Abstract
We hypothesized that initial treatment of acute graft-versus-host disease (GVHD) with low-dose glucocorticoids (prednisone-equivalent dose of 1 mg/kg per day) instead of standard-dose glucocorticoids (prednisone-equivalent dose of 2 mg/kg per day) does not compromise major transplantation outcomes. We retrospectively analyzed outcomes among 733 patients who received transplants between 2000 and 2005 according to initial treatment with low-dose (n = 347) versus standard-dose (n = 386) systemic glucocorticoids. The mean cumulative prednisone-equivalent doses at day 100 after starting treatment were 44 and 87 mg/kg for patients given low-dose and standard-dose glucocorticoids, respectively. Adjusted outcomes between the groups given low-dose versus standard-dose glucocorticoids were not statistically significantly different: overall mortality (hazard ratio [HR], 1.10; 95% confidence interval [CI], 0.9-1.4), relapse (HR, 1.22; 95% CI, 0.9-1.7), nonrelapse mortality (HR, 1.06; 95% CI, 0.8-1.5). The small number of patients with grades III/IV acute GVHD at onset precluded definitive conclusions for this subgroup. In multivariate analysis, the risks of invasive fungal infections (HR, 0.59; 95% CI, 0.3-1.0) and the duration of hospitalization (odds ratio, 0.62; 95% CI, 0.4-0.9) were reduced in the low-dose prednisone group. We conclude that initial treatment with low-dose glucocorticoids for patients with grades I-II GVHD did not compromise disease control or mortality and was associated with decreased toxicity.
Introduction
Successful treatment of malignant diseases by allogeneic hematopoietic cell transplantation (HCT) depends upon the ability to control acute graft-versus-host disease (GVHD), a complication associated with considerable morbidity and mortality.1-4 In most patients who present with acute GVHD, symptoms develop during prophylaxis with immunosuppressive medications, and systemic glucocorticoids are typically added as first-line treatment.5,6 In most patients, glucocorticoids effectively control inflammatory GVHD manifestations without the need for additional immunosuppressive agents, and treatment can eventually be withdrawn. Patients with persistent or recurrent GVHD symptoms despite glucocorticoid treatment have increased risks of morbidity and mortality related to uncontrolled GVHD, prolonged glucocorticoid exposure, and infections related to profound immune suppression.7,8
Prospective randomized studies have identified no demonstrable benefit for treatment of acute GVHD with methylprednisolone at doses higher than 2 mg/kg per day.9 The choice of initial therapy for acute GVHD still varies widely around the world, reflecting a paucity of dose-finding studies in the literature and at least 2 schools of thought. One school advocates that all patients with newly diagnosed acute GVHD should receive glucocorticoids at a methylprednisolone or prednisone-equivalent dose of at least 2 mg/kg per day (standard dose), based on a belief that without aggressive treatment, milder symptoms of GVHD will inevitably progress to more severe GVHD. The corollary of this approach is that initial treatment with a lower dose of glucocorticoids will endanger patient outcomes because of undertreatment.3 A gradual taper of the dose is typically instituted once manifestations abate.6,10-12
Another school of thought asserts that lower doses of glucocorticoids can safely and effectively control clinically milder presentations of GVHD. The corollary of this approach is that glucocorticoid doses in excess of those needed to control GVHD manifestations cause major morbidity and increase mortality.13-16 In support of this view, studies aimed at reducing treatment-related toxicities by limiting the exposure to systemic glucocorticoids have yielded promising results.17,18 In these studies, patients with mild to moderately severe acute GVHD (rash involving ≤ 50% body surface area and stool volumes ≤ 1.0 L/day with or without anorexia, nausea, and vomiting) who were initially treated with low-dose systemic glucocorticoids (prednisone-equivalent doses of 1 mg/kg per day) in combination with oral beclomethasone dipropionate (BDP), a topically active glucocorticoid with limited exposure in the systemic circulation, had lower rates of treatment failure and superior survival than those given low-dose systemic glucocorticoids alone. The authors attributed these findings to the sustained systemic glucocorticoid-sparing effect of oral BDP. These studies included only patients presenting with mild to moderate acute GVHD and did not address the question of whether initial therapy with low-dose systemic glucocorticoids can effectively and safely be used to control GVHD with more severe presenting manifestations.
To address this dichotomy in therapeutic approaches, we conducted a retrospective analysis of outcomes among 733 patients who received either standard-dose prednisone (2 mg/kg per day) or low-dose prednisone (≤ 1 mg/kg per day) for initial therapy of GVHD. The aims of this analysis were to determine whether standard-dose initial therapy was associated with excess treatment-related morbidity, and conversely, whether lower-dose initial therapy endangered patient outcomes because of undertreatment that might result in excess mortality.
Methods
Patients
All patients who had allogeneic HCT at the Fred Hutchinson Cancer Research Center between January 2000 and December 2005, were at least 18 years of age, and had initial treatment for acute GVHD with systemic glucocorticoids were included in this retrospective study. Patients had signed institutional review board–approved consent forms allowing the use of medical records for research related to outcomes after hematopoietic cell transplantation. Details regarding time to GVHD symptoms and treatment, GVHD grades at onset, donor types, type of conditioning regimen, postgrafting immunosuppression, and supportive care according to initial treatment with low-dose (prednisone-equivalent dose of 1 mg/kg per day; n = 347) versus standard-dose (prednisone-equivalent dose of 2 mg/kg per day; n = 386) systemic glucocorticoids are listed in Table 1.
Patient characteristics according to the initial prednisone-equivalent dose of glucocorticoids for treatment of acute GVHD
| Characteristic . | Prednisone-equivalent dose . | |
|---|---|---|
| 1 mg/kg per day . | 2 mg/kg per day . | |
| n | 347 | 386 |
| Age, y (range) | 47 (18-71) | 46 (18-72) |
| Patient sex, female (%) | 151 (44) | 169 (44) |
| Disease risk, n (%) | ||
| Standard | 178 (51) | 194 (50) |
| High | 169 (49) | 192 (50) |
| HLA-mismatch, n (%) | 48 (14) | 87 (23) |
| Donor, n (%) | ||
| Related | 173 (50) | 134 (35) |
| Unrelated | 174 (50) | 252 (65) |
| Conditioning, n (%) | ||
| Myeloablative | ||
| Bu/Cy | 138 (40) | 166 (43) |
| Cy/TBI | 102 (29) | 133 (34) |
| Other | 46 (13) | 31 (18) |
| Nonmyeloablative | 61 (18) | 56 (15) |
| Median days to therapy after GVHD diagnosis (range) | 2 (0-92) | 1 (0-77) |
| Median days to therapy after transplantation (range) | 30 (8-118) | 21 (5-114) |
| GVHD grade at onset, n (%) | ||
| I | 31 (9) | 38 (10) |
| IIa | 238 (69) | 118 (31) |
| IIb | 69 (20) | 167 (43) |
| III | 9 (3) | 62 (16) |
| IV | 0 (0) | 1 (< 1) |
| GVHD organ site involved at onset, n (%) | ||
| Skin | 143 (41) | 285 (74) |
| Gut | 288 (82) | 249 (65) |
| Liver | 26 (7) | 89 (23) |
| Limited to the skin | 54 (16) | 106 (27) |
| Limited to the gut | 192 (55) | 69 (18) |
| Biopsy before GVHD treatment, n (%) | 328 (95) | 303 (79) |
| Hospitalized at onset of GVHD treatment, n (%) | 164 (47) | 268 (69) |
| Year of transplantation, n (%) | ||
| 2000 | 58 (17) | 84 (22) |
| 2001 | 50 (14) | 87 (23) |
| 2002 | 61 (18) | 66 (17) |
| 2003 | 65 (19) | 57 (15) |
| 2004 | 61 (18) | 52 (13) |
| 2005 | 52 (15) | 40 (10) |
| Tacrolimus prophylaxis, n (%) | 54 (16) | 85 (22) |
| BDP use, n (%) | ||
| Never | 175 (50) | 302 (78) |
| Initiated with systemic therapy | 134 (39) | 27 (7) |
| Characteristic . | Prednisone-equivalent dose . | |
|---|---|---|
| 1 mg/kg per day . | 2 mg/kg per day . | |
| n | 347 | 386 |
| Age, y (range) | 47 (18-71) | 46 (18-72) |
| Patient sex, female (%) | 151 (44) | 169 (44) |
| Disease risk, n (%) | ||
| Standard | 178 (51) | 194 (50) |
| High | 169 (49) | 192 (50) |
| HLA-mismatch, n (%) | 48 (14) | 87 (23) |
| Donor, n (%) | ||
| Related | 173 (50) | 134 (35) |
| Unrelated | 174 (50) | 252 (65) |
| Conditioning, n (%) | ||
| Myeloablative | ||
| Bu/Cy | 138 (40) | 166 (43) |
| Cy/TBI | 102 (29) | 133 (34) |
| Other | 46 (13) | 31 (18) |
| Nonmyeloablative | 61 (18) | 56 (15) |
| Median days to therapy after GVHD diagnosis (range) | 2 (0-92) | 1 (0-77) |
| Median days to therapy after transplantation (range) | 30 (8-118) | 21 (5-114) |
| GVHD grade at onset, n (%) | ||
| I | 31 (9) | 38 (10) |
| IIa | 238 (69) | 118 (31) |
| IIb | 69 (20) | 167 (43) |
| III | 9 (3) | 62 (16) |
| IV | 0 (0) | 1 (< 1) |
| GVHD organ site involved at onset, n (%) | ||
| Skin | 143 (41) | 285 (74) |
| Gut | 288 (82) | 249 (65) |
| Liver | 26 (7) | 89 (23) |
| Limited to the skin | 54 (16) | 106 (27) |
| Limited to the gut | 192 (55) | 69 (18) |
| Biopsy before GVHD treatment, n (%) | 328 (95) | 303 (79) |
| Hospitalized at onset of GVHD treatment, n (%) | 164 (47) | 268 (69) |
| Year of transplantation, n (%) | ||
| 2000 | 58 (17) | 84 (22) |
| 2001 | 50 (14) | 87 (23) |
| 2002 | 61 (18) | 66 (17) |
| 2003 | 65 (19) | 57 (15) |
| 2004 | 61 (18) | 52 (13) |
| 2005 | 52 (15) | 40 (10) |
| Tacrolimus prophylaxis, n (%) | 54 (16) | 85 (22) |
| BDP use, n (%) | ||
| Never | 175 (50) | 302 (78) |
| Initiated with systemic therapy | 134 (39) | 27 (7) |
Bu indicates busulfan; Cy, cyclophosphamide; TBI, total body irradiation; and BDP, beclomethasone diproprionate.
Disease risk: standard refers to aplastic anemia, chronic myeloid leukemia in chronic phase, myelodysplastic syndromes without excess blasts, and leukemia and lymphoma in remission. High refers to all other hematologic malignancies.
Preparative regimens and posttransplantation immunosuppressive regimens
Myeloablative conditioning regimens included targeted oral busulfan (4 mg/kg per day for 4 consecutive days) and intravenous cyclophosphamide (60 mg/kg per day for 2 consecutive days) (41%); cyclophosphamide (60 mg/kg per day for 2 consecutive days) followed by fractionated total body irradiation (TBI; 12 Gy) (34%); and other regimens (11%). Patients treated with these conditioning regimens were given a calcineurin inhibitor (cyclosporine [CSP] or tacrolimus) in combination with methotrexate (MTX) or mycophenolate mofetil (MMF) after the transplantation.5 Nonmyeloablative conditioning regimens (16%) included low-dose TBI (2-3 Gy) alone or in combination with fludarabine (30 mg/m2 body surface area/day, for 3 consecutive days). Patients treated with these conditioning regimens were given a calcineurin inhibitor (CSP or tacrolimus) in combination with MMF.19,20 Tapering schedules of immunosuppressive agents were modified at the discretion of the attending physicians for treatment of GVHD or management of persistent or recurrent malignancy.
Supportive care and infection definitions
Throughout the period encompassed by the retrospective review, fluconazole 400 mg/day was administered until day 75 as standard antifungal prophylaxis. Nine patients (3%) in the low-dose prednisone group and 13 patients (3%) in the standard-dose prednisone group began mold-active prophylaxis with voriconazole within 3 weeks after the transplantation at the discretion of the attending physician. In addition, 18 patients (5%) in the low-dose prednisone group and 19 patients (5%) in the standard-dose prednisone group participated in a randomized study voriconazole versus fluconazole for antifungal prophylaxis. Patients received levofloxacin prophylaxis when the absolute neutrophil count decreased below 500/μL.21 Patients who developed neutropenic fever were treated with ceftazidime or imipenem, with the addition of an aminoglycoside and vancomycin when clinically indicated. If fever persisted for greater than 96 hours despite broad-spectrum antibiotics, amphotericin B lipid preparations, an echinocandin, or voriconazole were administered until resolution of fever and neutropenia. Cytomegalovirus prevention consisted of weekly antigenemia surveillance and preemptive therapy with ganciclovir.22 Patients with upper respiratory symptoms had nasal wash testing by direct fluorescent antibodies, shell vial culture, and conventional culture.23
Data regarding invasive fungal infections (IFI) were obtained by chart review and prospective surveillance (started in 2002). Only IFI documented as proven or probable according to standardized definitions24 were included in the analysis.
GVHD grading and treatment
Acute GVHD was diagnosed and graded according to established criteria.2,25 Patients who presented with grade II acute GVHD before initial treatment were further categorized as having grade IIa manifestations (rash involving ≤ 50% of body surface area and stool volumes ≤ 1.0 L/day with or without anorexia, nausea, and vomiting and no liver involvement) or IIb manifestations (rash involving > 50% of body surface, stool volumes > 1.0 L/day, or any liver involvement) at onset.18
Systemic glucocorticoids were used for initial treatment, and decisions regarding the initial dose (prednisone-equivalent dose of 1 or 2 mg/kg per day) were made at the discretion of the attending physician. Some patients with gastrointestinal GVHD were given oral BDP, 8 mg daily, in combination with systemic glucocorticoids. In addition, administration of calcineurin inhibitors or MMF was usually continued at full doses. Glucocorticoid doses were tapered as manifestations of GVHD resolved with more rapid tapering if patients were receiving oral BDP. Decisions regarding the timing and choice of secondary therapy for patients with an unsatisfactory response after initial glucocorticoid treatment were made at the discretion of the attending physician. For patients treated initially with standard-dose prednisone, the typical sequence of secondary therapy was to add a nonglucocorticoid agent. In contrast, for patients treated initially with low-dose prednisone, the typical sequence was first to increase the prednisone-equivalent dose from 1 to 2 mg/kg per day. Patients who had an inadequate response after increasing the glucocorticoid dose were then treated with a nonglucocorticoid agent. In the current analysis, administration of agents used in the original prophylaxis regimen was not considered as secondary treatment.
Statistical analysis
Survival and progression-free survival were estimated using the Kaplan-Meier method. Cumulative incidence curves for relapse, nonrelapse mortality (NRM), infection, and secondary therapy were estimated by methods previously described.26 Unadjusted and adjusted hazard ratios (HR) for time-to-event end points were estimated by Cox regression, treating death and recurrent malignancy as competing events when appropriate. Unadjusted and adjusted odds ratios for binary end points (prolonged hospitalization) were estimated using logistic regression. Cumulative glucocorticoid dose was calculated by cumulatively summing the mean daily dose among patients in each group until the onset of recurrent or progressive malignancy, death, return to the care of the referring physician, or day 100 after starting therapy, whichever occurred first. P values are 2-sided and were not adjusted for multiple comparisons.
Results
Patient characteristics
Among the 733 patients who required systemic glucocorticoid therapy for acute GVHD during the study period, 347 (47%) were given low-dose glucocorticoids and 386 (53%) were given standard-dose glucocorticoids at the initiation of treatment (Table 1). The median ages of patients given low-dose and standard-dose glucocorticoids were 47 years (range, 18-71) and 46 years (range, 18-72), respectively. Compared with those given standard-dose glucocorticoids, patients given low-dose glucocorticoids had lower proportions of human leukocyte antigen (HLA)–mismatched (14 vs 23%) and unrelated donors (50 vs 65%), longer median intervals from transplantation to GVHD therapy (30 vs 21 days) and from onset of GVHD symptoms to initiation of treatment (2 vs 1 day), and a higher proportion of patients with grades I-IIa GVHD (78 vs 41%). Patients given low-dose glucocorticoids were also more likely to be given concurrent therapy with oral BDP than those given standard-dose glucocorticoids (39 vs 7%).
Cumulative glucocorticoid dose
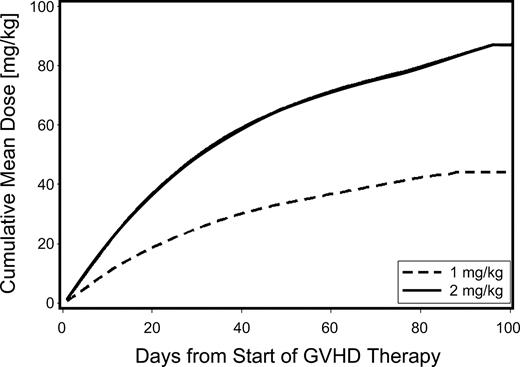
The mean cumulative prednisone-equivalent doses at day 100 after starting systemic immunosuppressive treatment for acute GVHD were 44 and 87 mg/kg for patients given low-dose and standard-dose glucocorticoids, respectively, representing a 48% reduction in cumulative dose for the low-dose group (Figure 1).
Cumulative use of glucocorticoids among patients given either low-dose glucocorticoids (prednisone-equivalent dose of 1 mg/kg per day; dashed line) or standard-dose glucocorticoids (prednisone-equivalent dose 2 mg/kg per day; solid line) for initial treatment of acute GVHD. In this analysis, follow-up was censored when patients returned to the care of the referring physicians.
Cumulative use of glucocorticoids among patients given either low-dose glucocorticoids (prednisone-equivalent dose of 1 mg/kg per day; dashed line) or standard-dose glucocorticoids (prednisone-equivalent dose 2 mg/kg per day; solid line) for initial treatment of acute GVHD. In this analysis, follow-up was censored when patients returned to the care of the referring physicians.
NRM, relapse, overall mortality and chronic GVHD according to initial glucocorticoid dose
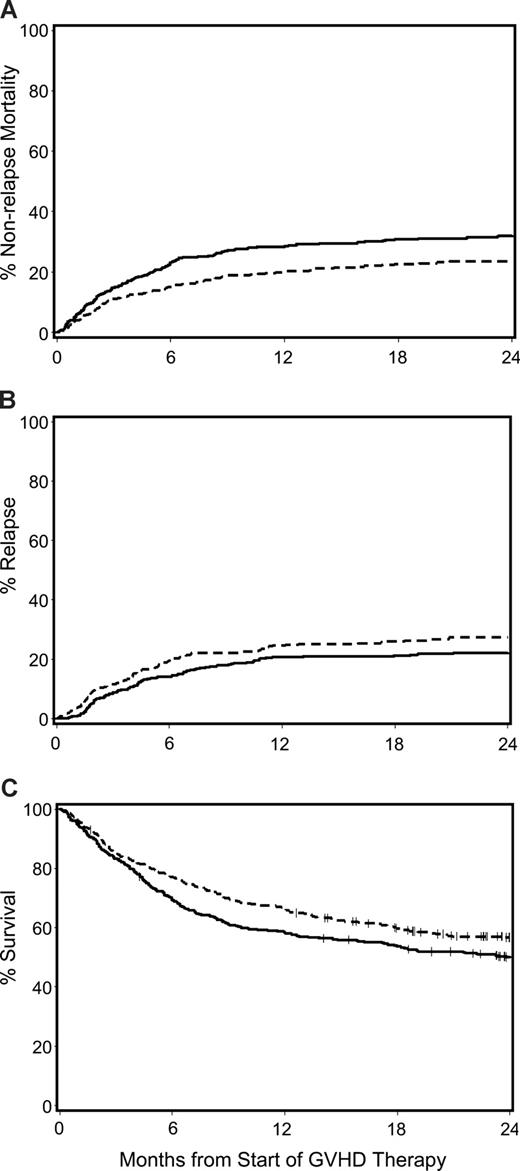
The unadjusted cumulative incidence of NRM, recurrent malignancy, and Kaplan-Meier estimates of overall survival for patients with acute GVHD given prednisone-equivalent doses of 1 mg/kg per day versus 2 mg/kg per day at the beginning of therapy are shown in Figure 2. After adjusting for GVHD grade at onset of treatment (I vs IIa vs IIB vs ≥ III), year of transplantation (continuous variable), patient age (continuous variable), donor-type (unrelated vs other), donor/recipient HLA-mismatch (any vs other) and sex-mismatch (female donor with male recipient vs other), conditioning intensity (myeloablative vs nonmyeloablative), use of tacrolimus as part of the GVHD prophylaxis, gut GVHD at onset (any vs none), concurrent use of BDP, and transplantation-to-treatment interval (continuous variable) in multivariate analysis, outcomes were not significantly different among patients initially treated with low-dose glucocorticoids compared with those treated with standard-dose glucocorticoids. The HR for NRM, recurrent or progressive malignancy, overall mortality and extensive chronic GVHD, respectively, were 1.06 (95% confidence interval [CI], 0.8-1.5), 1.22 (95% CI, 0.9-1.7), 1.10 (95% CI, 0.9-1.4), and 0.95 (95% CI, 0.8-1.2) (Table 2). Subgroup analyses of patients with grade IIb or greater acute GVHD and those with less than grade IIb acute GVHD at the beginning of glucocorticoid therapy did not change these conclusions.
Major transplantation outcomes for patients with acute GVHD given either low-dose glucocorticoids (prednisone-equivalent dose of 1 mg/kg per day; dashed line) or standard-dose glucocorticoids (prednisone-equivalent dose of 2 mg/kg per day; solid line) for initial treatment of acute GVHD. Cumulative incidence of NRM (A) and recurrent malignancy (B), and Kaplan-Meier estimates of overall survival (C).
Major transplantation outcomes for patients with acute GVHD given either low-dose glucocorticoids (prednisone-equivalent dose of 1 mg/kg per day; dashed line) or standard-dose glucocorticoids (prednisone-equivalent dose of 2 mg/kg per day; solid line) for initial treatment of acute GVHD. Cumulative incidence of NRM (A) and recurrent malignancy (B), and Kaplan-Meier estimates of overall survival (C).
Multivariate analysis of transplant outcomes according to prednisone-equivalent dose of glucocorticoids for initial treatment of acute GVHD
| . | 1 mg/kg per day versus 2 mg/kg per day . | |||||
|---|---|---|---|---|---|---|
| All patients . | Grades IIb-IV . | Grades I-IIa . | ||||
| HR (95% CI) . | P . | HR (95% CI) . | P . | HR (95% CI) . | P . | |
| Nonrelapse mortality | 1.06 (0.8-1.5) | .73 | 1.48 (0.9-2.3) | .09 | 0.82 (0.5-1.3) | .37 |
| Relapse | 1.22 (0.9-1.7) | .26 | 0.85 (0.5-1.6) | .60 | 1.54 (1.0-2.4) | .05 |
| Overall mortality | 1.10 (0.9-1.4) | .42 | 1.15 (0.8-1.7) | .46 | 1.07 (0.8-1.5) | .70 |
| Chronic GVHD | 0.95 (0.8-1.2) | .61 | 0.97 (0.7-1.4) | .88 | 0.95 (0.7-1.2) | .65 |
| . | 1 mg/kg per day versus 2 mg/kg per day . | |||||
|---|---|---|---|---|---|---|
| All patients . | Grades IIb-IV . | Grades I-IIa . | ||||
| HR (95% CI) . | P . | HR (95% CI) . | P . | HR (95% CI) . | P . | |
| Nonrelapse mortality | 1.06 (0.8-1.5) | .73 | 1.48 (0.9-2.3) | .09 | 0.82 (0.5-1.3) | .37 |
| Relapse | 1.22 (0.9-1.7) | .26 | 0.85 (0.5-1.6) | .60 | 1.54 (1.0-2.4) | .05 |
| Overall mortality | 1.10 (0.9-1.4) | .42 | 1.15 (0.8-1.7) | .46 | 1.07 (0.8-1.5) | .70 |
| Chronic GVHD | 0.95 (0.8-1.2) | .61 | 0.97 (0.7-1.4) | .88 | 0.95 (0.7-1.2) | .65 |
Adjusted for GVHD grade at onset of treatment (I vs IIa vs IIb vs ≥ III), gut GVHD at onset (any vs none), patient age (continuous variable), donor type (unrelated vs other), donor/recipient HLA mismatch (any vs other) and sex mismatch (female donor with male recipient vs other), conditioning intensity (myeloablative vs nonmyeloablative), use of tacrolimus as part of the GVHD prophylaxis, year of transplantation (continuous variable), concurrent use of BDP and systemic glucocorticoids, and transplantation-to-treatment interval (continuous variable).
Among patients with grade III acute GVHD at the beginning of glucocorticoid therapy, 42 of 62 (68%) treated with high-dose glucocorticoids and 7 of 9 (78%) treated with low-dose glucocorticoids had NRM. Five of the 9 patients who had grade III acute GVHD treated initially with low-dose glucocorticoids had documented or presumed infection at the onset of treatment. Multivariate hazards of NRM and overall survival were not significantly different between the 2 groups (P = .27 and P = .61, respectively). The small number of patients with grade III acute GVHD at the onset of glucocorticoid treatment, however, precludes definitive conclusions, and overall results may therefore not be applicable to this subgroup.
Secondary GVHD therapy according to initial glucocorticoid dose
In principle, the initiation of secondary therapy indicates progression or insufficient resolution of GVHD manifestations during primary therapy. For patients given standard-dose glucocorticoids as primary therapy, secondary therapy typically consisted of a nonglucocorticoid drug because further escalation of glucocorticoid doses has not been the standard practice at our institution. For patients given low-dose glucocorticoids as primary therapy, a dose escalation to a prednisone-equivalent dose of 2 mg/kg per day was one option. Among patients who had initial treatment with standard-dose glucocorticoids, 22% received secondary therapy (Figure 3). Among patients who had initial treatment with low-dose glucocorticoids, 16% had the prednisone-equivalent dose increased from 1 mg/kg per day to 2 mg/kg per day, and an additional 7% received secondary therapy with other agents. At 12 months after starting secondary therapy, the cumulative incidence of NRM was 49% (95% CI, 37%-60%) among patients in the low-dose group (n = 79) and 58% (95% CI, 47%-69%) among those in the standard-dose group (n = 84). The adjusted HR for NRM after starting secondary therapy for the low-dose group was 0.69 (95% CI, 0.4-1.1) compared with the standard-dose group.
Cumulative incidence of secondary therapy among patients with acute GVHD initially given either low-dose glucocorticoids (prednisone-equivalent dose of 1 mg/kg per day) or standard-dose glucocorticoids (prednisone-equivalent dose of 2 mg/kg per day). (Left panel) Nonglucocorticoid secondary therapy. (Right panel) Increased glucocorticoid dose or nonglucocorticoid secondary therapy.
Cumulative incidence of secondary therapy among patients with acute GVHD initially given either low-dose glucocorticoids (prednisone-equivalent dose of 1 mg/kg per day) or standard-dose glucocorticoids (prednisone-equivalent dose of 2 mg/kg per day). (Left panel) Nonglucocorticoid secondary therapy. (Right panel) Increased glucocorticoid dose or nonglucocorticoid secondary therapy.
Risk of infection according to initial glucocorticoid dose
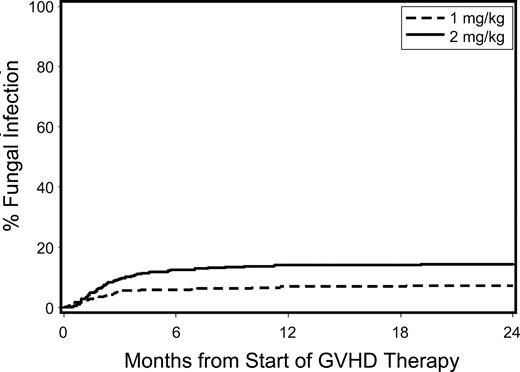
The unadjusted cumulative incidence of invasive fungal infections for patients with acute GVHD treated with low-dose versus standard-dose glucocorticoids is shown in Figure 4. Patients given low-dose treatment showed trends suggesting lower hazards of invasive fungal infection (HR, 0.59; 95% CI, 0.3-1.0) and bacteremia with Gram-positive organisms (HR, 0.78; 95% CI, 0.6-1.0), respectively, than those given standard-dose treatment (Table 3). We found no statistically significant differences in the risks of bacteremia with Gram-negative organisms, cytomegalovirus reactivation or disease, respiratory virus infections, and Epstein-Barr virus infections between the 2 groups.
Cumulative incidence of invasive fungal infections among patients given either low-dose glucocorticoids (prednisone-equivalent dose of 1 mg/kg per day; dashed line) or standard-dose glucocorticoids (prednisone-equivalent dose of 2 mg/kg per day; solid line) for initial treatment of acute GVHD.
Cumulative incidence of invasive fungal infections among patients given either low-dose glucocorticoids (prednisone-equivalent dose of 1 mg/kg per day; dashed line) or standard-dose glucocorticoids (prednisone-equivalent dose of 2 mg/kg per day; solid line) for initial treatment of acute GVHD.
Multivariate analysis of infections according to prednisone-equivalent dose of glucocorticoids for initial treatment of acute GVHD
| Type of infection . | 1 mg/kg per day versus 2 mg/kg per day . | |
|---|---|---|
| HR (95% CI) . | P . | |
| Invasive fungal (n = 82) | 0.59 (0.3-1.0) | .06 |
| Gram-positive (n = 291) | 0.71 (0.5-1.0) | .07 |
| Gram-negative (n = 61) | 1.55 (0.8-2.9) | .15 |
| CMV reactivation (n = 288) | 1.06 (0.8-1.4) | .69 |
| CMV disease (n = 38) | 0.68 (0.3-1.5) | .35 |
| Respiratory viruses (n = 66) | 1.21 (0.7-2.1) | .52 |
| EBV (n = 31) | 0.55 (0.2-1.4) | .21 |
| Type of infection . | 1 mg/kg per day versus 2 mg/kg per day . | |
|---|---|---|
| HR (95% CI) . | P . | |
| Invasive fungal (n = 82) | 0.59 (0.3-1.0) | .06 |
| Gram-positive (n = 291) | 0.71 (0.5-1.0) | .07 |
| Gram-negative (n = 61) | 1.55 (0.8-2.9) | .15 |
| CMV reactivation (n = 288) | 1.06 (0.8-1.4) | .69 |
| CMV disease (n = 38) | 0.68 (0.3-1.5) | .35 |
| Respiratory viruses (n = 66) | 1.21 (0.7-2.1) | .52 |
| EBV (n = 31) | 0.55 (0.2-1.4) | .21 |
Adjusted for GVHD grade at onset of treatment (I vs IIa vs IIb vs ≥ III), gut GVHD at onset (any vs none), patient age (continuous variable), donor type (unrelated versus other), donor/recipient HLA mismatch (any vs other) and sex mismatch (female donor with male recipient vs other), conditioning intensity (myeloablative vs nonmyeloablative), use of tacrolimus as part of the GVHD prophylaxis, year of transplantation (continuous variable), concurrent use of BDP and systemic glucocorticoids, and transplantation-to-treatment interval (continuous variable).
CMV indicates cytomegalovirus; and EBV, Epstein-Barr virus.
Duration of hospitalization according to initial glucocorticoid dose
Patients given initial treatment with low-dose and standard-dose glucocorticoids were hospitalized for a median of 2 (range, 0-144) and 10 (range, 0-183) days, respectively. Overall, 432 patients were hospitalized at the initiation of glucocorticoid treatment. Among these patients, 268 (69%) received standard-dose glucocorticoids and 164 (47%) received low-dose glucocorticoids. Subsequently, 43% of patients required hospitalization for more than 7 consecutive or nonconsecutive days after beginning glucocorticoid therapy for acute GVHD. In a multivariate analysis that included adjustment for “hospitalization at the initiation of glucocorticoid treatment,” the likelihood of hospitalization for more than 7 days was reduced by 38% in the group given low-dose glucocorticoids (odds ratio [OR], 0.62; 95% CI, 0.4-0.9) compared with the group given standard-dose glucocorticoids. The following additional factors were associated with hospitalization for more than 7 days: time to first GVHD therapy (OR, 0.81; 95% CI, 0.7-0.9; per week delayed), unrelated donor versus other donor (OR, 1.60; 95% CI, 1.1-2.3), and acute GVHD grade III/IV versus grade I/IIa at onset (OR, 4.94; 95% CI, 2.5-9.6). The following factors were not associated with hospitalization for more than 7 days: patient age, donor/recipient HLA-mismatch, donor sex, grade IIb acute GVHD at onset, and prophylactic use of ursodeoxycholic acid or BDP.
Discussion
This retrospective analysis of outcomes among 733 allograft recipients with newly diagnosed acute GVHD showed that, compared with patients given standard-dose systemic glucocorticoids as initial treatment, those given low-dose glucocorticoids had (1) nearly 50% lower cumulative glucocorticoid dose after 100 days of treatment; (2) similar risks of NRM, recurrent malignancy, overall mortality, and chronic GVHD; (3) nearly 50% reduction in the cumulative incidence of invasive fungal infections; and (4) a one-third reduction in the risk of hospitalization for more than 7 days. We found overall no evidence in the data that starting therapy with a lower than standard dose of glucocorticoids led to worse patient outcomes. None of the conclusions from this analysis changed when the evaluation was limited to patients with grade II GVHD at the onset of steroid treatment (see Tables S1,S2 and Figures S1Figure S2. Cumulative incidence of secondary therapy among patients with grade II acute GVHD initially given either “low-dose” glucocorticoids (prednisone-equivalent dose of 1 mg/kg/day) or “standard-dose” glucocorticoids (prednisone-equivalent dose of 2 mg/kg/day) for treatment of acute GVHD (JPG, 52.9 KB)–S3, available on the Blood website; see the Supplemental Materials link at the top of the online article), but the relatively small number of patients who presented with grade III or greater acute GVHD at the onset of glucocorticoid treatment precludes definitive conclusions for this subgroup.
Our analysis was prompted by the hypothesis that the majority of patients who present with newly diagnosed acute GVHD can be managed effectively and safely by initiating treatment with low-dose systemic glucocorticoids, reserving the option of a dose increase for patients who have an unsatisfactory response or progressive symptoms. Our analysis among patients treated at a single institution was possible because, over the past several years, low-dose glucocorticoids have increasingly been used as initial treatment of acute GVHD, even in patients with more severe manifestations at the time of presentation. It appears that this practice was influenced by a favorable experience with low-dose glucocorticoids as initial treatment for patients with mild to moderate GVHD, particularly among patients who received concurrent treatment with oral BDP for management of upper gastrointestinal manifestations.17,18 During our 5-year study period, low-dose glucocorticoids were used in 25% of patients who presented with grades IIb-IV GVHD, while high-dose glucocorticoids were used in 37% of patients who presented with less severe grades I-IIa GVHD.
Taken together, the similar incidence of secondary therapy and the absence of statistically significant differences in survival, NRM, risk of recurrent or progressive malignancy, or cumulative incidence of chronic GVHD, even after analyzing subgroups of patients according to GVHD severity at the time of treatment initiation, indicate that initial treatment with low-dose glucocorticoids adequately controls GVHD manifestations in most patients. Moreover, the adjusted risk of NRM among patients who started treatment with low-dose glucocorticoids and then required a glucocorticoid dose-escalation or nonglucocorticoid secondary therapy was not significantly different from that among patients who started treatment with high-dose glucocorticoids and required nonglucocorticoid secondary therapy.
At 100 days after the start of glucocorticoid therapy, the cumulative glucocorticoid dose was nearly 50% lower in the group initially given low-dose glucocorticoids compared with the group initially given standard-dose glucocorticoids. This result shows that the low-dose treatment approach could be sustained during at least the first 3 months of therapy and did not prompt an excessive risk of dose escalations because of insufficient control or progression of GVHD manifestations. Not surprisingly, decreased exposure to glucocorticoids translated into a nearly 50% reduction in the risk of invasive fungal infection and a nearly 30% reduction in the risk of Gram-positive bacteremia. The overall low incidence of these infections and their successful treatment with antifungal and antibiotic medications might explain why the reduced incidence of these infections did not improve survival among patients treated with low-dose glucocorticoids.27
In addition to profound immunosuppression with associated risks of infection, exposure to glucocorticoids may contribute to overall morbidity and impairment of quality of life by causing hyperglycemia, hypertension, myopathy, avascular necrosis, osteoporosis, and neuropsychologic alterations, among other complications. Glucocorticoid toxicity is related to both the average dose and cumulative duration of treatment, although for most toxicities a threshold dose or duration has not been established. Even though comprehensive assessment of glucocorticoid-related morbidity was beyond the scope of our retrospective study, we found that patients given low-dose glucocorticoids as initial therapy had a one-third lower likelihood of hospitalization for more than 7 days compared with those given high-dose glucocorticoids.
As with any retrospective analysis, our study, which included only adult patients, has important limitations and is therefore best viewed as hypothesis-generating. Certain clinical findings that might have influenced decisions to use either low-dose or high-dose glucocorticoids as initial treatment could not be taken into account. For example, it is likely that physicians considered not only the severity of organ involvement at the onset of treatment, but also the rapidity of symptom progression before treatment was started. We suspect that high-dose glucocorticoids were used preferentially in patients who had a more fulminant onset of GVHD. Conversely, some patients with more severe GVHD symptoms at presentation were treated with lower doses of glucocorticoids if they had contraindications such as presumed or documented infections. In our cohort, only a small number of patients who presented with grade III acute GVHD received low-dose glucocorticoids as initial treatment. Therefore, our data cannot adequately address whether initial treatment with low-dose glucocorticoids is adequate for patients with greater than or equal to grade III acute GVHD.
Thus, only a prospective trial can resolve the uncertainties resulting from the complexity of clinical decision-making and the variation in physician practices. The statistical significance of associations between glucocorticoid dose and risks of infection and duration of hospitalization should be interpreted with caution, because no adjustments were made for multiple comparisons. Nonetheless, these results are plausible, because previous studies have demonstrated associations between cumulative steroid dose and increased risk of infections.28,29
In conclusion, at least for patients who present with grade I-II acute GVHD, initial treatment with a prednisone-equivalent dose of 1 mg/kg per day did not compromise GVHD-control or mortality and was associated with decreased risks of invasive fungal infections and duration of hospitalization. Our data provide support for the concept that initial treatment with low-dose glucocorticoids reduces overall glucocorticoid exposure and the consequent toxicities among patients who have clinical improvement. Outcomes after secondary therapy were similar in the 2 groups, suggesting that initial treatment with low-dose glucocorticoids does not compromise the ability to control the disease when the initial treatment is not successful. A more comprehensive description of factors that potentially influence the choice of the appropriate glucocorticoid dose, a better assessment of nonfatal glucocorticoid-related complications and the definition of the lowest effective glucocorticoid dose for initial treatment of acute GVHD should be addressed in prospective trials.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Gary Schoch and Linda Glockling for data management support, the staff at the Long-Term Follow-Up Department for their invaluable assistance with data collection, and Helen Crawford and Bonnie Larson for typing the manuscript. We are also very thankful for the excellent care provided to patients and families by the inpatient and outpatient physicians, nursing teams, and support staff at the Fred Hutchinson Cancer Research Center and at the University of Washington Medical Center.
This work was supported in part by grant nos. CA78902, CA18221, CA18029, CA15704, HL36444, CA92058, and CA09515 from the National Institutes of Health (NIH), Department of Health and Human Services (DHHS), Bethesda, MD. M.M. was also supported by a Dana Foundation grant in Human Immunology.
National Institutes of Health
Authorship
Contribution: M.M. designed the study, extracted and analyzed data, and wrote the manuscript; P.J.M. helped design the study, analyzed data and edited the manuscript; B.E.S. conducted the statistical analysis; M.B. and K.A.M. provided and analyzed data pertaining to infectious disease end points and edited the manuscript; T.F. helped with data extraction and edited the manuscript; and M.M., P.J.M., P.C., G.B.M., J.D., R.N., M.E.D.F., K.D., S.L., R.S., and F.R.A. served as GVHD attending physicians and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Marco Mielcarek, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, D1-100, Seattle, WA 98109-1024; e-mail: mielcar@fhcrc.org.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal