Abstract
Engagement of endothelial protein C receptor (EPCR) by activated protein C (aPC) decreases expression of endothelial adhesion molecules implicated in tumor-endothelium interactions. We examined the role of the aPC/EPCR pathway on tumor migration and metastasis. In vitro, B16-F10 melanoma cells showed decreased adhesion to and transmigration through endothelium treated with recombinant human aPC (rhaPC). In murine B16-F10 metastasis models, transgenic EPCR overexpressing (Tie2-EPCR) mice exhibited marked reductions in liver (50%) and lung (92%) metastases compared with wild-type (WT) animals. Intravital imaging showed reduced B16-F10 entrapment within livers of Tie2-EPCR compared with WT mice. A similar reduction was observed in WT mice treated with rhaPC. Strikingly, rhaPC treatment resulted in a 44% reduction in lung metastases. This was associated with decreased lung P-selectin and TNF-α mRNA levels. These findings support an important role for the aPC/EPCR pathway in reducing metastasis via inhibition of tumor cell adhesion and transmigration.
Introduction
Metastasizing cancer cells are thought to overcome the endothelial barrier through mechanisms commonly observed in leukocyte trafficking, including the up-regulation of adhesion molecules, apoptosis induction, cytoskeletal reorganization, and disruption of normal endothelial intercellular contacts.1-5 Both cancer cell transmigration and leukocyte trafficking are increased by proinflammatory cytokines, such as interleukin-1β and tumor necrosis factor (TNF), which up-regulate adhesion molecules, including P-selectin,5-7 and E-selectin.1,8 Given the parallels between these processes, we hypothesized that a therapy directed at the endothelium to reduce leukocyte trafficking might also be beneficial in preventing tumor cell metastasis. The serine protease activated protein C (aPC) has been used to treat severe sepsis.9 It possesses anti-inflammatory and cytoprotective activities mediated through engagement of its receptor, endothelial protein C receptor (EPCR).10-12 This interaction down-regulates endothelial proinflammatory and proapoptotic gene expression and decreases vascular permeability.13,14 In this report, we assessed the ability of melanoma cells to adhere to and transmigrate through endothelial monolayers treated with recombinant human aPC (rhaPC). Furthermore, we investigated the role of the aPC/EPCR pathway in a murine model of hematogenous B16-F10 melanoma metastasis by characterizing liver and lung metastases in EPCR-overexpressing mice and by administering rhaPC to wild-type (WT) mice
Study design
Animals and cell lines
Female Tie2-EPCR transgenic mice were generated on a C57BL/6 background as previously described.15 Wild-type C57BL/6 mice from TheJackson Laboratory (Bar Harbor, ME) and transgene-negative littermates were used as controls. Experiments were performed in accordance with the Canadian Council on Animal Care guidelines. B16-F10 mouse melanoma and bEnd.3 mouse brain microvascular endothelial lines were purchased from ATCC (Manassas, VA), and rhaPC was purchased from Sigma-Aldrich (Oakville, ON). Research ethics approval was obtained for all animal studies from the Dalhousie University Committee on Laboratory Animals in accordance with the Canadian Council on Animal Care.
Adhesion and transendothelial migration assays
For adhesion assays, confluent bEnd.3 monolayers grown in 24-well polystyrene plates (BD Biosciences, Mississauga, ON) were pretreated with rhaPC (0, 5, 10, 25, 100 nM) for 3 hours then washed. B16-F10 cells (2 × 105) labeled with CFDA-SE (Invitrogen, Burlington, ON) were added to each well.4 One hour later, wells were washed and adherent fluorescent cells were counted. For transmigration assays, bEnd.3 monolayers on polycarbonate Transwell inserts (8-μm pore-size, 6.4-mm diameter; Corning-Costar, Corning, NY) were pretreated with rhaPC. To induce chemotaxis, DMEM supplemented with 15% FCS was added to the lower chambers. CFDA-SE–labeled B16-F10 cells (2 × 105) in DMEM were added to the upper chambers. After 16 hours at 37°C/5% CO2, fluorescent cells in the lower chambers were counted.
Metastasis models
Mice received intrasplenic or tail vein injections of B16-F10 cells (2.5 × 105 cells) in 50 μL PBS as previously described.6,7,16 Two weeks later, or sooner if moribund, mice were killed. Livers, spleens, and lungs were weighed, photographed, and biopsied for reverse transcription–polymerase chain reaction (RT-PCR). Liver or lung surface area covered by tumor nodules was calculated with the use of Simple PCI digital image analysis (Compix, Sewickley, PA). For drug treatments, tail vein injections of rhaPC (20 μg/mouse) or phosphate-buffered saline (PBS) were initiated 2 hours before B16-F10 cell inoculation and repeated every 4 hours for 48 hours then every 6 hours for 24 hours.
Intravital imaging
Intravital imaging was performed as previously described.17 Mice were anaesthetized, and the liver was exteriorized by right subcostal and midline incisions. Animals were placed in the right supine position, and the right liver lobe was positioned onto a Plexiglas microscope stage for image capture.
RT-PCR
Total lung RNA was isolated by TRIzol reagent (Invitrogen, Burlington, ON) and chloroform extraction, and cDNA was prepared with the use of Superscript III Reverse Transcriptase (Invitrogen). Quantitative polymerase chain reaction (PCR) was performed in duplicate on 1 μL of cDNA on a RG-3000 Rotor-Gene (Corbett Research, Sydney, Australia) with the use of iQ SYBR Green Supermix (Bio-Rad, Mississauga, ON). Primer pairs were designed for mouse P-selectin (forward: GTCCACGGAGAGTTTGGTGT; reverse: AAGTGGTGTTCGGACCAAAG), TNF-α (forward: TCTCATGCACCACCATCAAGGACT; reverse: ACCACTCTCCCTTTGCAGAACTCA), and E-selectin (forward: GCCTGCCATGTGGTTGAATGTGAA; reverse: GTCAAACGTGCATGTCGTGTTCCA), with 18S used as an internal normalizing standard (forward: TCAACTTTCGATGGTAGTCGCCGT; reverse: TCCTTGGATGTGGTAGCCGTTTCT).
Statistical analysis
Survival data were analyzed by log-rank (Mantel-Cox) analysis. All other data were analyzed by ANOVA with the Fisher post hoc test. All data are reported as mean with standard error. Statistical significance was set at P less than .05.
Results and discussion
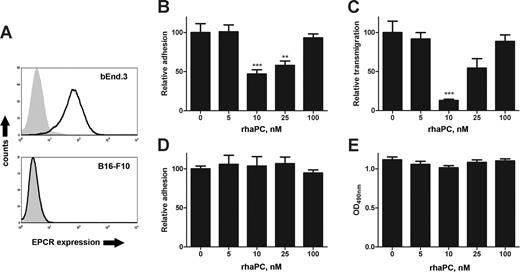
To assess the role of the endothelial aPC/EPCR pathway on melanoma/endothelial cell interactions, we performed tumor adhesion and transmigration assays with bEnd.3 endothelial monolayers pretreated with rhaPC. These cells express EPCR (Figure 1A) and adhesion molecules, including ICAM-1, P-selectin, and E-selectin.18 Pretreatment of endothelial monolayers with rhaPC concentrations (10-25 nM) known to be cytoprotective and barrier-enhancing in activated human endothelium,11,19 led to significantly decreased adhesion of B16-F10 melanoma cells (Figure 1B). These concentrations may be comparable to therapeutic doses attained in sepsis patients,9,20 in light of the decreased activity of rhaPC in mice.21 Similar inhibition was observed for transmigration (Figure 1C). In keeping with reports demonstrating increased endothelial permeability at higher rhaPC doses,11,19 a return to baseline adhesion and transmigration was observed with 100 nM rhaPC (Figure 1B and 1C, respectively). This narrow range of inhibitory action may in part be explained by cross-species differences in rhaPC potency.21 This effect was not due to direct actions of rhaPC on B16-F10 cells, because they did not express EPCR (Figure 1A). Furthermore, rhaPC pretreatment of B16-F10 cells did not affect their adhesion or viability by methyl thiazole tetrazolium (MTT) assay22 (Figure 1D,E). Direct rhaPC treatment of some human tumor cells can increase in vitro migration in an EPCR-dependent fashion, but only at concentrations 50- to 1000-fold higher than therapeutic levels.23,24
Treatment of endothelial cells with rhaPC prevents B16-F10 adhesion and transmigration. (A) Flow cytometric analysis of EPCR expression in bEnd.3 and B16-F10 cells. EPCR staining (solid line) performed with FITC-labeled anti–mouse mAb (clone RMEPCR1560; StemCell Technologies, Vancouver, BC). Isotype control (IgG2b) is represented by the shaded area. (B) Confluent bEnd.3 monolayers were pretreated with rhaPC (0, 5, 10, 25, 100 nM) for 3 hours and washed. CFDA-SE–labeled B16-F10 melanoma cells were then added to each well. After 1 hour, wells were washed and adherent fluorescent cells were counted. ***P < .001, **P < .01 compared with untreated group. Each dose was tested in quadruplicate. Data are representative of 3 independent experiments. (C) Confluent bEnd.3 monolayers on 8-μm pore Transwell inserts were pretreated with rhaPC for 3 hours. Chambers were washed, and DMEM was supplemented with 15% FCS added to each lower chamber. CFDA-SE–labeled B16-F10 melanoma cells were added to the upper chamber. Cells in the lower chamber were counted 16 hours later. ***P < .001, compared with the untreated group. Each dose was tested in quadruplicate. Data are representative of 3 independent experiments. (D) CFDA-SE–labeled B16-F10 melanoma cells were pretreated with rhaPC for 3 hours, washed, and then applied to confluent bEnd.3 monolayers. After 1 hour, wells were washed thoroughly and adherent fluorescent cells were counted. Each dose was tested in quadruplicate. Data represent 3 independent experiments. (E) B16-F10 melanoma cells were treated with rhaPC (0, 5, 10, 25, 100 nM) 5 times at 1-hour intervals and assessed for viability by MTT assay. Each dose was tested 16 times per experiment. Data are representative of 3 independent experiments. All data reported as mean with standard error.
Treatment of endothelial cells with rhaPC prevents B16-F10 adhesion and transmigration. (A) Flow cytometric analysis of EPCR expression in bEnd.3 and B16-F10 cells. EPCR staining (solid line) performed with FITC-labeled anti–mouse mAb (clone RMEPCR1560; StemCell Technologies, Vancouver, BC). Isotype control (IgG2b) is represented by the shaded area. (B) Confluent bEnd.3 monolayers were pretreated with rhaPC (0, 5, 10, 25, 100 nM) for 3 hours and washed. CFDA-SE–labeled B16-F10 melanoma cells were then added to each well. After 1 hour, wells were washed and adherent fluorescent cells were counted. ***P < .001, **P < .01 compared with untreated group. Each dose was tested in quadruplicate. Data are representative of 3 independent experiments. (C) Confluent bEnd.3 monolayers on 8-μm pore Transwell inserts were pretreated with rhaPC for 3 hours. Chambers were washed, and DMEM was supplemented with 15% FCS added to each lower chamber. CFDA-SE–labeled B16-F10 melanoma cells were added to the upper chamber. Cells in the lower chamber were counted 16 hours later. ***P < .001, compared with the untreated group. Each dose was tested in quadruplicate. Data are representative of 3 independent experiments. (D) CFDA-SE–labeled B16-F10 melanoma cells were pretreated with rhaPC for 3 hours, washed, and then applied to confluent bEnd.3 monolayers. After 1 hour, wells were washed thoroughly and adherent fluorescent cells were counted. Each dose was tested in quadruplicate. Data represent 3 independent experiments. (E) B16-F10 melanoma cells were treated with rhaPC (0, 5, 10, 25, 100 nM) 5 times at 1-hour intervals and assessed for viability by MTT assay. Each dose was tested 16 times per experiment. Data are representative of 3 independent experiments. All data reported as mean with standard error.
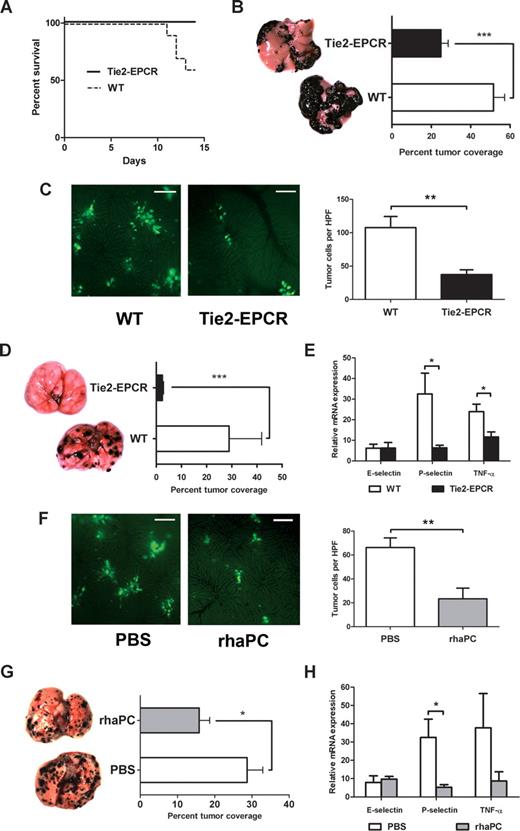
Next, we examined the effect of increased endothelial EPCR expression on hematogenous metastasis in well-established mouse models.6,7,16 First, we compared the rate of animal survival and extent of liver metastases after intrasplenic injection of B16-F10 melanoma cells in WT and Tie2-EPCR mice. Tie2-EPCR mice overexpress EPCR on all tissue endothelium and exhibit significant resistance to endotoxin-induced death.15 Two weeks after tumor inoculation, 4 of 10 WT animals succumbed to metastatic disease, whereas all Tie2-EPCR mice survived (Figure 2A; P = .03). A significantly greater percentage of total liver surface area was involved with tumor nodules in WT compared with Tie2-EPCR animals (Figure 2B; 51.6% ± 5.7% in WT versus 24.9% ± 3.7% in Tie2-EPCR; P < .001). Mean splenic tumor weights in WT and Tie2-EPCR mice did not differ significantly (data not shown), indicating that the difference in metastasis was not due to inhibition of primary tumor proliferation. In addition, intravital imaging showed decreased tumor cell entrapment within livers of Tie2-EPCR versus WT mice (Figure 2C; 66.2 ± 8.1 cells/high-power field [HPF] in WT versus 23.4 ± 8.9 cells/HPF in Tie2-EPCR; P < .01), further supporting our in vitro findings that decreased metastasis was attributable to changes in tumor cell trafficking.
EPCR overexpression and aPC administration protect mice from B16-F10 melanoma metastases. (A) Survival curves comparing WT and Tie2-EPCR mice over the 2 weeks after intrasplenic injection of B16-F10 melanoma cells, P = .03. For each group, n = 10. (B) Calculated percentage of total liver surface area involved with tumor nodules and corresponding photographs of representative liver surfaces for WT and Tie2-EPCR mice, ***P < .001, n = 10 per group. Images were visualized with a Leica S6D dissecting microscope equipped with a 10×/0.32 Plan Apo lens (Leica, Richmond Hill, ON) and a QImaging Micropublisher 3.3 RTV digital camera (QImaging, Surrey, BC). Images were acquired with QCapture Pro 5.0 software (QImaging) and were analyzed with the SimplePCI Imaging System (Compix). (C) Representative intravital images and cell counts per high-power field (HPF) of CFDA-SE–labeled B16-F10 melanoma cells in WT and Tie2-EPCR livers, **P < .01, n = 6 per group. One hour after intrasplenic B16-F10 inoculation, mice were anesthetized, and their livers were exteriorized for image capture. Images were visualized using a Leica DM5000B fluorescence microscope and Hamamatsu C9100 EM-CCD camera (Hamamatsu Photonics, Bridgewater, NJ). Images were acquired with Volocity 5.0.0 software (Improvision, Lexington, MA). Ten HPFs were captured per liver. Cell counts per HPF were performed by 2 blinded observers. Bar = 100 μm. (D) Calculated percentage of total lung surface area involved with tumor nodules and corresponding photographs of representative lung surfaces for WT and Tie2-EPCR mice, ***P < .001. A similar difference was observed if lung metastasis was measured with total nodule number or lung surface area (data not shown). For each group, n = 12. (E) Quantitation of E-selectin, P-selectin, and TNF-α transcript levels from WT and Tie2-EPCR lung specimens harvested 2 weeks after tail vein injection with B16-F10 cells, *P < .05, n = 6 per group. Each RT-PCR reaction was run in duplicate. Transcript levels expressed relative to mean value for nontumor-injected WT animals. In the absence of tumor injection, no significant differences in transcript levels were observed between WT and Tie2-EPCR mice. (F) Representative intravital images and cell counts per HPF of CFDA-SE–labeled B16-F10 melanoma cells in PBS- and rhaPC-treated WT animal livers, **P < .01, n = 6 per group. Bar = 100 μm. (G) Calculated percentage of total lung surface area involved with tumor nodules and corresponding photographs of representative lung surfaces for PBS- and rhaPC-treated WT mice, *P = .02. For PBS-treated group, n = 12; for rhaPC-treated group, n = 10. (H) Quantitation of E-selectin, P-selectin, and TNF-α transcript levels from PBS- and rhaPC-treated WT lung specimens harvested 12 hours after initiation of PBS or rhaPC injections (10 hours after inoculation with B16-F10 cells), *P = .02, n = 6 per group. Each RT-PCR reaction was run in duplicate. Transcript levels expressed relative to mean value for PBS-treated, nontumor-injected mice. In the absence of tumor injection, no significant differences in transcript levels were observed between PBS- and APC-treated mice. All data reported as mean with standard error.
EPCR overexpression and aPC administration protect mice from B16-F10 melanoma metastases. (A) Survival curves comparing WT and Tie2-EPCR mice over the 2 weeks after intrasplenic injection of B16-F10 melanoma cells, P = .03. For each group, n = 10. (B) Calculated percentage of total liver surface area involved with tumor nodules and corresponding photographs of representative liver surfaces for WT and Tie2-EPCR mice, ***P < .001, n = 10 per group. Images were visualized with a Leica S6D dissecting microscope equipped with a 10×/0.32 Plan Apo lens (Leica, Richmond Hill, ON) and a QImaging Micropublisher 3.3 RTV digital camera (QImaging, Surrey, BC). Images were acquired with QCapture Pro 5.0 software (QImaging) and were analyzed with the SimplePCI Imaging System (Compix). (C) Representative intravital images and cell counts per high-power field (HPF) of CFDA-SE–labeled B16-F10 melanoma cells in WT and Tie2-EPCR livers, **P < .01, n = 6 per group. One hour after intrasplenic B16-F10 inoculation, mice were anesthetized, and their livers were exteriorized for image capture. Images were visualized using a Leica DM5000B fluorescence microscope and Hamamatsu C9100 EM-CCD camera (Hamamatsu Photonics, Bridgewater, NJ). Images were acquired with Volocity 5.0.0 software (Improvision, Lexington, MA). Ten HPFs were captured per liver. Cell counts per HPF were performed by 2 blinded observers. Bar = 100 μm. (D) Calculated percentage of total lung surface area involved with tumor nodules and corresponding photographs of representative lung surfaces for WT and Tie2-EPCR mice, ***P < .001. A similar difference was observed if lung metastasis was measured with total nodule number or lung surface area (data not shown). For each group, n = 12. (E) Quantitation of E-selectin, P-selectin, and TNF-α transcript levels from WT and Tie2-EPCR lung specimens harvested 2 weeks after tail vein injection with B16-F10 cells, *P < .05, n = 6 per group. Each RT-PCR reaction was run in duplicate. Transcript levels expressed relative to mean value for nontumor-injected WT animals. In the absence of tumor injection, no significant differences in transcript levels were observed between WT and Tie2-EPCR mice. (F) Representative intravital images and cell counts per HPF of CFDA-SE–labeled B16-F10 melanoma cells in PBS- and rhaPC-treated WT animal livers, **P < .01, n = 6 per group. Bar = 100 μm. (G) Calculated percentage of total lung surface area involved with tumor nodules and corresponding photographs of representative lung surfaces for PBS- and rhaPC-treated WT mice, *P = .02. For PBS-treated group, n = 12; for rhaPC-treated group, n = 10. (H) Quantitation of E-selectin, P-selectin, and TNF-α transcript levels from PBS- and rhaPC-treated WT lung specimens harvested 12 hours after initiation of PBS or rhaPC injections (10 hours after inoculation with B16-F10 cells), *P = .02, n = 6 per group. Each RT-PCR reaction was run in duplicate. Transcript levels expressed relative to mean value for PBS-treated, nontumor-injected mice. In the absence of tumor injection, no significant differences in transcript levels were observed between PBS- and APC-treated mice. All data reported as mean with standard error.
The effect of EPCR overexpression on lung metastasis was even more striking. We observed a 92% relative decrease in the percentage of total lung surface area involved with tumor nodules in Tie2-EPCR compared with control animals (Figure 2D; 28.9% ± 3.9% in WT versus 2.4% ± 0.5% in Tie2-EPCR; P < .001). Importantly, transgene-free littermates and WT C57BL/6 mice did not differ in the extent of tumor metastasis (data not shown). The reduction in metastasis in Tie2-EPCR mice was associated with reduced lung mRNA levels of P-selectin and TNF-α (Figure 2E; 80.6% reduction for P-selectin, P = .03; 51.1% reduction in TNF-α relative to WT mice, P = .02). This supports previous reports showing a critical role for P-selectin in B16 melanoma's ability to metastasize to the lung.6,7
To investigate the effects of exogenous rhaPC administration on tumor metastasis, we first examined fluorescent B16-F10 cell entrapment in the livers of WT mice after rhaPC or PBS injection. Compared with PBS, rhaPC markedly decreased tumor cell entrapment within the liver (Figure 2F; 107.7 ± 13.6 cells/HPF in PBS-treated mice versus 37.3 ± 7.1 cells/HPF in rhaPC-treated mice; P < .01). This reduction was consistent with that observed in Tie2-EPCR mice. To examine the effect of rhaPC administration in the lung, WT mice received scheduled rhaPC tail vein injections for 72 hours, beginning 2 hours before inoculation with B16-F10 cells. A 44% reduction in lung surface area tumor involvement was observed in rhaPC-treated compared with control animals (Figure 2G; 28.7% ± 6.0% in PBS-treated mice vs 16.2% ± 4.0% in rhaPC-treated mice; P = .02). As in Tie2-EPCR animals, this was associated with significantly decreased P-selectin mRNA levels (Figure 2H; 83.9% reduction; P = .03), and a trend toward reduced TNF-α levels, 12 hours after rhaPC treatment initiation.
In summary, we provide direct evidence that EPCR overexpression or rhaPC administration protects against tumor metastasis. The greater protection against lung metastasis offered by EPCR overexpression compared with rhaPC treatment is probably secondary to the relatively short and intermittent course of rhaPC tested. Improved protection may be provided by prolonged, continuous administration of rhaPC, an approach currently feasible only in large animal models.25 Although thrombin inhibition by rhaPC may contribute to this protective effect in vivo,26,27 our in vitro experiments, in which coagulation proteins are heat-inactivated, clearly show that rhaPC can act directly on endothelium to prevent tumor cell adhesion and transmigration. Although our data support P-selectin inhibition as a possible mechanism for decreased tumor adhesion in the lung,6,7 specific target molecules responsible for rhaPC's effects on adhesion and transmigration may vary between tissue and tumor types. This study provides proof of concept for the strategy of using rhaPC in the prevention of tumor metastasis and underscores the complex interplay between inflammation and cancer progression.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank D. M. Conrad, L. L. Veinotte, A. K. Matheson, E. Rogerson, K. N. Graves, and A. Li for technical assistance.
This work was supported by grants from the Nova Scotia Health Research Foundation (M.B.), Cancer Research Training Program (R.C.), Capital Health Research Fund (K.A.W. and R.S.L.), and the Canadian Cancer Society (B.J.), all Halifax, NS.
National Institutes of Health
Authorship
Contribution: M.B. and R.S.L. developed the study hypothesis; M.B., B.J., and R.S.L. designed the experiments; C.T.E. generated the transgenic mice; M.B. and R.C. performed the research; M.B., R.C., S.F.M., K.A.W., B.J., and R.S.L. analyzed and interpreted the data; M.B. and R.S.L. wrote the manuscript; and M.B., C.T.E., S.F.M., K.A.W., B.J., and R.S.L. edited all drafts of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Robert S. Liwski, Department of Pathology, Dalhousie University, 5850 College St, Rm 11L1, Halifax, NS, Canada B3H 1X5; e-mail: rliwski@dal.ca; or Brent Johnston, e-mail: brent.johnston@dal.ca.
References
Author notes
*R.S.L., B.J., and K.A.W. are senior authors.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal