Abstract
Large granular lymphocyte (LGL) leukemia, or LGLL, is characterized by increased numbers of circulating clonal LGL cells in association with neutropenia, anemia, rheumatoid arthritis, and pulmonary artery hypertension (PAH). Emerging evidence suggests that LGLL cells with a CD8+CD28null phenotype induce these clinical manifestations through direct destruction of normal tissue. Compared with CD8+CD28null T cells from healthy controls, CD8+CD28null T cells from LGLL patients have acquired the ability to directly lyse pulmonary artery endothelial cells and human synovial cells. Here, we show that LGLL cells from patients possess enhanced cytotoxic characteristics and express elevated levels of activating natural killer receptors as well as their signaling partners, DAP10 and DAP12. Moreover, downstream targets of DAP10 and DAP12 are constitutively activated in LGLL cells, and expression of dominant-negative DAP10 and DAP12 dramatically reduces their lytic capacity. These are the first results to show that activating NKR-ligand interactions play a critical role in initiating the DAP10 and DAP12 signaling events that lead to enhanced lytic potential of LGLL cells. Results shown suggest that inhibitors of DAP10 and DAP12 or other proteins involved in this signaling pathway will be attractive therapeutic targets for the treatment of LGLL and other autoimmune diseases and syndromes.
Introduction
Large granular lymphocytic leukemia (LGLL) is a clonal disorder marked by increased numbers of circulating LGLs that have the ability to invade bone marrow, spleen, liver, and lung.1 LGL proliferations are clonally derived from either CD3−/CD56+ or CD3/CD8+ LGLs2-5 and are designated natural killer LGLL (NK-LGLL) and T-cell LGLL (T LGLL), respectively.1,6,7 T LGLL represents roughly 85% of all reported LGLL cases and the clinical course in these patients is generally characterized by recurrent bacterial infections, anemia, neutropenia, rheumatoid arthritis, and occasionally by pulmonary artery hypertension (PAH).2,5,6,8,9 Although aberrant immune tolerance as a result of the malignant cytotoxic CD8+ T cells has been suggested, the molecular mechanisms underlying this pathobiology have not been elucidated.10
Recent studies in LGLL showed that the infiltrating leukemic cells have an association with direct tissue destruction.5,8,11,12 Moreover, activated CD8+CD28null and CD4+CD28null T lymphocytes are commonly overexpressed in autoimmune diseases.9,13-16 By microarray analysis, CD4+CD28null T cells overexpressed perforin and several natural killer receptors (NKRs). Acquisition of non-MHC–restricted direct cytotoxicity against known NK tumor targets, normal tissue epithelial cells, and normal endothelial cells after activation in vitro suggested that these NKRs were functional.17 Activating NKRs typically recognize and bind specific molecules on target cells in the absence of MHC class I or II antigen presentation.18 Activating NKRs such as those of the killer immunoglobulin-like receptor (KIR), NK cytotoxicity receptor (NCR), and CD94-NKG2 families as well as NKG2D mediate NK-cell direct cytotoxicity and may also impart cytotoxic function to these effector T cells.
The binding of activating NKR ligands stimulates a cytoplasmic signaling cascade leading to NK- and T-cell activation and cytotoxicity.19 Activating NKRs typically partner with and signal via membrane-bound adapter proteins that possess canonical cytoplasmic activation motifs. DAP10 and DAP12 are the adapters that partner with most activating NKRs expressed in NK cells and all NKRs expressed in T cells. DAP12 possesses a cytoplasmic immunoreceptor tyrosine-based activation motif (ITAM; D/ExxYxxL/Ix6-12YxxL/I)20 and signals by activating Syk protein tyrosine kinase, phosphoinositide 3-kinase (PI3K), and extracellular signal-regulated kinase (ERK/MAPK). This signaling pathway results in granule mobilization, target cell lysis, and cytokine production.19 DAP10 partners exclusively with NKG2D through interaction with a cytoplasmic PI3K binding motif (YxxM), which recruits PI3K after NKG2D recognizes its specific ligands (eg, MICA/MICB) leading to the phosphorylation of AKT and subsequent target cell lysis and cytokine release.21 Evidence is provided in this study that LGLL cells are CD8+CD28null T cells that constitutively express elevated levels of multiple NKRs as well as DAP10 and DAP12 and display constitutive lytic activity and secrete inflammatory cytokines after ligation to normal epithelial and endothelial cell lines. Moreover, our results suggest that signals initiated by NKRs through DAP10 and DAP12 activation control these potentially harmful events. We conclude that constitutive activation of NKR signals in vivo may be involved in the breakdown of immune tolerance that results in damage to normal tissue. Because DAP10 and DAP12 represent common intermediates of this pathway, they may serve as attractive therapeutic targets for the treatment of LGLL and possibly other autoimmune diseases and syndromes linked to the expansion of autoreactive cytotoxic T cells.
Methods
Patients and preparation of peripheral blood CD8+ T cells and NK cells
Ten untreated patients were enrolled into a national LGLL registry located at the Penn State Cancer Center, College of Medicine. Local approval for use of peripheral blood samples from this registry was granted by the University of South Florida (Tampa, FL) Institutional Review Board committee. Based on increased total numbers of circulating LGL cells in the peripheral blood and presence of T-cell receptor clonality, these patients were confirmed to have the diagnosis of LGLL.4 The absolute lymphocyte counts (ALCs) ranged from 1485 to 12 453 × 109/L (mean = 6873 × 109/L) with the percentage of LGL cells ranging from 34% to 83%. In addition to lymphocytosis, these patients had other clinical symptoms such as anemia, neutropenia, and/or rheumatoid arthritis. Three patients had a clinical course characterized by pulmonary artery hypertension (PAH). Two patients met World Health Organization (WHO) criteria for the diagnosis of PAH but displayed no expansion of LGL cells.22 Informed consent was obtained from all patients to allow the use of their cells in accordance with the Declaration of Helsinki. Leukocyte buffy coats, obtained from healthy volunteers from the Southwest Florida Blood Services (Tampa, FL), were used as controls. Peripheral blood mononuclear cells (PBMCs) were isolated from whole blood of LGLL patients and buffy coats by Ficoll-Hypaque gradient separation, as previously described.23 CD8+ cells were purified using a negative selection process, RosetteSep, as recommended by the manufacturer (Stem Cell Separation Systems, Vancouver, BC). By flow cytometric analysis, we found that more than 90% of the cells isolated using this procedure stained positively for CD8 and CD3 with fewer than 3% CD56+ or CD4+ and 5% CD19+ B cells (Figure 1A). NK cells (CD56+) from healthy blood donors were enriched using a negative selection process, RosetteSep, as recommended by the manufacturer (Stem Cell Separation Systems). Purity of the NK population was found to be at least 95% pure after negative selection based on flow cytometry analysis (data not shown). To sort CD8+CD28null cells from healthy blood donors, 5 × 107 PBMCs were labeled with anti-CD28 conjugated with PE, anti-CD3 conjugated with APC, and anti-CD8 conjugated with APCCY7 antibodies from BD Biosciences (San Jose, CA). DAPI (Invitrogen, Carlsbad, CA) was used as a viability dye to exclude dead cells. T cells were gated by selecting CD3/CD8 double positives and cells were sorted into 2 populations based on CD28 expression.
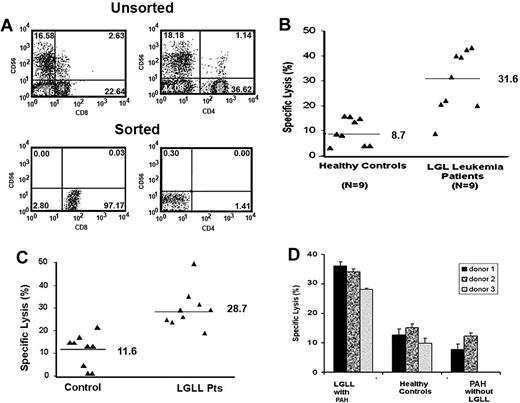
CD8+ T cells from LGLL patients display enhanced lysis of a normal pulmonary artery endothelial cell line. (A) Percentage of CD56+, CD8+, and CD4+ T cells by flow cytometric analysis in unsorted and sorted populations after negative selection using RosetteSep. (B) CD8+ T cells were purified from LGLL patients and healthy blood donors. Direct cytotoxicity was performed using 5-hour 51Cr release assays. CD8+ T cells were incubated with 51Cr-labeled CRL-2598 endothelial cells at an E/T ratio of 50:1, and the CD8+ T-cell–mediated cytolysis of CRL-2598 cells was compared among LGLL patients (n = 9) and healthy controls (n = 9). (C) CD8+ T cells from LGLL patients are cytotoxic toward synovial cells. CD8+ T cells were purified from LGLL patients and healthy blood donors. Direct cytotoxicity assays were performed using 5-hour 51Cr release assays. CD8+ T cells were incubated with 51Cr-labeled HTB293 synovial cells at an E/T ratio of 50:1. CD8+ T-cell–mediated cytolysis of HTB293 cells was compared among 9 LGLL patients and 9 healthy controls. (D) CD8+ T cell–mediated cytolysis of CRL-2598 cells was compared among patients presenting with LGLL and PAH (n = 3), PAH without LGLL (n = 2), and healthy controls (n = 3). The mean and SEM of triplicate wells are shown.
CD8+ T cells from LGLL patients display enhanced lysis of a normal pulmonary artery endothelial cell line. (A) Percentage of CD56+, CD8+, and CD4+ T cells by flow cytometric analysis in unsorted and sorted populations after negative selection using RosetteSep. (B) CD8+ T cells were purified from LGLL patients and healthy blood donors. Direct cytotoxicity was performed using 5-hour 51Cr release assays. CD8+ T cells were incubated with 51Cr-labeled CRL-2598 endothelial cells at an E/T ratio of 50:1, and the CD8+ T-cell–mediated cytolysis of CRL-2598 cells was compared among LGLL patients (n = 9) and healthy controls (n = 9). (C) CD8+ T cells from LGLL patients are cytotoxic toward synovial cells. CD8+ T cells were purified from LGLL patients and healthy blood donors. Direct cytotoxicity assays were performed using 5-hour 51Cr release assays. CD8+ T cells were incubated with 51Cr-labeled HTB293 synovial cells at an E/T ratio of 50:1. CD8+ T-cell–mediated cytolysis of HTB293 cells was compared among 9 LGLL patients and 9 healthy controls. (D) CD8+ T cell–mediated cytolysis of CRL-2598 cells was compared among patients presenting with LGLL and PAH (n = 3), PAH without LGLL (n = 2), and healthy controls (n = 3). The mean and SEM of triplicate wells are shown.
Cytotoxicity assays
Cytotoxicity assays were performed as described previously.24 Briefly, 5-hour 51Cr release assays were performed using CRL-2598, a primary normal human pulmonary artery endothelial cell line, as well as HTB-93, a human synovial cell line, as targets for CD8+ T cells from healthy donors and patients with LGLL. All experiments were performed in triplicate, and the standard deviations (SDs) of all assays were calculated, which were typically approximately 5% of the mean or less.
Western blot analyses
Freshly purified CD8+ T cells from either LGLL patients or healthy blood donors were used for Western blot analysis using previously published methods.23 Proteins were separated by 10% SDS polyacrylamide gel electrophoresis and Western blot analysis was performed with either anti–phosphorylated-ERK1/2 (New England Biolabs, Beverly, MA) or anti–phosphorylated-AKT (Cell Signaling Technology, Danvers, MA). Anti–total ERK, and anti–total AKT antibodies (Cell Signaling Technology) were used to show equal loading.
Surface phenotyping by flow cytometry
Determination of CD28 and NKR expression on CD8+ T cells was carried out using healthy and patient LGL cells by 3-color flow cytometry with anti–CD8-CyChrome, anti–CD3-FITC, and anti-NKR antibodies. The following NKR antibodies were used conjugated to phycoerythrin (PE): anti-CD158a (KIR2DL1, KIR2DS1); anti-CD158b (KIR2DL2, KIR2DL3, KIR2DS2); anti-NKB1 (KIR3DL1), anti-KARp50 (KIR2DS4), anti-NKG2C, and anti-NKG2D, which were all obtained from BD Biosciences. Data acquisition and analysis was carried out on a FACScan flow cytometer (BD Biosciences) using the Cell Quest software program (BD Biosciences).
Analysis of mRNA expression by real-time quantitative RT-PCR
Total RNA was prepared from CD8+ T cells from healthy control and LGLL patients using Trizol-Reagent according to the manufacturer's instructions (Life Technologies, Bethesda, MD). Reverse-transcription (RT) reactions were performed using iScriptTM cDNA Synthesis kit (Bio-Rad, Hercules, CA). Oligonucleotide primers for amplifying DAP10 were DAP10-F (5′-GGC TGC AGC TCA GAC GAC-3′) and DAP10-R (5′-AGG AGC GGC AGA GAG AGG-3′). Primers for amplifying DAP12 were DAP12-F (5′-GAG ACC GAG TCG CCT TAT C-3′) and DAP12-R (5′-ATA CGG CCT CTG TGT GTT G-3′). Quantitative RT–polymerase chain reaction (qRT-PCR) reactions were performed by iQ SYBR Green Supermix of Bio-Rad. A negative control without cDNA template was run with every assay. Transcript copy number per subject was calculated by normalization to GAPDH expression.
Vaccinia viral delivery of dominant-negative proteins
Recombinant expression cassettes encoding FLAG-tagged human DAP12 and DAP10 were constructed in the pLF plasmid (a derivative of pcDNA3; Invitrogen) as described25 and mutagenized with the QuickChange Site Directed Mutagenesis Kit (Stratagene, La Jolla, CA). Vaccinia virus expressing CD56, a LGL-specific surface marker,26 was used as a control for nonspecific effects of viral infection. The generation of and infection with vaccinia virus have been described previously.21,27-29
Immunostaining
CD8+ T cells from healthy donors and from LGLL patients were incubated with CRL-2598 cells at a 1:1 ratio. Cell-Tracker Orange (Molecular Probes, Eugene, OR) was used to prestain CRL-2598 cells. An antigranzyme antibody and secondary goat anti–mouse Ig FITC (Sigma-Aldrich, St Louis, MO) was used to visualize granzyme in lytic granules. Samples were viewed with a Leica DMLB upright fluorescence microscope with a 100×/1.3 NA oil-immersion objective (Leica Microsystems, Germany), and DAPI, FITC, and Texas Red filter cubes. Images were produced using the Retiga 1300 CCD camera (QIamging, Surrey, BC) and Iplab version 3.1 software suite (BD Bioscience Imaging, Rockville, MD). On each slide, 100 cells were counted for polarized granule movement. Nonspecific binding with secondary antibody alone was not detected (data not shown).
ELISAs
Concentrations of RANTES and IL-8 were determined by enzyme-linked immunosorbent assay (ELISA) as recommended by the manufacturers (Human RANTES ELISA Construction Kit [Antigenix America, Huntington Station, NY]; IL-8 BD OptEIA ELISA Kit [BD Bioscience]).
Statistical analysis
The nonparametric Mann-Whitney-Wilcoxon rank sum test was used to compare the median expression of cell lyses in patients and in healthy donors at each target. The null hypothesis of same median expression level in the healthy donors and in the patients was tested against a 2-sided alternative. The same test was conducted to compare the median expression of the patients with that of the healthy donors for each of the 10 NK receptors. Our study is exploratory in nature and the false discovery rate is controlled at 5% to adjust for multiple hypothesis testing.
Results
CD8+ T cells from LGLL patients display enhanced lysis of pulmonary artery endothelial cells
Unknown mechanisms generate anemia, neutropenia, rheumatoid arthritis, and PAH in patients with LGLL. We hypothesize that leukemic T LGL cells infiltrate into bone marrow, joint synovium, arteries, and lung where they mediate the direct autoimmune destruction of normal tissue.5,8,11,12 In this study, we examined the ability of CD8+ T cells (effector) from T LGLL patients and healthy donors to lyse CRL-2598 cells (target), which were derived from normal pulmonary artery endothelial cells. Lytic function against CRL-2598 cells was assessed in 5-hour 51Cr release assays using effector-to-target (E/T) cell ratios of 6:1, 12:1, 25:1, and 50:1. At a 50:1 E/T ratio, CRL-2598 cell lysis was significantly greater by CD8+ T cells from LGLL patients (median, 31.6%; range, 10.3% to 42.5%) compared with healthy donors (median, 8.7%; range, 3.2%-15.8%; P = .002; Figure 1B). This difference in lyses was also observed at 25:1 and 12:1 E/T ratios (data not shown). To assess whether this was an unusual property of the CRL-2598 cells, parallel studies were conducted using the synovial cell line HTB293 and similar results were observed between patients (median, 28.7%; range, 25.5% to 47.8%) and healthy donors (median, 11.6%; range, 1.1-21.2%; P < .001; Figure 1C).
There is an increased risk of PAH in patients with LGLL and previous studies suggested that the leukemic cells may have an association with tissue injury in LGLL.5,8,11,12 Therefore, we investigated whether the observed increase in T-cell lytic function is specific for LGLL or also associates with PAH. The cytotoxicity of CD8+ T cells toward CRL-2598 cells was compared among patients presenting with both LGLL and PAH, patients presenting with PAH (without LGLL), and healthy donors. A greater percentage of CRL-2598 killing was observed using T cells from patients presenting with both LGLL and PAH (Figure 1D), suggesting that CD8+ T cells from LGLL patients have an overall elevated cytotoxicity against CRL-2598 monitored by a 5-hour 51Cr release assay.
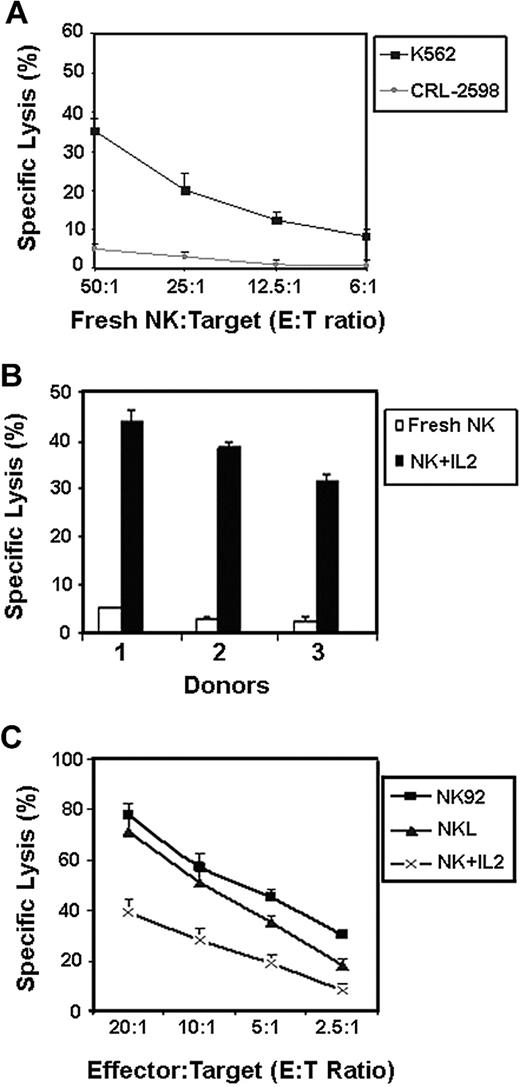
Stress signals from tissue epithelial and endothelial cells have been shown to trigger cytotoxicity by NK cells.9,30,31 To determine whether pulmonary artery endothelial cells serve as targets for normal NK cells, we compared the lysis of CRL-2598 cells with that of K562 cell line. In this assay, K562 cells represent a well-known NK-cell target and were used as a control for NK-cell function. In 5-hour 51Cr release assays, we found that freshly isolated NK cells efficiently lyse K562 cells, but failed to lyse the CRL-2598 cells (Figure 2A). Activation of the normal NK cells, by addition of interleukin-2 (IL-2) for 3 days before the cytotoxicity assays, resulted in greater cytolysis of the CRL-2598 cells (Figure 2B). Similarly, the IL-2–dependent NK-cell lines NK92 and NKL displayed effective lysis of CRL-2598 cells (Figure 2C). Collectively, these results showed that the CRL-2598 cell line is not a natural target for healthy NK or CD8+ T cells (shown in Figures 1 and 2) under nonactivating conditions but develops lytic susceptibility after activation.
Activated NK cells, but not freshly isolated NK cells, lyse CRL-2598 cells. NK cells isolated from healthy blood donors were either untreated (fresh) or treated with 100 U/mL IL-2 for 3 days and then tested for their ability to lyse 51Cr-labeled CRL-2598 cells or K562 (NK-sensitive target) tumor cells. (A) Fresh NK cells were used in direct cytotoxicity assays against K562 and CRL-2598 at the E/T ratios indicated. Results from 1 representative experiment are shown; results from 2 different control subjects yielded similar results (not shown). (B) Percentage specific lysis of CRL-2598 cells was compared between IL-2–treated and fresh NK cells using an E/T ratio of 50:1. Data include results from 3 healthy control subjects. The mean and SEM of triplicate wells are shown. (C) Percentage specific lysis of CRL-2598 cells by the activated NK-cell lines, NK92 or NKL, at E:T ratios of 20:1, 10:1, 5:1, and 2.5:1. The mean and SEM of triplicate wells are shown.
Activated NK cells, but not freshly isolated NK cells, lyse CRL-2598 cells. NK cells isolated from healthy blood donors were either untreated (fresh) or treated with 100 U/mL IL-2 for 3 days and then tested for their ability to lyse 51Cr-labeled CRL-2598 cells or K562 (NK-sensitive target) tumor cells. (A) Fresh NK cells were used in direct cytotoxicity assays against K562 and CRL-2598 at the E/T ratios indicated. Results from 1 representative experiment are shown; results from 2 different control subjects yielded similar results (not shown). (B) Percentage specific lysis of CRL-2598 cells was compared between IL-2–treated and fresh NK cells using an E/T ratio of 50:1. Data include results from 3 healthy control subjects. The mean and SEM of triplicate wells are shown. (C) Percentage specific lysis of CRL-2598 cells by the activated NK-cell lines, NK92 or NKL, at E:T ratios of 20:1, 10:1, 5:1, and 2.5:1. The mean and SEM of triplicate wells are shown.
Constitutive activation of the PI3K and ERK pathways in CD8+ T cells from LGLL patients
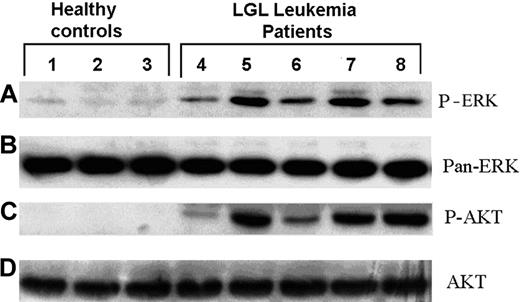
Our previous studies showed that NK cells display PI3K and ERK phosphorylation, and thus activation, immediately after target ligation by Raji tumor cells. These early phosphorylation events are necessary for cytotoxic granule redistribution and serve a nonredundant role in direct tumor lysis.21,24 There is a possibility that T LGLL cells kill normal CRL-2598 cells and the synovial cell line HTB293 by the same molecular mechanism that is constitutively activated in vivo. To determine the activation of PI3K and ERK pathways within T cells from LGLL patients, the constitutive phosphorylation status of ERK and AKT was assessed. Because PI3K is an upstream signaling factor for Ser473 AKT phosphorylation, phosphorylation of AKT was used as a surrogate marker of PI3K activation. Freshly isolated CD8+ T cells were removed from blood samples of healthy donors and LGLL patients and examined for ERK and AKT phosphorylation. Similar to previous reports,23 T cells from LGLL patients possessed greater amounts of constitutively phosphorylated ERK and AKT by Western blot analysis in the absence of NKR or target cell cross-linking (Figure 3A,C). Although the level of phosphorylated ERK and AKT is increased in cells from patients, there was no difference detected in the total level of these proteins between patients and healthy controls (Figure 3B,D). These results demonstrate that the ERK and AKT/PI3K pathways are constitutively activated in LGLL cells that may reflect exposure to activating signals in vivo.
CD8+ T cells from LGLL patients maintain constitutively activated ERK1/2 and PI3K. CD8+ T-cell lysates were prepared from LGLL patients and healthy donors. The activity of ERK and PI3K in these cells was assessed by Western blot analyses using antibodies specific for (A) phosphorylated ERK1/2 (pERK) and (C) phosphorylated AKT. Membranes were stripped and reprobed with antibodies that detect (B) pan-ERK and (D) pan-AKT to demonstrate total protein levels.
CD8+ T cells from LGLL patients maintain constitutively activated ERK1/2 and PI3K. CD8+ T-cell lysates were prepared from LGLL patients and healthy donors. The activity of ERK and PI3K in these cells was assessed by Western blot analyses using antibodies specific for (A) phosphorylated ERK1/2 (pERK) and (C) phosphorylated AKT. Membranes were stripped and reprobed with antibodies that detect (B) pan-ERK and (D) pan-AKT to demonstrate total protein levels.
CD8+ CD28null phenotype characterizes leukemic LGL cells
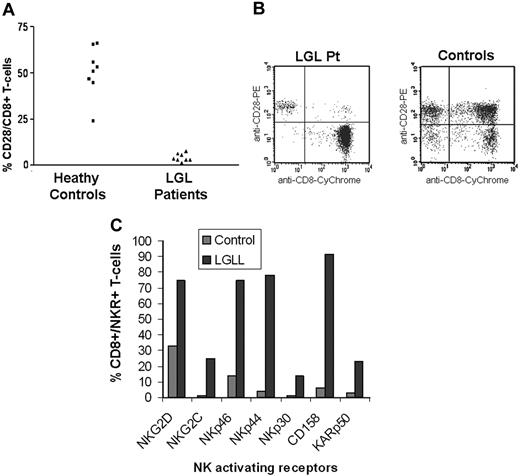
Our observation that CD8+ T cells in LGLL are responsible for increased cytotoxicity suggests that there is an accumulation and expansion of activated effector cells. One of the major changes that occurs after chronic T-cell activation and cancerous conditions is the down-regulation of CD28 and expansion of CD8+CD28null T cells.26,32-36 Consistent with previous findings, CD8+CD28null T cells were dramatically overrepresented in the peripheral blood of LGLL patients compared with healthy donors, as shown in Figure 4A,B. The absolute number of CD8+CD28null T cells present in the blood was greater in LGLL patients compared with healthy donors and nearly all of the patients' CD8+ T cells lacked CD28 expression. There are small numbers of CD8+CD28null present in the healthy subjects and it is therefore possible that the elevated cytotoxicity of CD8+ T LGLL cells against CRL2598 may be due simply to the number of CD8+CD28null cells in the peripheral blood. To address this question, we sorted CD8+CD28null T cells from 5 healthy blood donors and examined their cytolytic function against CRL2598. CD8+CD28null cells display only background levels of cytotoxicity even at high E/T ratios (50:1) without in vitro stimulation. Moreover, there was no significant difference in cytotoxicity observed among purified CD8+ cells, CD8+CD28+ cells, and CD8+CD28null T cells (data not shown) in healthy donors. Results of these studies indicate that a unique molecular mechanism, rather than overrepresentation of CD8+CD28null T cells, generates the aggressive cytotoxic phenotype observed in LGLL patients.
A CD8+CD28null phenotype and increased NKR expression characterizes T-LGLL cells. (A) CD8+ T cells isolated from LGLL patients and healthy donors were analyzed by 2-color flow cytometry for the cell surface expression of CD28. (B) Dot plots of CD8+/CD28+ expression are shown for 1 control and 1 LGLL patient. (C) The presence of activating NKRs on CD8+ cells were analyzed using flow cytometry. Results from 1 LGLL patient and 1 control are shown. Results from 10 patients are summarized in Table 1.
A CD8+CD28null phenotype and increased NKR expression characterizes T-LGLL cells. (A) CD8+ T cells isolated from LGLL patients and healthy donors were analyzed by 2-color flow cytometry for the cell surface expression of CD28. (B) Dot plots of CD8+/CD28+ expression are shown for 1 control and 1 LGLL patient. (C) The presence of activating NKRs on CD8+ cells were analyzed using flow cytometry. Results from 1 LGLL patient and 1 control are shown. Results from 10 patients are summarized in Table 1.
CD8+ T-LGLL cells express elevated levels of multiple activating NKRs
Both CD4+ and CD8+ T lymphocytes are able to mediate lysis of normal tissue epithelial cells and endothelial cells in certain chronic infectious and inflammatory diseases using NK activating pathways.9,13-15 This activation can be initiated by activating NKRs that are expressed on both NK and T cells. Tissue epithelial or endothelial cells respond to stress by the display of cell surface ligands for activating NKRs leading to the recognition of activated NK cells and effector T cells. There is the possibility that CD8+CD28null T cells in LGLL patients acquire non-MHC–restricted target cell cytotoxicity through the induction of activating NKR expression. Our data indicate that CRL-2598 cells trigger NKR-associated signaling pathways within activated NK cells to stimulate granule redistribution and cytotoxicity (Figures 2,3). Therefore, to determine whether the CD8+CD28null T-cell populations from LGLL patients display elevated levels of NKRs, we examined the surface expression of NKG2D, NKG2C, NKp46, NKp44, NKp30, CD158b (KIR2DL2/3/KIR2DS2), and KARp50 (KIR2DS4) on CD8+ T cells from LGLL patients (which are primarily CD28null) and healthy controls using flow cytometry analysis. Because the percentage of NKR-expressing T cells may change with age, we compared the percentage of NKR+ T cells from LGLL patients with healthy donors of a similar age that ranged from 60 to 70 years. NKRs that were displayed at 2-times greater than the mean plus the standard deviation of the control population are shown in Table 1. CD8+ T cells from 10 LGLL patients displayed at least 1 and often several activating NKRs on the cell surface (Table 1). The result from 1 representative patient is shown in Figure 4C.
Expression of NK-activating receptors on CD8+ T LGLL patients
| NKR . | Patient 1 . | Patient 2 . | Patient 3 . | Patient 4 . | Patient 5 . | Patient 6 . | Patient 7 . | Patient 8 . | Patient 9 . | Patient 10 . | Mean controls,* n = 10 . | SD . | P† . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NKG2D | 76 | 76 | 67 | 92 | 43 | 98 | 81 | 28.1 | 5.2 | .001 | |||
| CD94 | 28 | 34 | 62 | 39 | 90 | 61 | 45 | 15.6 | 5.6 | .049 | |||
| NKG2C | 25 | 38 | 86 | 6 | 6 | 4.3 | 2.1 | .162 | |||||
| NKp44 | 5 | 10 | 1.2 | 1.0 | .241 | ||||||||
| NKp46 | 11 | 35 | 3 | 33 | 14 | 1.7 | 1.1 | .005 | |||||
| NKp30 | 7 | 5 | 73 | 4 | 6 | 7 | 1.4 | 0.8 | .006 | ||||
| KIR2DL1/2DS1‡ | 8 | 4 | 4 | 0.6 | 0.5 | .677 | |||||||
| KIR2DL2/3 | |||||||||||||
| 2DS2§ | 10 | 91 | 89 | 2.8 | 1.6 | .821 | |||||||
| KIR2DS4‖ | 14 | 23 | 2.1 | 0.9 | .496 | ||||||||
| KIR3DL1¶ | 0.9 | 0.7 | .406 |
| NKR . | Patient 1 . | Patient 2 . | Patient 3 . | Patient 4 . | Patient 5 . | Patient 6 . | Patient 7 . | Patient 8 . | Patient 9 . | Patient 10 . | Mean controls,* n = 10 . | SD . | P† . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NKG2D | 76 | 76 | 67 | 92 | 43 | 98 | 81 | 28.1 | 5.2 | .001 | |||
| CD94 | 28 | 34 | 62 | 39 | 90 | 61 | 45 | 15.6 | 5.6 | .049 | |||
| NKG2C | 25 | 38 | 86 | 6 | 6 | 4.3 | 2.1 | .162 | |||||
| NKp44 | 5 | 10 | 1.2 | 1.0 | .241 | ||||||||
| NKp46 | 11 | 35 | 3 | 33 | 14 | 1.7 | 1.1 | .005 | |||||
| NKp30 | 7 | 5 | 73 | 4 | 6 | 7 | 1.4 | 0.8 | .006 | ||||
| KIR2DL1/2DS1‡ | 8 | 4 | 4 | 0.6 | 0.5 | .677 | |||||||
| KIR2DL2/3 | |||||||||||||
| 2DS2§ | 10 | 91 | 89 | 2.8 | 1.6 | .821 | |||||||
| KIR2DS4‖ | 14 | 23 | 2.1 | 0.9 | .496 | ||||||||
| KIR3DL1¶ | 0.9 | 0.7 | .406 |
CD8+/CD3+ mean percentage positive and SD of the reference control group (n = 10) of a similar age range. (Note: Where percentage is shown, the percentage of CD3+/CD8+/ NKR+ cells was more than 2 SD above the mean of CD3+/CD8+ T cells from the control group.)
Median difference between the patient and the control groups was tested using Mann-Whitney-Wilcoxon statistics.
Antibody reaction with anti-CD158a that recognizes KIR2DL1 (inhibitory) and KIR2DS1 (activating) receptors.
Antibody reaction with anti-CD158b that recognizes KIR2DL2, KIR2DL3 (both inhibitory), and KIR2DS2 (activating) receptors.
Antibody reaction with anti-KARp50 that recognizes KIR2DS4.
Antibody reaction with anti-NKB1 that recognizes KIR3DL1.
CD8+ T LGLL cells express elevated levels of DAP12 and DAP10
To further investigate the signaling potential of activating NKRs within T LGLL cells, we assessed the constitutive mRNA expression of DAP10 (encoded by the HCST gene) and DAP12 (encoded by the TYROBP gene) in patients compared with healthy controls. Activating NKRs lack cytoplasmic signaling motifs and partner with the transmembrane adaptor proteins DAP12 or DAP10.19 Recent studies have shown DAP12 and DAP10 regulation in T cells occurs primarily at the transcriptional level.37,38 Given the critical roles of DAP12 and DAP10 in NK-mediated signaling, we quantified the mRNA expression of these molecules by qPCR using CD8+ T cells from LGL leukemia patients and healthy controls. compared with healthy controls, our results showed that DAP12 expression is higher in CD8+ T cells from LGLL patients. Similarly, DAP10 was expressed at higher levels in CD8+ T cells from 5 patients examined (Figure 5).
CD8+ T cells from LGLL patients display elevated levels of DAP10 and DAP12. Quantitative RT-PCR was used to determine the relative levels of (A) DAP12 and (B) DAP10 expression in CD8+ T cells from 5 LGLL patients compared with healthy donors (controls). The mean and SEM of 5 healthy donors and LGLL patients are shown.
CD8+ T cells from LGLL patients display elevated levels of DAP10 and DAP12. Quantitative RT-PCR was used to determine the relative levels of (A) DAP12 and (B) DAP10 expression in CD8+ T cells from 5 LGLL patients compared with healthy donors (controls). The mean and SEM of 5 healthy donors and LGLL patients are shown.
Dominant-negative DAP12 and DAP10 block NKR-mediated signaling, cytotoxicity, and granule redistribution
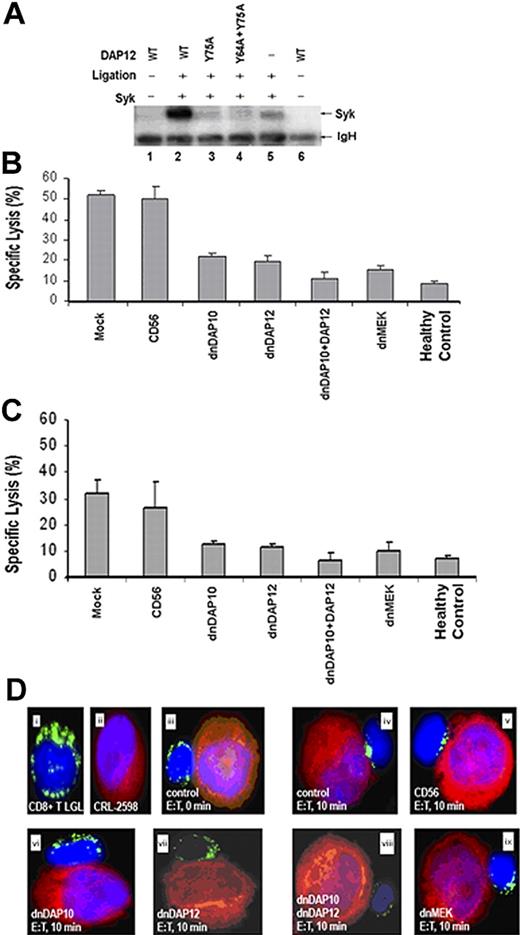
Our data showed that CD8+ T cells in LGLL patients lysed pulmonary artery endothelial and synovial cells and express elevated levels of activating NKRs, DAP10, and DAP12 compared with healthy donors. To further examine whether signaling by DAP10 and DAP12 controls target cell lysis, dominant-negative (dn) constructs were generated for these proteins.
A single cytoplasmic ITAM domain is present in DAP12 that functionally relies on residues Y64 and Y75. As mutations at either of these sites have been shown to block ITAM signaling,39-42 Y→A mutations were introduced at the Y75 and Y64 sites. Single and double mutations were constructed to create the DAP12Y75A and DAP12Y64A;Y75A dnDAP12 expression constructs (plasmids). The dominant-negative functionality of these constructs was confirmed by transfecting them into AD293 cells and demonstrating that they are unable to stimulate the phosphorylation of the well-characterized downstream target Syk (Figure 6A).28 Using the same strategy, a dnDAP10 expression construct (plasmid) was constructed containing a Y→A mutation within the cytoplasmic YxxM motif and demonstrated to also have a dominant-negative affect (data not shown). The dnDAP12 and dnDAP10 expression constructs were then shuttled into vaccinia viral vectors and used to generate recombinant vaccinia viruses for infection into primary CD8+ T cells from LGLL patients and healthy controls. Vaccinia virus gene delivery was used because of an efficient rate of infection without cytokine exposure and infectivity of diverse cells including all subtypes of primary T cells. Moreover, the DNA replicative cycle of the virus takes place in the cytoplasm of the infected host cell, and the recombinant protein is produced in high quantities through viral-mediated transcription. A short period of incubation is therefore required to achieve significant foreign protein expression (4-6 hours) independent of the biosynthetic capacity of the target cell.43
Expression of dnDAP12 and/or dnDAP10 in CD8+ T cells from LGLL patients reduces adaptor signaling, cytotoxicity toward CRL-2598 cells, and granule redistribution. (A) Expression of dnDAP12 blocks Syk phosphorylation. AD293 cells were cotransfected with plasmids encoding Syk and 1 of the following FLAG-tagged DAP12 constructs: wild type (WT, lanes 2), DAP12Y75A (lane 3), or DAP12Y64A;Y75A (lane 4). Single transfections with Syk and WT DAP12 are included as negative controls (lanes 1, 5, and 6). After cross-linking with anti-Flag antibody, DAP12 was immunoprecipitated before Western blot analyses with an antiphosphotyrosine antibody (4G10). The blot was subsequently stripped and reprobed with an anti-Syk antibody confirming that the phosphorylated protein is Syk (data not shown). (B) Expression of dnDAP10 and dnDAP12 reduce the cytotoxic potential of CD8+ T cells from LGLL patients. The cytotoxic properties of genetically modified CD8+ T cells from LGLL patients were evaluated in 5-hour 51Cr release assays. CD8+ cells were infected with recombinant vaccinia virus encoding CD56 (negative control), dnDAP10, dnDAP12, or a combination of dnDAP10 and dnDAP12(Y75A), as well as dnMEK (positive control) and mixed at an E/T ratio of 50:1 with CRL-2598 target cells. Mock-infected CD8+ T cells from LGLL patients and healthy controls are included for comparison. Representative results from 1 LGLL patient are shown: cells from 5 other patients yielded similar results. The inhibitory effects of dnDAP10 and dnDAP12 occurred at E/T cell ratios ranging from 50:1 to 6:1 (data not shown). The mean and SEM of triplicate wells are shown. (C) Expression of dnDAP12 and/or dnDAP10 in CD8+ T cells from LGLL patients reduces cytotoxicity toward synovial cells (HTB293). Direct cytotoxicity was performed using 5-hour 51Cr release assays as described in Figure 7B. Results from 1 representative patient and healthy control are shown: similar results were obtained from 5 additional patients. The mean and SEM of triplicate wells are shown. (D) Target cell–induced granule redistribution is blocked in CD8+ T LGLL expressing dnDAP10 and/or dnDAP12. A proportion of the cells described in panel B were mixed at 1:1 E/T ratio and examined for granule redistribution. Cells were cytospun onto slides and lytic granules visualized with (i) a FITC-conjugated anti–granzyme B antibody (green). (ii) CRL-2598 target cells were prestained with Cell-Tracker Orange (red: Molecular Probes). DAPI was used for nuclear staining (blue) of both cell types. Results using untreated CD8+ T LGLL cells are shown at (iii) 0 and (iv) 10 minutes after mixing. Representative results using CD8+ T LGLL cells expressing (v) CD56 (negative control), (vi) dnDAP10, (vii) dnDAP12(Y75A), (viii) dnDAP10, and dnDAP12(Y75A) and (ix) dnMEK are shown. Results from 1 representative experiment are shown: results from 4 other patients yielded similar results (data not shown).
Expression of dnDAP12 and/or dnDAP10 in CD8+ T cells from LGLL patients reduces adaptor signaling, cytotoxicity toward CRL-2598 cells, and granule redistribution. (A) Expression of dnDAP12 blocks Syk phosphorylation. AD293 cells were cotransfected with plasmids encoding Syk and 1 of the following FLAG-tagged DAP12 constructs: wild type (WT, lanes 2), DAP12Y75A (lane 3), or DAP12Y64A;Y75A (lane 4). Single transfections with Syk and WT DAP12 are included as negative controls (lanes 1, 5, and 6). After cross-linking with anti-Flag antibody, DAP12 was immunoprecipitated before Western blot analyses with an antiphosphotyrosine antibody (4G10). The blot was subsequently stripped and reprobed with an anti-Syk antibody confirming that the phosphorylated protein is Syk (data not shown). (B) Expression of dnDAP10 and dnDAP12 reduce the cytotoxic potential of CD8+ T cells from LGLL patients. The cytotoxic properties of genetically modified CD8+ T cells from LGLL patients were evaluated in 5-hour 51Cr release assays. CD8+ cells were infected with recombinant vaccinia virus encoding CD56 (negative control), dnDAP10, dnDAP12, or a combination of dnDAP10 and dnDAP12(Y75A), as well as dnMEK (positive control) and mixed at an E/T ratio of 50:1 with CRL-2598 target cells. Mock-infected CD8+ T cells from LGLL patients and healthy controls are included for comparison. Representative results from 1 LGLL patient are shown: cells from 5 other patients yielded similar results. The inhibitory effects of dnDAP10 and dnDAP12 occurred at E/T cell ratios ranging from 50:1 to 6:1 (data not shown). The mean and SEM of triplicate wells are shown. (C) Expression of dnDAP12 and/or dnDAP10 in CD8+ T cells from LGLL patients reduces cytotoxicity toward synovial cells (HTB293). Direct cytotoxicity was performed using 5-hour 51Cr release assays as described in Figure 7B. Results from 1 representative patient and healthy control are shown: similar results were obtained from 5 additional patients. The mean and SEM of triplicate wells are shown. (D) Target cell–induced granule redistribution is blocked in CD8+ T LGLL expressing dnDAP10 and/or dnDAP12. A proportion of the cells described in panel B were mixed at 1:1 E/T ratio and examined for granule redistribution. Cells were cytospun onto slides and lytic granules visualized with (i) a FITC-conjugated anti–granzyme B antibody (green). (ii) CRL-2598 target cells were prestained with Cell-Tracker Orange (red: Molecular Probes). DAPI was used for nuclear staining (blue) of both cell types. Results using untreated CD8+ T LGLL cells are shown at (iii) 0 and (iv) 10 minutes after mixing. Representative results using CD8+ T LGLL cells expressing (v) CD56 (negative control), (vi) dnDAP10, (vii) dnDAP12(Y75A), (viii) dnDAP10, and dnDAP12(Y75A) and (ix) dnMEK are shown. Results from 1 representative experiment are shown: results from 4 other patients yielded similar results (data not shown).
Vaccinia viruses encoding recombinant Flag-tagged dnDAP10, dnDAP12, or an irrelevant gene (CD56) were used to infect CD8+ T cells before 5-hour 51Cr release assays. Using our vaccinia viral gene delivery system, Flag-tagged DAP10 and DAP12 expression was routinely detected in 80% of the CD8+ cells after 6 hours of infection as determined by flow cytometry (data not shown).44 Using these recombinant viruses, expression of both dnDAP12 and dnDAP10 in CD8+ T LGLL cells reduced the percentage of CRL-2598 lysed by at least 50% compared with mock- and CD56 control virus–infected T cells (Figure 6B). Coexpression of both dnDAP12 and dnDAP10 further reduced cytolysis of target cells by CD8+ T cells from patients. Moreover, lytic function by LGLL cells in the presence of dnDAP12 combined with dnDAP10 was similar to lysis by T cells from healthy donors, suggesting more than 1 type of activating NKR contributes to this lysis. Expression of dnMEK,45 a signaling component downstream of DAP10 and DAP12, in T LGLL cells also blocked lyses of CRL-2598 cells, suggesting that the signaling pathway used by T LGL cells is similar to that of NK-mediated cytotoxicity against tumor cells and CRL-2598 (Figure 6B). In addition to these results in CRL-2598, we found that expression of dnDAP12, dnDAP10, and dnMEK also reduced T LGLL–mediated cytotoxicity of HTB293 synovial target cells (Figure 6C).
Next, the dependence of DAP10 and DAP12 signaling for granule mobilization within T LGLL cells in response to target conjugation was assessed. T LGLL cells were mixed at a 1:1 ratio with CRL-2598 target cells and granzyme B polarization examined by immunofluorescent staining (Figure 6D). Immediately after T LGLL conjugation to CRL-2598 cells, granzyme B–containing granules were evenly distributed within the cytoplasm of T LGLL cells from patients (Figure 6Diii). After a 10-minute incubation period, granzyme B demonstrated rapid redistribution to the site of target contact (Figure 6Div). Expression of dnDAP10, dnDAP12, or a combination of both dnDAP10 and dnDAP12 in CD8+ T LGLL cells effectively blocked granzyme B redistribution, suggesting that multiple activating NKRs control granule movement (Figure 6Dvi-viii). Expression of the functionally unrelated CD56 control virus in T LGLL cells failed to block granule redistribution. As a positive control, expression of dnMEK successfully blocked redistribution of cytolytic granzyme B–containing granules (Figure 6Dv,ix). To obtain quantitative data, the number of effector-target conjugates with mobilized granules were directly counted out of 100 cell pairs after 10 minutes of incubation. We found that 41% of CD56vv-treated compared with 25% infected with dnDAP12, 24% infected with dnDAP10, and 17% infected with dnDAP12+dnDAP10 displayed mobilized granules. Granule mobilization in cells infected with dnMEK was 23% of 100 effector-target conjugates, which was used here as a positive control. These results show that disruption of early signaling events mediated by NKR effectively blocked the redistribution of lytic granules within T LGLL cells.
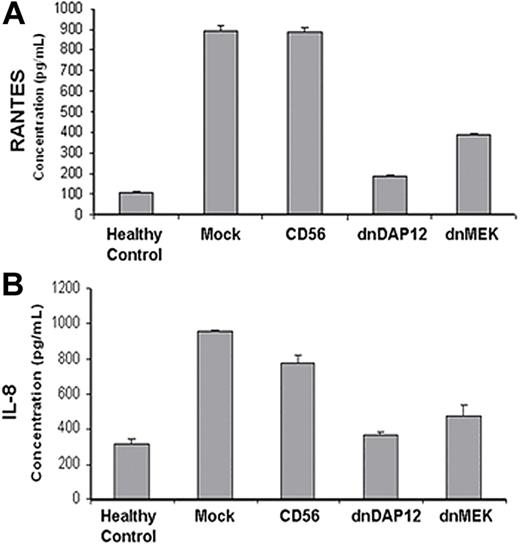
PBMCs from LGLL patients release elevated levels of chemokines that can be inhibited by dnDAP12 expression
LGLL is characterized by constitutive production of proinflammatory cytokines that may potentially contribute to the local damage within the lung, synovial tissue, and bone marrow.46 DAP12 is not only expressed in NK and T cells but also expressed in monocytes, dendritic cells, and neutrophils where activation leads to a signaling cascade that culminates in chemokine production of RANTES (regulated on activation, normal T expressed and secreted) and IL-8 among others.47-49 PBMCs isolated from LGLL patients were infected with recombinant viruses and cultured for 24 hours. Culture supernatants from each patient and healthy control were then analyzed for the release of RANTES and IL-8 using ELISA from triplicate samples. The amount of RANTES and IL-8 was higher in PBMCs from LGLL patients compared with healthy donors (Figure 7A,B). The functional contribution of DAP12 and MEK activation was assessed by comparing the release of chemokines after infection with dnDAP12 and dnMEK compared with mock-infected and CD56 control virus–infected cells in patient PBMCs before chemokine analysis. Our data showed that expression of dnDAP12 or dnMEK suppressed RANTES and IL-8 release by PBMCs from LGLL patients (Figure 7A,B). Collectively, these results show that DAP12- and DAP10-initiated signaling events are necessary not only for granzyme B–containing granule mobilization and cytotoxicity by LGLL cells but also chemokine release by PBMCs from LGLL patients.
Expression of dnDAP12(Y75A) in PBMCs from LGLL patients reduces their production of chemokines. PBMCs from LGLL patients were infected with recombinant retrovirus as described in panel B and supernatants collected at 24 hours and analyzed for (A) RANTES and (B) IL-8 production by ELISA. Viability of PBMCs was confirmed to be more than 80% at time of ELISA analyses by annexin-V–FITC labeling. ELISA results are shown from 1 representative LGLL patient; PBMCs from 2 other patients were assessed yielding similar results. The mean and SEM of triplicate wells are shown.
Expression of dnDAP12(Y75A) in PBMCs from LGLL patients reduces their production of chemokines. PBMCs from LGLL patients were infected with recombinant retrovirus as described in panel B and supernatants collected at 24 hours and analyzed for (A) RANTES and (B) IL-8 production by ELISA. Viability of PBMCs was confirmed to be more than 80% at time of ELISA analyses by annexin-V–FITC labeling. ELISA results are shown from 1 representative LGLL patient; PBMCs from 2 other patients were assessed yielding similar results. The mean and SEM of triplicate wells are shown.
Discussion
Autoimmune disease, particularly rheumatoid arthritis and PAH, is often a central clinical feature of LGLL.2,4,50 Such an exacerbated immune reaction against self-tissue is the pathogenic mechanism underlying a variety of human diseases and is positively linked to the expansion of CD4+ and/or CD8+CD28null T cells.9,51-54 Data presented here showed that CD8+CD28null leukemic T LGL cells constitutively killed a pulmonary artery endothelial target cell line and a synovial target cell line in vitro, without requiring activation by IL-2. Cytotoxicity exhibited by T LGLL cells was inherently different from purified CD8+CD28null cells from healthy donors, suggesting that a unique mechanism may mediate the cytotoxicity and may contribute to the disease pathogenesis.
CD8+ cytotoxic T lymphocytes (CTLs) share a common killing mechanism with NK cells. Our data demonstrated that the CD8+ T cells from LGLL patients possess higher levels of multiple NKRs on their cell surface, express higher levels of DAP10 and DAP12 mRNAs, and display constitutive activity of multiple signaling intermediates within the NKR/DAP10/DAP12 signaling cascade compared with CD8+ T cells from healthy control subjects. These observations suggest that NKR and their associated activating adaptors must play a unique role in cytotoxicity by this cell population compared with CD8+CD28null T cells that are normally present in the peripheral blood of healthy subjects. Blockade of DAP10/DAP12 signaling pathways in CD8+ T LGLL cells with dominant-negative forms of each protein demonstrated that these pathways are directly linked to PI3K and ERK1/2 activation, granule polarization, cytotoxicity, and cytokine production. Our data support the observation that activating NKR signaling in T cells occurs via DAP10/DAP12 and can occur independently from TCR engagement or antigen presentation.53 Therefore, the NKR-DAP10/DAP12 signaling pathways, which are essential events for NK-cell function, also play critical roles during T-cell activation in patients with LGLL. Abridged tolerance through this NKR-generated signaling pathway may represent the key event in inflammation and tissue destruction associated with autoimmunity.
The fact that DAP10 and DAP12 play key regulatory roles in CD8+ T cell–mediated lysis of endothelial and synovial cells in LGLL suggests that pharmacologic therapies targeting these adaptor proteins may provide clinical benefit to patients with other inflammatory diseases. Pharmacologic inhibition of the ubiquitous signaling molecules such as PI3K and ERK1/2 that control numerous vital cell functions such as proliferation, differentiation, and survival, are less than ideal as therapeutic targets. Identification of the signaling pathways responsible for inflammatory cytokine production, granule release, and cytolysis of noncancerous target cells by CD8+CD28null or CD4+CD28null T cells represents a significant advancement in our understanding of autoimmune pathobiology.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by National Institutes of Health (NIH, Bethesda, MD) grant AI056213, Veterans' Administration Hospital (Tampa, FL), and National Cancer Institute (NCI, Bethesda, MD) R01 grants CA098080, CA11211201, and CA94872.
National Institutes of Health
Authorship
Contribution: S.W. designed research and wrote the paper; X.C. and P.K.E.-B. performed research; L.S., T.P.L., Y.A.C., and J.Y.D. helped to edit the paper; F.B., J.Z., A.R., J.S.P., and D.A.S. also contributed to some of the experiments; and J.L. and J.A.Y. contributed vital reagents.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sheng Wei, H. Lee Moffitt Cancer Center & Research Institute, MRC 4 East, Room 4072C, 12902 Magnolia Dr, Tampa, FL 33612; e-mail: sheng.wei@moffitt.org.
References
Author notes
*X.C. and F.B. share equally in the preparation of this paper.
†J.Y.D., T.P.L., P.K.E.-B. and S.W. share equally in senior authorship of this paper.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal