Abstract
To determine whether inhibition of Syk would be useful in FcγR-dependent cytopenias such as immune thrombocytopenic purpura (ITP) or autoimmune hemolytic anemia, mouse models were used to evaluate efficacy of R406, an inhibitor of Syk function, in treating cytopenia. Both disease models responded favorably to treatment, with amelioration of ITP being more dramatic. Thus, phase 2 clinical trial was initiated to study the effects of Syk inhibition in humans with ITP. Sixteen adults with chronic ITP were entered into an open-label, single-arm cohort dose-escalation trial beginning with 75 mg and escalating as high as 175 mg twice daily. Doses were increased until a persistent response was seen, toxicity occurred, or 175 mg twice daily was reached. Eight patients achieved persistent responses with platelet counts greater than 50 × 109/L (50 000 mm3) on more than 67% (actually 95%) of their study visits, including 3 who had not persistently responded to thrombopoietic agents. Four others had nonsustained responses. Mean peak platelet count exceeded 100 × 109/L (100 000 mm3) in these 12 patients. Toxicity was primarily GI-related with diarrhea (urgency) and vomiting; 2 patients had transaminitis. In conclusion, inhibition of Syk was an efficacious means of increasing and maintaining the platelet count in half the patients with chronic refractory ITP. (ClinicalTrials.gov, no. NCT00706342).
Introduction
Immune thrombocytopenic purpura (ITP) is an autoimmune disorder characterized by antibody-mediated destruction of platelets. Patients with ITP have accelerated clearance of circulating IgG-coated platelets via Fcγ receptor (FcγR)–bearing macrophages in the spleen and liver, leading to different levels of thrombocytopenia and variable degrees of mucocutaneous bleeding.1 Recent studies of the pathophysiology of ITP have suggested that decreased platelet production may also be an important component of the thrombocytopenia.2,3 This reduction in platelet production is thought to be mediated by the antibodies that destroy the platelets having a similar effect on megakaryocytes.3,4
Many patients exhibit responses to established therapies, including corticosteroids, intravenous immunoglobulin (IVIg), anti-D, splenectomy, and rituximab. Nonetheless, there are a significant minority of patients who retain persistently low platelet counts despite treatment. They consequently remain at risk of intracranial hemorrhage as well as other bleeding complications.
The number of medications currently being studied for their ability to safely and stably increase the platelet count in patients with ITP is a testament to the current unmet need for better treatments in these patients. For example, several novel thrombopoietic agents have each shown promise in ameliorating thrombocytopenia in some patients with ITP.5,6
Syk is a protein tyrosine kinase that associates with the FcγR in various inflammatory cells, including macrophages, which are thought to be the cells responsible for platelet clearance in ITP. Binding of FcγRs I, IIA, and IIIA to their ligands induces activation of the receptor complex and phosphorylation of the immunoreceptor-activating motifs (ITAMs) with the downstream recruitment and activation of Syk. This in turn leads to activation of various genes, resulting in a cytoskeletal rearrangement that then mediates phagocytosis in cells of the monocyte/macrophage lineage. Given its central role in FcγR-mediated signal transduction and propagation of the inflammatory response, Syk has been considered a reasonable target for inhibition in various autoimmune and malignant conditions, including rheumatoid arthritis and lymphoma.7,8
The underlying hypothesis of this study is that blocking FcγR signaling by inhibiting Syk would ameliorate platelet destruction in patients with ITP. R788 (R935788), a small molecule prodrug of the biologically active R406, is a potent and relatively selective, orally available inhibitor of Syk.9 In animal models, treatment with R406/R788 was shown to be safe10 and effective in reducing inflammation and joint damage in immune-mediated rheumatoid arthritis.11
This study shows that in mice injected with an antibody to integrin αIIb, a murine model of ITP, pretreatment with R788 was protective against the development of thrombocytopenia. In addition, mice injected with an anti–red cell antibody as a model of autoimmune hemolytic anemia (AHA) also successfully responded to treatment. Because initial human studies have shown that R788 has good oral bioavailability, has biologic activity, is well tolerated, and does not inhibit collagen or ADP-induced platelet aggregation,9 the efficacy of R788 in treating adults with ITP was explored, and these results are reported here in the first pilot study of the safety and efficacy of R788 treatment in a cohort of adult patients with chronic refractory ITP.
Methods
Induction and treatment of murine cytopenias
Thrombocytopenia.
Mice received the indicated doses (in 0.25 mL) of the R406 prodrug R788 or vehicle (0.1% carboxymethylcellulose, 0.1% methylparaben–0.02% propylparaben–99.78% water) once on the first day, then twice (∼8 hours apart) on the following day by the oral route with an 18-gauge ball-tipped feeding needle. For the IVIg group, mice were injected intraperitoneally with approximately 2 g/kg IVIg (Gamimune 10%; Bayer, Elkhart, IL) once on the first day only. On the second day, mice were injected with 2 μg anti-CD41 antibody (MWReg30; PharMingen, Mississauga, ON) in 200 μL PBS. Mice were bled the following day for platelet enumeration by flow cytometry as previously described.12
Anemia.
Mice were treated as described in “Thrombocytopenia” with the following exception: mice received 50 μg anti-Ly76 (TER-119; PharMingen) in 200 μL PBS on the second day instead of anti-CD41. The use of mice in this study was approved by the St Michael's Hospital Animal Care Committee.
Patient eligibility
Sixteen male and female patients 18 years of age or older with chronic refractory ITP were enrolled at a single center in the United States in an open-label fashion. The study protocol was approved by the Human Subjects Committee at Weill Cornell Medical Center, and all patients gave written informed consent before enrollment in accordance with the Declaration of Helsinki. According to study criteria, chronic refractory ITP was defined as platelet count of less than 30 × 109/L (30 000/mm3) consistently for at least 3 months, except for transient, nonsustained responses to various therapeutic regimens. All patients had to have at least 3 separate documented platelet counts of less than 30 × 109/L (30 000/mm3), with at least one extending back to 3 months or more before patient entry into the study. Patients must have tried at least 2 typical regimens for the treatment of ITP.13-15 The following conditions were excluded either by history or, if necessary, by laboratory investigation: HIV, HBV, or HCV infection; lymphoproliferative disorders, myelodysplasia, systemic lupus erythematosus, drug-induced thrombocytopenia, and hypoglobulinemia or dysglobulinemia. In addition, patients with a history of any of the following were excluded: substance abuse, alcoholism or drug addiction, use of any investigational drug within 3 months before the first dose, transfusion with blood or blood products within 2 weeks before first dosing, and increase in the dose of, or added, treatment of ITP, that is, prednisone, within 2 weeks before first dosing. All patients were free of significant infection, inflammatory process, and acute gastrointestinal (GI) symptoms (nausea, vomiting, diarrhea) at the time of screening. Required laboratory values included a leukocyte count greater than 2.5 × 109/L (2500/mm3), neutrophil count greater than 1.8 × 109/L (1800/mm3), lymphocyte count greater than 0.75 ×109/L (750/mm3), hemoglobin greater than 100 g/L (10 g/dL), and transaminase levels (alanine aminotransferase [ALT], aspartate aminotransferase [AST]) less than 1.5 times the upper limit of normal.
Study drugs
R788 was supplied as white to off-white plain oval tablets in 2 tablet strengths, 25 mg and 100 mg. In addition to the active agent, each tablet contained microcrystalline cellulose, sodium starch glycolate, copovidone, and magnesium stearate. The appropriate number of tablets was dispensed at each study visit to ensure continuous dosing during the 1- to 4-week interval period between visits. Patients returned unused tablets at each study visit to be counted to monitor compliance.
Drug dosing
Patients were enrolled in cohorts of 3 to 4 and treated with escalating doses of R788. After a cohort completed 4 weeks, a subsequent cohort at a dose 25 mg higher could be initiated. Dosing was initiated at 75 mg to 150 mg orally twice daily 12 hours apart. Patients were required to complete a minimum of 2 weeks at a given dose before the dose could be increased, in increments of 25 mg twice daily. Patients who responded and then had their platelet counts decline, or else did not respond well initially, could have their dose increased, to a maximum of 175 mg twice daily. An independent data safety monitor reviewed all the safety and efficacy data before patients were enrolled in a higher dose cohort.
Study procedures
Patients were screened at an initial study visit up to 28 days before the first dose of the study drug. Initial evaluation included viral screen (HIV, HBV, and HCV serologic tests), urine pregnancy test (for women of childbearing potential), complete blood cell (CBC) count, prothrombin time/partial thromboplastin time, serum chemistry, urinalysis, and 12-lead ECG.
On day 1 of treatment, before the first dose of the study drug, baseline values were established for vital signs, CBC count, urinalysis, and liver function tests (LFTs), including ALT, AST, alkaline phosphatase, and bilirubin. Patients were followed weekly for the first 7 weeks of study, with vital signs, CBC count, and LFTs performed at every visit. Adverse events were also recorded at each study visit. A responder assessment was completed on each visit. After completion of 7 weeks of treatment, further visits were scheduled every 1 to 4 weeks, depending on the response status and clinical needs of each patient.
Statistical analysis
Response was defined before study start as an increase in platelet count by greater than 20 × 109/L (20 000/mm3) from baseline to at least 30 × 109/L (30 000/mm3) with no rescue treatment, for example, with IVIg, in the preceding 2 weeks. Baseline was defined as the lowest platelet count within 1 month of the first dose of the study drug. A sustained platelet response was defined post hoc as a platelet count increase by greater than 20 × 109/L (20 000/mm3) to at least 30 × 109/L (30 000/mm3) on at least 66% of study visits. Data are summarized with the use of descriptive statistics. When noted, values before and after are compared with the use of the Student t test, with an α value of .05.
Results
Successful amelioration of murine cytopenia models by inhibition of Syk
ITP.
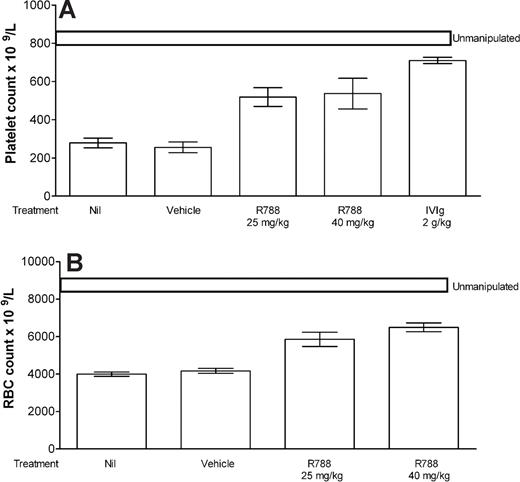
With the use of a well-described murine model of ITP,12 we tested the ability of R788 (the prodrug of R406) to ameliorate thrombocytopenia. Mice injected with an antibody directed to integrin αIIb were profoundly thrombocytopenic when platelets were enumerated 24 hours after injection (Figure 1A column 1). However, mice pretreated with 25 or 40 mg/kg R788 were protected from thrombocytopenia (Figure 1A columns 3 and 4, respectively; P < .05 for both compared with Nil). Mice treated with IVIg responded as expected (Figure 1A column 5), whereas those pretreated with the vehicle alone (Figure 1A column 2) displayed no protection, that is, profound thrombocytopenia.
Inhibition of Syk prevents murine antibody-mediated cytopenias. (A) CD1 mice were pretreated with nothing (Nil), vehicle, R788 (at the dose indicated), or IVIg on day 1. Mice in the vehicle and R788 groups were injected again (8 hours apart) on day 2. All mice except for the unmanipulated group were injected with antiplatelet antibody on day 2. Mice were bled on day 3 for platelet counting as detailed in “Methods.” n = 6 mice per group. (B) Mice were treated as in panel A, except that anemia was induced by injection of antierythrocyte (TER119) antibody. All data are expressed as mean plus or minus SEM (n = 6 mice per group).
Inhibition of Syk prevents murine antibody-mediated cytopenias. (A) CD1 mice were pretreated with nothing (Nil), vehicle, R788 (at the dose indicated), or IVIg on day 1. Mice in the vehicle and R788 groups were injected again (8 hours apart) on day 2. All mice except for the unmanipulated group were injected with antiplatelet antibody on day 2. Mice were bled on day 3 for platelet counting as detailed in “Methods.” n = 6 mice per group. (B) Mice were treated as in panel A, except that anemia was induced by injection of antierythrocyte (TER119) antibody. All data are expressed as mean plus or minus SEM (n = 6 mice per group).
AHA.
To determine whether the inhibition of Syk could ameliorate a different cytopenia, a mouse model of AHA was used. Mice were treated as described in “ITP” with the following exception: an antibody directed to red cells was used instead of an antiplatelet antibody. Mice injected with the anti–red cell antibody displayed anemia 24 hours after injection (Figure 1B column 1). Mice pretreated with 25 or 40 mg/kg R788 were significantly protected from anemia (Figure 1B columns 3 and 4, respectively; P < .05 for both compared with Nil). Mice pretreated with the vehicle alone displayed no protection (Figure 1B column 2).
Patients and disposition
Sixteen patients, 10 of whom were women, and ranging from 31 to 81 years of age with 5 older than 70 years, enrolled in this study (Table 1; Figure 2A). Baseline platelet counts ranged from 3 × 109/L (3000/mm3) to 28 × 109/L (28 000/mm3). The mean number of prior ITP treatments was 5, of which the most common were corticosteroids (100% of patients), IVIg (93%), rituximab (88%), and splenectomy (69%). Only 1 patient had received as few as 2 prior treatments of ITP and 5 patients (31%) had failed treatment with a thrombopoietic agent.
Baseline characteristics for 16 enrolled patients
| Baseline characteristic . | Value . |
|---|---|
| Median age, y (range) | 66 (31-81) |
| Sex, n, male/female | 6/10 |
| Race, n | |
| White | 13 |
| Black | 0 |
| Hispanic or Latino | 1 |
| Asian | 1 |
| Other | 1 |
| Median duration of ITP, y (range) | 9 (1 to ≥ 29) |
| Comorbidities | |
| n, median | 3 |
| At least 3 comorbidities, n (%) | 8 (50) |
| History of ≥ 3 prior treatments, n (%) | 15 (94) |
| Prior treatments, n (%) | |
| Steroids | 16 (100) |
| IVIg | 15 (94) |
| Rituximab | 14 (88) |
| Splenectomy | 11 (69) |
| Anti-D | 9 (56) |
| Danazol | 8 (50) |
| Azathioprine | 6 (38) |
| Thrombopoietic agents | 5 (31) |
| Vincristine | 5 (31) |
| GMA161 | 3 (19) |
| Anti-CD40L | 2 (13) |
| Helicobacter pylori | 1 (6) |
| Median baseline platelet count, n × 109/L, (range) | 16 (2-28) |
| Baseline characteristic . | Value . |
|---|---|
| Median age, y (range) | 66 (31-81) |
| Sex, n, male/female | 6/10 |
| Race, n | |
| White | 13 |
| Black | 0 |
| Hispanic or Latino | 1 |
| Asian | 1 |
| Other | 1 |
| Median duration of ITP, y (range) | 9 (1 to ≥ 29) |
| Comorbidities | |
| n, median | 3 |
| At least 3 comorbidities, n (%) | 8 (50) |
| History of ≥ 3 prior treatments, n (%) | 15 (94) |
| Prior treatments, n (%) | |
| Steroids | 16 (100) |
| IVIg | 15 (94) |
| Rituximab | 14 (88) |
| Splenectomy | 11 (69) |
| Anti-D | 9 (56) |
| Danazol | 8 (50) |
| Azathioprine | 6 (38) |
| Thrombopoietic agents | 5 (31) |
| Vincristine | 5 (31) |
| GMA161 | 3 (19) |
| Anti-CD40L | 2 (13) |
| Helicobacter pylori | 1 (6) |
| Median baseline platelet count, n × 109/L, (range) | 16 (2-28) |
Baseline platelet count is defined as lowest count within 1 month of starting treatment. The most common comorbidities included hypertension (n = 5), dyslipidemia (n = 3), arthritis (n = 3), and diabetes (n = 3).
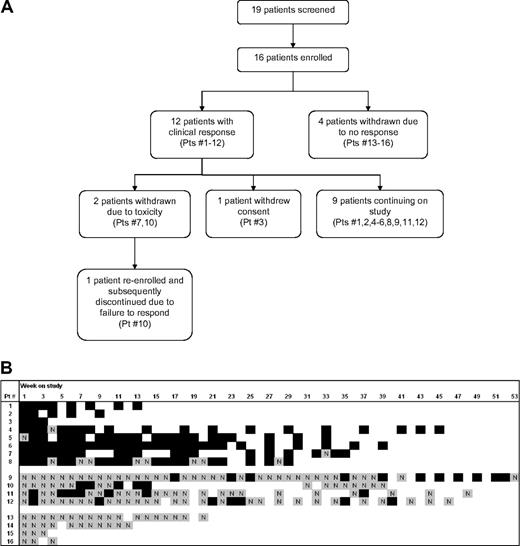
Study flowchart and patient response. (A) Study flowchart, with all patients enrolled in study as of January 31, 2008. Patient numbers correspond to panel B. (B) Graphical representation of response for each study patient at each study visit. At each visit every patient was classified as either responder (Y, black) or nonresponder (N, gray). Response was defined as platelet count increased by at least 20 × 109/L (20 000/mm3) to greater than 30 × 109/L (30 000/mm3) with no IVIg treatment in the preceding 2 weeks. Blank space indicates no study visit in a given week.
Study flowchart and patient response. (A) Study flowchart, with all patients enrolled in study as of January 31, 2008. Patient numbers correspond to panel B. (B) Graphical representation of response for each study patient at each study visit. At each visit every patient was classified as either responder (Y, black) or nonresponder (N, gray). Response was defined as platelet count increased by at least 20 × 109/L (20 000/mm3) to greater than 30 × 109/L (30 000/mm3) with no IVIg treatment in the preceding 2 weeks. Blank space indicates no study visit in a given week.
All patients completed at least 3 weeks on study. As of January 31, 2008, the longest duration of (ongoing) study treatment was 53 weeks, and the average duration on treatment for the 9 active patients was 36 weeks (Figure 2B). Four patients were taken off study after 3 to 20 weeks because of failure to respond. Three patients withdrew because of concerns about toxicity.
Efficacy
The platelet response at each study visit for each patient enrolled in the study is summarized in Figure 2B. For the 12 patients who responded to R788, the median platelet count increased from 16 × 109/L (16 000/mm3) at baseline to a median peak of 105 × 109/L (105 000/mm3) while on the study drug. Twelve patients (75%) achieved at least one substantial platelet increase (response) which was sustained in 8 patients (50%), as evidenced by increased platelet counts on at least 67% of occasions, reduced need for IVIg treatment, or tapering of steroids while on the study drug (Table 1).
Sustained responders.
Eight patients (50%) maintained a platelet response (Table 2) at a median R788 dose of 125 mg twice daily, requiring one or no dose increases to maintain platelet counts above 30 × 109/L (30 000/mm3). They maintained platelet counts above 50 × 109/L (50 000/mm3) on a median 95% of study visits (Table 2) and were thus able to avoid other therapy while on R788. The median peak platelet count for the 8 patients who developed sustained platelet responses on R788 was 188 × 109/L (188 000/mm3). All patients, as indicated in Table 1, had been on multiple prior therapies. Two patients with a sustained response (and one with a transient response) to R788 had previously failed treatment with a thrombopoietic agent.
Platelet response of enrolled patients
| Patient ID . | Visits as responder, %* . | Visits with plt count > 30 × 109/L, % . | Visits with plt count > 50 × 109/L, % . | Visits IVIg given, % . | Baseline plt count, ×109/L† . | Max plt count, ×109/L‡ . | Med plt count, ×109/L§ . | No. of dose changes . | Steroids tapered‖ . |
|---|---|---|---|---|---|---|---|---|---|
| Maintained response¶ | |||||||||
| 1 | 100 | 100 | 100 | 0 | 6 | 458 | 216 | 0 | No |
| 2 | 100 | 100 | 100 | 0 | 12 | 154 | 100 | 0 | Yes |
| 3 | 100 | 100 | 100 | 0 | 22 | 159 | 106 | 1 | Yes |
| 4 | 95 | 100 | 95 | 0 | 22 | 190 | 104 | 1 | Yes |
| 5 | 94 | 100 | 94 | 0 | 16 | 197 | 127 | 0 | Yes |
| 6 | 91 | 95 | 95 | 5 | 2 | 394 | 317 | 0 | No |
| 7 | 77 | 96 | 58 | 0 | 20 | 111 | 55 | 0 | No |
| 8 | 68 | 68 | 45 | 0 | 11 | 186 | 43 | 1 | No |
| Median | 91 | 96 | 94 | 0 | 16 | 190 | 104 | 0 | |
| Nonmaintained response# | |||||||||
| 9 | 23 | 32 | 17 | 4 | 12 | 195 | 23 | 4 | No |
| 10 | 25 | 45 | 35 | 20 | 6 | 98 | 14 | 4 | Yes |
| 11 | 24 | 72 | 24 | 0 | 28 | 158 | 36 | 4 | No |
| 12 | 20 | 49 | 14 | 0 | 22 | 222 | 27 | 4 | No |
| Median | 24 | 47 | 21 | 2 | 17 | 177 | 25 | 4 | |
| No response** | |||||||||
| 13 | 0 | 6 | 0 | 78 | 11 | 16 | 10 | 4 | No |
| 14 | 0 | 0 | 0 | 73 | 18 | 13 | 12 | 2 | No |
| 15 | 0 | 0 | 0 | 0 | 16 | 15 | 14 | 0 | No |
| 16 | 0 | 0 | 0 | 25 | 3 | 13 | 9 | 0 | No |
| Median | 0 | 0 | 0 | 49 | 14 | 14 | 11 | 1 |
| Patient ID . | Visits as responder, %* . | Visits with plt count > 30 × 109/L, % . | Visits with plt count > 50 × 109/L, % . | Visits IVIg given, % . | Baseline plt count, ×109/L† . | Max plt count, ×109/L‡ . | Med plt count, ×109/L§ . | No. of dose changes . | Steroids tapered‖ . |
|---|---|---|---|---|---|---|---|---|---|
| Maintained response¶ | |||||||||
| 1 | 100 | 100 | 100 | 0 | 6 | 458 | 216 | 0 | No |
| 2 | 100 | 100 | 100 | 0 | 12 | 154 | 100 | 0 | Yes |
| 3 | 100 | 100 | 100 | 0 | 22 | 159 | 106 | 1 | Yes |
| 4 | 95 | 100 | 95 | 0 | 22 | 190 | 104 | 1 | Yes |
| 5 | 94 | 100 | 94 | 0 | 16 | 197 | 127 | 0 | Yes |
| 6 | 91 | 95 | 95 | 5 | 2 | 394 | 317 | 0 | No |
| 7 | 77 | 96 | 58 | 0 | 20 | 111 | 55 | 0 | No |
| 8 | 68 | 68 | 45 | 0 | 11 | 186 | 43 | 1 | No |
| Median | 91 | 96 | 94 | 0 | 16 | 190 | 104 | 0 | |
| Nonmaintained response# | |||||||||
| 9 | 23 | 32 | 17 | 4 | 12 | 195 | 23 | 4 | No |
| 10 | 25 | 45 | 35 | 20 | 6 | 98 | 14 | 4 | Yes |
| 11 | 24 | 72 | 24 | 0 | 28 | 158 | 36 | 4 | No |
| 12 | 20 | 49 | 14 | 0 | 22 | 222 | 27 | 4 | No |
| Median | 24 | 47 | 21 | 2 | 17 | 177 | 25 | 4 | |
| No response** | |||||||||
| 13 | 0 | 6 | 0 | 78 | 11 | 16 | 10 | 4 | No |
| 14 | 0 | 0 | 0 | 73 | 18 | 13 | 12 | 2 | No |
| 15 | 0 | 0 | 0 | 0 | 16 | 15 | 14 | 0 | No |
| 16 | 0 | 0 | 0 | 25 | 3 | 13 | 9 | 0 | No |
| Median | 0 | 0 | 0 | 49 | 14 | 14 | 11 | 1 |
Subjects 1-3, 5, 6, 8-10, and 12-14 had undergone splenectomy; subjects 6, 8, 9, 13, and 16 had failed a thrombopoietic agent.
Plt indicates platelet; max, maximum; and med, median.
Responder was defined as increase in platelet count by more than 20 × 109/L to greater than 30 × 109/L.
Baseline value is within 1 month before first dose of study drug.
Max value is while on study drug with no IVIg treatment in preceding 2 weeks.
Median value is while on study drug with no IVIg treatment in preceding 2 weeks.
Steroids include prednisone, methylprednisolone, and danazol.
Platelets increased by greater than 20 × 109/L to greater than 30 × 109/L for at least 66% of visits.
Platelets increased by greater than 20 × 109/L to greater than 30 × 109/L for less than 66% of study visits.
Platelets did not increase by greater than 20 × 109/L to greater than 30 × 109/L.
Transient responses.
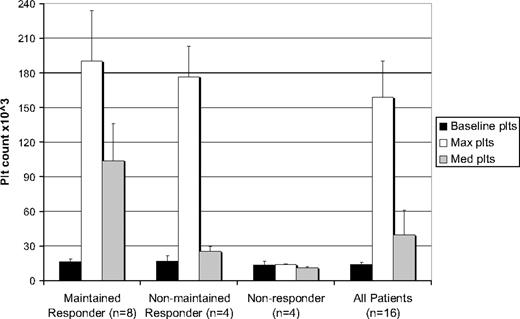
Four patients with a transient response (25%) developed an increase in platelet count from a median minimum of 17 × 109/L (17 000/mm3) at baseline to a median maximum of 177 × 109/L (177 000/mm3) while on R788, similar to that seen in the 8 patients who sustained their response (Figure 3). This platelet response was not sustained, however, despite increasing the median R788 dose of 156 mg, with platelet responses on less than 25% of study visits. These patients continued R788 because of improvement in clinical parameters, for example, less bleeding, avoiding rescue medications (median of 2% of study visits), and tapering of steroids.
Baseline, maximal, and median platelet counts for maintained responders, nonmaintained responders, nonresponders, and all patients in study. Maintained response is defined as platelet count increased by at least 20 × 109/L (20 000/mm3) to greater than 30 × 109/L (30 000/mm3) on at least 66% of the study visits. Nonmaintained response is defined as platelet counts increased by at least 20 × 109/L (20 000/mm3) to greater than 30 × 109/L (30 000/mm3) on less than 66% of the study visits. Nonresponders did not achieve any increases in platelet counts as per the above definition. Baseline platelet counts represent the lowest count within 1 month of study start. Maximum and average platelet counts were calculated as the median of peak and average counts, respectively, achieved by each patient in a given group.
Baseline, maximal, and median platelet counts for maintained responders, nonmaintained responders, nonresponders, and all patients in study. Maintained response is defined as platelet count increased by at least 20 × 109/L (20 000/mm3) to greater than 30 × 109/L (30 000/mm3) on at least 66% of the study visits. Nonmaintained response is defined as platelet counts increased by at least 20 × 109/L (20 000/mm3) to greater than 30 × 109/L (30 000/mm3) on less than 66% of the study visits. Nonresponders did not achieve any increases in platelet counts as per the above definition. Baseline platelet counts represent the lowest count within 1 month of study start. Maximum and average platelet counts were calculated as the median of peak and average counts, respectively, achieved by each patient in a given group.
Nonresponders.
Four patients (25%) had no significant increases in platelet counts in response to treatment with R788, despite a median dose of 138 mg twice daily. These patients were enrolled at starting dose cohorts of 75 mg twice daily to 125 mg twice daily and had their dose increased 0 to 4 times, without a resultant increase in platelet counts. These patients required rescue medications on a median 53% of study visits.
No significant differences or suggestive trends were observed between the clinical parameters of these nonresponding patients and patients who exhibited a clinical response to treatment with R788 when age, sex, duration of ITP, splenectomy status, baseline platelet count, and number of previous treatments were considered.
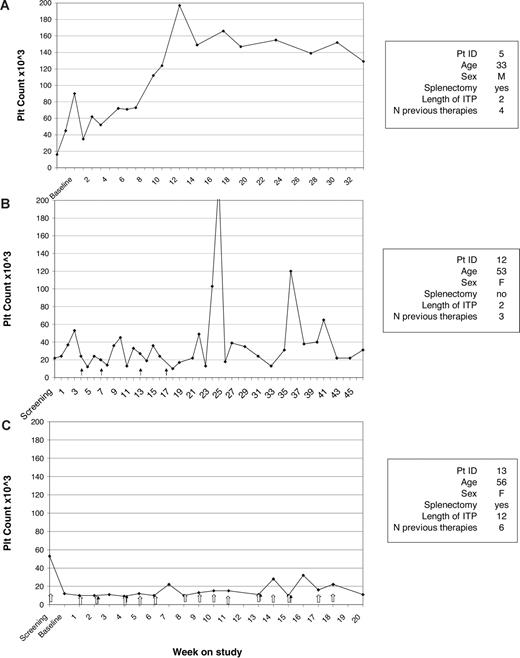
Sample platelet responses for a representative patient from each of the 3 groups (sustained response, nonmaintained response, no response) are shown in Figure 4.
Sample platelet responses. Sample platelet response for maintained responder (A), nonmaintained responder (B), and nonresponder (C). No significant difference was observed in the baseline characteristics between the 3 groups.  indicates dose increase; and
indicates dose increase; and  , rescue treatment.
, rescue treatment.
Sample platelet responses. Sample platelet response for maintained responder (A), nonmaintained responder (B), and nonresponder (C). No significant difference was observed in the baseline characteristics between the 3 groups.  indicates dose increase; and
indicates dose increase; and  , rescue treatment.
, rescue treatment.
Safety
Three patients withdrew because of toxicity. One 80-year-old patient withdrew consent because of hospitalization for a urinary tract infection (UTI) and subsequent development of deep vein thrombosis. The UTI was unrelated to the study drug, and the thrombosis was also considered unrelated (although R788 increased the platelet count into the normal range in this patient). One patient withdrew consent, despite adequate platelet response, because of GI toxicity (nausea and vomiting). He was reenrolled several months later and received R788 in conjunction with antinausea medications. This time, however, he failed to respond and was discontinued after an additional 9 weeks of treatment. One patient withdrew from the study secondary to preexisting elevated transaminase levels that worsened on the study drug, preventing further increase in the dose (Figure 2A).
The most commonly reported adverse events were GI-related reactions (Table 3). Six patients reported diarrhea. This was generally characterized by increased urgency rather than increased frequency or more liquid consistency of stool. Four patients reported nausea, and 3 patients developed episodes of vomiting. In most patients, these adverse effects were mild to moderate in severity, self-limited, and did not lead to discontinuation of medication. One patient was hospitalized for dehydration secondary to vomiting. R788 was discontinued in this patient while he was hospitalized. He was subsequently reenrolled with concomitant administration of antinausea agents, with good relief of symptoms. In general, GI reactions developed in patients at doses of 125 mg twice daily or higher.
All adverse reactions reported by patients on study
| Adverse reaction . | Patients reporting reaction, n . | Reaction probably related to R788 . |
|---|---|---|
| Drowsiness/fatigue | 7 | No |
| Diarrhea | 6 | Yes |
| SBP increased by > 10 mm Hg | 5 | Yes |
| Nausea | 4 | Yes |
| Headache | 4 | Yes |
| Weight gain > 5 kg | 3 | Yes |
| Vomiting | 3 | Yes |
| Abdominal pain | 3 | Yes |
| Shortness of breath | 3 | No |
| Dizziness | 2 | No |
| Constipation | 2 | Yes |
| ALT > 2× ULN | 2 | Yes |
| UTI | 1 | No |
| DVT | 1 | No |
| Chest tightness | 1 | No |
| Fever | 1 | No |
| Blurry vision | 1 | No |
| Lung hemangioma | 1 | No |
| LAD | 1 | No |
| Adverse reaction . | Patients reporting reaction, n . | Reaction probably related to R788 . |
|---|---|---|
| Drowsiness/fatigue | 7 | No |
| Diarrhea | 6 | Yes |
| SBP increased by > 10 mm Hg | 5 | Yes |
| Nausea | 4 | Yes |
| Headache | 4 | Yes |
| Weight gain > 5 kg | 3 | Yes |
| Vomiting | 3 | Yes |
| Abdominal pain | 3 | Yes |
| Shortness of breath | 3 | No |
| Dizziness | 2 | No |
| Constipation | 2 | Yes |
| ALT > 2× ULN | 2 | Yes |
| UTI | 1 | No |
| DVT | 1 | No |
| Chest tightness | 1 | No |
| Fever | 1 | No |
| Blurry vision | 1 | No |
| Lung hemangioma | 1 | No |
| LAD | 1 | No |
SBP indicates systolic blood pressure; ALT, alanine aminotransferase; ULN, upper limit of normal; UTI, urinary tract infection; DVT, deep vein thrombosis; and LAD, lymphadenopathy.
A number of patients had mild elevations in liver function tests (ALT, AST, alkaline phosphatase). Two patients developed elevations in ALT greater than 2 times the upper limit of normal. One of those patients had a preexisting elevated transaminase level. Liver function returned to normal in this patient on tapering of R788.
Five patients had elevations in systolic blood pressure greater than 10 mm Hg, 3 of whom had underlying hypertension. Three patients had significant weight gain while on the study (> 5 kg). Other patients, however, had no change in weight, and no significant weight gain was observed across the cohort.
Other reactions reported while on R788 included fatigue which was common, drowsiness, headache, dizziness, and shortness of breath. These seemed unlikely to be related to R788 and seemed to be caused by comorbidities in the study population, including the ITP itself.
Patients on R788 had a small but statistically significant decrease in white blood cell count. No significant changes were observed in the percentage of lymphocytes or neutrophils after starting R788, and there was no effect on the red blood cell counts. None of these findings correlated with increased rates of infection. The only serious infection was the UTI in patient number 13; she was not neutropenic at this time.
Discussion
R406, an inhibitor of Syk, increased platelet and red cell counts in murine models of ITP and AHA, respectively. On the basis of these findings, a pilot study with R788, an orally administered Syk kinase inhibitor that is a prodrug of R406, was initiated to explore the safety and efficacy of treatment for patients with chronic ITP. R788 was effective in maintaining adequate platelet counts and in reducing the need for rescue medications in most patients despite preexisting refractory ITP. Three-quarters of the patients on this study achieved a substantial clinical response, and one-half of the 16 patients had a sustained response on R788. The median peak platelet count was greater than 100 × 109/L (100 000/mm3) in the 16 patients despite all patients starting at a baseline of less than 30 × 109/L (30 000/mm3).
Patients included in this study had been thrombocytopenic for many years, in some cases living at platelet counts less than 20 × 109/L (20 000/mm3). They had failed most standard treatments for ITP as well as several investigational agents and required “rescue medications” on a frequent basis. Fourteen of the 16 patients had failed to respond to rituximab, 11 failed splenectomy, and 5 failed thrombopoietic agents. Six sustained and 3 transient responders responded to R788 after failing splenectomy, whereas 2 sustained and 1 transient responded to R788 after failing to sustain a response to a thrombopoietic agent. The patients who had sustained responses to R788 after not responding to a thrombopoietic agent support the hypothesis that certain patients may respond best to treatments such as R788 which are believed to primarily inhibit antibody-mediated platelet destruction. The mouse model not only provided the impetus to proceed but also helps to validate that the primary effect of Syk inhibition is to ameliorate antibody-mediated platelet (and red cell) destruction. Other patients,5,6 perhaps the majority, may respond best to treatments that primarily stimulate platelet production.
Variability of response could have been due to individual differences in the degree of inhibition of Syk. This hypothesis was preliminarily tested with a basophil activation–based assay for syk inhibition, as shown in the supplemental data (available on the Blood website; see the Supplemental Materials link at the top of the online article). Unfortunately, the assay for the degree of inhibition of Syk was available only at the end of the study, so nonresponders could not be studied because they had already discontinued R788. Testing in the responders suggested that a key feature was the duration of inhibitory effect after ingestion of R788. Patients who had an almost identical inhibition of Syk 12 hours after taking R788 to 1 to 2 hours after taking it (peak inhibition = trough inhibition) had the best platelet response to R788. Discontinuing R788 for several days in 2 patients showed a markedly lower degree of inhibition accompanied by a marked fall in platelet count. In the sustained responder but not the transient responder, inhibition of Syk could be seen after ingestion of a single dose of R788 but not to the level seen with repeated twice-daily dosing. Because only responders were studied, whether effective inhibition was achieved in patients who did not respond could not be evaluated. The indirect Basotest relying on CD63 expression was used because of the expected rapid off rate of R788 in vitro and the inability to selectively study its effects in whole blood, preventing a rapid, direct assessment of the degree of inhibition of Syk.9
Most patients tolerated R788, but 3 of 16 patients discontinued the study drug for reasons of toxicity. The reasons were different for each of the 3 patients: deep vein thrombosis (unrelated), vomiting and diarrhea, and elevated transaminase levels in 1 patient each. Other patients experienced mild-to-moderate primarily GI adverse events that generally did not prevent them from continuing R788. Individual GI symptoms were quite variable, both in type and degree, ranging from a mild increase in urgency for bowel movements to frank diarrhea or constipation. The GI toxicity seen in this study and the extent of adverse reactions to R788 are consistent with the toxicities seen with other tyrosine kinase inhibitors used at optimal biologic doses.16 GI toxicity is particularly common and has been attributed to poor specificity of the agents. These effects are both variable from patient to patient and poorly understood. Bone marrow biopsies were not performed in this study but may be part of future trials. A more complete assessment of the safety of R788 may be obtained from larger placebo-controlled studies, that is, in rheumatoid arthritis and lymphoma.
Appropriate dosing on R788 also needs to be further investigated. In this study population, doses of at least 150 mg twice daily were associated with better platelet response rates. However, toxicity appeared to be increased at these doses as well. It is likely that lower doses would be sufficient to maintain a platelet response in less refractory patients and that some patients who achieve a response at a high dose may be able over time to taper R788 to a more tolerable dose as was done in 3 responding patients in this study.
R788 can be beneficial for certain patients with refractory ITP even though not all patients with refractory ITP will respond to it. In the future, it may be an alternative to treatment with newer thrombopoiesis-stimulating agents or be combined with them to take advantage of the different mechanisms of therapeutic effect. Additional studies are required to further evaluate the safety and confirm the optimal dosing of R788 especially in view of the GI toxicity seen in this study of patients with ITP. Future randomized larger trials in patients with ITP are planned. Because R788 was also successful at treating a murine model of AHA, this drug would probably hold promise for human patients with AHA as well.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Tara Patten, Study Nurse; Allison Schindler, Data Control Coordinator; Doug Cines, MD, Data Safety Monitor; and the patients who participated in the study. Chris Yurchuck assisted in the preparation of the manuscript.
This work was supported by clinical research funding from Rigel (South San Francisco, CA).
Authorship
Contribution: J.B.B. and E.G. designed the study; J.B.B. performed the clinical part of the human research, helped to analyze the data, and wrote the paper; A.P. performed the research on degree of inhibition, analyzed the data, and wrote the paper; A.R.C. and A.H.L. designed and performed the animal research, analyzed the animal data, wrote the animal section of the paper, and edited the rest of the paper; and E.G. contributed to the patient management in the protocol and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Anna Podolanczuk, Department of Medicine, New York Presbyterian Hospital, Columbia University Medical Center, 177 Fort Washington Ave, 6th Fl Center Rm 12, New York, NY 10032; e-mail: ajp2158@columbia.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal