Abstract
Although the inhibitory receptor CTLA-4 (CD152) has been implicated in peripheral CD4 T-cell tolerance, its mechanism of action remains poorly defined. We analyzed mechanisms of CD4 cell tolerance in a model of tolerance induction involving establishment of mixed hematopoietic chimerism in recipients of fully MHC-mismatched allogeneic bone marrow cells with anti-CD154 mAb. Animals lacking CD80 and CD86 failed to achieve chimerism. We detected no T cell–intrinsic requirement for CD28 for chimerism induction. However, a CD4 T cell–intrinsic signal through CTLA-4 was shown to be essential within the first 48 hours of exposure to alloantigen for the establishment of tolerance and mixed chimerism. This signal must be provided by a recipient CD80/86+ non–T-cell population. Donor CD80/86 expression was insufficient to achieve tolerance. Together, our findings demonstrate a surprising role for interactions of CTLA-4 expressed by alloreactive peripheral CD4 T cells with CD80/86 on recipient antigen-presenting cells (APCs) in the induction of early tolerance, suggesting a 3-cell tolerance model involving directly alloreactive CD4 cells, donor antigen-expressing bone marrow cells, and recipient antigen-presenting cells. This tolerance is independent of regulatory T cells and culminates in the deletion of directly alloreactive CD4 T cells.
Introduction
The ultimate goal in transplantation research is the induction of donor-specific tolerance, in which genetically disparate organs or tissues are regarded by the recipient's immune system as “self,” ensuring life-long graft survival without chronic immunosuppressive therapies. Unfortunately, although many protocols and therapeutic agents have led to either prolonged graft acceptance or functional tolerance in rodent models, most have been unsuccessful in more stringent tests of tolerance in rodents (eg, long-term acceptance of fully MHC-mismatched skin grafts by euthymic recipients) and have not been successful in large animal models or humans. In contrast, the establishment of mixed hematopoietic chimerism has been shown to lead to transplantation tolerance, including the most stringent test grafts, not only in rodent models, but also in large animal models and recently in patients.1-3 Although these results are promising, the widespread clinical application of hematopoietic cell transplantation (HCT) for the induction of organ graft tolerance remains elusive, as regimens for achieving donor chimerism are often too toxic for therapeutic use.4 Over the past decade, new protocols have been developed that use reagents that markedly decrease the potential toxicity of conditioning by reducing or eliminating the need for irradiation treatments and host T-cell depletion. Specifically, blockade of the CD40-CD154 and CD28-CD80/86 pathways has been shown to facilitate the induction of high levels of long-term stable donor hematopoietic chimerism across full MHC barriers.5-9
Currently, the mechanisms implicated in graft prolongation using costimulatory blockade range from deletion of donor-reactive T cells, anergy, and a role for regulatory T cells (Tregs; reviewed in Wekerle et al10 ). In models of bone marrow transplantation (BMT) with costimulatory blockade, the major mechanism for maintenance of long-term tolerance is intrathymic clonal deletion of alloreactive T cells.5,8,11 Regulatory T cells play a role in maintaining tolerance only when low levels of hematopoietic chimerism and incomplete deletion of peripheral donor-reactive T cells are achieved.12-16 Within the peripheral T-cell pool, thymus-independent early deletion of both donor-reactive CD4 and CD8 T cells in the peripheral lymphoid tissues occurs in recipients of BM transplant with costimulatory blockade.5,17,18 At least for CD4 cells, this deletion is not the result of anti-CD154 antibody-targeted depletion of activated cells, as mixed chimerism and tolerance can be readily achieved without anti-CD154 mAb in CD8 cell-depleted recipients lacking the CD154 gene.17
Clinical application of BMT with costimulatory blockade will require a detailed understanding of the important cellular and molecular pathways involved. We have previously shown that BMT with blockade of the CD40/CD154 pathway by an anti-CD154 mAb is sufficient for the induction of CD4 T-cell tolerance,11 whereas the addition of CTLA-4Ig5 or the correctly timed use of low-dose total body irradiation (TBI)19 was required to also achieve tolerance of preexisting donor-reactive CD8 cells.
We have now investigated the roles of the CD80/86–CTLA-4 and CD80/86-CD28 pathways in the induction of peripheral CD4 T-cell tolerance with BMT and anti-CD154 mAb. CD28 promotes T-cell activation and proliferation by enhancing cytokine production, promoting cell division, and up-regulating antiapoptotic proteins.20 However, CD28-mediated signaling has, paradoxically, also been associated with T-cell tolerance induction in some models.21,22 CTLA-4 inhibits T-cell activation through poorly defined mechanisms that may include delivery of an inhibitory signal to the T cell, ligand competition (with CD28 for CD80/86 molecules), and a role in Treg function.23
Our findings suggest that a CD28 signal is not required, and that an early signal through CTLA-4 (within 48 hours of BMT) is critical for preventing the CD4-mediated rejection of allogeneic bone marrow cells (BMCs) when administered with anti-CD154 mAb. Through the use of various knockout and reconstitution models, we show that this signal appears to be CD4 T-cell intrinsic. Furthermore, the population that provides the necessary CD80/86-mediated signal is of host origin, and cannot be replaced by CD80/86+ donor BMCs. Given the potential for clinical application of mixed chimerism for tolerance induction, these findings identify important pathways that may be targeted for future therapeutic intervention.
Methods
Animals
C57BL/6 (B6; H-2b), C57BL/6 CD45.1 congenic, B10.A (H-2a), B10.S (H-2s), C57BL/6-CD28−/−, and C57BL/6-TCRβ−/− mice were purchased from Frederick Cancer Research Center (Frederick, MD) or The Jackson Laboratory (Bar Harbor, ME). CD80−/−CD86−/−CTLA-4−/− (TKO) and CD80−/−CD86−/− (DKO) mice on either the C57BL/6 or 129 background were kindly provided by Dr Arlene Sharpe (Harvard Medical School) and were bred in our facility. In one experiment, C57BL/6 DKO mice were purchased from The Jackson Laboratory. Mice were housed in a specific pathogen-free microisolator environment, as described.24 Animal studies were approved by the institutional animal care and use committee of Massachusetts General Hospital, per National Institutes of Health (NIH, Bethesda, MD) guidelines.
Conditioning and allogeneic BMT
Recipient mice were treated with a nonmyeloablative dose of total body irradiation (TBI, 300 cGy) on day 0 or day −1, followed by an intravenous tail injection of 15 to 25 × 106 non–T cell–depleted bone marrow cells (BMCs) from indicated donors. Hamster antimouse CD154 mAb (MR1; 2 mg) was administered intraperitoneally on day 0 with respect to BMT. The MR1 hybridoma was kindly provided to us by Dr Randolph Noelle (Dartmouth University, Hanover, NH). Depleting anti-CD8 mAb (2.43; 1.44 mg/mouse) and anti-CD4 mAb (GK1.5; 1.7 mg/mouse) were administered intraperitoneally as indicated. Hybridomas producing the anti-CD28 mAb (37.51) and anti–CTLA-4 (9H10) were kindly provided by Dr James Allison (Memorial Sloan-Kettering Cancer Center, New York, NY), and purified mAbs were administered intraperitoneally as indicated.
Creation of syngeneic WT/KO chimeras
For the creation of the “mixed” CD28± recipients, B6-CD45.1 mice received 300 cGy TBI followed by various doses of B6-CD28−/− (CD45.2+) BMCs (1 × 106, 5 × 106, or 25 × 106) administered intravenously. Mice were then allowed to recover for more than 5 weeks before allogeneic BMT. “CTLA-4±” recipients were prepared by lethally irradiating T cell–depleted B6-CD45.1 mice (1025 cGy TBI; anti-CD4 and anti-CD8 on days −7 and −1) followed by administration of either 3 × 106 B6-WT (CD45.2+ Ly9.2+), 3 × 106 129-TKO (CD80−/− CD86−/− CTLA-4−/− CD45.2+ Ly9.1+), or a mixture of 2.5 × 106 B6-WT and 7.5 × 106 129-TKO BMCs. Mice were allowed to recover for more than 5 weeks before allogeneic BMT. “WT,” “T-null,” “T-WT,” “T-DKO,” and “T-TKO” recipients were prepared by lethally irradiating B6-CD45.1 mice (1025 cGy) and administering T cell–depleted BMCs from B6-WT (5 × 106) or B6-TCRβ−/− (5 × 106) mice, or from TCRβ−/− plus B6-WT, B6-DKO, or B6-TKO mice (7.5 × 106 TCRβ−/− BMCs + 0.5 × 106 WT/DKO/TKO BMCs). Mice were allowed to recover for more than 8 weeks before allogeneic BMT.
Flow cytometric analysis of multilineage chimerism in white blood cells
Flow cytometric (FCM) analysis was performed as previously described.25 In recipients of B10.A BM transplant, donor-derived cells were identified by anti-H2Dd mAb 34-2-12, and cell lineages were distinguished by anti-CD4, anti-CD8, anti-B220, anti-CD19 (all purchased from Pharmingen, San Diego, CA), and anti-Mac1 (purchased from Caltag, San Francisco, CA). Determination of mixed chimerism for all experiments was set at more than 5% donor-positive cells in the CD4, CD8, B-cell, monocyte, and granulocyte lineages. In syngeneic WT/KO chimeras, reconstitution of various lineages was assessed by anti-CD45.1, anti-CD45.2, or anti-Ly9.1 staining (Pharmingen). Nonspecific FcγR binding was blocked by anti–mouse FcγR mAb 2.4G2. Dead cells were excluded using propidium iodide staining. Three- and 4-color FCM was used to distinguish donor and host cells of particular lineages, and the percentages of donor cells were calculated as previously described.25
Results
Role for CTLA-4 in peripheral CD4 tolerance induction
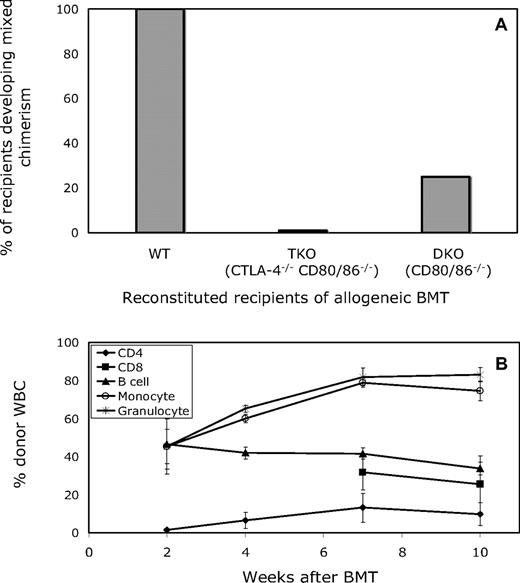
We wished to use gene knockout mice to investigate the role of CTLA-4 in CD4 T-cell tolerance in our model. However, mice deficient in CTLA-4 develop a severe lymphoproliferative disorder and do not survive past 6 weeks of age,26 precluding their use as BM transplant recipients. In “triple knockout” (TKO) mice, however, that are also deficient in CD80 and CD86 in addition to CTLA-4, autoimmunity does not occur.27 We therefore wished to use TKO mice to investigate the role of CTLA-4 expression on recipient CD4 T cells in peripheral tolerance induction. However, since these mice lack CTLA-4 in addition to CD80/86, it was important to compare the achievement of chimerism in mice lacking all 3 molecules with those lacking only CD80 and CD86 (DKO; “double knockout”). In recipients of allogeneic (B10.A) BM transplant with anti-CD154, anti-CD8, and 3 Gy total body irradiation (TBI), all wild-type (WT) B6 recipients developed mixed chimerism (6 of 6; 8.09% ± 3.41% and 29.48% ± 13.55% donor chimerism in the CD19 and Mac1 lineage, respectively, at 2 weeks after BMT), whereas none of either the TKO or DKO recipients (also on the B6 background; 0/7 and 0/6, respectively) had any detectable donor white blood cells (WBCs) at 1 or 2 weeks after BMT (data not shown). With this surprising result and a view to developing a model whereby varying proportions of recipient CD4 T cells express CTLA-4, we used a bone marrow reconstitution model, in which wild-type B6 (CD45.2, Ly9.2) mice were lethally irradiated and reconstituted with either wild-type (B6; CD45.1, Ly9.2), TKO (129; CD45.2, Ly9.1), or DKO (129; CD45.2, Ly9.1) marrow, then treated 6 weeks later with the anti-CD154/TBI regimen and allogeneic BMT. Five weeks after lethal irradiation and reconstitution, 100% of the peripheral B-cell and Mac1+ cell populations were of WT, DKO, or TKO origin, in accordance with the type of marrow they were given. As shown in Figure 1A, 100% of the WT-reconstituted recipients developed mixed chimerism, whereas only 25% of the DKO-reconstituted mice and none of the TKO-reconstituted mice developed mixed chimerism. Donor repopulation was observed in all white blood cell (WBC) lineages (CD4, CD8, B cell, monocyte, and granulocyte) and persisted for the duration of follow-up (> 10 weeks, WT recipients shown, Figure 1B). These data demonstrated a role for recipient hematopoietically derived CD80 and/or CD86 and possibly for CTLA-4 in achievement of CD4 tolerance.
Both CTLA-4−/− CD80/86−/− and CD80/86−/− reconstituted recipients are resistant to the induction of mixed chimerism in mice treated with anti-CD154. (A) Lethally irradiated B6 (CD45.2, Ly9.2) mice reconstituted with BM from B6-wild type (CD45.1, Ly9.2), 129-TKO (CTLA-4−/− CD80/86−/−; CD45.2, Ly9.1), or 129-DKO (CD80/86−/−; CD45.2, Ly9.1) mice were treated with anti-CD8, 3 Gy TBI, anti-CD154, and allogeneic BMT 6 weeks after reconstitution. Among recipients reconstituted with WT BMCs, 100% (5 of 5) developed and maintained long-term mixed chimerism. In contrast, all recipients of TKO-derived BMCs (5 of 5) and most recipients of DKO-derived BMCs (3 of 4) failed to establish mixed chimerism at 4 weeks after allogeneic BMT. (B) The mean percentage of donor WBCs in WT recipients of BM transplant with anti-CD154 among CD4, CD8, B-cell, monocyte, and granulocyte lineages was determined by FCM at various time points after BMT. Results are shown as mean plus or minus SEM for each lineage. Similar results were obtained in the single chimeric DKO recipient (data not shown).
Both CTLA-4−/− CD80/86−/− and CD80/86−/− reconstituted recipients are resistant to the induction of mixed chimerism in mice treated with anti-CD154. (A) Lethally irradiated B6 (CD45.2, Ly9.2) mice reconstituted with BM from B6-wild type (CD45.1, Ly9.2), 129-TKO (CTLA-4−/− CD80/86−/−; CD45.2, Ly9.1), or 129-DKO (CD80/86−/−; CD45.2, Ly9.1) mice were treated with anti-CD8, 3 Gy TBI, anti-CD154, and allogeneic BMT 6 weeks after reconstitution. Among recipients reconstituted with WT BMCs, 100% (5 of 5) developed and maintained long-term mixed chimerism. In contrast, all recipients of TKO-derived BMCs (5 of 5) and most recipients of DKO-derived BMCs (3 of 4) failed to establish mixed chimerism at 4 weeks after allogeneic BMT. (B) The mean percentage of donor WBCs in WT recipients of BM transplant with anti-CD154 among CD4, CD8, B-cell, monocyte, and granulocyte lineages was determined by FCM at various time points after BMT. Results are shown as mean plus or minus SEM for each lineage. Similar results were obtained in the single chimeric DKO recipient (data not shown).
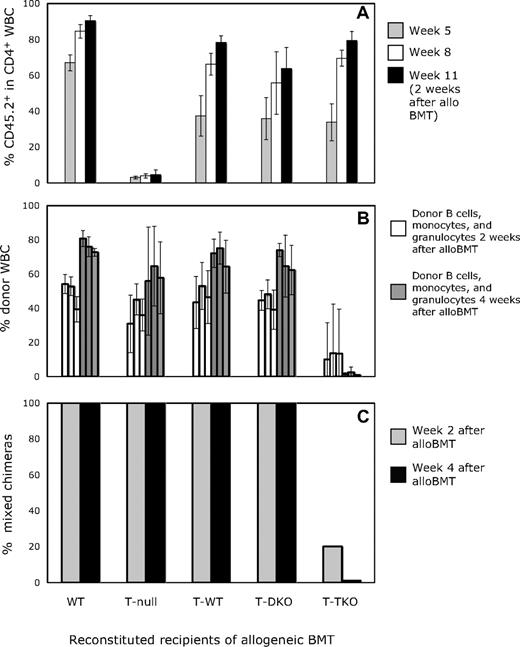
The observed molecular requirements for achievement of CD4 tolerance and chimerism in our model could reflect a requirement for interactions of T cells with CD80/86 molecules on T cells or on APCs for peripheral tolerance induction. To identify the requirements on T cells for peripheral tolerance induction, we developed a model whereby recipient CD4 T cells would be derived from either WT, DKO, or TKO marrow, but the majority of all remaining hematopoietic populations (B cells, monocytes, dendritic cells, etc) would be of WT origin, and therefore capable of expressing CD80/86. To create such animals, we used a B6 syngeneic chimera model system in which CD45.1 mice were lethally irradiated and reconstituted with CD45.2+ BMCs from WT (WT) or TCRβ−/− mice, or from TCRβ−/− mice in combination with marrow from WT (T-WT), DKO (T-DKO), or TKO (T-TKO) mice in a 15:1 ratio. In this experiment, all mouse strains were on the B6 background. In preliminary experiments involving reconstitution of lethally irradiated recipients with a mixture of WT and DKO or TKO BMCs in a 3:1 ratio, WT-derived cells made up greater than 90% of the peripheral B-cell pool and more than 75% of the Mac1+ granulocyte population as early as 3 weeks after BMT (data not shown). Therefore, in mice reconstituted with a 15:1 ratio of TCRβ−/− and either WT, DKO, or TKO BMCs, αβ T cells would be derived from the WT, DKO, or TKO progenitors, respectively, whereas the majority of other cell lineages would originate mainly from TCRβ−/− marrow, and thus be capable of expressing CD80/86. As shown in Figure 2A, the recovery of the peripheral CD4 population with WT-, DKO-, or TKO-derived T cells in mice that also received TCRβ−/− BMCs was comparable with CD4 recovery in recipients of WT BMC alone. The increase observed from approximately 40% marrow-derived CD4 T cells at week 5 to 60% at week 8 and more than 80% by week 11 reflects reconstitution of the peripheral CD4 pool by newly generated T cells and a decrease in the proportion of residual host CD4 T cells after lethal TBI. In recipients of only TCRβ−/− BMCs, no CD4 T-cell recovery above the residual host CD4 pool was observed, demonstrating that TCRβ−/− BMCs did not contribute to the recovery of the peripheral T-cell pool and no residual host thymopoiesis occurred after lethal TBI.
Peripheral CD4 T cells deficient in CTLA-4 resist establishment of mixed chimerism in mice receiving CD8 depletion, 3 Gy TBI, anti-CD154 mAb, and allogeneic BM transplant. (A) The percentage of CD4 cells derived from WT, DKO, or TKO progenitors (CD45.2) is shown at 5, 8, and 11 weeks after lethal irradiation for lethally irradiated B6 (CD45.1) recipients reconstituted with (CD45.2) WT, DKO, or TKO BMCs in combination with TCRβ−/− (CD45.2) BMCs. The 11-week time point is 2 weeks after allogeneic BMT, before allogeneic donors contribute to T-cell chimerism. (B) The mean percentage of donor cells in WBC B-cell, monocyte, and granulocyte lineages (from left to right) is shown in reconstituted recipients at 2 and 4 weeks after alloBMT with anti-CD154. Results are shown as mean plus or minus SEM for each lineage. (C) Nine weeks after lethal irradiation and reconstitution, mice received allogeneic B10.A BM transplant with anti-CD154, anti-CD8, and 3 Gy TBI and were assessed for chimerism by flow cytometry. In WT (5 of 5), TCRβ−/− (5 of 5), T-WT (5 of 5), and T-DKO (9 of 9) mice, donor-derived cells were detected in the CD4, CD8, B-cell, monocyte, and granulocyte populations, whereas none of the T-TKO recipients (0 of 5) developed lasting mixed chimerism.
Peripheral CD4 T cells deficient in CTLA-4 resist establishment of mixed chimerism in mice receiving CD8 depletion, 3 Gy TBI, anti-CD154 mAb, and allogeneic BM transplant. (A) The percentage of CD4 cells derived from WT, DKO, or TKO progenitors (CD45.2) is shown at 5, 8, and 11 weeks after lethal irradiation for lethally irradiated B6 (CD45.1) recipients reconstituted with (CD45.2) WT, DKO, or TKO BMCs in combination with TCRβ−/− (CD45.2) BMCs. The 11-week time point is 2 weeks after allogeneic BMT, before allogeneic donors contribute to T-cell chimerism. (B) The mean percentage of donor cells in WBC B-cell, monocyte, and granulocyte lineages (from left to right) is shown in reconstituted recipients at 2 and 4 weeks after alloBMT with anti-CD154. Results are shown as mean plus or minus SEM for each lineage. (C) Nine weeks after lethal irradiation and reconstitution, mice received allogeneic B10.A BM transplant with anti-CD154, anti-CD8, and 3 Gy TBI and were assessed for chimerism by flow cytometry. In WT (5 of 5), TCRβ−/− (5 of 5), T-WT (5 of 5), and T-DKO (9 of 9) mice, donor-derived cells were detected in the CD4, CD8, B-cell, monocyte, and granulocyte populations, whereas none of the T-TKO recipients (0 of 5) developed lasting mixed chimerism.
After 9 weeks had passed to allow reconstitution of the various hematopoietic lineages, these animals received 3 Gy TBI, and anti-CD154 and anti-CD8 mAbs followed by BMT from fully MHC-mismatched B10.A donors. As expected, 100% of the mice that had been originally reconstituted with either WT or TCRβ−/− BMCs (T null) alone developed and maintained mixed chimerism, as did all T-WT–reconstituted mice (Figure 2C). By 2 weeks after allogeneic BMT, the levels of donor-derived cells within the B-cell, monocyte, and granulocyte populations were comparable (Figure 2B). Similar results were observed in the T-DKO–reconstituted mice, as all recipients developed mixed chimerism, demonstrating that CD80/86 was not required on the T cells that were being rendered tolerant. Since studies in Figure 1 show that CD80 and/or CD86 expression on radiosensitive (since B7 on residual host T cells that resist irradiation was insufficient) recipient hematopoietic cells is needed for tolerance induction, we can conclude from these studies together that expression of CD80/86 on recipient non-T cells is important for the reliable induction of tolerance in this model. In contrast to T-DKO mice, only 20% of the T-TKO recipients achieved initial mixed allogeneic chimerism. By 4 weeks after allogeneic BMT, none of the mice with TKO-derived CD4 T cells maintained their chimerism, in contrast to all recipients in the other groups. These data indicate that, unlike CD80/86 expression, CTLA-4 expression on recipient CD4 T cells is required for the induction of donor-specific tolerance with this regimen.
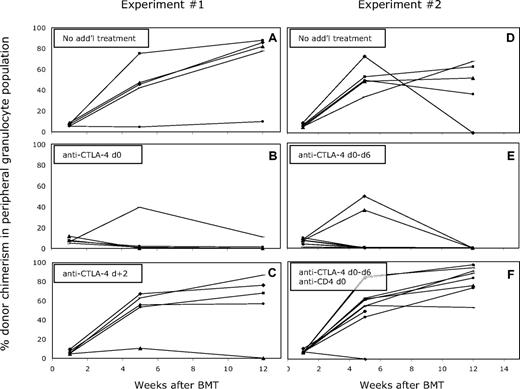
To determine the timing of the requirement for a signal through CTLA-4 in permitting induction of peripheral CD4 T-cell tolerance, we performed experiments with blocking anti–CTLA-4 mAb. Mixed allogeneic chimeras were prepared with anti-CD8, anti-CD154, and 3 Gy TBI, with or without administration of anti–CTLA-4 mAb intraperitoneally on day 0 (clone 9H10; 2 mg). As shown in Figure 3A, all of the mice that received 3 Gy TBI, anti-CD8, and anti-CD154 developed long-term mixed chimerism, whereas the majority of the mice that received anti–CTLA-4 in addition to this protocol did not develop chimerism (Figure 3B). These mice showed initial chimerism, but most animals lost it over time (between 4-7 weeks), failing to maintain long-term multilineage chimerism. A signal through CTLA-4 was required only within the first 48 hours after BMT, as mice that received anti–CTLA-4 on day 2 after BMT did not lose chimerism over time (Figure 3C).
Administration of anti–CTLA-4 on day 0 impairs the establishment of mixed chimerism in mice receiving CD8 depletion, 3 Gy TBI, anti-CD154 mAb, and BM transplant. The mean percentage of donor cells among CD4, CD8, B-cell, monocyte, and granulocyte lineages in WBCs was determined by FCM at various time points after BMT. The percentage of donor-derived cells within the granulocyte population is illustrated as a representative lineage, and results for individual mice within each group are shown (A-C). Five of 5 control mice achieved and maintained multilineage chimerism more than 26 weeks after BMT (A), in contrast to only 1 of 5 recipients that received anti–CTLA-4 on day 0 (B). Four of 5 mice that received anti–CTLA-4 on day +2 established long-term mixed chimerism (C). (D-F) In mice that received BM transplant with anti-CD154 on day 0 and anti–CTLA-4 on days 0, +2, +4, and +6, long-term mixed chimerism was not achieved (E; 0 of 10 mice). Depletion of peripheral CD4+ cells (9 of 10) reversed the effect of the anti–CTLA-4 (F), and permitted levels of chimerism similar to those in mice that received only anti-CD154 with alloBMT (D; 4 of 5).
Administration of anti–CTLA-4 on day 0 impairs the establishment of mixed chimerism in mice receiving CD8 depletion, 3 Gy TBI, anti-CD154 mAb, and BM transplant. The mean percentage of donor cells among CD4, CD8, B-cell, monocyte, and granulocyte lineages in WBCs was determined by FCM at various time points after BMT. The percentage of donor-derived cells within the granulocyte population is illustrated as a representative lineage, and results for individual mice within each group are shown (A-C). Five of 5 control mice achieved and maintained multilineage chimerism more than 26 weeks after BMT (A), in contrast to only 1 of 5 recipients that received anti–CTLA-4 on day 0 (B). Four of 5 mice that received anti–CTLA-4 on day +2 established long-term mixed chimerism (C). (D-F) In mice that received BM transplant with anti-CD154 on day 0 and anti–CTLA-4 on days 0, +2, +4, and +6, long-term mixed chimerism was not achieved (E; 0 of 10 mice). Depletion of peripheral CD4+ cells (9 of 10) reversed the effect of the anti–CTLA-4 (F), and permitted levels of chimerism similar to those in mice that received only anti-CD154 with alloBMT (D; 4 of 5).
Since initial chimerism was achieved in mice receiving anti–CTLA-4 on day 0, it was important to rule out the possibility that the mAb affected only intrathymic tolerance, resulting in the delayed loss of chimerism. Therefore, an additional experiment was performed with mice receiving the standard treatment and anti–CTLA-4 mAb, but peripheral CD4+ cells were depleted with anti-CD4 mAb injection on day 0. This treatment depletes peripheral CD4 cells but not mature alloreactive CD4+ thymocytes.28 In this experiment, anti–CTLA-4 was administered for an extended period after BMT (1 mg on day 0, 0.5 mg on days 2, 4, and 6). As shown in Figure 3F, recipients depleted of peripheral CD4 T cells (in addition to CD8 cells) achieved durable mixed chimerism despite treatment with anti–CTLA-4 mAb, similar to results in the group of mice that did not receive anti–CTLA-4 mAb (Figure 3D). Similar to the previous experiment, no mice that received anti–CTLA-4 without CD4 depletion maintained long-term chimerism (Figure 3E). From these data, we conclude that CTLA-4 is required for the tolerization of preexisting peripheral alloreactive CD4 cells.
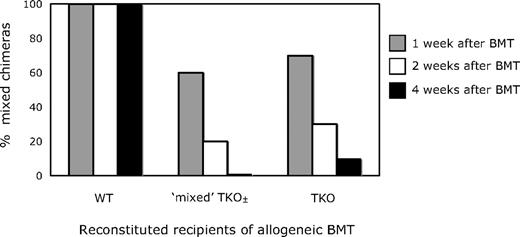
To investigate whether the presence of WT CD4 T cells could provide a regulatory or suppressive function to inhibit the rejection of donor BMCs by CTLA-4−/− CD4 T cells, we created mixed TKO± recipients by lethally irradiating B6 CD45.1 recipients and reconstituting them with BMCs from WT (B6; CD45.2, Ly9.2) or TKO (129; CD45.2, Ly9.1) mice, or with a mixture of the 2 (1:3 ratio of WT:TKO BMCs). After 6 weeks, recipients of either the WT or TKO BMCs alone showed more than 90% reconstitution of peripheral CD4+ cells of WT or TKO origin, respectively, whereas recipients of mixed BMCs had a mixture of TKO and WT (55.1% ± 8.5% TKO-derived) cells within this population. These mice were then treated with 3 Gy TBI, anti-CD154 and anti-CD8, and allogeneic B10.A BMT, and assessed for the establishment of mixed chimerism and tolerance. As shown in Figure 4, recipients with only WT CD4 T cells all developed chimerism, whereas those with either predominantly TKO or a mixture of WT and TKO CD4 cells did not achieve durable mixed allogeneic chimerism. In other experiments in which we varied the ratio of WT:TKO CD4 cells (data not shown), it was not until less than 10% of the peripheral CD4 pool was CTLA-4−/− that mixed chimerism could be established. In the presence of substantial numbers of WT CD80/86+ APCs, chimerism was detected at 4 weeks in only 2 of 8 mice with 10% to 40% TKO recipient CD4 cells. In contrast, 4 of 5 animals with DKO recipient CD4 cells in the same range (10%-40%) achieved chimerism. This suggests that even when the majority of the peripheral CD4 cells express CTLA-4, they are insufficient to prevent the rejection of donor BMCs by the relatively small population of CTLA-4−/− CD4 cells, and the presence of CTLA-4–negative CD4 cells dominantly blocked the achievement of tolerance. This result is consistent with the interpretation that a CD4 T cell–intrinsic signal through CTLA-4 is required for the tolerization of peripheral CD4 T cells.
WT CD4 T cells are unable to prevent the rejection of allogeneic BMCs by CTLA-4–deficient CD4 cells in mice treated with anti-CD8, 3 Gy TBI, and anti-CD154. In recipients in which all peripheral CD4 cells were derived from WT progenitors, 100% (5 of 5) developed and maintained long-term mixed chimerism. In contrast, recipients containing CD4 cells that were either entirely TKO-derived or included an approximately 1:1 mixture of TKO- and WT-derived cells failed to establish durable mixed chimerism (0/10 for WT-TKO and 1/10 for TKO).
WT CD4 T cells are unable to prevent the rejection of allogeneic BMCs by CTLA-4–deficient CD4 cells in mice treated with anti-CD8, 3 Gy TBI, and anti-CD154. In recipients in which all peripheral CD4 cells were derived from WT progenitors, 100% (5 of 5) developed and maintained long-term mixed chimerism. In contrast, recipients containing CD4 cells that were either entirely TKO-derived or included an approximately 1:1 mixture of TKO- and WT-derived cells failed to establish durable mixed chimerism (0/10 for WT-TKO and 1/10 for TKO).
Lack of a role for T cell–intrinsic signaling through CD28 in peripheral CD4 cell tolerance with anti-CD154 and BMT
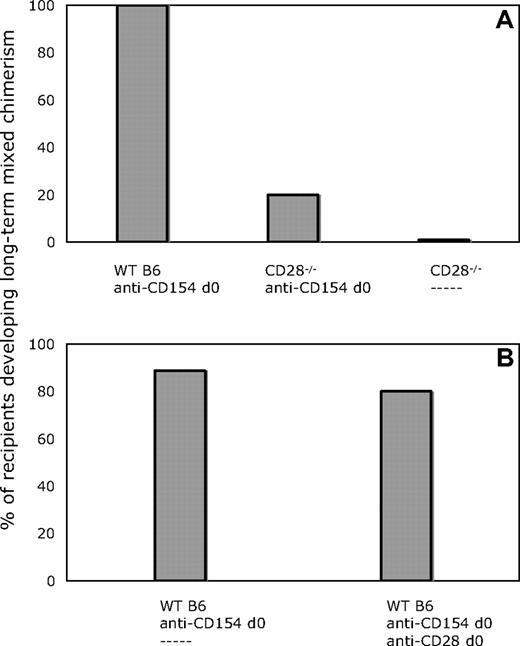
Given the requirement for CD80/86 expression on non-T cells for the achievement of peripheral CD4 T-cell tolerance with our protocol (Figures 1,2), we considered the possibility that CD80/86 interactions with CD28 on T cells, in addition to interactions with CTLA-4, also played a role in tolerance induction. To address this possibility, we compared CD28−/− and WT mice on the B6 background as recipients of BM transplant from B10.A donors with our conditioning regimen. As shown in Figure 5A, all WT recipients developed lasting multilineage chimerism, whereas only a minority of CD28−/− mice developed measurable chimerism. These data suggested that the establishment of chimerism by BMT in combination with costimulatory blockade might be dependent on an early signal to CD4+ cells through CD28.
CD28−/− recipients do not develop chimerism with anti-CD154 and allogeneic BMT. Blocking anti-CD28 mAb does not impair the establishment of chimerism in WT recipients. (A) All animals received 3 Gy TBI on day 0, anti-CD8 mAb on day − 1, and 20 × 106 B10.A BMCs. In contrast to 100% (5 of 5) of WT recipients, only 1 of 5 CD28−/− recipient mice developed chimerism when treated with anti-CD154 and CD8-depleting mAb. None of 5 CD28−/− mice receiving CD8-depleting mAb alone developed chimerism. (B) In WT recipients of anti-CD154 and CD8-depleting mAb, 8 of 10 mice that also received anti-CD28 developed lasting chimerism (> 30 weeks after BMT), similar to mice that received only anti-CD154 (8 of 9).
CD28−/− recipients do not develop chimerism with anti-CD154 and allogeneic BMT. Blocking anti-CD28 mAb does not impair the establishment of chimerism in WT recipients. (A) All animals received 3 Gy TBI on day 0, anti-CD8 mAb on day − 1, and 20 × 106 B10.A BMCs. In contrast to 100% (5 of 5) of WT recipients, only 1 of 5 CD28−/− recipient mice developed chimerism when treated with anti-CD154 and CD8-depleting mAb. None of 5 CD28−/− mice receiving CD8-depleting mAb alone developed chimerism. (B) In WT recipients of anti-CD154 and CD8-depleting mAb, 8 of 10 mice that also received anti-CD28 developed lasting chimerism (> 30 weeks after BMT), similar to mice that received only anti-CD154 (8 of 9).
In an attempt to further address the possibility that a signal through CD28 was necessary for the induction of CD4+ T-cell tolerance, we used an in vivo blocking mAb specific for CD28.29 In these experiments, WT mice received anti-CD8, anti-CD154, 3 Gy TBI, and BM transplant, and one group received an additional 2-mg injection of anti-CD28 on day 0, an amount in excess of that previously reported to have in vivo efficacy.29 As shown in Figure 5B, the administration of anti-CD28 did not have any measurable effect on the achievement of long-term mixed chimerism compared with mice receiving only anti-CD154. Similar results were obtained in 2 additional experiments.
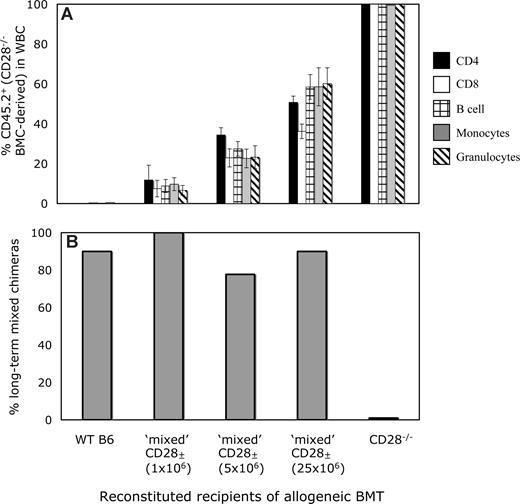
The conflicting data in Figure 5 might reflect incomplete CD28 blocking by the mAb in vivo, allowing a minimal but sufficient signal to be transmitted to the CD4 T cells, or could reflect CD4 cell depletion by the anti-CD28 mAb, although no significant depletion of CD4+ cells was observed at 1 week after BMT (data not shown), suggesting that this explanation is unlikely. Alternatively, CD28−/− mice have been shown to have subtle defects in the CD4+CD25+ Treg subset,30,31 and this or another undefined developmental defect might be responsible for the resistance to mixed chimerism induction in CD28−/− mice. To distinguish between these possibilities, we devised a mixed CD28± recipient mouse model, whereby varying percentages of the peripheral CD4+ T-cell pool would be deficient in CD28. B6 (CD45.1) mice received 3 Gy TBI and various numbers (1 × 106, 5 × 106, or 25 × 106) of BMCs from syngeneic B6 (CD45.2) CD28−/− mice. By 5 weeks after syngeneic BMT, the mixed CD28± recipients had achieved stable levels of CD45.2+ chimerism within CD4, CD8, B-cell, monocyte, and granulocyte populations that correlated with the dose of CD28−/− BMCs (Figure 6A). Thus, if a signal through CD28 to donor-reactive CD4 T cells was required for the induction of tolerance, mixed CD28± recipients containing a significant fraction of CD28−/− T cells would be resistant to the establishment of mixed chimerism, since the CD28−/− CD4 T cells would not be tolerized and would reject the donor cells. Conversely, if a T cell–intrinsic signal through CD28 is not required for the induction of CD4 tolerance and the resistance observed in CD28−/− mice is due to an undefined environmental or developmental defect due to lack of CD28, then the WT component of reconstitution could provide the necessary environment to allow tolerization of both WT and CD28−/− T cells.
Lack of a T cell–intrinsic requirement for CD28 for the tolerization of CD4 T cells. (A) Peripheral levels of CD28−/−-derived WBCs in mixed CD28± mice. In B6-CD45.1 mice treated with 3 Gy TBI, the administration of increasing doses of syngeneic B6 CD28−/− (CD45.2) BMCs (1 × 106, 5 × 106, and 25 × 106) led to increasing numbers of peripheral CD45.2+ cells in the CD4, CD8, B-cell, monocyte, and granulocyte lineages. Mean levels of CD45.2+ cells in each lineage 5 weeks after syngeneic BMT are shown. (B) Mixed CD28± mice develop long-term mixed chimerism in most instances after treatment with anti-CD8, 3 Gy TBI, anti-CD154 mAb, and allogeneic BMT. WT B6 (CD45.1), B6-CD28−/− (CD45.2), and CD28± mice all received B10.A BM transplant with anti-CD8, anti-CD154, and 3 Gy TBI. Although none of the CD28−/− recipients developed chimerism (0 of 5), all remaining groups, regardless of level of CD28−./− CD4+ T cells in the periphery, developed chimerism at similar rates (9 of 10 for WT; 8 of 8 for CD28± created with 1 × 106, 7 of 9 for CD28± created with 5 × 106, and 9 of 10 for CD28± created with 25 × 106 CD28−/− BMCs). Chimerism was detected in CD4, CD8, B-cell, monocyte, and granulocyte lineages by FCM analysis for more than 25 weeks after BMT.
Lack of a T cell–intrinsic requirement for CD28 for the tolerization of CD4 T cells. (A) Peripheral levels of CD28−/−-derived WBCs in mixed CD28± mice. In B6-CD45.1 mice treated with 3 Gy TBI, the administration of increasing doses of syngeneic B6 CD28−/− (CD45.2) BMCs (1 × 106, 5 × 106, and 25 × 106) led to increasing numbers of peripheral CD45.2+ cells in the CD4, CD8, B-cell, monocyte, and granulocyte lineages. Mean levels of CD45.2+ cells in each lineage 5 weeks after syngeneic BMT are shown. (B) Mixed CD28± mice develop long-term mixed chimerism in most instances after treatment with anti-CD8, 3 Gy TBI, anti-CD154 mAb, and allogeneic BMT. WT B6 (CD45.1), B6-CD28−/− (CD45.2), and CD28± mice all received B10.A BM transplant with anti-CD8, anti-CD154, and 3 Gy TBI. Although none of the CD28−/− recipients developed chimerism (0 of 5), all remaining groups, regardless of level of CD28−./− CD4+ T cells in the periphery, developed chimerism at similar rates (9 of 10 for WT; 8 of 8 for CD28± created with 1 × 106, 7 of 9 for CD28± created with 5 × 106, and 9 of 10 for CD28± created with 25 × 106 CD28−/− BMCs). Chimerism was detected in CD4, CD8, B-cell, monocyte, and granulocyte lineages by FCM analysis for more than 25 weeks after BMT.
At 7 weeks after CD28−/− BMT, the CD28± mice, as well as WT and CD28−/− mice, received anti-CD8, 3 Gy TBI, anti-CD154, and BM transplant from B10.A donors. As shown in Figure 6B, only CD28−/− recipients failed to develop mixed chimerism, whereas mice with low, medium, or high proportions of CD28−/− CD4 T cells accepted allogeneic marrow at similar rates as WT recipient mice. This chimerism persisted long term and was similar in all lineages in all groups of mice (data not shown). In addition, the early deletion of both WT and CD28−/− donor-reactive CD4 T cells (as measured by analysis of CD4 cells bearing Vβ that recognize endogenous superantigens presented by donor MHC17 ) was similar and progressed over time equally (data not shown). Taken together, these data indicate that a signal through CD28 is not required for either the induction of tolerance or for the deletion of donor-reactive CD4 T cells, and that the inability to induce mixed chimerism in CD28−/− recipients is most likely due to a developmental defect.
CD80/86 is required on recipient but not donor hematopoietic cells for achievement of CD4 tolerance
The data in Figure 2 demonstrate that T-cell CD80/86 expression is not necessary for the tolerization of peripheral CD4 cells, since animals with only DKO T cells reliably achieved durable mixed chimerism. In contrast, the results in Figure 1 indicate that a recipient hematopoietically derived CD80/86+ APC population is required to achieve CD4 tolerance in the majority of mice. To address whether CD80/86 expression was also required on the donor bone marrow inoculum for the induction of CD4 T-cell tolerance and the establishment of mixed chimerism, WT B6 and DKO mice were used as donors into recipient B10.S mice treated with 3 Gy TBI, anti-CD8, and anti-CD154 mAb. In this experiment, all mice developed comparable levels of multilineage mixed chimerism regardless of donor BMC origin (Figure 7), demonstrating that CD80/86 expression by the donor BMC is not required for the induction of recipient CD4 cell tolerance. Thus, we conclude that CD80/86 from a recipient non–T cell is required to signal to CTLA-4 on recipient CD4 cells to tolerize them.
CD80/86 expression is not required on donor BMCs for the induction of mixed chimerism in mice treated with anti-CD154. B6 WT and CD80/86−/− mice were used as bone marrow donors into B10.S recipient mice that received anti-CD8, 3 Gy TBI, and anti-CD154. In both groups, all of the mice (7 of 7 for each group) developed long-term mixed chimerism (> 10 weeks) in all lineages. Donor chimerism (mean ± SD shown) was detected in CD4, CD8, B-cell, monocyte, and granulocyte lineages by FCM analysis for more than 13 weeks after BMT.
CD80/86 expression is not required on donor BMCs for the induction of mixed chimerism in mice treated with anti-CD154. B6 WT and CD80/86−/− mice were used as bone marrow donors into B10.S recipient mice that received anti-CD8, 3 Gy TBI, and anti-CD154. In both groups, all of the mice (7 of 7 for each group) developed long-term mixed chimerism (> 10 weeks) in all lineages. Donor chimerism (mean ± SD shown) was detected in CD4, CD8, B-cell, monocyte, and granulocyte lineages by FCM analysis for more than 13 weeks after BMT.
Discussion
We demonstrate here that an early, CD4 T cell–autonomous CTLA-4 interaction with CD80/86 molecules on recipient APCs promotes tolerance of alloreactive CD4 cells in recipients of BM transplant with anti-CD154.
Although CD28−/− mice failed to achieve mixed chimerism with our regimen, treatment with blocking anti-CD28 did not prevent chimerism induction, and animals with mixtures of wild-type and CD28−/− T cells readily achieved chimerism and tolerance. Thus, a CD4 cell–autonomous signal through CD28 is not required for tolerance induction, and the inability to induce mixed chimerism in CD28−/− recipients most likely reflects an undefined developmental defect. CD28 has been implicated in the homeostasis and function of Tregs.30,32,33 However, our previous studies have ruled out a major role for regulatory cells in the induction of peripheral CD4 T-cell tolerance in this model.12 Other T-cell developmental defects present in CD28−/− mice34-38 may account for our results. Indeed, several novel rejection pathways have been identified in CD28−/− mice that are not effective in WT mice, including more prominent roles for natural killer (NK) cells39 and CD8 T cells.40
In contrast to CD28, CTLA-4 ligation of CD80/86 molecules was found to play a critical role in CD4 tolerance induction in our model, as indicated by the failure to achieve durable mixed chimerism in recipients of anti–CTLA-4 mAb. CTLA-4 appears to be required within the first 48 hours after BMT, since anti–CTLA-4 given on day +2 did not impair the establishment of mixed chimerism. Whereas expression of CTLA-4 on the surface of T cells is not detected until 24 to 48 hours after activation,41 downstream effects of CTLA-4–mediated signaling have been observed as early as 1 to 4 hours after T-cell activation.42 Our data are the first, to our knowledge, to demonstrate that an early and not later CTLA-4 signal plays an important intrinsic role in CD4 T-cell tolerance induction.
Several lines of evidence suggest that the lymphoproliferative disorder that characterizes CTLA-4−/− mice is not effector T-cell autonomous.43,44 These data are consistent with a role for CTLA-4 predominantly on regulatory T cells, which constitutively express this molecule and dominantly down-modulate immune responses. In contrast, our studies in mice reconstituted with mixtures of WT and TKO marrow suggest an effector CD4 T cell–intrinsic requirement for CTLA-4 expression for tolerance induction, as mice in which only approximately one half of the peripheral CD4 T cells lacked CTLA-4 failed to become tolerized. This ratio of 1:1 was similar to that described in previously published studies that demonstrated effector cell–extrinsic CTLA-4–mediated immune modulation.43,44 Consistently, our previous studies ruled out a major role for regulatory mechanisms in the induction or maintenance of tolerance in this model, and the initial peripheral alloreactive CD4 tolerance involves anergy followed by deletion over a period of 3 to 4 weeks.12 Long-term maintenance of tolerance is primarily through central deletion within the thymus.14 Thus, in contrast to previous results,45 a requirement for CTLA-4+ regulatory T cells is unlikely to explain the role of CTLA-4 in tolerizing CD4 cells and our data point to a role for CTLA-4 on the CD4 T cell being tolerized in the tolerization process.46 This, to our knowledge, is the first in vivo study to specifically point to an effector T cell–intrinsic requirement for CTLA-4 in the induction of transplantation tolerance.
There are several possible mechanisms by which CTLA-4 may function besides its role in Treg development and function.32 CTLA-4 regulates T cell–cycle progression,47-49 blocks activation of cell-cycle progression–associated proteins Cdk-4, Cdk-6, and cyclin D3,49 blocks IL-2 production, and modulates lipid raft function and TCR accumulation at the immunologic synapse,49-52 promoting anergy.53-57 CTLA-4 signaling also triggers TGF-β production58-60 and apoptosis of T cells,61,62 and has recently been shown to affect T-cell migration through the lymph node and shorten T cell–APC contact periods.63 In our model, donor-reactive CD4 T cells are rendered nonresponsive to donor antigen 1 week after BMT, before their deletion, and this anergy cannot be overcome by the addition of exogenous IL-2,64 consistent with CTLA-4–dependent anergy.53 Blockade of CTLA-4 signaling has also been shown to negatively affect the induction of tolerance in other models using costimulatory blockade, but in these cases the effects were attributed to enhanced CD8 T-cell responses.65,66
Mixed chimerism could not be established in mice with only CD80/86−/− (DKO) hematopoietic cells. However, in animals in which all T cells were derived from DKO BMCs whereas most non-T cells originated from TCRβ−/− marrow and expressed normal levels of CD80/86, mixed allogeneic chimerism was readily achieved. Therefore, neither T cell–T cell CD80/86–CTLA-4 interactions, which have been implicated in other systems,67,68 nor the recently described T-cell CD80-APC PD-L1 interaction69 play a requisite role in tolerance induction in this model. Together, our studies show that CD80/86 expression on a recipient non–T-cell population is required to achieve tolerance. This could reflect a developmental requirement for these molecules or a requirement for them during the process of tolerizing alloreactive CD4 cells with our regimen. Given the early, T cell–intrinsic requirement for CTLA-4 signaling for CD4 tolerance induction with our regimen, CD80 and/or CD86 must also be required in this process. However, donor CD80/86 expression was insufficient to permit tolerance induction in recipients lacking these molecules, and was not even required for the achievement of chimerism and tolerance. Thus, there is an absolute requirement for recipient CD80/86 expression for CD4 tolerance induction in this model, although direct allorecognition is the major pathway likely to cause early rejection of donor marrow. One possible explanation for this paradox would be that indirect allorecognition by CD4 cells is required to tolerize directly alloreactive CD4 cells. Indeed, a requirement for indirect CD4 allorecognition has been implicated in several tolerance protocols involving anti-CD154 administration without BMT.70-72 However, studies we have performed in class II+ recipients reconstituted with class II knockout marrow have shown clearly that CD4 T-cell interactions with recipient class II+ APCs are not required to prevent rejection of allogeneic bone marrow by directly alloreactive CD4 cells in mice receiving anti-CD154.73 These data suggest that indirect allorecognition is not required to overcome the directly alloreactive CD4 T-cell barrier to alloengraftment in this model. Therefore, our data suggest that the requisite CTLA-4 signal needed to tolerize directly alloreactive CD4 cells is not delivered by the same cell that expresses donor alloantigens and must be provided in trans by a recipient-derived cell population. Although early studies of anergy induction in vitro showed that anergy could be blocked by costimulation provided in trans,74 our study is the first, to our knowledge, to demonstrate that tolerance is induced by a costimulatory ligand expressed in trans. We speculate that allogeneic bone marrow may lack mature APCs with sufficient CD80/86 expression to provide the requisite early CTLA-4 signal to recipient CD4 T cells, which therefore can receive this signal only from recipient APCs. Another possible explanation for the requirement for CD80/86 expression by recipient APCs is that these cells might capture and present intact donor MHC molecules.75 These potential mechanisms are currently under investigation.
Thus, early engagement of CTLA-4 on CD4 T cells at the time of BMT is necessary for the induction of donor-specific tolerance. Strategies for inducing tolerance through the use of costimulatory blockade might be most effective if they do not interfere with this pathway.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Thomas Wekerle and Dr Emmanuel Zorn for critical review of the paper, Joia Spinelli for assistance with DKO/TKO experiments, and Ms Kelly Walsh for assistance in paper preparation.
This work was supported by NIH grant R01HL49915.
National Institutes of Health
Authorship
Contribution: J.K. designed research, analyzed data, and wrote the paper; F.R., C.V., and J.D. performed research and analyzed data; and M.S. designed research, interpreted data, wrote the paper, and oversaw all aspects of the work.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Megan Sykes, Bone Marrow Transplantation Section, Transplantation Biology Research Center, Massachusetts General Hospital/Harvard Medical School, MGH-East Bldg 149-5102, 13th St, Boston, MA 02129; e-mail: megan.sykes@tbrc.mgh.harvard.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal