Abstract
The D-dimer antigen is a unique marker of fibrin degradation that is formed by the sequential action of 3 enzymes: thrombin, factor XIIIa, and plasmin. First, thrombin cleaves fibrinogen producing fibrin monomers, which polymerize and serve as a template for factor XIIIa and plasmin formation. Second, thrombin activates plasma factor XIII bound to fibrin polymers to produce the active transglutaminase, factor XIIIa. Factor XIIIa catalyzes the formation of covalent bonds between D-domains in the polymerized fibrin. Finally, plasmin degrades the crosslinked fibrin to release fibrin degradation products and expose the D-dimer antigen. D-dimer antigen can exist on fibrin degradation products derived from soluble fibrin before its incorporation into a fibrin gel, or after the fibrin clot has been degraded by plasmin. The clinical utility of D-dimer measurement has been established in some scenarios, most notably for the exclusion of VTE. This article consists of 2 sections: in the first, the dynamics of D-dimer antigen formation is discussed and an overview of commercially available D-dimer assays is provided. The second section reviews available evidence for the clinical utilization of D-dimer antigen measurement in VTE, as well as emerging areas of D-dimer utilization as a marker of coagulation activation in other clinical settings.
Introduction
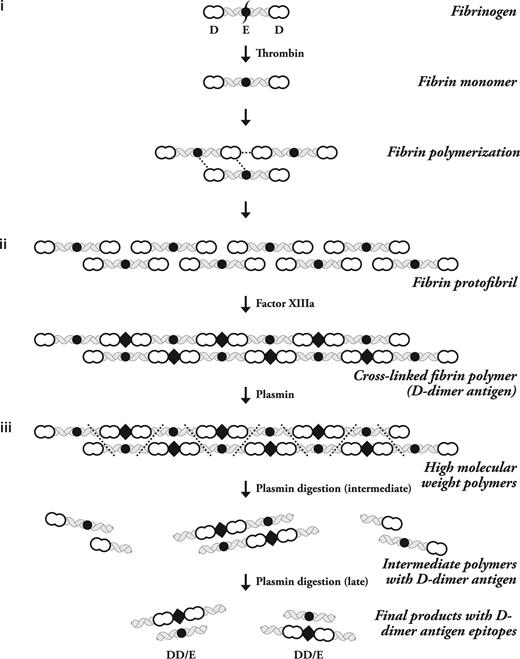
Fibrinogen is a soluble plasma glycoprotein that is transformed into highly self-adhesive fibrin monomers after thrombin cleavage.1 A detailed overview of the process of fibrin formation was recently published.2 In brief, in the first step of D-dimer formation, thrombin cleavage exposes a previously cryptic polymerization site on fibrinogen that promotes the binding of either another fibrinogen or a monomeric fibrin molecule.3 Fibrin monomers then bind to one another in an overlapping manner to form 2 molecule thick protofibrils (Figure 1).4,5
The stepwise process of Fibrin polymerization. The 3 major steps of D-dimer antigen formation are shown. (i) The fibrinogen molecule is cleaved by thrombin to produce fibrin monomers. These monomers associate with fibrinogen or fibrin to form protofibrils. They are held together by noncovalent forces shown as dotted lines between the intermolecular D-domain and D-E domains. (ii) Factor XIIIa formed by thrombin on fibrin polymers then covalently attaches D domains and inserts a covalent intermolecular linkage designated by the diamond-shaped figure. (iii) Plasmin must degrade fibrin at multiple sites to release fibrin degradation products, which then expose the D-dimer antigen epitope. The initial fragments are high-molecular-weight complexes followed by further degradation to produce the terminal D-dimer–E complex, which contains the dimer antigen. The 3 phases of this process are labeled on the right side of the diagram, and the different molecular forms of fibrinogen and its subsequent transformation by thrombin, factor XIIIa, and plasmin are shown on the left side of the diagram. This is a schematic representation of just one protofibril. Multiple protofibrils are aligned side by side and undergo branching to make a fibrin gel.
The stepwise process of Fibrin polymerization. The 3 major steps of D-dimer antigen formation are shown. (i) The fibrinogen molecule is cleaved by thrombin to produce fibrin monomers. These monomers associate with fibrinogen or fibrin to form protofibrils. They are held together by noncovalent forces shown as dotted lines between the intermolecular D-domain and D-E domains. (ii) Factor XIIIa formed by thrombin on fibrin polymers then covalently attaches D domains and inserts a covalent intermolecular linkage designated by the diamond-shaped figure. (iii) Plasmin must degrade fibrin at multiple sites to release fibrin degradation products, which then expose the D-dimer antigen epitope. The initial fragments are high-molecular-weight complexes followed by further degradation to produce the terminal D-dimer–E complex, which contains the dimer antigen. The 3 phases of this process are labeled on the right side of the diagram, and the different molecular forms of fibrinogen and its subsequent transformation by thrombin, factor XIIIa, and plasmin are shown on the left side of the diagram. This is a schematic representation of just one protofibril. Multiple protofibrils are aligned side by side and undergo branching to make a fibrin gel.
Plasma remains fluid until 25% to 30% of plasma fibrinogen is cleaved by thrombin,6 allowing time for fibrin to polymerize while simultaneously promoting thrombin activation of plasma factor XIII.7 Thrombin remains associated with fibrin,8 and as additional fibrin molecules polymerize, it activates plasma factor XIII bound to fibrinogen.9 The complex between soluble fibrin polymers, thrombin, and plasma factor XIII promotes the formation of factor XIIIa before a fibrin gel is detected.6
In the second step of D-dimer formation, factor XIIIa covalently cross links fibrin monomers via intermolecular isopeptide bonds formed between lysine and glutamine residues within the soluble protofibrils and the insoluble fibrin gel.10
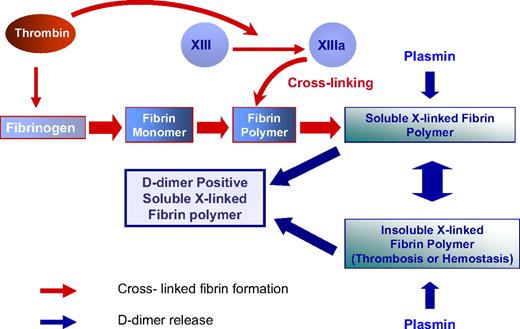
D-dimer antigen remains undetectable until it is released from crosslinked fibrin by the action of plasmin. In the final step of D-dimer formation, plasmin formed on the fibrin surface by plasminogen activation cleaves substrate fibrin at specific sites (Figure 1).11 Fibrin degradation products are produced in a wide variety of molecular weights, including the terminal degradation products of crosslinked fibrin containing D-dimer and fragment E complex (Figure 1).12,13 It is uncommon to detect circulating terminal fibrin degradation products (D-dimer–E complex) in human plasma, whereas soluble high-molecular-weight fragments that contain the “D-dimer antigen” are present in patients with DIC and other thrombotic disorders.14 These fragments may be derived from soluble fibrin before it has been incorporated into a fibrin gel, or alternatively may be derived from high-molecular-weight complexes released from an insoluble clot (Figure 2).15,16
The dynamics of D-dimer formation. Thrombin converts plasma fibrinogen to fibrin monomers. Thrombin then remains associated with fibrin and activates factor XIII, which circulates bound to fibrinogen. Factor XIIIa is formed as fibrin polymerizes and continues after fibrin has formed an insoluble gel. The D-dimer antigen is formed by the sequential action of thrombin, FXIIIa, and plasmin. Plasmin releases D-dimer antigen from fibrin polymers before and after fibrin gels. Thus, D-dimer antigen as detected by commercially available assays can either be derived from the soluble fibrin polymers before their uptake in the clot or be the product of plasmin cleavage of the fibrin clot.
The dynamics of D-dimer formation. Thrombin converts plasma fibrinogen to fibrin monomers. Thrombin then remains associated with fibrin and activates factor XIII, which circulates bound to fibrinogen. Factor XIIIa is formed as fibrin polymerizes and continues after fibrin has formed an insoluble gel. The D-dimer antigen is formed by the sequential action of thrombin, FXIIIa, and plasmin. Plasmin releases D-dimer antigen from fibrin polymers before and after fibrin gels. Thus, D-dimer antigen as detected by commercially available assays can either be derived from the soluble fibrin polymers before their uptake in the clot or be the product of plasmin cleavage of the fibrin clot.
“D-dimer antigen” detection requires specific monoclonal antibodies
Modern commercial D-dimer assays measure an epitope on degradation products of factor XIIIa–crosslinked fibrin by one of several methods. All assays use monoclonal antibodies that detect an epitope that is present in the factor XIIIa–crosslinked fragment D domain of fibrin (Figure 1), but not in fibrinogen degradation products or noncrosslinked fibrin degradation products. It is noteworthy that each detecting monoclonal antibody has its own unique specificity.17 Several monoclonal antibodies have been epitope-mapped, and the antigenic determinant recognized is a portion of the polypeptides in the D-domain that are conformationally reactive after factor XIIIa and plasmin have modified the protein.18
D-dimer antigen versus the terminal digest of fibrin
Initially, the term “fragment D-dimer” was used to describe a terminal plasmin digestion product of a factor XIIIa–crosslinked fibrin clot.19 The terminal digest of a crosslinked fibrin clot contains the fragment D-dimer–fragment E complex (Figure 1).20 However, the actual “D-dimer antigen” detected by contemporary clinical assays is not necessarily the terminal digestion product of fibrin (ie, the fragment D-dimer–E complex), and for some clinical disorders has been shown to be high-molecular-weight soluble fibrin fragments that either have not entered a fibrin gel or are released before complete plasmin degradation has occurred (Figure 2).17 Detailed studies conducted by Francis et al21 have shown that the fragment D-dimer–E complexes are formed after high-molecular-weight crosslinked fibrin complexes are released from an insoluble fibrin clot.
D-dimer laboratory assays
The impetus to develop a D-dimer assay came from the fact that clinical laboratory tests for fibrin degradation were incapable of distinguishing between fibrinogen and fibrin degradation products.22 Numerous clinical studies were initiated once commercially available assays for fibrin related D-dimer antigen were developed.23
Despite their ability to measure a fibrin-specific product of thrombin, factor XIIIa and plasmin action, these assays have limitations related to both their specificity and their sensitivity. Currently available D-dimer assays are not identical because the D-dimer antigen is present on different size degradation products, the monoclonal antibodies recognize different epitopes, and the assay format, assay calibration standards, and instrumentation vary. A comprehensive comparison of clinical laboratory performance characteristics of different assays has recently been presented.24 These results emphasize the need for physicians to recognize that D-dimer assays have unique performance characteristics. Clinicians need to be aware of the performance characteristics of the particular D-dimer test in use at their institution, because the D-dimer analyte is not a simple structure with a uniform composition. The cutoff value used to exclude venous thromboembolism (VTE) needs to be confirmed by the clinical laboratory. Alternatively, the institution should use an assay that has been previously validated in clinical studies. This topic has been discussed extensively by Dempfle and is the subject of an International Society of Thrombosis and Hemostasis Scientific Standardization Subcommittee study.17 Efforts to standardize assay results have not been successful so far, because the D-dimer analyte is not uniform across the different assays. Accordingly, there were attempts to harmonize assay performance through the interconversion of results from different assays using specific mathematical formulas,25 but this has yet to be accepted as universal practice.
The first generation of D-dimer assays were performed on plasma using latex beads coated with the DD-3B6 antibody.23 In this assay, the D-dimer epitope was characterized and was found to be a unique portion of the D-domain that underwent a conformational change upon covalent ligation by factor XIIIa, thereby becoming reactive with the monoclonal antibody after plasmin degradation.26 Latex agglutination assays rely upon the presence of sufficient bivalent D-dimer antigen on fibrin degradation products to initiate agglutination. Early assays required laboratory personnel to visually read and report the magnitude of the agglutination response.27 The assay could be performed in plasma and would only detect D-dimer antigen after fibrin had been subjected to factor XIIIa–mediated crosslinking, and plasmin had degraded the crosslinked protein.23 Subsequently, other monoclonal antibodies and automated assay detection methods were developed.28,29
Automated latex agglutination assays that recorded the rate at which antibody-coated particles aggregated in response to the D-dimer antigen were developed for use on specialized analyzers.30 The specificities of these antibodies are not identical, and they may react differently with high- and low-molecular-weight fibrin degradation products. Studies conducted with standards produced from partially digested and fully digested fibrin clots have shown that each assay may have a distinct sensitivity to various size degradation products. The calibrator for the assays should thus contain various D-dimer containing fibrin compounds, simulating the analyzed samples.31
Enzyme-linked immunosorbent assay (ELISA) methods were initially developed for research purposes before the latex agglutination assays, and they relied upon antibody capture of the D-dimer antigen on the plate, followed by tagging of the antigen with an antibody detection system for fibrin-related antigen. Although this assay format was extremely sensitive, it required more time to perform, and until recently, was not easily automated for clinical use. Several technologic advances in assay format and instrumentation led to ELISA-based assays that have increased sensitivity and are capable of detecting elevated D-dimer antigen associated with a variety of clinical disorders.32,33 Assays using fluorescence end point detection methods had equivalent sensitivity and specificity, with the added advantage of speed and a wide linear range that could detect D-dimer levels between 0 and 1000 μg/mL.34 Tests using immunofiltration were subsequently developed that further shortened laboratory turnaround times while maintaining excellent sensitivity, specificity, and negative and positive predictive values compared with the gold standard ELISA.35 Immunofiltration tests yield results within 2 minutes, allowing for prompt reporting and clinical management.36 Automated techniques that quantitate latex agglutination rates also have excellent sensitivity and have been demonstrated to maintain good correlation with ELISA.37 ELISA and latex turbidimetric methods have both been approved by the US Food and Drug Administration for the exclusion of venous thromboembolism and are used worldwide for this purpose.
Whole-blood agglutination tests that do not require sophisticated instrumentation were also subsequently developed,38 allowing for prompt clinical decision making with limited need for advanced laboratory equipment.39 Although these assays are less sensitive and cannot detect low levels of D-dimer antigen, they show sufficient specificity to allow exclusion of the diagnosis of VTE in the correct clinical setting.
Comparing the performance of various D-dimer assays leads to better understanding of their potential role in diagnosis of VTE (Table 1). The ELISA and fluorescence assay (ELFA), the microplate ELISA, and the automated quantitative turbidimetric assays have a higher sensitivity than the whole-blood agglutination assay (95% compared with 85%, respectively), but a lower specificity (50% vs 70%, respectively). This trade-off increases the need for further imaging to establish a diagnosis of VTE in the case of the ELISA-based assays. The whole-blood agglutination assays have a higher negative predictive value in populations with a low prevalence of VTE.40
Comparison of commercially available D-dimer assays
| Technique . | Commercial kits . | Sample type . | Sensitivity . | Specificity . | Automation . | Series . |
|---|---|---|---|---|---|---|
| Microplate ELISA* | Asserachrom DDI (Stago); Enzygnost (Dade-Behring)* | Plasma | High | Low | Manual | Indik et al,109 Bournameaux et al110 |
| ELISA and fluorescence (ELFA) | Vidas DD (bioMérieux); AxSym D-dimer (Abbott); Stratus D-dimer (Dade-Behring) | Plasma | High | Low | Automated | Mountain et al,28 van Belle et al60 |
| ELISA and chemiluminescence | Immulite (Siemens); Pathfast (Mitsubishi) | Plasma | High | Low | Automated | Fukuda et al,111 Dempfle et al112 |
| Immunofiltration and sandwich-type | NycoCard (Nycomed); Cardiac D-dimer (Roche) | Plasma | High High | Low-intermediate High | Automated | Scarano et al,35 Killick et al113 |
| Semi-quantitative latex agglutination | Dimertest latex (IL); Fibrinosticon (bioMérieux); DDI latex (Stago) | Plasma | Intermediate | Intermediate | Manual | Veitl et al,114 Sukhu et al115 |
| Manual whole-blood agglutination | SimpliRED (Agen); Clearview Simplify D-dimer (Agen) | Whole blood | High- intermediate | Intermediate | Manual | de Groot et al,39 Toulon et al116 |
| Second-generation latex agglutination (immunoturbidimetric) | TinaQuant (Roche); Liatest (Stago); Automated Dimertest (Agen); MDA D-dimer (bioMérieux); Turbiquant (Dade-Behring) | Plasma | High | Intermediate | Automated | Froehling et al,30 Curtin et al37 |
| Technique . | Commercial kits . | Sample type . | Sensitivity . | Specificity . | Automation . | Series . |
|---|---|---|---|---|---|---|
| Microplate ELISA* | Asserachrom DDI (Stago); Enzygnost (Dade-Behring)* | Plasma | High | Low | Manual | Indik et al,109 Bournameaux et al110 |
| ELISA and fluorescence (ELFA) | Vidas DD (bioMérieux); AxSym D-dimer (Abbott); Stratus D-dimer (Dade-Behring) | Plasma | High | Low | Automated | Mountain et al,28 van Belle et al60 |
| ELISA and chemiluminescence | Immulite (Siemens); Pathfast (Mitsubishi) | Plasma | High | Low | Automated | Fukuda et al,111 Dempfle et al112 |
| Immunofiltration and sandwich-type | NycoCard (Nycomed); Cardiac D-dimer (Roche) | Plasma | High High | Low-intermediate High | Automated | Scarano et al,35 Killick et al113 |
| Semi-quantitative latex agglutination | Dimertest latex (IL); Fibrinosticon (bioMérieux); DDI latex (Stago) | Plasma | Intermediate | Intermediate | Manual | Veitl et al,114 Sukhu et al115 |
| Manual whole-blood agglutination | SimpliRED (Agen); Clearview Simplify D-dimer (Agen) | Whole blood | High- intermediate | Intermediate | Manual | de Groot et al,39 Toulon et al116 |
| Second-generation latex agglutination (immunoturbidimetric) | TinaQuant (Roche); Liatest (Stago); Automated Dimertest (Agen); MDA D-dimer (bioMérieux); Turbiquant (Dade-Behring) | Plasma | High | Intermediate | Automated | Froehling et al,30 Curtin et al37 |
Adapted from Righini et al117 with permission.
ELISA indicates enzyme-linked immunosorbent assay.
This assay can only be performed in batches and has a relatively longer turnaround time.
These compromises make it imperative that clinicians understand the nature of the assay and the performance characteristics of the particular D-dimer test used by the laboratory at their hospitals. Collaborative efforts between clinicians and laboratory personnel should be directed toward offering tests that can aid clinicians to effectively exclude VTE in their patient population. Clinicians should request D-dimer assays in the appropriate clinical context, and laboratories should use assays that have been tested and validated in clinical studies. Furthermore, because the cutoff value plays a critical role in determining clinical decisions, it is imperative that the laboratory provide established and reliable cutoff values to help interpret the results accurately. Corresponding cutoff values for unique patient populations, based on clinical evidence, should be available to apply the results in a relevant manner.
The clinical utility of D-dimer measurement
Several clinical scenarios might prompt a practitioner to measure or monitor D-dimer levels. In general, the D-dimer test may be ordered to ascertain to what extent fibrin formation has been initiated or to learn whether there is any change in this process in the course of a specific therapy or disease process.41,42 In practice, D-dimer measurement has been most comprehensively validated in (1) the exclusion of VTE in certain patient populations and (2) the diagnosis and monitoring of coagulation activation in disseminated intravascular coagulation (DIC). More recently, D-dimer assays have also begun to find clinical utility in the prediction of recurrent VTE and risk stratification of patients for VTE recurrence. We will briefly review the data supporting the utility of D-dimer assays in each of these clinical settings.
Clinical prediction rules and D-dimer measurement in the exclusion of VTE
D-dimer assays may be used in the initial evaluation of patients suspected of having VTE (deep vein thrombosis [DVT] and/or pulmonary embolism [PE]) because the exclusion of VTE cannot be made on clinical grounds alone.43 The fact that only a small portion of circulating fibrinogen needs to be converted to crosslinked fibrin to generate a detectable D-dimer antigen signal after plasmin digestion in plasma44 confers the sensitivity required. Thus, a normal D-dimer in the appropriate clinical context denotes that there is no major ongoing activation of intravascular coagulation, and serves as a reliable tool for the exclusion of VTE. In a large meta-analysis, Stein et al45 demonstrated that a negative D-dimer test by the rapid ELISA method is as diagnostically useful as a negative computed tomography (CT) or a negative compression ultrasonography study (CUS) in excluding PE and DVT, respectively. However, recent surveys indicate that D-dimer assays are often not used appropriately for the exclusion of VTE in clinical practice.46
Upon presentation, all patients should be carefully evaluated for clinical pretest probability of VTE using a validated clinical prediction rule (CPR) and then stratified into probability groups. Several CPRs are available for the assessment of the clinical pretest probability for VTE including the Emperic, Wells, modified Wells, Charlotte, Geneva, and modified Geneva. A recently validated simplified CPR dichotomizes patients into 2 groups according to the likelihood of VTE (Figures 3,4).47,48 For a CPR to reliably determine pretest probability, it must be reproducible. In prospective assessments of these CPRs in the diagnosis of PE, the interrater agreement on the Wells score ranged from moderate to excellent (0.47-0.86), whereas for the revised Wells score, it was 0.72. Two assessments of the Charlotte rule showed excellent agreement (0.83 and 0.85). No published prospective studies address interrater agreement of the Geneva score to date.49
DVT indicates deep venous thrombosis; and CUS, compression ultrasonography. Patients with a score of less than 2 were considered unlikely and those with a score of 2 or more were considered likely to have DVT.108 *Sometimes as in the case of morbid obesity, CUS is not feasible.
DVT indicates deep venous thrombosis; and CUS, compression ultrasonography. Patients with a score of less than 2 were considered unlikely and those with a score of 2 or more were considered likely to have DVT.108 *Sometimes as in the case of morbid obesity, CUS is not feasible.
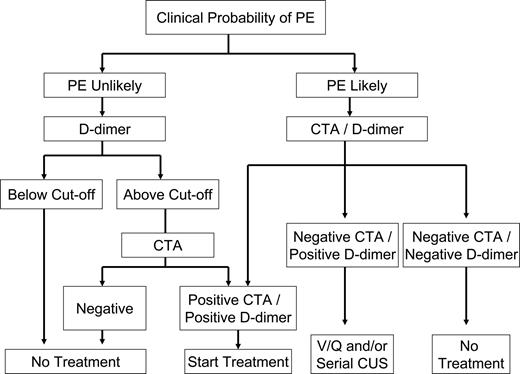
Clinical algorithm for diagnosis of pulmonary embolism. The diagnosis of PE was unlikely in patients scoring 4 or less, using the Wells Simplified Clinical Prediction Model, whereas the diagnosis of PE was likely in those scoring more than 4.47 CTA indicates contrast-enhanced computed tomographic angiography; and V/Q Scan, ventilation/perfusion scan.
Clinical algorithm for diagnosis of pulmonary embolism. The diagnosis of PE was unlikely in patients scoring 4 or less, using the Wells Simplified Clinical Prediction Model, whereas the diagnosis of PE was likely in those scoring more than 4.47 CTA indicates contrast-enhanced computed tomographic angiography; and V/Q Scan, ventilation/perfusion scan.
D-dimer and DVT
Most patients referred for evaluation of suspected DVT are outpatients, where the prevalence of DVT is relatively low. In this setting, D-dimer measurements combined with CPR and CUS have a high negative predictive value in the diagnosis of DVT, and are used to limit the use of more expensive and invasive studies.43
The incidence of DVT in hospitalized patients has reportedly increased from 0.8% to 1.3% of admissions over a period of 20 years,50 making this a growing healthcare problem. However, the diagnosis of thrombosis in this setting is complicated by the fact that D-dimer antigen levels are commonly elevated for various reasons in hospitalized patients, which limits its value for exclusion of VTE. In one representative study, only 22% of hospitalized patients without DVT had plasma D-dimer levels below the normal cutoff value that was used to exclude venous thrombosis in outpatients.51 The higher baseline values of D-dimer in hospitalized patients may reflect any one of several underlying disease processes that initiate intravascular fibrin formation but do not necessarily result in overt thrombosis.52 Because fibrin may be crosslinked before it gels, D-dimer antigen may be generated in the absence of overt thrombosis.53 Activation of blood coagulation is often promoted by inflammatory responses, resulting in elevated plasma D-dimer antigen levels.54 Furthermore, during the aging process, inflammation and activation of blood coagulation may also be enhanced and account for elevated baseline D-dimer levels seen in the elderly.55 This effect alters the reference range in the elderly population and negates the clinical value of the standard D-dimer antigen cutoff threshold as an exclusionary test in this age group.56
Thus, in the appropriate setting, D-dimer testing can be a valuable screening and diagnostic tool for the clinician. The pretest probability of DVT is a major determinant of the potential value of D-dimer measurement. A working algorithm for the diagnosis of DVT should guide the clinician to a prompt and confident diagnosis (Figure 3). This topic has been widely investigated, and excellent reviews have recently been published.40,45 However, the lack of standardization of the measurement, the most appropriate cutoff values, and the units used to report D-dimer antigen levels have led to some confusion regarding the utility of D-dimer in clinical practice. In addition, many manufacturers of the D-dimer assay recommend that the optimal cutoff values for excluding deep vein thrombosis should be determined for each population of patients tested.57 In general, when performed by a qualified laboratory on non-anticoagulated outpatients suspected of having DVT, the assay can be effectively incorporated into evidence-based clinical algorithms to exclude DVT.43 Conversely, the use of D-dimer assays for exclusion of recurrent DVT in patients already on anticoagulation may be of limited value.58
D-dimer and pulmonary embolism
Clinical trials have demonstrated that D-dimer measurements used in isolation are insufficient to make diagnostic decisions in VTE. However, in PE as with DVT, D-dimer testing, when used as part of a diagnostic algorithm that incorporates the determination of pretest probability, may obviate the need for more costly evaluation (Figure 4).33 According to a recent report using Bayesian analysis, the diagnosis of PE relies on the clinical pretest probability as well as on the sensitivity and specificity of the diagnostic tests used.59 In a study of 3306 patients, the combination of a low clinical probability and a negative D-dimer test effectively excluded PE, with a 3-month follow-up incidence of VTE of only 0.5%.60 Other studies have subsequently confirmed this finding.33,61 In patients with a high clinical probability of PE, a prospective study recently found that D-dimer had a higher negative predictive value (NPV) than the Wells CPR and that the combination of both further improved the diagnostic algorithm.62 Analysis of the data from the Christopher study showed that the false negative rate of PE diagnosis by CT in patients with high probability of PE was 5.3%.63 Likewise, in the PIOPED II study, 6 of 15 patients with a high clinical probability of PE and a negative CT had PE.64 Therefore, further testing in this setting seems justified.
The plasma levels of D-dimer were found to be directly related to the severity of PE and could predict adverse outcomes assessed by radiologic, biochemical, and clinical criteria.65 Furthermore, markedly elevated D-dimer levels were recently found to increase the likelihood of PE diagnosis.66 Thus, high D-dimer levels upon presentation may potentially prompt a more intense diagnostic approach, irrespective of pretest probability.
Effect of anticoagulation on D-dimer levels in acute VTE
Elevated plasma levels of D-dimer antigen gradually normalize in patients receiving anticoagulant therapy for acute VTE. In an attempt to study these changes, patients were randomized to receive either dose-adjusted unfractionated heparin (UFH) or low-molecular-weight heparin (LMWH).67 In this instance, no significant difference in the rate of normalization of markers of coagulation activation (including D-dimer) was noted. This observation suggests that the use of agents with predominantly anti-Xa activity (LMWH) or agents with combined anti-Xa and antithrombin activity (UFH), results in equivalent changes in fibrin formation and degradation after acute thrombosis. In theory, plasma “D-dimer antigen” levels could thus be used to monitor the response to therapy using either agent. However, on the basis of the current evidence, there is generally no compelling reason to track D-dimer antigen levels during the initial phase of anticoagulation.
D-dimer and recurrent VTE
It has been shown previously that a negative D-dimer test can exclude recurrent DVT and that heparin therapy can also be safely withheld in patients with negative serial compression ultrasonography, even if their D-dimer is positive. This approach can be safely and effectively used to manage approximately 70% of patients with suspected recurrent DVT.68 Furthermore, D-dimer levels were recently proposed to be useful in establishing the risk of recurrent thrombosis, thereby assisting in determining the proposed duration of anticoagulation for VTE patients.69 In this study, 608 patients with a first unprovoked VTE, who had received at least 3 months of oral anticoagulation, were followed up prospectively. D-dimer levels were obtained 1 month after cessation of anticoagulation. Patients with normal D-dimer did not resume anticoagulation. Patients with an abnormal D-dimer were randomized to resume or not to resume anticoagulation. During follow-up of this group, the incidence of recurrent VTE was 15% and 2.9% in patients who discontinued and those who resumed anticoagulation, respectively. Among patients who stopped anticoagulation, the adjusted hazard ratio for VTE recurrence among those with an abnormal D-dimer level was 2.27 (95% CI, 1.15-4.46; P = .02). Data from this trial were recently analyzed by different quantitative ELISA D-dimer assays to determine the risk for VTE recurrence.57 Different cutoff values were assigned according to patient characteristics and the nature of the assay.
D-dimer testing for exclusion of DVT in patients with cancer
The validity of available DVT diagnostic algorithms in patients with cancer is compromised by several factors. First, D-dimer levels may be elevated in patients with cancer in the absence of thrombosis. Second, none of the diagnostic algorithms devised for diagnosis of DVT have been validated in patients with cancer. Moreover, the NPV of D-dimer testing in this population is lower than in patients without cancer as a consequence of the higher prevalence of DVT in patients with cancer.70 A large study reported that 7.8% of patients with cancer with a negative D-dimer test had acute DVT compared with 3.5% in patients without cancer.71 The prevalence of DVT in patients with cancer was twice that in patients without cancer. This study also found that 88% to 94% of patients with cancer required further investigations beyond D-dimer testing, potentially negating the value of D-dimer testing as a screening tool for DVT in this population. In the case of PE diagnosis, data analysis from 1721 patients showed that an ELISA D-dimer assay could be used to exclude PE in patients with cancer, although it was recommended that a higher cutoff value be used.72 A recent prospective study of oncology patients found D-dimer levels to have a high negative predictive value and a high sensitivity in the diagnosis of PE.73
D-dimer for excluding VTE in pregnancy
In normal pregnancy, D-dimer levels steadily increase until the time of delivery,74 and there are no specific cutoff values for diagnosis of VTE in pregnancy. Furthermore, the CPRs currently in use have not been validated in pregnancy. A prospective study found D-dimer measurements helpful in excluding DVT in the first 2 trimesters of pregnancy, although its value in diagnosis of PE was limited.75 It is, however, recommended to perform a D-dimer test and a proper clinical assessment for suspected VTE in pregnancy.76 If the D-dimer is positive, then radiologic assessment should be performed.
D-dimer antigen and the DIC syndrome
DIC is a serious complication of sepsis, malignancy, obstetric mishaps, and other inflammatory disorders in which there is a persistent stimulus for the activation of blood coagulation. DIC is characterized by persistent intravascular thrombin generation and fibrin formation in the microvasculature, ultimately leading to depletion of coagulation factors and their inhibitors to produce a bleeding and/or thrombotic disorder.77 Early diagnosis of DIC is pivotal to initiation of appropriate management (which generally includes an aggressive attempt to remove the underlying cause) and achieving better outcomes. The lack of standard diagnostic criteria made it difficult to study treatment options and design clinical studies, but in 1987, a Japanese scoring system for DIC was introduced,78 and a few years later, the International Society on Thrombosis and Haemostasis (ISTH) introduced 2 separate scoring systems for overt and nonovert DIC.77 A prospective comparison of the ISTH and the Japanese scoring systems reported equal effectiveness in identifying DIC.79
The ISTH scoring system for overt DIC has been shown to be a valid predictor of outcome and fatality associated with DIC.80 The laboratory parameters for assessment of the overt DIC score are the platelet count, prothrombin time, and a fibrin-related marker (FRM), as well as fibrinogen concentration. D-dimers or fibrin degradation products are the most common fibrin-related markers used by clinical laboratories in this context.
Dempfle et al81 reported that the choice of FRM can affect the performance of the overt DIC score. These investigators concluded that using soluble fibrin marker as an FRM was more prognostically relevant than D-dimers in a cohort of ICU patients. Although additional studies are needed to confirm this finding, the observation is biologically plausible; because D-dimer assays detect relatively small proteolytic fragments of fibrin clots, a positive result can reflect both extravascular and intravascular fibrin generation. On the other hand, soluble fibrin is a more specific measure of intravascular fibrin formation.82
The emerging role of D-dimer measurements in other clinical settings
In addition to the established uses described above, D-dimer measurements have increasingly been studied as markers of coagulation activation in several other clinical scenarios.
As evidence evolves that some of these clinical settings are prothrombotic, there is an increasing need to explore the possible role of D-dimer in the assessment of VTE risk in these settings. Some of these disorders will be briefly reviewed here.
D-dimer for the diagnosis of acute aortic dissection
Sodeck et al concluded from a study of 65 patients that D-dimer can exclude acute aortic dissection (AAD) with a 100% sensitivity.83 However, because AAD is a life-threatening condition, D-dimer measurement should not preclude the use of diagnostic imaging. Clinical risk assessment in combination with D-dimer may prove useful and requires further study.
D-dimer antigen and hematopoietic growth factors
The increased use of hematopoietic growth factors to treat cytopenias and mobilize stem cells has led to the recognition of thrombosis as a complication of these agents.84 It remains unclear whether the growth factor alone or the treatment regimen in combination with the underlying disease process lead to thrombosis. The need to monitor patients for thrombotic risk and to identify those who may benefit from pharmacologic thromboprophylaxis has led to the assessment of D-dimer levels in patients on growth factors.
Recombinant erythropoietin
The recognition of several adverse effects of erythropoietin therapy, including thrombosis, hyperviscosity, hypertension, and possibly promotion of cancer progression, has led to more stringent guidelines on the use of this growth factor in the treatment of anemia in patients on hemodialysis, with cancer/chemotherapy-related anemia, and in other disorders.85,86 A markedly elevated hematocrit has been shown to activate coagulation, but the elevation of the hematocrit to levels within the normal range in patients with a history of anemia has not been uniformly demonstrated to activate blood coagulation.87
Several studies have examined coagulation activation markers during recombinant erythropoietin therapy. In patients on hemodialysis, D-dimer levels initially increased upon achievement of target hemoglobin levels, with a decline 3 months after stabilization.88 Multiple factors may lead to thrombosis in patients on hemodialysis, and interpretation of D-dimer measurements requires an assessment of all the underlying clinical covariables.
Granulocyte colony-stimulating factor
Canales et al89 examined the effect of granulocyte colony-stimulating factor (G-CSF) on the coagulation system in 25 healthy peripheral blood stem cell donors. They reported significant increases in D-dimer and in thrombin-antithrombin complex (TAT) levels associated with decreases of antithrombin and protein C levels in plasma. The authors concluded that the use of G-CSF results in a prothrombotic state in healthy volunteer donors of peripheral blood stem cells and should be used with caution in patients with a known hypercoagulable state. These findings were later reproduced by other investigators.90
Granulocyte macrophage colony-stimulating factor
Granulocyte macrophage colony-stimulating factor (GM-CSF) administration, whether alone or in combination with other agents, such as G-CSF, has also been reported to be associated with thrombosis.91,92
Clinicians should thus be aware of the possibility that growth factors may enhance the risk of thrombosis, particularly in patients with prior thrombosis or a strong family history of thrombosis or in the presence of other known risk factors. D-dimer measurements may prove to be valuable for the prediction of thrombosis and should certainly be considered to rule out thrombosis should relevant symptoms develop.
Hemolysis, thrombosis, and D-dimer measurement
Hemolysis is a well-recognized risk factor for the development of thrombosis in several hematologic disorders.93
Sickle cell disease
Sickle cell disease has been described as a hypercoagulable state, and although it is uncertain whether activation of coagulation is a cause or effect of many vasoocclusive complications in sickle cell disease, there is growing evidence that the sickle hemoglobinopathies are associated with an increased risk of clinically overt thrombosis.94 The mechanism of hypercoagulability is probably multifactorial and involves abnormalities in platelet function, thrombin generation and regulation, and fibrinolysis.95 Furthermore, we have recently demonstrated that even patients with sickle trait may be at increased risk for DVT.96
Early studies reported changes in the formation of factor XIIIa-crosslinked fibrin(ogen) during pain crises in sickle cell disease.97 Subsequently, D-dimer levels were discovered to be increased at baseline and during pain crisis in sickle cell disease.98 This hypercoagulable state has been associated with increases in plasma levels of markers of endothelial activation, such as soluble E-selectin and von Willebrand factor, as well as markers of inflammation such as interleukin 6 (IL-6), which promotes tissue factor and thrombin generation.99
Thalassemia
Thrombosis is a well-described complication in thalassemia, particularly in the “thalassemia intermedia” clinical phenotype.100 Direct and indirect markers of thrombin generation, such as prothrombin F1 plus 2 and D-dimers, were found to be increased, particularly after splenectomy.101 Other studies have reported elevated D-dimers in splenectomized hemoglobin E thalassemia and in double heterozygotes for hemoglobin E/β-thalassemia.102 Clinical trials focusing on prevention and treatment of thrombosis in these disorders are limited thus far, and efforts to develop risk-stratification models are needed.
Cancer therapy and D-dimer measurement
Chemotherapy
A study examining the association of thrombosis with thalidomide treatment in multiple myeloma reported a relationship between high D-dimer levels at presentation and the subsequent development of VTE. It has been suggested that such patients may benefit from prolonged prophylactic anticoagulation.103 However, additional studies are needed to validate whether an elevated D-dimer could be used to guide the type and duration of prophylactic anticoagulation in this situation.
Immunotherapy
Various cytotoxic chemotherapeutic agents and immunotherapies have been reported to increase the risk of thrombosis in cancer.104 A report described a cytokine-release syndrome associated with elevated D-dimers, lactate dehydrogenase, and liver transaminases in a population of patients with B-cell chronic lymphocytic leukemia receiving monoclonal anti-CD20 (rituximab).105 Weitz et al106 demonstrated that repeated cycles of systemic chemotherapy administered to patients with breast and lung cancer led to elevation in circulating levels of TAT and D-dimer and that a single dose of LMWH administered before chemotherapy prevented this phenomenon. It is possible that D-dimer could be used as an adjunctive biomarker to predict the risk of chemotherapy-induced thrombosis, although additional studies are required before this approach can be endorsed.
Stem cell transplantation
In stem cell transplantation, D-dimer levels were significantly elevated in patients with posttransplantation complications.107 The risk of thrombosis in the posttransplantation setting may vary depending upon the conditioning regimen, nature of the transplant, underlying disease, and posttransplantation therapy. Whether or not these clinical predictors of VTE are negated by the presence of normal plasma D-dimer levels at a given time point remains to be determined.
Conclusions
The D-dimer antigen is generated as a result of fibrin formation and fibrinolysis. The enzymes that function to generate this antigen are thrombin, factor XIIIa, and plasmin. Fibrin molecules that contain the D-dimer antigen are formed in both intravascular and extravascular spaces during hemostasis, thrombosis, and tissue repair. The D-dimer antigen is a specific marker of fibrin clot formation and fibrinolysis, which serves as a clinically useful marker for exclusion of VTE, and evaluation of the risk of VTE recurrence in select populations. Clinicians need to be aware of the heterogeneous nature of the D-dimer antigen and the different performance characteristics of the available assays, to make safe and timely therapeutic decisions. There is growing evidence that D-dimer antigen measurements can assist clinicians in numerous other clinical scenarios. Further standardization and accurate communication of assay performance characteristics will allow for effective utilization of this test.
Acknowledgments
The authors thank Ben Hulkower and Mariam Ezz for their help with the figures.
S.S.A. is an Assistant Professor at King Abdul Aziz University in Jeddah, Saudi Arabia.
Authorship
Contribution: S.S.A. wrote the manuscript; N.S.K. revised and edited the manuscript; and C.S.G. cowrote and revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Soheir S. Adam, The University of North Carolina at Chapel Hill, Division of Hematology/Oncology, 170 Manning Dr, CB#7305, Chapel Hill, NC 27599-7305; e-mail: soheir_adam@med.unc.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal