Abstract
Emerging evidence suggests that progression of hematologic malignancies is associated with angiogenesis. Dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) can provide global and functional imaging of tumor angiogenesis. In this study, we performed bone marrow DCE-MRI prospectively at diagnosis and after induction chemotherapy in 78 de novo acute myeloid leukemia (AML) patients and correlated it with treatment outcome. An algorithm to assess bone marrow angiogenesis by measuring the DCE-MRI time-intensity curve pixel by pixel was developed using 3 distinct parameters: peak enhancement ratio (Peak) to indicate tissue blood perfusion; amplitude (Amp) to reflect vascularity; and volume transfer constant (K trans) to indicate vascular permeability. The Peak and Amp decreased significantly at remission status after induction chemotherapy. Patients with higher Peak or Amp at diagnosis had shorter overall survival and disease-free survival than others. Cox multivariate analysis identified higher Peak value (hazard ratio, 9.181; 95% confidence interval, 1.740-48.437; P = .009) as an independent predictor for overall survival in addition to unfavorable karyotype and old age. Our findings provide evidence that increased bone marrow angiogenesis measured by DCE-MRI can predict adverse clinical outcome in AML patients. DCE-MRI may help to select high-risk phenotype AML patients for tailored antiangiogenic therapy and to monitor treatment response.
Introduction
Personalized medicine is the final goal of translational research in the postgenomic era.1 Numerous biomarkers have been identified by genomic or proteomic approach that can subcategorize the disease phenotype and predict clinical outcome.2 Defining a specific disease phenotype, however, does not necessarily depend on sophisticated molecular or genomic technologies. In this study we report that by using dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI), we are able to measure the global bone marrow angiogenesis in vivo and independently predict the clinical outcome of patients with acute myeloid leukemia (AML).
It is well recognized that angiogenesis plays an important role in tumor development, progression, and metastasis.3-5 Angiogenesis is one of the significant factors that correlate with the tumor aggressiveness and clinical outcome of patients with melanoma,6 prostate cancer,7 breast cancer,8 and recently in leukemia or lymphomas9,10 and multiple myeloma.11-13
Microvessel density (MVD) is an assumed “gold standard” for quantification of angiogenesis in tumor tissue. MVD is increased in patients with AML with active disease in comparison to healthy controls or patients in remission.14-17 However, MVD can be measured only in the limited area of the bone marrow biopsy specimen; thus, it cannot be used to assess the global or in vivo tumor angiogenesis. Neither the functional status of the tumor blood vessel (eg, permeability, elimination rate) could be determined by the above-mentioned immunohistochemical methods.
New technologies for cancer image with functional assessment are now possible in vivo by Doppler sonography, DCE-MRI, DCE computed tomography, and fluorodeoxyglucose-18 positron emission tomography.18-20 DCE-MRI is a noninvasive quantitative method of investigating microvascular structure and function by tracking the pharmacokinetics of injected low-molecular-weight contrast agents as they pass through the tumor vasculature.21 This technique provides a direct quantification of blood vessel density, vascular flow, and permeability.22-24 In addition, functional image biomarkers may potentially replace anatomic studies by assessing treatment response earlier.25 There have been increasing attempts to use this approach to assess spatial and temporal heterogeneity in tumor angiogenesis and to predict tumor biologic aggressiveness and treatment response in solid tumors,26-29 multiple myeloma,30 and myelodysplastic syndrome.31
Tumor angiogenesis imaging assessment in patients with AML was reported in our previous, preliminary study.32 Our findings clearly showed that increased bone marrow angiogenesis, evaluated by DCE-MRI, can predict adverse outcome. Thus, we conducted a prospective study to evaluate the in vivo angiogenesis by DCE-MRI on a large cohort of patients with newly diagnosed AML before chemotherapy and after complete remission (CR); we then correlated the results with the clinical outcome.
Methods
Subjects
This study was approved by the institutional review board of the National Taiwan University Hospital; written informed consent was obtained from all participants in accordance with the Declaration of Helsinki. From November 2004 to March 2007, a total of 78 adult patients with newly diagnosed de novo AML at National Taiwan University Hospital and were suitable and agreed to receive conventional induction chemotherapy were enrolled consecutively. Diagnosis and classification of AML were made according to the French-American-British Cooperative Group Criteria.33 Demographic data (eg, age and sex) and clinical information (eg, white blood cell count, hemoglobin level, platelet count, lactate dehydrogenase [LDH] level, chromosome change, and survival) were collected.
All participants received the first DCE-MRI (as initial MRI) immediately after diagnosis of AML. All patients with non-M3 subtypes of AML were then treated with standard induction chemotherapy (idarubicin 12 mg/m2 per day on days 1-3 and Ara-C 100 mg/m2 per day on days 1-7). The patients with acute promyelocytic leukemia (M3 subtype) received concurrent all-trans retinoic acid and chemotherapy. After CR was achieved, the patients received consolidation therapy with high-dose cytarabine, with or without an anthracycline. Nineteen patients received hematopoietic stem cell transplant (HSCT). Follow-up DCE-MRI (as remission status MRI) was performed after the first consolidation chemotherapy (before second consolidation chemotherapy) in patients who obtained CR. The choice of salvage chemotherapy in non-CR patients was decided by the attending physician. Every patient was followed up until August 31, 2007.
DCE-MRI of bone marrow
MR imaging of the bone marrow was performed with a 1.5-Tesla superconducting system (Sonata; Siemens, Erlangen, Germany) at the lumbar spine of the 78 patients as previously described,32,34,35 with some modification. DCE-MRI was performed (section thickness, 10 mm; field of view, 28 cm) at the midsection of the vertebral body and covered the area from T11 to the sacrum.34,35 An injection of 0.15 mmol/kg body weight of gadopentetate dimeglumine (Omiscan Injection; GE Healthcare, Little Chalfont, United Kingdom) was administrated by the power injector through a 21-gauge intravenous catheter that was inserted in the right antecubital vein. A brief constant injection rate of 2.0 mL/second was used, as in our preliminary study.32 The pulse sequence used for the dynamic scanning was a turbo fast low-grade shot gradient-echo sequence (repetition time/echo time, 500/1.37 msec; prepulse inversion time, 230 msec; flip angle, 8°; acquisition matrix, 157 × 256; average, 4). Acquisition time was 2.0 seconds per contiguous frame. In total, 300 dynamic images were obtained within 600 seconds (one frame per 2 seconds) in each subject. The data derived from DCE-MRI of the lumbar spine were used to evaluate the bone marrow perfusion.
Algorithm for acquisition of bone marrow MR imaging angiogenesis
Signal intensity values were measured by a radiologist in regions of interest, which covered the entire vertebral body. The signal intensity values were then plotted as a time-intensity curve.36
In our previous pilot study,32 we used the peak enhancement ratio (Peak) and slope to represent the bone marrow angiogenesis. In this prospective study, the perfusion parameters were calculated quantitatively from the time-intensity curve according to the bicompartmental model proposed by Brix et al,24 Toft et al,37 and Tofts and Kermode,37,38 using the Peak, vascularity parameter amplitude (Amp), and permeability parameter (K trans). The Amp is similar to slope but has better quantification of vascularity; it is measured with the curve-fitting model and reflects the vascularity and extravascular extracellular space volume. The K trans is the parameter that represents the permeability from intravascular to interstitial and reflects the volume transfer constant of exchange of tracers between extravascular extracellular space volume and intravascular plasma.37,39 The Peak is calculated for each region of interest as (SImax − SIbase)/SIbase, which indicates the concentration of contrast material in the intravascular and extravascular interstitial spaces and represents as the tissue perfusion. We measured the perfusion parameters of vertebral bodies from L2 to L4 and took the average to represent the bone marrow angiogenesis of the patient.
A computer program for producing a color-coded map of the DCE-MRI parameters was developed. The software architecture has several layers, including an interface layer to visualize images, an analysis layer to compute statistical data, a graphics processing layer to convert image format, and an in/out (I/O) layer to retrieve and store information. Algorithms to generate statistical data for analysis were implemented; computational results were delivered to the interface layer for visualization.
Statistical analysis
Data were expressed as mean and SD. Cutoffs for high and low bone marrow angiogenesis were determined by the Classification and Regression Tree (CART) method.40 Overall survival was measured from the date of first diagnosis to the date of last follow-up or death from any cause, whereas disease-free status indicated that the patient had achieved CR and had not relapsed by the end of this study. Kaplan-Meier estimation was used to plot survival curves, and 2-sided log-rank tests were used to test the difference between groups.
We used multivariate Cox regression analysis to investigate whether the angiogenetic parameter is an independent prognostic factor of overall survival and disease-free survival in patients with AML. The concept of multivariate analysis is that the estimated hazard ratio of the risk parameter is adjusted by the effects of potential confounding variables (eg, karyotype). Multivariate Cox proportional hazard regression analysis was used to evaluate the contribution of independent prognostic factors to patient survival. Angiogenetic parameters, age, sex, karyotype, and LDH were used as covariates. The data were analyzed using STATISTICA Data Miner software (version 8.0; StatSoft, Tulsa, OK) and SPSS software (release 13; SPSS, Chicago, IL).
Results
The demographic and clinical features of the 78 patients with AML are shown in Table 1. Cytogenetic results were available in 76 of the 78 patients. Of these, 14 (18.4%) had favorable-risk cytogenetics, 52 (68.4%) had intermediate-risk cytogenetics, and 10 (13.2%) had unfavorable-risk cytogenetics. Fifty-seven (73.1%) patients achieved CR. With a median follow-up duration of 16.2 months, the median overall survival time was 11.3 months and CR duration was 9 months. A median of 4 courses of chemotherapy (range, 1-7 courses) were given.
Characteristics of the 78 patients with acute myeloid leukemia (AML) receiving dynamic contrast-enhanced magnetic resonance image (DCE-MRI)
| Variable . | No. of patients (%) . |
|---|---|
| Male | 37 (47) |
| Female | 41 (53) |
| Age, y* | 49 (17-76) |
| FAB classification* | |
| M0 | 1 (1.3) |
| M1 | 16 (20.5) |
| M2 | 31 (39.8) |
| M3 | 5 (6.4) |
| M4 | 16 (20.5) |
| M5 | 4 (5.1) |
| M6 | 4 (5.1) |
| Unclassified | 1 (1.3) |
| Karyotype† | |
| Favorable | 14 (18.0) |
| Intermediate | 52 (66.7) |
| Unfavorable | 10 (12.8) |
| Unknown | 2 (2.5) |
| Follow-up, mo* | 16.2 (4-34.3) |
| CR | 57 (73.1) |
| CR duration, mo* | 9.0 (1-33.4) |
| Overall survival, mo* | 11.3 (0.4-34.3) |
| Variable . | No. of patients (%) . |
|---|---|
| Male | 37 (47) |
| Female | 41 (53) |
| Age, y* | 49 (17-76) |
| FAB classification* | |
| M0 | 1 (1.3) |
| M1 | 16 (20.5) |
| M2 | 31 (39.8) |
| M3 | 5 (6.4) |
| M4 | 16 (20.5) |
| M5 | 4 (5.1) |
| M6 | 4 (5.1) |
| Unclassified | 1 (1.3) |
| Karyotype† | |
| Favorable | 14 (18.0) |
| Intermediate | 52 (66.7) |
| Unfavorable | 10 (12.8) |
| Unknown | 2 (2.5) |
| Follow-up, mo* | 16.2 (4-34.3) |
| CR | 57 (73.1) |
| CR duration, mo* | 9.0 (1-33.4) |
| Overall survival, mo* | 11.3 (0.4-34.3) |
FAB indicates French-American-British classification; and CR, complete remission.
Data are shown as median (range).
Favorable, t(8;21), inv(16), t(15;17); unfavorable, −7, del(7q), −5, del(5q), 3q abnormality; complex; intermediate, normal karyotype, and other abnormalities.
Global assessment of bone marrow angiogenesis by DCE-MRI in patients with AML
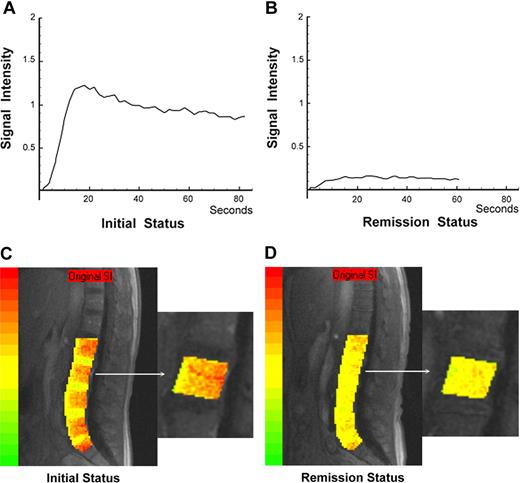
The time-intensity curve derived from DCE-MRI and the color-coded angiogenesis map of the vertebral bone marrow in a 54-year-old female patient at initial diagnosis are shown in Figure 1A and C; those in remission are shown in Figure 1B and D.
The time-intensity curves derived from DCE-MRI and color-coded angiogenesis maps of vertebral bone marrow in a 54-year-old female patient with de novo AML are shown. She achieved complete remission after induction chemotherapy. Her remission duration until the end of August 2007 was 1002 days. The time-intensity curve (A) and color-coded angiogenesis map (C) at initial diagnosis are shown; those in complete remission are shown (B,D), respectively.
The time-intensity curves derived from DCE-MRI and color-coded angiogenesis maps of vertebral bone marrow in a 54-year-old female patient with de novo AML are shown. She achieved complete remission after induction chemotherapy. Her remission duration until the end of August 2007 was 1002 days. The time-intensity curve (A) and color-coded angiogenesis map (C) at initial diagnosis are shown; those in complete remission are shown (B,D), respectively.
The clinical parameters, including age, sex, hemogram, LDH, karyotype, and bone marrow angiogenesis, as well as Peak, Amp, and K trans measured by DCE-MRI, were compared between AML patients with and without disease control. Patients who remained in disease-free status had significantly lower Peak and Amp values than did those who did not (P = .009 and .010, respectively). There was no difference in other parameters between these 2 groups.
Comparison of bone marrow angiogenesis MRI at diagnosis with that in complete remission
Follow-up DCE-MRI was performed after first consolidation chemotherapy in 51 patients who achieved CR, remained disease-free, and agreed to receive the examination. The MR angiogenesis parameters Peak and Amp at remission status were significantly reduced compared with those in the initial DCE-MRI (P < .001 for both). However, no significant difference was observed in the permeability parameter K trans between these 2 examinations (P = .538).
Bone marrow angiogenesis MRI predicts outcome of patients with AML
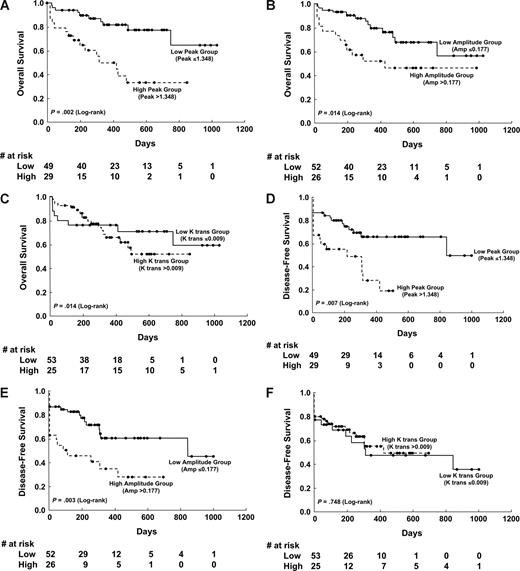
The cutoffs for high and low levels of initial DCE-MRI parameters by the CART analysis were set at 1.3475 for Peak (≤ 1.348, n = 49; > 1.348, n = 29), 0.1769 for Amp (≤ 0.177, n = 52; > 0.1777, n = 26), and 0.0096 for K trans (≤ 0.009, n = 55; > 0.009, n = 23). Those patients with lower Peak or Amp at diagnosis had a higher chance to enter CR than did others (81.6% vs 62.1%; P = .06 and 84.6% vs 61.5%; P = .04, respectively). Univariate analysis showed that unfavorable karyotype, higher Peak, and higher Amp values were associated with shorter disease-free survival (DFS) and overall survival (OS; Table 2), but the K trans value was not. Kaplan-Meier survival curves for DFS and OS stratified by Peak, Amp, and K trans values, respectively, are shown in Figure 2.
Univariate analysis of the effect of risk factors on disease-free survival and overall survival in patients with acute myeloid leukemia
| Variable . | Disease-free survival . | Overall survival . | ||
|---|---|---|---|---|
| No. of patients . | P . | No. of patients . | P . | |
| Sex | .595 | .384 | ||
| Male | 38 | 38 | ||
| Female | 40 | 40 | ||
| Age, y | .430 | .005* | ||
| Younger than 50 | 41 | 41 | ||
| Older than 50 | 37 | 37 | ||
| Karyotype | .026* | .009* | ||
| Favorable | 14 | 14 | ||
| Intermediate | 52 | 52 | ||
| Unfavorable | 10 | 10 | ||
| WBC count, ×109/L | .875 | .915 | ||
| Less than 30 | 55 | 55 | ||
| 30 or more | 23 | 23 | ||
| LDH | .806 | .932 | ||
| Less than 2× | 42 | 42 | ||
| Greater than 2× | 36 | 36 | ||
| Peak | .007* | .002* | ||
| Low | 49 | 49 | ||
| High | 29 | 29 | ||
| Amp | .003* | .014* | ||
| Low | 52 | 52 | ||
| High | 26 | 26 | ||
| K trans | .748 | .246 | ||
| Low | 23 | 23 | ||
| High | 55 | 55 | ||
| Variable . | Disease-free survival . | Overall survival . | ||
|---|---|---|---|---|
| No. of patients . | P . | No. of patients . | P . | |
| Sex | .595 | .384 | ||
| Male | 38 | 38 | ||
| Female | 40 | 40 | ||
| Age, y | .430 | .005* | ||
| Younger than 50 | 41 | 41 | ||
| Older than 50 | 37 | 37 | ||
| Karyotype | .026* | .009* | ||
| Favorable | 14 | 14 | ||
| Intermediate | 52 | 52 | ||
| Unfavorable | 10 | 10 | ||
| WBC count, ×109/L | .875 | .915 | ||
| Less than 30 | 55 | 55 | ||
| 30 or more | 23 | 23 | ||
| LDH | .806 | .932 | ||
| Less than 2× | 42 | 42 | ||
| Greater than 2× | 36 | 36 | ||
| Peak | .007* | .002* | ||
| Low | 49 | 49 | ||
| High | 29 | 29 | ||
| Amp | .003* | .014* | ||
| Low | 52 | 52 | ||
| High | 26 | 26 | ||
| K trans | .748 | .246 | ||
| Low | 23 | 23 | ||
| High | 55 | 55 | ||
WBC indicates white blood cell; LDH, lactate dehydrogenase; 2×, 2 times normal range; Peak, peak enhancement ratio; Amp, amplitude; and K trans, volume transfer constant.
Relative risk that is statistically significant (P < .05).
Kaplan-Meier survival curves of disease-free survival and overall survival stratified by different angiogenetic changes at diagnosis. The low versus high Peak group showed significant differences in overall survival (P = .002; A) and disease-free survival (P = .007; D). The Amp also showed significant implication in overall survival (P = .014; B) and disease-free survival (P = .003; E). However, the permeability parameter K trans showed no influence on overall and disease-free survival (C,F).
Kaplan-Meier survival curves of disease-free survival and overall survival stratified by different angiogenetic changes at diagnosis. The low versus high Peak group showed significant differences in overall survival (P = .002; A) and disease-free survival (P = .007; D). The Amp also showed significant implication in overall survival (P = .014; B) and disease-free survival (P = .003; E). However, the permeability parameter K trans showed no influence on overall and disease-free survival (C,F).
Subgroup analysis showed that among patients with higher Peak values, those receiving HSCT survived longer than did those without (median, 516 days vs 371 days; P = .018). There was also a trend of survival benefit of HSC transplantation in patients with higher Amp values (median, 647 vs 322 days; P = .095). On the contrary, HSC transplantation did not offer survival benefit for patients with lower Peak or Amp values.
Bone marrow angiogenesis MRI as an independent prognostic factor of patients with AML
Furthermore, multivariate Cox proportional hazards analysis identified that higher Peak value (hazard ratio [HR], 9.181; 95% CI, 1.740-48.437; P = .009; Table 3) was an independent poor prognostic factor for OS, in addition to the unfavorable karyotype (HR, 6.894; 95% CI, 1.728-27.498; P = .006) and old age (HR, 6.494; 95% CI, 2.454-17.181; P < .001).
Multivariate analysis (Cox regression) on the overall survival in patients with acute myeloid leukemia
| Variable . | RR . | 95% CI . | P . |
|---|---|---|---|
| Karyotype* | 6.894 | 1.728-27.498 | .006 |
| Peak* | 9.181 | 1.740-48.437 | .009 |
| Amp | 5.064 | 0 to > 1000 | .751 |
| Sex | 1.705 | 0.689-4.22 | .248 |
| LDH | 0.786 | 0.328-1.923 | .610 |
| K trans | 0 | 0 to > 1000 | .640 |
| Age*† | 6.494 | 2.454-17.181 | < .001 |
| Variable . | RR . | 95% CI . | P . |
|---|---|---|---|
| Karyotype* | 6.894 | 1.728-27.498 | .006 |
| Peak* | 9.181 | 1.740-48.437 | .009 |
| Amp | 5.064 | 0 to > 1000 | .751 |
| Sex | 1.705 | 0.689-4.22 | .248 |
| LDH | 0.786 | 0.328-1.923 | .610 |
| K trans | 0 | 0 to > 1000 | .640 |
| Age*† | 6.494 | 2.454-17.181 | < .001 |
CI indicates confidence interval; RR, relative risk; Peak, peak enhancement ratio; Amp, amplitude; LDH, lactate dehydrogenase; and K trans, volume transfer constant.
Relative risk is statistically significant (P < .05).
Age at least 50 years relative to age younger than 50 years (the reference).
Bone marrow angiogenesis MRI predicts outcome of patients with intermediate-risk cytogenetics
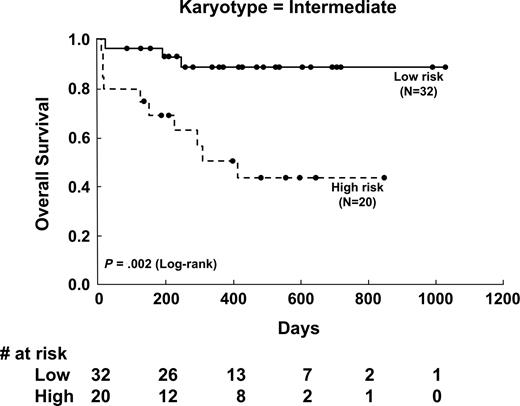
To further show the effect of Peak value on OS in different cytogenetic groups, Kaplan-Meier survival curves of 3 cytogenetic-risk groups stratified for Peak value were analyzed. Peak value had a significant effect on OS in patients with intermediate-risk karyotype (P = .002; Figure 3), in the whole population (P = .002), and in those with favorable-risk cytogenetics (P = .046), but not in patients with unfavorable-risk cytogenetics (P = .786). If only the patients with non–acute promyelocytic leukemia aged 18 to 65 years were analyzed, Peak value had even more significant implication in OS (P = .001).
Kaplan-Meier survival curves of overall survival stratified by initial DCE-MRI angiogenetic parameter (Peak) in patients with intermediate-risk cytogenetics. The low versus high Peak group showed significant differences in overall survival (P = .002).
Kaplan-Meier survival curves of overall survival stratified by initial DCE-MRI angiogenetic parameter (Peak) in patients with intermediate-risk cytogenetics. The low versus high Peak group showed significant differences in overall survival (P = .002).
Discussion
This is the first large-scale prospective study that used DCE-MRI to evaluate the functional bone marrow angiogenesis in patients with AML. We found that pretreatment Peak, reflecting bone marrow perfusion, and Amp, reflecting vascularity, of functional bone marrow angiogenesis MRI can predict overall survival and disease-free survival of patients with newly diagnosed AML. More intriguingly, higher Peak value was an independent predictor for poor overall survival. We also showed that Peak and Amp decreased significantly at CR, compared with those at diagnosis. These findings suggest that functional MRI of bone marrow angiogenesis is a useful biomarker to predict clinical outcome of patients with AML, and tumor angiogenesis may play an important role in the pathogenesis of AML.17,41
Increased bone marrow MVD was reported in patients with AML, but its association with patients' survival is unclear and inconclusive,16,42,43 probably because the MVD detected by conventional immunocytochemical technique is unable to assess the global and dynamic angiogenesis of the bone marrow. Vascularity within a tumor can be spatially or temporally heterogeneous; and tumor vessels are much more permeable than are normal blood vessels. Thus, assessment of angiogenesis in the bone marrow by traditional MVD poses special challenges.40,44,45 DCE-MRI may be a better alternative than microvessel density in bone marrow biopsy specimens because it is noninvasive and can evaluate much bigger bone volumes, thus better representing the overall disease burden.
Recently, DCE-MRI has become a promising, attractive method to evaluate tumor microvasculature in solid tumors.25,46,47 A consensus publication in 199937 about the estimation of 2-compartment kinetic parameters from DCE-MRI described the standardized quantities and symbols. DCE-MRI measurements of bone marrow angiogenesis, which analyzed the total vascularity, tissue perfusion, and vasculature functional status (permeability), may give a more informative evaluation of global and dynamic bone marrow angiodiagnosis in AML and can more reliably predict the outcome of the patients.
There is increasing evidence that AML may be an angiogenesis-dependent disease with progressive recruitment of blood vessels in the bone marrow.15,16,41 This has prompted novel antiangiogenic approaches in AML.41,48-50 However, to assess the angiogenic changes in patients with AML and monitor clinical effects of such antiangiogenic therapy requires a new and promising strategy. The current study's finding that DCE-MRI assessment of patients with AML with increased bone marrow angiogenesis can predict poor clinical outcome also indicates that antiangiogenesis treatment may be beneficial for these patients. DCE-MRI can provide noninvasive, convenient, and reproducible serial evaluations of global bone marrow angiogenesis diagnostics with only 600 seconds of scanning time and total preparation time of less than 20 minutes. This is more practical than repeated bone marrow biopsies and MVD studies. In short, DCE-MRE may be an important tool to help physicians both identify the most appropriate patients for antiangiogenic therapies for the best treatment results and to monitor the response to treatment. Moreover, the finding that patients receiving HSCT survive longer than those without in the group with higher Peak value (P = .018) implies that HSC transplantation may be a therapeutic option for those with higher bone marrow angiogenesis. Further studies in more patients are needed to clarify this point.
In bone marrow MRI, the contrast enhancement is to determine the concentration of contrast agent in the macrovascular factors (eg, from paired segmental arteries to intravertebral small arteriole) and microvascular factors (from arterial capillary to sinusoid), as well as those in the extravascular and interstitial compartments. In this study, 3 parameters were used to quantify the hemodynamics of the bone marrow: Peak, Amp, and K trans. Peak reflects the contrast concentration at extracellular compartment; Amp, the microvascular density and leakage space; and K trans, the vascular permeability. Our data showed that the Peak (represents tissue perfusion) played as one of the most important factors in angiogenesis and also appeared to be most reproducible.19 The Peak has to be regarded as a complex process, including blood inflow, outflow, and transit factors that affect gadolinium concentration at the extracellular compartment, which indicated the summation of vessel density and permeability factors.
This report also provides color-coded image presentations of DCE-MRI bone marrow angiogenesis, using an algorithm that measures pixel by pixel from the time-intensity curve (Figure 1). The color-coded map of bone marrow angiogenesis in AML is user friendly and easily interpretable by physicians.
In our previous research,35 we have shown that the rate of vertebral bone marrow perfusion decreased significantly in subjects older than 50 years, but it decreased in a relatively slow speed. However, the differences of bone marrow perfusion between patients with AML and age- and sex-matched healthy subjects were drastic.32 The patients with AML had much higher Peak values (the key parameter in the current research) than did the age- and sex-matched controls (2.04 ± 0.69 vs 0.42 ± 0.21; an almost 4.8-fold difference), and the distribution box plots had no overlap between these 2 groups.32 Thus, we can postulate that, although the age factor has influence on the Peak of the bone marrow, it is minor and can be overridden by the great effect of tumor angiogenesis. Therefore, although the angiogenesis was not adjusted by the age in this study, we suggest that it would not influence analysis results.
There was one potential flaw and limitation in our analysis. The microvessel density shown by immunohistochemical staining was not routinely performed in our patients because of limited biopsy specimen and nonuniversal staining technique. Thus, no data correlations of the MR angiogenetic parameter with MVD were made. However, we believed that angiogenesis by traditional MVD method in patients with AML may not reflect the global angiogenesis or functional and dynamic changes. On the contrary, DCE-MRI is a noninvasive and reproducibly quantitative method with serial evaluations.
In conclusion, bone marrow angiogenesis can be measured by DCE-MRI. Increased bone marrow perfusion as reflected by higher Peak value can independently predict adverse clinical outcome in patients with AML. DCE-MRI may provide dynamic and functional tumor angiogenesis and may help identify high-risk patients for tailored antiantigenic therapy and monitor treatment response.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Professor Timothy K. Shih, Department of Computer Engineering, Tamkang University, Taipei, Taiwan, for the designing of the computer program used for color-coding angiogenesis image.
This work was supported by a grant from the National Science Council (NSC 94-2314-B-002-182; NSC 95-2314-B-002-061) and the National Taiwan University Hospital (NTUH.94A19-1).
Authorship
Contribution: T.T.-F.S. was responsible for coordinating the study, integrating the whole research, managing and interpreting data, and writing the manuscript; H.-A.H. was responsible for literature collection, study design, and writing the manuscript; C.-Y.L. and H.-Y.C. were responsible for the statistical analysis and interpretation of the statistical findings; B.-B.C. calculated the dynamic MRI results and interpreted the data; S.-Y.W. participated in the patient care and data collection; J.-L.T., M.Y., S.-Y.H., W.-C.C., S.-C.H., and W.T. participated in study design, patient care, and laboratory collection; C.-W.Y. and C.-Y.H. collected and managed the MR raw data; H.-F.T. and P.-C.Y. planned, designed, and coordinated the study over the entire period; and all authors took part in revising the report and saw and approved the final version.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Hwei-Fan Tien, No 7, Chung-Shan South Rd, Department of Internal Medicine, National Taiwan University Hospital and College of Medicine, Taipei, Taiwan; e-mail: hftien@ntu.edu.tw; and Pan-Chyr Yang, No 7, Chung-Shan South Rd, Department of Internal Medicine, National Taiwan University Hospital and College of Medicine, Taipei, Taiwan; e-mail: pcyang@ntu.edu.tw.
References
Author notes
*H.-F.T. and P.-C.Y. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal