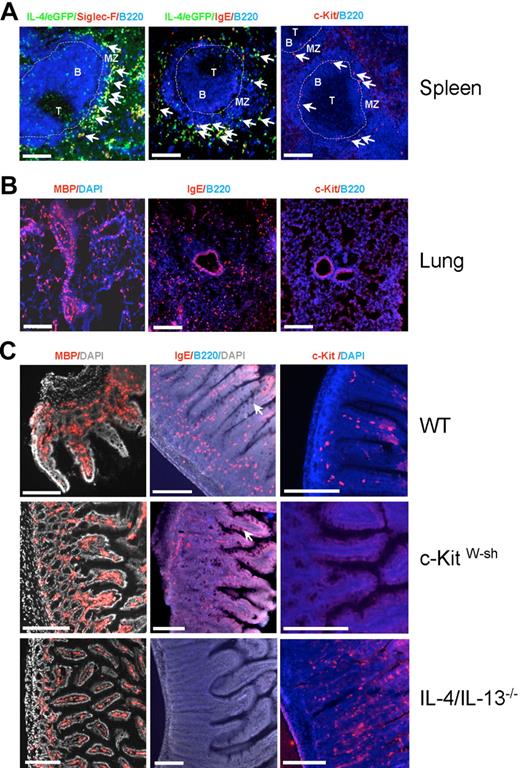
It is well known that eosinophils accumulate at the perivascular and peribronchial regions of the lung during allergic airway inflammation,28 whereas the distribution of basophils in the lung has not been described. Mast cells are essentially absent in the lung of mice subjected to acute allergic inflammation models, and their numbers only increase during chronic allergen challenge.29 Although it has been demonstrated that eosinophil and mast cell numbers increase in the small intestine after infection with the helminth N brasiliensis,30,31 it remains unclear whether basophils are also present in this organ. Furthermore, it is unclear whether eosinophils, basophils, and mast cells in the spleen are randomly distributed throughout the red pulp or concentrated at specific structures. Therefore, we determined the localization of basophils, eosinophils, and mast cells in the lung, spleen, and small intestine of N brasiliensis–infected 4get mice by immunofluorescence staining. In the spleen, double-staining for IL-4/eGFP and IgE allowed the discrimination between IgE-producing plasma cells (IL-4/eGFP−IgE+) and mast cells or basophils (IL-4/eGFP+IgE+), which both bind IgE via FcϵRI. The localization and frequency of mast cells could then be determined by staining for c-Kit, which is not expressed on mature basophils. Using this staining strategy, basophils and eosinophils could be located in the red pulp in close proximity to the marginal zone, whereas mast cells were mainly located in or next to the marginal sinus (Figure 3A).
Localization of basophils, eosinophils, and mast cells in the spleen, lung, and intestine after N brasiliensis infection. (A) Arrows indicate the localization of eosinophils (left panel; Siglec-F+IL-4/eGFP+B220−), basophils/mast cells (middle panel; IgE+IL-4/eGFP+B220−), and mast cells (right panel; c-Kit+B220−) in the spleen of 4get mice that had been infected with N brasiliensis 10 days before. T indicates T cell zone; B, B-cell follicle; and MZ, marginal zone. The dashed line indicates the marginal sinus. (B) Localization of eosinophils (left panel; MBP+ cells), basophils (middle panel; IgE+B220− cells), and lack of mast cells (right panel; c-Kit+ cells) in the lung of infected 4get mice. (C) Localization of eosinophils (left panel; MBP+ cells), basophils/mast cells (middle panel; IgE+B220− cells), and mast cells (right panel; c-Kit+ cells) in the jejunum of day-10 infected wild-type (WT), mast cell–deficient c-KitW-sh, and IL-4/IL-13–deficient mice. Arrows indicate goblet cell hyperplasia. Scale bars indicate 200 μm.
Localization of basophils, eosinophils, and mast cells in the spleen, lung, and intestine after N brasiliensis infection. (A) Arrows indicate the localization of eosinophils (left panel; Siglec-F+IL-4/eGFP+B220−), basophils/mast cells (middle panel; IgE+IL-4/eGFP+B220−), and mast cells (right panel; c-Kit+B220−) in the spleen of 4get mice that had been infected with N brasiliensis 10 days before. T indicates T cell zone; B, B-cell follicle; and MZ, marginal zone. The dashed line indicates the marginal sinus. (B) Localization of eosinophils (left panel; MBP+ cells), basophils (middle panel; IgE+B220− cells), and lack of mast cells (right panel; c-Kit+ cells) in the lung of infected 4get mice. (C) Localization of eosinophils (left panel; MBP+ cells), basophils/mast cells (middle panel; IgE+B220− cells), and mast cells (right panel; c-Kit+ cells) in the jejunum of day-10 infected wild-type (WT), mast cell–deficient c-KitW-sh, and IL-4/IL-13–deficient mice. Arrows indicate goblet cell hyperplasia. Scale bars indicate 200 μm.
In the lung and small intestine we could not stain for IL-4/eGFP due to a high background signal. Therefore, sections were stained for IgE and B220 to detect both basophils and mast cells (IgE+B220−) and in parallel with c-Kit to specifically localize mast cells. Eosinophils were detected by an eosinophil-specific anti-MBP antibody. In the lung, eosinophils were accumulated at perivascular and peribronchial areas, whereas basophils were equally distributed in parenchyma and mast cells were essentially absent (Figure 3B). Eosinophils and basophils were also found in large numbers in the lamina propria of the small intestine in N brasiliensis–infected wild-type and mast cell–deficient c-Kit W-sh mice (Figure 3C). To exclude that the IgE+B220− cells represent IgE-producing plasma cells, we stained lung and small intestine with anti-CD200R3 or anti-FcϵRI mAb, which bind exclusively to basophils and mast cells, and observed a comparable staining pattern (Figure S6). Hyperplasia of mucus-producing goblet cells was observed in N brasiliensis–infected wild-type and c-KitW-sh mice but not in IL-4/IL-13–deficient mice, where worm expulsion is severely impaired (Figure 3C). It remains to be determined whether basophils, eosinophils, or T cells are required for IL-4/IL-13–induced goblet cell hyperplasia in the intestines.
Secreted antigens of N brasiliensis activate basophils in an antigen-specific manner
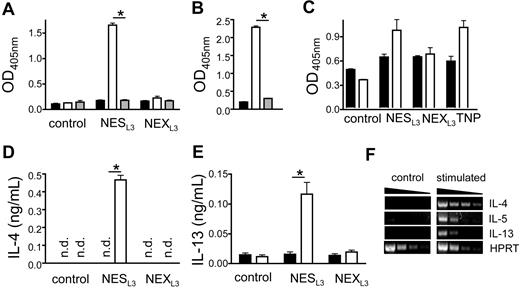
Although basophils can be activated by cross-linking of IgE or IgG1 bound to receptors on the cell surface, it is not clear whether pathogen-specific IgE or IgG1 are required for basophil activation during helminth infection. To address this question, the basophil-like cell line IC-2 was sensitized with serum from N brasiliensis–infected wild-type mice as source for antigen-specific IgE and IgG1 or with serum from N brasiliensis–infected IL-4/IL-13–deficient mice that served as control, since these mice are defective in class-switching to both isotypes. Then, whole larval extract (NEXL3) or secreted larval antigens (NESL3) of N brasiliensis larval stage L3 were added, and the release of β-hexosaminidase was analyzed to determine antigen-induced degranulation. Degranulation was only observed when cells had been sensitized with serum from wild-type mice and challenged with NESL3, whereas NEXL3 had no degranulating activity, suggesting that IL-4/IL-13–dependent parasite-specific IgE or IgG1 are required for activation and that the response is mainly directed against secreted antigens (Figure 4A). Degranulation was antigen-specific, since IC-2 cells sensitized with anti-TNP–specific IgE could not be degranulated with NESL3 (Figure 4B). NESL3 also caused degranulation and release of IL-4 and IL-13 from primary bone marrow–derived basophils, whereas NEXL3 had no effect (Figure 4C-E). Furthermore, NESL3 induced the expression of IL-5 (Figure 4F), and IgE-specific activation of basophils resulted in IL-5 protein secretion (Figure S7), suggesting that activated basophils might mobilize and prolong the survival of eosinophils since IL-5 exerts potent antiapoptotic activity on eosinophils.32
Basophil activation by secreted substances from N brasiliensis. (A,C) β-hexosaminidase release from the basophil-like murine cell line IC-2 (A) or bone marrow–derived basophils (C) before (■) or after sensitization with serum from N brasiliensis–infected wild-type mice (□) or IL-4/IL-13–deficient mice (▩). Cultures were stimulated with antigen from total larval extract (NEXL3) or secreted larval antigen (NESL3), which were generated as described in Document S1. Control indicates samples that were sensitized but not exposed to antigen; TNP, samples which were sensitized with anti-TNP IgE mAb (□) or not (■) and stimulated with TNP-BSA as positive control. *P < .005. (B) β-hexosaminidase release from IC-2 cells that were either sensitized with serum from N brasiliensis–infected wild-type mice (□) or with anti-TNP IgE (▩) and stimulated with NESL3. ■ indicates untreated controls. (D,E) IL-4 or IL-13 release from bone marrow–derived basophils after sensitization and stimulation as described in panel C. *P < .005. Bars show the mean plus SD from triplicate samples. n.d. indicates not detectable. The results are representative of 3 independent experiments. (F) Semiquantiative RT-PCR analysis of IL-4, IL-5, and IL-13 expression in bone marrow–derived basophils that were left untreated (control) or stimulated for 6 hours with NESL3 (stimulated) after cells had been sensitized with serum from N brasiliensis–infected mice.
Basophil activation by secreted substances from N brasiliensis. (A,C) β-hexosaminidase release from the basophil-like murine cell line IC-2 (A) or bone marrow–derived basophils (C) before (■) or after sensitization with serum from N brasiliensis–infected wild-type mice (□) or IL-4/IL-13–deficient mice (▩). Cultures were stimulated with antigen from total larval extract (NEXL3) or secreted larval antigen (NESL3), which were generated as described in Document S1. Control indicates samples that were sensitized but not exposed to antigen; TNP, samples which were sensitized with anti-TNP IgE mAb (□) or not (■) and stimulated with TNP-BSA as positive control. *P < .005. (B) β-hexosaminidase release from IC-2 cells that were either sensitized with serum from N brasiliensis–infected wild-type mice (□) or with anti-TNP IgE (▩) and stimulated with NESL3. ■ indicates untreated controls. (D,E) IL-4 or IL-13 release from bone marrow–derived basophils after sensitization and stimulation as described in panel C. *P < .005. Bars show the mean plus SD from triplicate samples. n.d. indicates not detectable. The results are representative of 3 independent experiments. (F) Semiquantiative RT-PCR analysis of IL-4, IL-5, and IL-13 expression in bone marrow–derived basophils that were left untreated (control) or stimulated for 6 hours with NESL3 (stimulated) after cells had been sensitized with serum from N brasiliensis–infected mice.
Basophil depletion reveals their effector functions in vivo
Direct assessment of the role of basophils in vivo requires their selective and efficient depletion. Previous reports used anti-FcϵRIα9 or anti-CD200R3 treatment33,34 to deplete basophils. However, both molecules are also expressed by mast cells, and it remains possible that mast cells are activated and/or depleted after antibody administration. Since basophils express the Thy1.2 marker, which is not expressed on eosinophils or mast cells (Figure 1E20 ), we sought to deplete basophils by anti-Thy1.2 mAb treatment. Basophil depletion in wild-type Thy1.2 mice was not successful (data not shown), which is most likely due to the large number of T cells that express higher levels of Thy1.2 and therefore serve as sink for the anti-Thy1.2 mAb. However, basophils were efficiently depleted for up to 10 days by a single injection of 0.5 mg anti-Thy1.2 in naive 4get/rag−/− mice (Figure 5A,B). No depletion of eosinophils was observed during this treatment, indicating that anti-Thy1.2 mAb did not deplete a potential common precursor population of eosinophils and basophils (Figure 5C). To be able to activate basophils in vivo in an antigen-specific manner, we sensitized basophils in 4get/rag−/− mice by transfer of serum from N brasiliensis–infected wild-type mice (Figure 5D,E). The depletion of such passively “armed” basophils by anti-Thy1.2 treatment allowed us to determine their role during the effector phase of a type 2 immune response.
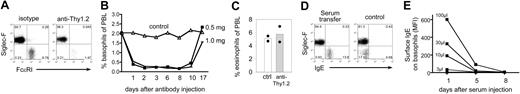
Basophil depletion by anti-Thy1.2 mAb administration and basophil sensitization by serum transfer. (A) Dot plot of blood samples from 4get/rag−/− mice before (left) or 2 days after (right) anti-Thy1.2 treatment. Plots are gated on total autofluorescence−IL-4/eGFP+ cells and display eosinophils (Siglec-F+FcϵRI−) and basophils (Siglec-F−FcϵRI+). (B) Kinetics of basophil depletion after administration of 0.5 mg or 1 mg anti-Thy1.2 mAb. Results show the mean of 2 mice per time point and condition. (C) Frequency of eosinophils in the peripheral blood of 2 individual basophil-depleted (anti-Thy1.2) or control mice on day 2 after mAb administration. Each dot represents 1 mouse. (D) Dot plots of blood samples from 4get/rag−/− mice before (control) or 1 day after (serum transfer) transfer of serum from N brasiliensis–infected wild-type mice. Plots are gated on autofluorescence−IL-4/eGFP+ cells and display eosinophils (Siglec-F+) and basophils (Siglec-F−). For panels A and D, the numbers in quadrants indicate the frequency of each population. (E) Kinetics of IgE clearance. Indicated amounts of serum from N brasiliensis–infected mice were transferred to 4get/rag−/− mice, and the mean fluorescence staining intensity (MFI) of IgE bound to the cell surface of basophils was determined on days 1, 5, and 8 after transfer.
Basophil depletion by anti-Thy1.2 mAb administration and basophil sensitization by serum transfer. (A) Dot plot of blood samples from 4get/rag−/− mice before (left) or 2 days after (right) anti-Thy1.2 treatment. Plots are gated on total autofluorescence−IL-4/eGFP+ cells and display eosinophils (Siglec-F+FcϵRI−) and basophils (Siglec-F−FcϵRI+). (B) Kinetics of basophil depletion after administration of 0.5 mg or 1 mg anti-Thy1.2 mAb. Results show the mean of 2 mice per time point and condition. (C) Frequency of eosinophils in the peripheral blood of 2 individual basophil-depleted (anti-Thy1.2) or control mice on day 2 after mAb administration. Each dot represents 1 mouse. (D) Dot plots of blood samples from 4get/rag−/− mice before (control) or 1 day after (serum transfer) transfer of serum from N brasiliensis–infected wild-type mice. Plots are gated on autofluorescence−IL-4/eGFP+ cells and display eosinophils (Siglec-F+) and basophils (Siglec-F−). For panels A and D, the numbers in quadrants indicate the frequency of each population. (E) Kinetics of IgE clearance. Indicated amounts of serum from N brasiliensis–infected mice were transferred to 4get/rag−/− mice, and the mean fluorescence staining intensity (MFI) of IgE bound to the cell surface of basophils was determined on days 1, 5, and 8 after transfer.
First, 4get/rag−/− mice were sensitized with serum from N brasiliensis–infected mice or from naive control mice and either depleted of basophils or not. These mice were then infected with N brasiliensis and analyzed on day 10 after infection, which marks the peak of the immune response in wild-type mice. Mice which were sensitized with serum from N brasiliensis–infected mice showed pronounced increase in eosinophils and basophils compared with mice that received control serum. Anti-Thy1.2 treatment of sensitized mice resulted in almost complete depletion of basophils from the lung, spleen, and blood (Figure 6A). Interestingly, sensitized and basophil-depleted mice also showed significantly reduced numbers of eosinophils in the lung, spleen, and blood, whereas eosinophil and mast cell numbers in the peritoneum, where basophils are generally absent,28 were not affected (Figure 6A,B). In addition, RT-PCR analysis of total lung tissue on day 4 after infection revealed that the increased levels of IL-4, IL-5, and IL-13 were dependent on the presence of sensitized basophils (Figure 6C).
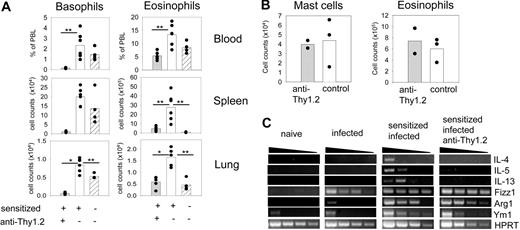
Basophil depletion during infection of sensitized 4get/rag−/− mice. (A) Percentages and total numbers of basophils (left) and eosinophils (right) in the blood, spleen, and lung of anti-Thy1.2 treated (▩) or control (□) 4get/rag−/− mice that had been sensitized with serum from N brasiliensis–infected wild-type mice and infected with N brasiliensis 10 days before analysis. As control, mice were treated with serum from naive wild-type mice (▨). Plots show combined results from 2 independent experiments with 5 to 6 mice total. *P < .001; **P < .05 by Mann-Whitney U test. (B) Number of mast cells and eosinophils in the peritoneum of sensitized and infected 4get/rag−/− mice that had been treated with anti-Thy1.2 or not. Each dot represents 1 mouse. (C) Semiquantitative RT-PCR analysis of total lung tissue for Th2-associated cytokines and markers for alternatively activated macrophages. The following samples were compared: naive (noninfected 4get/rag−/−) mice, infected (day 4 N brasiliensis–infected 4get/rag−/−) mice, sensitized and infected mice (4get/rag−/− mice that had been sensitized with serum from N brasiliensis–infected wild-type mice before infection), and sensitized, infected, and anti-Thy1.2 mice (sensitized and infected 4get/rag−/− mice that were depleted of basophils by anti-Thy1.2 treatment).
Basophil depletion during infection of sensitized 4get/rag−/− mice. (A) Percentages and total numbers of basophils (left) and eosinophils (right) in the blood, spleen, and lung of anti-Thy1.2 treated (▩) or control (□) 4get/rag−/− mice that had been sensitized with serum from N brasiliensis–infected wild-type mice and infected with N brasiliensis 10 days before analysis. As control, mice were treated with serum from naive wild-type mice (▨). Plots show combined results from 2 independent experiments with 5 to 6 mice total. *P < .001; **P < .05 by Mann-Whitney U test. (B) Number of mast cells and eosinophils in the peritoneum of sensitized and infected 4get/rag−/− mice that had been treated with anti-Thy1.2 or not. Each dot represents 1 mouse. (C) Semiquantitative RT-PCR analysis of total lung tissue for Th2-associated cytokines and markers for alternatively activated macrophages. The following samples were compared: naive (noninfected 4get/rag−/−) mice, infected (day 4 N brasiliensis–infected 4get/rag−/−) mice, sensitized and infected mice (4get/rag−/− mice that had been sensitized with serum from N brasiliensis–infected wild-type mice before infection), and sensitized, infected, and anti-Thy1.2 mice (sensitized and infected 4get/rag−/− mice that were depleted of basophils by anti-Thy1.2 treatment).
Since IL-4 and IL-13 are known to induce the generation of alternatively activated macrophages (AAMs), a recently identified cell type that might be involved in antihelminth immune responses,35,36 we determined the expression level of 3 characteristic markers for AAM, namely Ym1, Fizz1, and Arg I, by RT-PCR of total lung tissue. Increased expression levels of AAM markers were observed in infected Rag-deficient mice compared with naive mice even without serum sensitization, confirming a previous report37 (Figure 6C). However, the expression levels further increased when mice had been sensitized with serum from N brasiliensis–infected wild-type mice. Basophil depletion did not ablate this effect, suggesting that remaining basophils and eosinophils, which are the only IL-4/eGFP–expressing cells in the lung of 4get/rag−/− mice (Figure S8), provide enough IL-4/IL-13 to induce AAM differentiation in the lung (Figure 6C), since IL-4/IL-13 is indeed essential for AAM differentiation (Figure S9).
We and others have previously shown that efficient eosinophil and basophil recruitment to effector sites and worm expulsion is dependent on CD4+ T cells; however, IL-4/IL-13–producing Th2 cells are not required for this effect.38,39 To determine the role of basophils in eosinophilia and worm expulsion in mice that have an adaptive immune system but lack Th2 cells, we reconstituted 4get/rag−/− mice with total splenocytes and lymph node cells from IL-4/IL-13–deficient Thy1.1-congenic mice. Using this approach, we could deplete basophils by anti-Thy1.2 treatment while leaving T cells, eosinophils, and mast cells untouched. As expected N brasiliensis infection of such reconstituted 4get/rag−/− mice resulted in massive eosinophilia and basophilia in the lung and spleen (Figure 7A). Basophil depletion ablated eosinophilia similar to the effect seen in nonreconstituted 4get/rag−/− mice (Figures 6A, 7A, S10). Interestingly, no eosinophilia was observed when basophilia was caused by transfer of T cells transfected with IL-3–encoding retroviruses suggesting that basophils needed to be activated by foreign antigen(s) to cause eosinophilia (Figures 7B, S11).
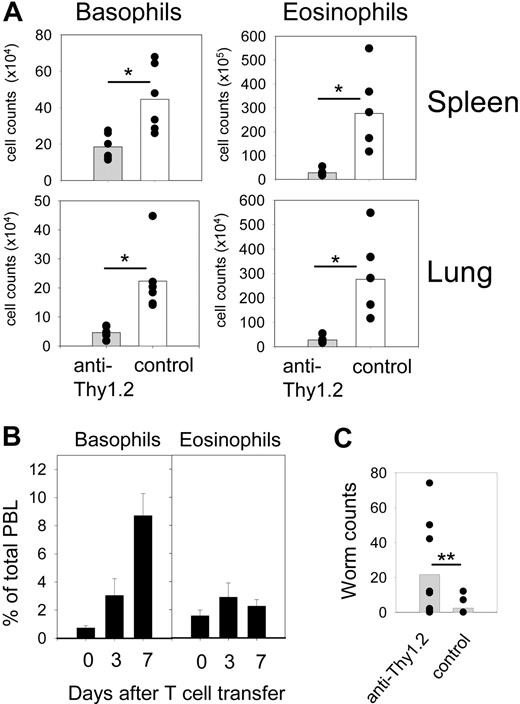
Reduced eosinophilia and impaired worm expulsion in basophil-depleted mice. (A) 4get/rag−/− mice were infected with N brasiliensis after they had been reconstituted with leukocytes from IL-4/IL-13−/− mice, sensitized with serum from N brasiliensis–infected wild-type mice, and either depleted of basophils (▩) or not (□). The number of basophils and eosinophils was determined in the lung and spleen on day 10 after infection. Plots show combined results from 2 independent experiments with 6 mice per group total. *P < .01 by Mann-Whitney U test. (B) IL-3–induced basophilia does not cause eosinophilia. The graphs show the frequency of basophils (left) and eosinophils (right) among total peripheral blood leukocytes (PBLs) of normal 4get mice at indicated days after transfer of polyclonal T cells that had been transfected with IL-3 cDNA-encoding retroviruses. (C) Number of adult worms in the small intestine of 4get/rag−/− mice that had been reconstituted with leukocytes from IL-4/IL-13−/− mice, sensitized with serum from N brasiliensis–infected wild-type mice, and either depleted of basophils (▩) or not (□). The graph shows pooled results from 3 independent experiments with 8 to 9 mice per group total. **P = .021 by Mann-Whitney U test. For panels A and C, each dot presents 1 mouse. The horizontal bars show the statistical comparison.
Reduced eosinophilia and impaired worm expulsion in basophil-depleted mice. (A) 4get/rag−/− mice were infected with N brasiliensis after they had been reconstituted with leukocytes from IL-4/IL-13−/− mice, sensitized with serum from N brasiliensis–infected wild-type mice, and either depleted of basophils (▩) or not (□). The number of basophils and eosinophils was determined in the lung and spleen on day 10 after infection. Plots show combined results from 2 independent experiments with 6 mice per group total. *P < .01 by Mann-Whitney U test. (B) IL-3–induced basophilia does not cause eosinophilia. The graphs show the frequency of basophils (left) and eosinophils (right) among total peripheral blood leukocytes (PBLs) of normal 4get mice at indicated days after transfer of polyclonal T cells that had been transfected with IL-3 cDNA-encoding retroviruses. (C) Number of adult worms in the small intestine of 4get/rag−/− mice that had been reconstituted with leukocytes from IL-4/IL-13−/− mice, sensitized with serum from N brasiliensis–infected wild-type mice, and either depleted of basophils (▩) or not (□). The graph shows pooled results from 3 independent experiments with 8 to 9 mice per group total. **P = .021 by Mann-Whitney U test. For panels A and C, each dot presents 1 mouse. The horizontal bars show the statistical comparison.
Expulsion of N brasiliensis has been shown to occur in the absence of mast cells,40 while it is dependent on IL-4/IL-13 expression from noneosinophil cells of the innate immune system.39 These findings point toward a major role for basophils in worm expulsion. Hence, we analyzed worm expulsion in basophil-depleted 4get/rag−/− mice that had been reconstituted with IL-4/IL-13–deficient leukocytes. Basophil-depleted mice showed significantly impaired worm expulsion on day 10 after infection, whereas worm expulsion was efficient in nondepleted mice (Figure 7C). A total of 2 of 9 anti-Thy1.2–treated mice were still able to completely expel the worms, suggesting that either basophil depletion was not efficient enough in these mice, or that other cell types were sufficient for worm expulsion. Hopefully, future studies with genetically basophil-deficient mice will help to clarify this point. Taken together, our results demonstrate that basophils provide important effector functions to promote eosinophilia, differentiation of AAM, and worm expulsion during helminth infection.
Discussion
Basophils might play an important role in type 2 immune responses, especially during a memory response when basophils have been sensitized by antigen-specific IgE produced during the primary immune response.8,12 We could demonstrate that basophil-like cells are present in the fetal liver of 4get mice, where they already express IL-4/eGFP but lack expression of FcϵRI. Our attempts to isolate these cells and reconstitute the basophil lineage by transfer into irradiated recipient mice failed (data not shown), which suggests that in contrast to immature eosinophils, which are also present in the fetal liver,26 these cells lack the potential to establish the basophil lineage in chimeric mice. A potential basophil-specific precursor cell type (BaP; Lin−CD34+FcϵRIαhic-Kit−) has been identified in the bone marrow of adult mice, and the transcription factor c/EBPα appears to regulate BaP development but seems to be dispensable for terminal basophil differentiation.41 Helminth infection increased the number of BaPs in the bone marrow which correlates with our results demonstrating increased frequencies of BrdU+ basophils in the bone marrow of N brasiliensis–infected mice41 (Figure 2D). Analysis of dendritic cells (DCs) revealed that they did not express IL-4/eGFP, FcϵRI, or Thy1, and although some DCs expressed c-Kit at a low level, they were not picked up by anti–c-Kit immunofluorescence staining of the spleen since the T-cell zone, where most splenic DCs are located, was not stained (Figures S12, 3A).
Eosinophilia and basophilia in helminth-infected mice appear to be regulated by different mechanisms. Eosinophilia in end organs is not the result of increased de novo generation of eosinophils in the bone marrow, but rather reflects a reduced death rate.27 In contrast, as we show here, basophilia is caused by increased production of basophils in the bone marrow. Eosinophils have a much broader tissue distribution compared with basophils. Activated eosinophils but essentially no basophils are found in the bronchoalveolar lavage, peritoneum, thymus, and lymph nodes of helminth-infected mice26 (data not shown). However, both cell types are present in the spleen, lung parenchyma, and small intestine of infected mice.
We could show here that splenic eosinophils and basophils accumulate in close proximity to the marginal zone. Interestingly, mast cells were mainly found in the marginal sinus. Since the blood drains into the marginal sinus of the spleen, mast cells would be ideally positioned to serve as sentinels for bloodborne pathogens. Furthermore, it has been shown that mast cell–derived carboxypeptidase A is essential for degradation of snake and honeybee venoms, and therefore mast cells might be strategically positioned in the marginal sinus to provide efficient inactivation of venoms that had been injected into the bloodstream.42,43
Our study provides in vitro and in vivo evidence that basophils produce IL-5 in an antigen-specific manner and that activation of basophils promotes eosinophilia independently of Th2 cells and mast cells. Similar observations have been made in a mouse model for IgE-dependent chronic allergic inflammation, where basophils appear to play a critical role in regulating eosinophil accumulation in the skin during the late phase of the allergic reaction.33,44 We could demonstrate here for the first time that reducing the number of basophils by antibody-mediated depletion resulted in impaired worm expulsion compared with nondepleted mice. Future studies on basophil development and function will help to better understand basophil physiology and hopefully pave new avenues for treatment of helminth infections and allergic diseases.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank J. Lee (Mayo Clinic, Scotsdale, AZ) for providing the anti-MBP mAb, H. Karasuyama (University of Tokyo, Tokyo, Japan) for providing the anti-CD200R3 mAb, M. Mack (University of Regensburg, Regensburg, Germany) for providing the anti-CCR2 mAb, S. Kaul (Paul Ehrlich Institute, Langen, Germany) for providing TNP-OVA and the protocol for the β-hexosaminidase assay, H.-M. Jaeck (University of Erlangen, Erlangen, Germany) for providing the anti-TNP IgE mAb IgEL b4, A. Pullner for excellent technical assistance, and J. Johnson, R. Obst, and T. Brocker for helpful comments on the manuscript.
This work was supported by an Emmy Noether grant from the Deutsche Forschungsgemeinschaft (Vo944/2).
Authorship
Contribution: C.O. and D.V. designed experiments and wrote the paper; and C.O. performed experiments.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: David Voehringer, Institute for Immunology, Goethestrasse 31, 80336 Munich, Germany; e-mail: david.voehringer@med.uni-muenchen.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal