Abstract
Continuous expression of activated factor VII (FVIIa) via gene transfer is a potential therapeutic approach for hemophilia patients with or without inhibitory antibodies to human factor VIII (FVIII) or IX (FIX). Here, we investigate whether gene transfer of an engineered canine FVIIa (cFVIIa) transgene can affect hemostasis in a canine model of hemophilia, a good predictor of efficacy of hemophilia treatments. Purified recombinant cFVIIa exhibited 12-fold higher tissue factor–dependent activity than purified recombinant zymogen cFVII. Subsequently, we generated a serotype 8 recombinant adeno-associated viral vector expressing cFVIIa from a liver-specific promoter. Vector delivery via the portal vein in hemophilia A and B dogs was well tolerated, and long-term expression of cFVIIa resulted in a shortening of the prothrombin time, partial correction of the whole blood clotting time and thromboelastography parameters, and a complete absence of spontaneous bleeding episodes. No evidence of hepatotoxicity, thrombotic complications, or inhibitory immune response was found. These data provide the first evidence for in vivo efficacy and safety of continuously expressed FVIIa as a FVIII/FIX-bypassing agent in a large animal model of hemophilia, avoiding the risk of inhibitor formation associated with bolus FVIII or FIX infusion.
Introduction
Despite the availability of plasma-derived and recombinant human factor VIII (rhFVIII) or factor IX (rhFIX) as therapeutics for hemophilia, inhibitor formation (20%-30% in severe hemophilia A; ∼ 5% in severe hemophilia B1,2 ) remains a serious complication in a substantial fraction of these patients. Pharmacologic doses of activated recombinant human factor VII (rhFVIIa) administered in multiple doses3 or a single high dose4 has been successful in effecting hemostasis in such patients as well as in patients with platelet disorders5 and FVII deficiency.6,7 Dosing of rhFVIIa has also demonstrated efficacy in short-term secondary prophylaxis.8 However, the need for repeated injections (daily dosing) because of the short plasma half-life may limit its convenience for prolonged periods of prophylaxis
In an effort to bypass these drawbacks, we have previously demonstrated that a factor VII transgene engineered to be secreted in its activated form (FVIIa) can correct the hemostatic parameters in mouse models of hemophilia A (HA) and B (HB), after either adeno-associated viral (AAV) vector delivery9 or in transgenic animals.10 However, a necessary step preceding human application is the demonstration of safety and efficacy in a large animal (canine) model. This important hemophilia model closely resembles the human disorder in terms of size, physiology, and the genetic determinants of immune response because it is outbred instead of inbred.11 In addition, it has been a good predictor of efficacy in hemophilia treatments.12-15 Viral-mediated gene transfer of factor VIII (FVIII) or factor IX (FIX) in this model demonstrated that the antigenicity of the transgene product remains a concern16,17 because these animals may not be fully tolerant to wild-type FVIII or FIX. On the other hand, continuous expression of murine FVIIa in immunocompetent hemophilic mice after gene transfer did not result in a transgene-specific immune response, as evidenced by the long-term expression.9 Therefore, FVIIa gene transfer may not only effect hemostasis and bypass an inhibitor (functional advantage), but, at the same time, evade a transgene-specific immune response resulting from tolerance to the introduced transgene (immunologic advantage).
To demonstrate whether this advantage carried through to an outbred large animal, we used the canine hemophilia model and evaluated gene-based, AAV-mediated continuous expression of canine factor VIIa (cFVIIa) as a treatment for hemophilia. We show long-term efficacy with a marked improvement in the bleeding diathesis with no evidence of hepatotoxicity, thrombotic complications, or an inhibitory immune response to cFVIIa. Thus, our results provide the first evidence for efficacy and safety of the gene-based FVIII/FIX bypassing agent FVIIa for the treatment of hemophilia in a large animal model. This strategy can also be applied in platelet disorders, FVII deficiency or for prophylaxis in congenital hemophilia complicated by inhibitor development.
Methods
Plasmid construction, protein purification, and AAV production
A pcDNA3-based (Invitrogen, Carlsbad, CA) plasmid vector containing the canine factor VII (cFVII) cDNA18 was used to generate cFVIIa by polymerase chain reaction mutagenesis (by insertion of the Arg-Lys-Arg-Arg-Lys-Arg [RKRRKR] sequence at position 152 in the mature cFVII sequence), as previously described.9 To purify cFVII or cFVIIa, a C-terminal epitope tag (HPC4) was added to the translated peptide sequence, as previously described,9 thus generating cFVII-HPC4 and cFVIIa-HPC4. Stable cell lines based on human embryonic kidney cells (HEK-293) were generated by transfection and selection in G418, and proteins were purified from vitamin K–supplemented conditioned medium using a 1-step immunoaffinity column (against the HPC4 epitope), as previously described.9 Protein concentration was determined spectrophotometrically using a molecular weight of 50 000 and an extinction coefficient (E2800.1%) of 1.39. The AAV plasmid used for vector production-directed expression of cFVIIa (without the HPC4 tag) from a liver-specific promoter (human α1 antitrypsin [hAAT]) and enhancer (4 copies of the apolipoprotein E [apoE] enhancer), as previously described.9 Production of serotype 8 AAV vector was performed by triple transfection, essentially as previously described.9
Clotting assays for purified proteins
Prothrombin time (PT) assays using purified recombinant protein were performed in duplicate using a fibrometer (STart Instrument; Diagnostica Stago, Parsippany, NJ) by recording the clot time of recombinant protein (25 μL in Tris-buffered saline [TBS]; 50 mM Tris, 150 mM NaCl, 0.1% bovine serum albumin, pH 7.5; TBS–bovine serum albumin [BSA]) using equal volumes of canine FVII-deficient plasma18 and, after incubation at 37°C, 100 μL of relipidated recombinant human tissue factor (TF; Innovin; Dade Behring, Deerfield, IL) as a source of TF. Activated partial thromboplastin time (aPTT) assays of recombinant proteins were performed in duplicate by diluting recombinant protein in a total of 25 μL of canine factor VIII– or canine factor IX–deficient plasma and adding 1-in-4 dilution of aPTT reagent (25 μL total volume in imidazole buffer; Biomerieux, Durham, NC). After incubation at 37°C for 3 minutes, 25 μL of 25 mM CaCl2 was added and time to clot was recorded. For both PT and aPTT, purified recombinant proteins were compared using a reference standard curve of rhFVIIa. In these experimental assay systems, normalization of canine FVII-deficient plasma PT was observed using approximately 70 ng/mL cFVIIa and approximately 200 ng/mL rhFVIIa. Normalization of the HA and HB aPTT was observed at 20 μg/mL of either cFVIIa or rhFVIIa.
Clotting assays for mouse and dog experiments
PT assays for mouse or canine plasma samples were performed by recording the time to clot after mixing 25 μL of sample (diluted 40-fold [canine] or 160-fold [mouse] in TBS-BSA), 25 μL of human FVII-deficient plasma and, after incubation at 37°C, addition of 100 μL Innovin. aPTT assays for mouse plasma samples were performed as described above (see “Clotting assays for purified proteins”), but using undiluted aPTT reagent. Whole blood clotting time (WBCT) was performed essentially as previously described.16 Thromboelastography was performed as previously described19 using freshly drawn blood and initiating coagulation using 1 in 200 000 dilution of Innovin. Comparison in thromboelastography parameters between the AAV-treated dogs and normal or untreated HA/HB dogs was performed using data points from at least 1 week after the last normal canine plasma infusion, after gene transfer.
cFVIIa antigen determination
The concentration of the expressed cFVIIa in canine plasma was determined from clotting activity using the STAclot kit (Diagnostica Stago), as previously described for rhFVIIa.20 Briefly, a standard curve of increasing cFVIIa concentration (50 μL sample) in dilution buffer (50 mM 1,4-piperazinediethanesulfonic acid, 100 mM NaCl, 2 mM ethylenediaminetetraacetic acid, 0.1% BSA, pH 7.2) was generated using 50 μL of human FVII-deficient plasma and 50 μL of recombinant soluble human TF/phospholipid mixture. After a 3-minute incubation at 37°C, 50 μL of 25 mM CaCl2 was added and the time to clot was recorded. A typical standard curve had a correlation coefficient (r2) of more than 0.97 (log10 [concentration] vs log10 [clot time]). In this assay, zymogen cFVII exhibited negligible activity (∼ 3% relative to cFVIIa). Citrated plasma samples from treated, untreated HA or HB, or control (normal) dogs were diluted 10- or 50-fold in dilution buffer, and clot time was determined. Concentration of cFVIIa in those samples was derived from the obtained standard curve. Canine FVIIa levels in normal, untreated HA or HB dogs were determined using at least 4 individual samples (in the case of HA or HB) or using a pooled sample from at least 4 dogs (in the case of normal dogs).
D-dimer, fibrinogen, and thrombin-antithrombin assays
D-dimer determination was performed in duplicate on citrated plasma dog samples using the enzyme-linked immunosorbent assay-based Asserachrom D-Di kit (Diagnostica Stago).21 Fibrinogen levels in citrated dog plasma samples were determined in duplicate using a clotting-based assay (Clauss method; Dade Behring) that used a human plasma standard as a reference.21 Assays were performed according to the manufacturer's instructions but using half the volume of each reagent. Thrombin-antithrombin (TAT) assays were performed in duplicate on citrated plasma using the Enzygnost TAT micro kit (Dade Behring). D-dimer, fibrinogen, and TAT levels in normal, untreated HA or HB dogs were determined using at least 4 individual samples (in HA or HB) or using a pooled sample from at least 4 dogs (in the case of normal dogs).
AAV vector administration in hemophilia mice and dogs
All animal experiments were approved by the Institutional Animal Care and Use Committee at the Children's Hospital of Philadelphia and the University of North Carolina at Chapel Hill. Administration of vector in HA mice was performed in 200 μL volume (vector in phosphate-buffered saline–5% sorbitol) via the tail vein. Plasma samples were collected in 3.8% citrate via sectioning of the tail. Liver-directed, portal vein vector infusion in 3 HA (HA-1-J55, HA-2-J57, and HA-3-E66) and 1 HB (HB-1-J10) dogs of the University of North Carolina at Chapel Hill colony was performed as previously described.22 Antibodies against AAV8 or cFVIIa were detected by enzyme-linked immunosorbent assay using either AAV8 or recombinant cFVIIa-coated plates, essentially as previously described.16 Canine serum chemistries (gamma-glutamyltranspeptidase, aspartate aminotransferase, alanine aminotransferase, creatine phosphokinase, alkaline phosphatase, serum glutamic pyruvic transaminase, serum glutamic oxaloacetic transaminase, total bilirubin, and platelet counts) were analyzed in an automated clinical pathology laboratory. Normal range of values for each test was derived from 18 clinically healthy dogs on a mixed genetic background. Monitoring was done every 2 days for the first 1 to 2 weeks after AAV-cFVIIa administration, then every 1 to 3 months thereafter.
Statistical analysis
An unpaired 2-tailed Student t test or a 2-sided Fisher exact test (for analysis of bleeding episodes) was used for statistical analysis, with P less than .05 indicating statistical significance.
Results
Purification of recombinant zymogen cFVII and cFVIIa
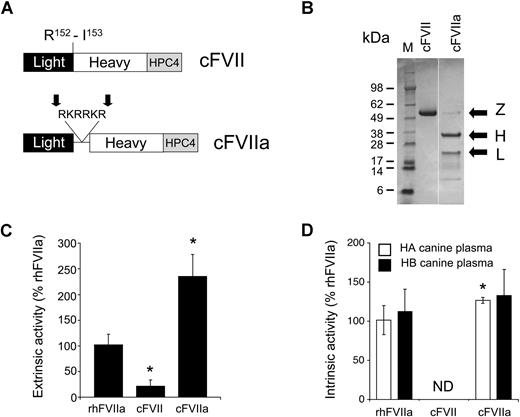
Initially, we generated HEK-293–based stable cell lines expressing zymogen cFVII or cFVIIa, both proteins containing a C-terminal epitope tag (HPC4) for immunoaffinity purification (Figure 1A).9 Recombinant zymogen cFVII and cFVIIa were successfully purified from vitamin K–supplemented conditioned medium. In contrast to zymogen cFVII, which was purified in a single-chain form (indicating lack of autoactivation during purification), cFVIIa was purified almost exclusively in the 2-chain, fully activated form (Figure 1B). The lower molecular weight bands observed at 10 to 16 kDa are probably degradation products, as have been previously observed in human recombinant FVIIa preparations.23,24 Although the heavy and light chains of cFVIIa exhibited differential staining with Coomassie blue, this is commonly found in human23,24 as well as murine FVIIa preparations.9,25
Construction and in vitro characterization of canine FVII and canine FVIIa. (A) The canine FVII and FVIIa constructs contained a C-terminal epitope tag (HPC4) for immunoaffinity purification. To generate canine FVIIa, we introduced a short amino acid sequence (RKRRKR) at the normal site of cleavage of canine FVII (Arg152-Ile153, indicated), which is recognized by an intracellular protease of the PACE/furin type. Cleavage at this sequence (indicated by  ) results in the secretion of a 2-chain, activated molecule. (B) Polyacrylamide gel electrophoresis of zymogen (Z) cFVII and cFVIIa under reducing conditions. The molecular size marker (M) bands in kDa are indicated as well as the heavy (∼35 kDa, H) and light (∼20 kDa, L) chains. (C) TF-dependent activity of cFVII and cFVIIa in a PT-based clotting assay using canine FVII-deficient plasma relative to rhFVIIa (100%). *P < .05 versus rhFVIIa. (D) TF-independent activity of cFVII and cFVIIa in an aPTT-based clotting assay using either canine hemophilia A (HA) or B (HB) plasma, relative to rhFVIIa (100%). ND indicates nondetectable activity.
) results in the secretion of a 2-chain, activated molecule. (B) Polyacrylamide gel electrophoresis of zymogen (Z) cFVII and cFVIIa under reducing conditions. The molecular size marker (M) bands in kDa are indicated as well as the heavy (∼35 kDa, H) and light (∼20 kDa, L) chains. (C) TF-dependent activity of cFVII and cFVIIa in a PT-based clotting assay using canine FVII-deficient plasma relative to rhFVIIa (100%). *P < .05 versus rhFVIIa. (D) TF-independent activity of cFVII and cFVIIa in an aPTT-based clotting assay using either canine hemophilia A (HA) or B (HB) plasma, relative to rhFVIIa (100%). ND indicates nondetectable activity.
Construction and in vitro characterization of canine FVII and canine FVIIa. (A) The canine FVII and FVIIa constructs contained a C-terminal epitope tag (HPC4) for immunoaffinity purification. To generate canine FVIIa, we introduced a short amino acid sequence (RKRRKR) at the normal site of cleavage of canine FVII (Arg152-Ile153, indicated), which is recognized by an intracellular protease of the PACE/furin type. Cleavage at this sequence (indicated by  ) results in the secretion of a 2-chain, activated molecule. (B) Polyacrylamide gel electrophoresis of zymogen (Z) cFVII and cFVIIa under reducing conditions. The molecular size marker (M) bands in kDa are indicated as well as the heavy (∼35 kDa, H) and light (∼20 kDa, L) chains. (C) TF-dependent activity of cFVII and cFVIIa in a PT-based clotting assay using canine FVII-deficient plasma relative to rhFVIIa (100%). *P < .05 versus rhFVIIa. (D) TF-independent activity of cFVII and cFVIIa in an aPTT-based clotting assay using either canine hemophilia A (HA) or B (HB) plasma, relative to rhFVIIa (100%). ND indicates nondetectable activity.
) results in the secretion of a 2-chain, activated molecule. (B) Polyacrylamide gel electrophoresis of zymogen (Z) cFVII and cFVIIa under reducing conditions. The molecular size marker (M) bands in kDa are indicated as well as the heavy (∼35 kDa, H) and light (∼20 kDa, L) chains. (C) TF-dependent activity of cFVII and cFVIIa in a PT-based clotting assay using canine FVII-deficient plasma relative to rhFVIIa (100%). *P < .05 versus rhFVIIa. (D) TF-independent activity of cFVII and cFVIIa in an aPTT-based clotting assay using either canine hemophilia A (HA) or B (HB) plasma, relative to rhFVIIa (100%). ND indicates nondetectable activity.
In vitro activity of zymogen cFVII and cFVIIa
Subsequently, we investigated the proteolytic activity of zymogen cFVII and cFVIIa, using rhFVIIa as a reference standard. Using pooled canine plasma from FVII-deficient dogs,18 we determined the TF-dependent activity of cFVIIa using relipidated recombinant human TF (Innovin). In this system, zymogen cFVII exhibited very low activity (∼ 20% of rhFVIIa, P < .001 vs rhFVIIa; Figure 1C) in contrast to cFVIIa, which exhibited approximately 240% of rhFVIIa (P < .001 vs rhFVIIa; Figure 1C), approximately 12-fold higher activity than zymogen cFVII. In a TF-independent aPTT-based assay using HA or HB canine plasma, cFVIIa exhibited a slightly increased (HA, P = .044) or comparable (HB, P = .44) activity relative to rhFVIIa (Figure 1D). In that assay, zymogen cFVII had negligible activity (Figure 1D). It is possible that the disparity in extrinsic and intrinsic activity of cFVIIa relative to rhFVIIa is the result of the use of human relipidated TF. Collectively, these data demonstrate that cFVII can be engineered so that it is secreted in a 2-chain, biologically active form.
Efficacy of AAV-mediated cFVIIa gene transfer in hemophilic mice and dogs
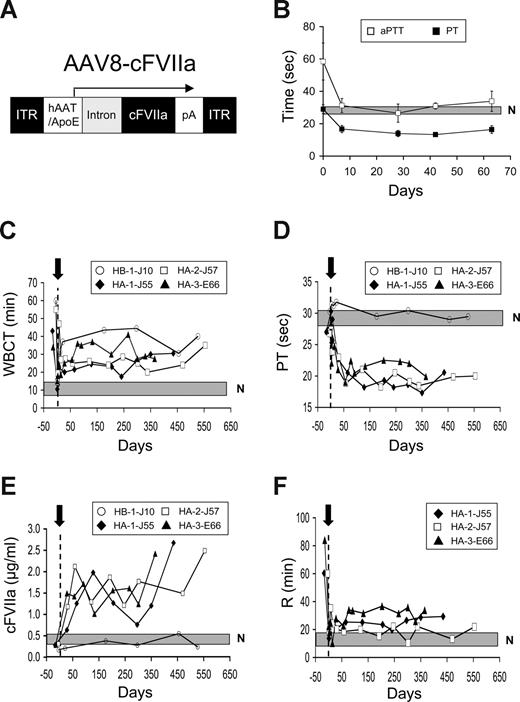
We generated a serotype 8 AAV vector (AAV8-cFVIIa, Figure 2A) directing expression of cFVIIa via a liver-specific promoter, similar to our previous studies in mice.9,10 The cFVIIa transgene in this AAV vector did not contain the C-terminal epitope tag (HPC4). In preliminary experiments designed to test the functionality of the AAV vector, we performed tail vein vector administration in hemophilic mice at a dose of 0.3 to 1.2E12 vector genomes/mouse (1.2-4.8E13 vector genomes/kg). After gene transfer, expression of cFVIIa resulted in long-term, cross-species, sustained hemostatic functionality (Figure 2B), as demonstrated by normalization of the hemophilic aPTT (P > .06 vs normal mice, except for day 42 [P = .01]) and concurrent supraphysiologic shortening in the PT (P < .001 vs normal mice). These experiments thus confirmed the functionality of the vector subsequently used in the dog experiments and suggested that cFVIIa expression in HA but otherwise immunocompetent mice did not induce an inhibitory immune response.
In vivo efficacy of AAV-mediated gene transfer of cFVIIa in hemophilia A and B dogs. (A) AAV8 vector used for infusion in hemophilia mice and dogs. Inverted terminal repeats (ITRs) flank the expression cassette composed of an hAAT/apoE promoter/enhancer, a synthetic intron, the cFVIIa cDNA (with 5′ UTR and absence of the HPC4 tag) and a polyadenylation signal (pA) from bovine growth hormone. (B) PT and aPTT in HA mice (n = 6) after tail vein administration of AAV8-cFVIIa. A shaded box represents the range of values for aPTT in normal (N) mice, determined from at least 4 mice. (C) WBCT after portal vein AAV8-cFVIIa vector administration ( ) in HA and HB dogs. A shaded box represents the range of values for normal dogs. A vertical dotted line represents day 0. Prophylactic plasma treatment was administered on days 2 to 4 (HA-1-J55, HA-2-J57), 2 to 5 (HA-3-E66), and 1 to 5 as well as for 2 days after nonspontaneous bleeding episodes in the HB dog (occurring on days 11, 159, and 228 after AAV administration). (D) PT after portal vein AAV8-cFVIIa vector administration (
) in HA and HB dogs. A shaded box represents the range of values for normal dogs. A vertical dotted line represents day 0. Prophylactic plasma treatment was administered on days 2 to 4 (HA-1-J55, HA-2-J57), 2 to 5 (HA-3-E66), and 1 to 5 as well as for 2 days after nonspontaneous bleeding episodes in the HB dog (occurring on days 11, 159, and 228 after AAV administration). (D) PT after portal vein AAV8-cFVIIa vector administration ( ) in HA and HB dogs. A shaded box represents the range of values for normal dogs. A vertical dotted line represents day 0. (E) cFVIIa expression after portal vein AAV8-cFVIIa vector administration (
) in HA and HB dogs. A shaded box represents the range of values for normal dogs. A vertical dotted line represents day 0. (E) cFVIIa expression after portal vein AAV8-cFVIIa vector administration ( ) in HA and HB dogs. A shaded box represents the range of values for normal, HA, and HB dogs. (F) Reaction time (time to initial fibrin formation) in thromboelastography analysis of whole blood in the treated HA dogs after AAV8-cFVIIa vector infusion (
) in HA and HB dogs. A shaded box represents the range of values for normal, HA, and HB dogs. (F) Reaction time (time to initial fibrin formation) in thromboelastography analysis of whole blood in the treated HA dogs after AAV8-cFVIIa vector infusion ( ). A shaded box represents the range of values for normal dogs.
). A shaded box represents the range of values for normal dogs.
In vivo efficacy of AAV-mediated gene transfer of cFVIIa in hemophilia A and B dogs. (A) AAV8 vector used for infusion in hemophilia mice and dogs. Inverted terminal repeats (ITRs) flank the expression cassette composed of an hAAT/apoE promoter/enhancer, a synthetic intron, the cFVIIa cDNA (with 5′ UTR and absence of the HPC4 tag) and a polyadenylation signal (pA) from bovine growth hormone. (B) PT and aPTT in HA mice (n = 6) after tail vein administration of AAV8-cFVIIa. A shaded box represents the range of values for aPTT in normal (N) mice, determined from at least 4 mice. (C) WBCT after portal vein AAV8-cFVIIa vector administration ( ) in HA and HB dogs. A shaded box represents the range of values for normal dogs. A vertical dotted line represents day 0. Prophylactic plasma treatment was administered on days 2 to 4 (HA-1-J55, HA-2-J57), 2 to 5 (HA-3-E66), and 1 to 5 as well as for 2 days after nonspontaneous bleeding episodes in the HB dog (occurring on days 11, 159, and 228 after AAV administration). (D) PT after portal vein AAV8-cFVIIa vector administration (
) in HA and HB dogs. A shaded box represents the range of values for normal dogs. A vertical dotted line represents day 0. Prophylactic plasma treatment was administered on days 2 to 4 (HA-1-J55, HA-2-J57), 2 to 5 (HA-3-E66), and 1 to 5 as well as for 2 days after nonspontaneous bleeding episodes in the HB dog (occurring on days 11, 159, and 228 after AAV administration). (D) PT after portal vein AAV8-cFVIIa vector administration ( ) in HA and HB dogs. A shaded box represents the range of values for normal dogs. A vertical dotted line represents day 0. (E) cFVIIa expression after portal vein AAV8-cFVIIa vector administration (
) in HA and HB dogs. A shaded box represents the range of values for normal dogs. A vertical dotted line represents day 0. (E) cFVIIa expression after portal vein AAV8-cFVIIa vector administration ( ) in HA and HB dogs. A shaded box represents the range of values for normal, HA, and HB dogs. (F) Reaction time (time to initial fibrin formation) in thromboelastography analysis of whole blood in the treated HA dogs after AAV8-cFVIIa vector infusion (
) in HA and HB dogs. A shaded box represents the range of values for normal, HA, and HB dogs. (F) Reaction time (time to initial fibrin formation) in thromboelastography analysis of whole blood in the treated HA dogs after AAV8-cFVIIa vector infusion ( ). A shaded box represents the range of values for normal dogs.
). A shaded box represents the range of values for normal dogs.
Because the AAV8-cFVIIa vector dose that would result in hemostatic efficacy in hemophilic dogs was unknown, we initially infused an HB male dog (HB-1-J10) with 2.06E13 vector genomes/kg via the portal vein (Table 1). This dose only resulted in a modest reduction in WBCT and no change in the PT, a finding that was not the result of an immune response to cFVIIa (Figure 2C,D; and data not shown). These observations were similar throughout the observation period of this animal (beyond day 527 depicted, up to 34 months, ongoing). Based on this result, we infused progressively higher doses of AAV8-cFVIIa (3- or 6-fold) in HA dogs (Table 1). Both HA and HB dogs respond similarly to infusions of rhFVIIa (Brinkhous et al12 ; T.C.N., personal communication, August 2008) and thus were expected to perform similarly after AAV8-cFVIIa gene transfer. The AAV8-cFVIIa–treated HA dogs exhibited an initial reduction in WBCT and PT because of prophylactic normal canine plasma infusions (daily for the first 4-5 days after vector administration, Figure 2C and D, respectively). Subsequently, we observed a long-term, stable reduction, well below preinfusion times for both WBCT and PT (Figure 2C and D, respectively). This was a result of sustained cFVIIa gene expression that ranged between 1.3 and 2.6 μg/mL (P < .03 vs normal or untreated HA dogs; Figure 2E). As expected, the treated HB-1-J10 dog that did not exhibit a measurable reduction in the PT did not exhibit a change in cFVIIa antigen levels (Figure 2E).
Hemophilia dogs used in this study
| Dog . | Phenotype . | Sex . | Age at vector infusion . | Weight at vector infusion, kg . | Vector dose, vector genomes/kg . | Total vector, vector genomes . |
|---|---|---|---|---|---|---|
| HB-1-J10 | HB | Male | 3 mo | 8.1 | 2.06E13 | 1.67E14 |
| HA-1-J55 | HA | Male | 11 mo | 19.3 | 6.25E13 | 1.25E15 |
| HA-2-J57 | HA | Female | 1.2 y | 21.1 | 1.25E14 | 2.6E15 |
| HA-3-E66 | HA | Male | 6.5 y | 20.0 | 1.25E14 | 2.5E15 |
| Dog . | Phenotype . | Sex . | Age at vector infusion . | Weight at vector infusion, kg . | Vector dose, vector genomes/kg . | Total vector, vector genomes . |
|---|---|---|---|---|---|---|
| HB-1-J10 | HB | Male | 3 mo | 8.1 | 2.06E13 | 1.67E14 |
| HA-1-J55 | HA | Male | 11 mo | 19.3 | 6.25E13 | 1.25E15 |
| HA-2-J57 | HA | Female | 1.2 y | 21.1 | 1.25E14 | 2.6E15 |
| HA-3-E66 | HA | Male | 6.5 y | 20.0 | 1.25E14 | 2.5E15 |
To further demonstrate efficacy, we performed thromboelastography analysis of whole blood from the treated HA dogs. We observed a greatly improved clot dynamics profile (data not shown) and a sustained, near-normalization of the reaction time (time to initial fibrin formation26 ; P = .03 [HA-1-J55], P = .08 [HA-2-J57], P = .004 [HA-3-E66] vs normal dogs; Figure 2F), well below HA values (P < .001 vs HA). The treated HA dogs also exhibited near-normalization of the maximum clot amplitude (data not shown). Corroborating its WBCT, PT, and cFVIIa antigen levels, the treated HB-1-J10 dog showed a modest improvement in these thromboelastography parameters compared with untreated HB controls (data not shown).
AAV-cFVIIa–treated hemophilic dogs do not exhibit spontaneous bleeding episodes
Because dogs with hemophilia exhibit approximately 5 or 6 spontaneous bleeding episodes per year,27,28 we used the number of spontaneous bleeds as a clinically relevant efficacy endpoint. Control untreated HA and HB dogs that were observed concurrently within this study exhibited 12 and 21 spontaneous bleeds in 36 and 60 cumulative months of observation, respectively (Table 2). In a cumulative 34 months (HB) and 45 months (HA) of observation, the AAV8-cFVIIa treated dogs did not exhibit any spontaneous bleeding episodes, in contrast to the expected 15 (HB) and 21 (HA) episodes (P < .002 vs historical data in this dog colony27,28 or the paired concurrent controls; Table 2). The lowest dosed HB dog exhibited 3 nonspontaneous bleeds within the first 8 months after AAV vector administration and each after a hemostatic challenge (the initial surgery for vector delivery, a liver biopsy performed to study cFVIIa expression, and dog fighting; normal canine plasma was administered daily for each episode for up to 3 days). Remarkably, despite the lack of appreciable changes in WBCT, PT, or cFVIIa expression in this dog (up to 34 months of observation, ongoing), AAV8-cFVIIa administered at vector doses that did not raise the circulating levels of cFVIIa resulted in a measurable phenotypic impact.
Spontaneous bleeding episodes in the hemophilia-treated dogs and untreated HA and HB controls recorded concurrently with this study
| Dog . | Vector, vector genomes/kg . | Spontaneous bleeds, observed/expected . | Months observed . | Untreated HA, bleeds/observation period (mo) . | Untreated HB, bleeds/observation period (mo) . |
|---|---|---|---|---|---|
| HB-1-J10 | 2.06E13 | 0/15* | 34 | Dog 1 (8/24) | Dog 1 (4/12) |
| HA-1-J55 | 6.25E13 | 0/8 | 18 | Dog 2 (4/12) | Dog 2 (9/24) |
| HA-2-J57 | 1.25E14 | 0/7 | 15 | Dog 3 (8/24) | |
| HA-3-E66 | 1.25E14 | 0/6 | 12 | ||
| Cumulative spontaneous bleeds | 12 in 36 mo | 21 in 60 mo | |||
| HB | 0 in 34 mo | ||||
| HA | 0 in 45 mo | ||||
| Expected spontaneous bleeds | |||||
| HB | 15 in 34 mo | ||||
| HA | 21 in 45 mo | ||||
| Dog . | Vector, vector genomes/kg . | Spontaneous bleeds, observed/expected . | Months observed . | Untreated HA, bleeds/observation period (mo) . | Untreated HB, bleeds/observation period (mo) . |
|---|---|---|---|---|---|
| HB-1-J10 | 2.06E13 | 0/15* | 34 | Dog 1 (8/24) | Dog 1 (4/12) |
| HA-1-J55 | 6.25E13 | 0/8 | 18 | Dog 2 (4/12) | Dog 2 (9/24) |
| HA-2-J57 | 1.25E14 | 0/7 | 15 | Dog 3 (8/24) | |
| HA-3-E66 | 1.25E14 | 0/6 | 12 | ||
| Cumulative spontaneous bleeds | 12 in 36 mo | 21 in 60 mo | |||
| HB | 0 in 34 mo | ||||
| HA | 0 in 45 mo | ||||
| Expected spontaneous bleeds | |||||
| HB | 15 in 34 mo | ||||
| HA | 21 in 45 mo | ||||
The HB (male) dog exhibited 3 nonspontaneous bleeding episodes within the first 8 months of observation.
Safety of long-term cFVIIa expression in the AAV-treated hemophilic dogs
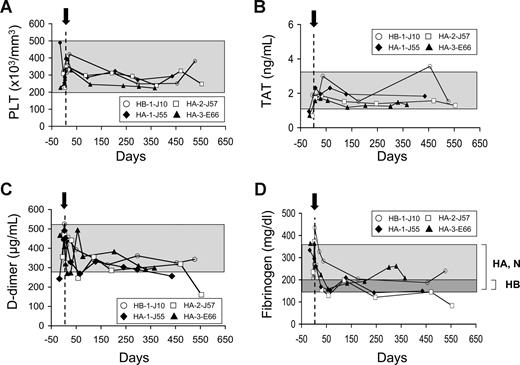
Serum chemistries for liver and kidney functions before and after gene transfer were closely monitored throughout the study. Values of serum creatine phosphokinase, AP, total bilirubin, aspartate aminotransferase, alanine aminotransferase, serum glutamyl pyruvic transaminase, serum glutamyl oxaloacetic transaminase, and gamma glutamyl transpeptidase were determined and found to be within the normal range in all dogs (Table 3). AP levels for the HB-1-J10 dog were marginally elevated for the first 2 weeks, but this dog also had slightly raised AP levels even before AAV infusion (Table 3). Platelet counts were within the normal range throughout the study (Figure 3A). Because mice continuously expressing mFVIIa at levels more than 2 μg/mL exhibited premature mortality,10 to further address the safety of this gene transfer approach, we used 3 different assays: levels of TAT complex, D-dimer levels (elevated levels of which are indicators of thromboembolic disease and disseminated intravascular coagulation [DIC] in dogs29,30 ), and fibrinogen levels, elevated levels of which are associated with cardiovascular disease.31 We did not detect any significant changes in TAT or D-dimer levels relative to pre-AAV administration (P > .2) in any of the vector doses used in AAV-treated dogs throughout the observation period (Figure 3B and C, respectively). Fibrinogen levels displayed some variability with time and 2 of 4 dogs (HB-1-J10 and HA-1-J55) exhibited a transient (∼ 1 week in duration) elevation, possibly because of a postoperative response to the administered analgesia before and after surgery, as previously described.32 In any case, analysis of postoperative samples starting 1 week after the last prophylactic normal canine plasma infusion revealed fibrinogen levels that were within the normal range for HA and HB dogs (P > .05 vs untreated HB for the HB dog; P > .12 vs untreated HA for the HA-1-J55, HA-2-J57, and HA-3-E66 dogs; Figure 3D). This was in agreement with the lack of significant changes in D-dimer levels observed (Figure 3C). Lastly, as expected, all AAV-treated dogs exhibited sustained anti-AAV8 antibodies, which developed within the first 4 weeks after vector infusion that were predominantly IgG2 (data not shown). It is noted that there is an initial drop in the WBCT and after AAV administration; however, this is probably the result of prophylaxis with normal plasma that is administered at the time of vector infusion. Only one dog (HA-3-E66) exhibited a transient rise in anti-cFVIIa IgG above background at day 7 after AAV infusion, returning to baseline at day 28 (data not shown). This antibody was not inhibitory because the WBCT was still below its baseline throughout that period even though the dog was no longer receiving normal canine plasma prophylaxis. Collectively, these findings further corroborate the long-term hemostatic and phenotypic effects observed after AAV8-cFVIIa gene transfer and indicate that continuous cFVIIa expression has an excellent safety profile throughout the duration of this study (34 months for the HB dog; 18 months for HA dogs [longest individual observation]).
Serum chemistries in the AAV-treated hemophilia dogs
| Day . | CPK 59-895 U/L . | AP 5-131 U/L . | SGPT 12-118 U/L . | SGOT 15-66 U/L . | Total bilirubin 0.1-0.3 mg/dL . | GGTP 1.0-12 U/L . |
|---|---|---|---|---|---|---|
| HB-1-J10 dog | ||||||
| Baseline | 340 | 134 | 37 | 33 | 0.1 | < 5 |
| 3 | 344 | 163 | 101 | 27 | 0.1 | < 5 |
| 14 | 283 | 136 | 32 | 27 | 0.1 | < 5 |
| 108 | 253 | 67 | 46 | 25 | 0.1 | < 5 |
| 297 | 145 | 45 | 45 | 23 | 0.2 | < 5 |
| 358 | 87 | 25 | 40 | 24 | 0.1 | < 5 |
| 527 | 104 | 32 | 36 | 19 | 0.3 | < 5 |
| HB-1-J10 dog | ||||||
| Baseline | 13 | 79 | 28 | 32 | 0.1 | < 5 |
| 3 | 207 | 81 | 65 | 37 | 0.2 | < 5 |
| 93 | 133 | 48 | 72 | 41 | 0.2 | < 5 |
| 296 | 80 | 44 | 41 | 31 | 0.2 | < 5 |
| 435 | 100 | 29 | 71 | 31 | 0.2 | < 5 |
| HA-2-J57 dog | ||||||
| Baseline | 100 | 46 | 34 | 35 | 0.2 | < 5 |
| 3 | 103 | 91 | 46 | 22 | 0.2 | < 5 |
| 86 | 116 | 41 | 47 | 44 | 0.2 | < 5 |
| 275 | 80 | 22 | 31 | 24 | 0.2 | < 5 |
| 470 | 107 | 41 | 47 | 27 | 0.3 | < 5 |
| 554 | 131 | 33 | 87 | 35 | 0.2 | < 5 |
| HA-3-E66 dog | ||||||
| Baseline | 152 | 20 | 73 | 45 | 0.1 | < 5 |
| 3 | 243 | 47 | 98 | 49 | 0.1 | < 5 |
| 6 | 177 | 41 | 68 | 36 | 0.1 | < 5 |
| 105 | 131 | 15 | 55 | 33 | 0.1 | < 5 |
| 301 | 153 | 15 | 44 | 32 | 0.1 | < 5 |
| 365 | 93 | 16 | 53 | 34 | 0.1 | < 5 |
| Day . | CPK 59-895 U/L . | AP 5-131 U/L . | SGPT 12-118 U/L . | SGOT 15-66 U/L . | Total bilirubin 0.1-0.3 mg/dL . | GGTP 1.0-12 U/L . |
|---|---|---|---|---|---|---|
| HB-1-J10 dog | ||||||
| Baseline | 340 | 134 | 37 | 33 | 0.1 | < 5 |
| 3 | 344 | 163 | 101 | 27 | 0.1 | < 5 |
| 14 | 283 | 136 | 32 | 27 | 0.1 | < 5 |
| 108 | 253 | 67 | 46 | 25 | 0.1 | < 5 |
| 297 | 145 | 45 | 45 | 23 | 0.2 | < 5 |
| 358 | 87 | 25 | 40 | 24 | 0.1 | < 5 |
| 527 | 104 | 32 | 36 | 19 | 0.3 | < 5 |
| HB-1-J10 dog | ||||||
| Baseline | 13 | 79 | 28 | 32 | 0.1 | < 5 |
| 3 | 207 | 81 | 65 | 37 | 0.2 | < 5 |
| 93 | 133 | 48 | 72 | 41 | 0.2 | < 5 |
| 296 | 80 | 44 | 41 | 31 | 0.2 | < 5 |
| 435 | 100 | 29 | 71 | 31 | 0.2 | < 5 |
| HA-2-J57 dog | ||||||
| Baseline | 100 | 46 | 34 | 35 | 0.2 | < 5 |
| 3 | 103 | 91 | 46 | 22 | 0.2 | < 5 |
| 86 | 116 | 41 | 47 | 44 | 0.2 | < 5 |
| 275 | 80 | 22 | 31 | 24 | 0.2 | < 5 |
| 470 | 107 | 41 | 47 | 27 | 0.3 | < 5 |
| 554 | 131 | 33 | 87 | 35 | 0.2 | < 5 |
| HA-3-E66 dog | ||||||
| Baseline | 152 | 20 | 73 | 45 | 0.1 | < 5 |
| 3 | 243 | 47 | 98 | 49 | 0.1 | < 5 |
| 6 | 177 | 41 | 68 | 36 | 0.1 | < 5 |
| 105 | 131 | 15 | 55 | 33 | 0.1 | < 5 |
| 301 | 153 | 15 | 44 | 32 | 0.1 | < 5 |
| 365 | 93 | 16 | 53 | 34 | 0.1 | < 5 |
The normal range (derived from 18 clinically healthy dogs) is stated for each test. CPK indicates creatine phosphokinase; AP, alkaline phosphatase; SGPT, serum glutamic pyruvic transaminase; SGOT, serum glutamic oxaloacetic transaminase; and GGPT, gamma glutamyl transpeptidase.
Safety of continuous expression of cFVIIa. Platelet counts (PLT) (A), levels of TAT (B), D-dimer (C), and fibrinogen (D) are shown as a function of time, after AAV8-cFVIIa administration (arrow). Day 0 is indicated by a dotted line. A shaded box represents the range of values for normal (N), HA, or HB dogs (except in panel D where it is indicated for HA, N, and HB separately).
Safety of continuous expression of cFVIIa. Platelet counts (PLT) (A), levels of TAT (B), D-dimer (C), and fibrinogen (D) are shown as a function of time, after AAV8-cFVIIa administration (arrow). Day 0 is indicated by a dotted line. A shaded box represents the range of values for normal (N), HA, or HB dogs (except in panel D where it is indicated for HA, N, and HB separately).
Discussion
Gene therapy has focused on targeting monogenic disorders in which the relationship between gene transfer and phenotypic improvement is straightforward. In particular, hemophilia has been very well characterized, both biochemically and molecularly, and extensive knowledge exists in terms of therapeutic interventions. However, despite several gene transfer clinical trials using a variety of vectors and routes of administration,15 none has yet addressed the possibility of such an approach in patients with inhibitors, where preexisting immunity would diminish the human FVIII or FIX therapeutic expression from a delivery vector. To address this, we have previously demonstrated that gene transfer of a FVII transgene engineered for secretion in an activated form can normalize the hemophilic phenotype in mice.9 Such an approach would be particularly beneficial in hemophilia patients with inhibitors because patients would be tolerant to the introduced transgene (FVIIa). Therefore, continuous expression of FVIIa would afford functional and immunologic advantages, effecting hemostasis in both HA and HB with or without inhibitors as well as other coagulation disorders, as observed with bolus dosing of rhFVIIa.
Here, we provide proof-of-concept for this gene transfer approach using continuous expression of canine FVIIa in hemophilic dogs. This large animal model has been a good predictor for efficacy in hemophilia using a range of products.12-15 However, because FVIIa is a protease (in contrast to previous gene transfer approaches using zymogen FVIII or FIX), the issue of safety, especially with respect to thrombosis, is pertinent. Hence, our study focused on demonstrating both efficacy and safety, and we report 3 major findings in hemophilic dogs: (1) canine FVII can be engineered for secretion in a biologically active, 2-chain form; (2) continuous expression of FVIIa at levels of approximately 2 μg/mL (∼40 nM) can result in a marked, long-term improvement in the bleeding diathesis, despite only partial correction of in vitro hemostatic parameters; and (3) at these expression levels and administered AAV8 vector doses, we observed no hepatotoxicity or increased risk of thrombosis.
With respect to levels of expression necessary for efficacy, our results are similar to those obtained in hemophilic mice transgenic for murine FVIIa (mFVIIa) or after AAV-mFVIIa-mediated gene transfer.9,10 An administered dose in the range of 6.25 to 12.5E13 vector genomes/kg led to sustained cFVIIa expression of 1.5 to 2.5 μg/mL, resulting in supraphysiologic reduction in the PT (as expected) and a partial or near-correction of the WBCT and thromboelastography parameters, respectively. This suggests that, in both animal models of hemophilia, hemostatic efficacy can be achieved at levels comparable with bolus rhFVIIa infusion in humans. However, the effective vector dose in hemophilic dogs was 2- to 5-fold higher than doses required in mice, resembling similar findings with cFVIII or cFIX.33,34 This observation thus justifies the use of this large animal model in preclinical gene transfer studies using FVIIa, as a predictor of efficacy in humans; it is worth noting that dogs, in contrast to mice, predicted the correct dosing in a human liver trial using an AAV-human FIX vector.35 Moreover, the lack of clinically relevant endpoints in mouse studies, such as the spontaneous bleeding episodes observed in hemophilic dogs, further underlines the value of the canine model of hemophilia. Indeed, we observed a complete lack of spontaneous bleeds in the AAV-treated hemophilic dogs throughout this study, a clear improvement in the bleeding diathesis. Comparable results would be expected after AAV-mediated gene transfer of cFVIIa in hemophilic dogs with inhibitors,36 similar to the phenotypic improvement demonstrated in hemophilic mice with hFVIII inhibitors, after transplantation of genetically modified platelets expressing mFVIIa.37
The evident improvement in the bleeding diathesis has particular clinical importance with respect to a human application of this gene transfer approach. The major morbidity in hemophilia patients results from repeated bleeds into the joints.38 If these result from damage in the joint microenvironment, it is conceivable that continuous expression of FVIIa may result in a reduced number of joint bleeds. We have previously shown that continuous expression of mFVIIa at levels of 0.5 to 1.5 μg/mL in hemophilic mice resulted in restoration of hemostasis after a hemostatic challenge in the microcirculation, but not a challenge in the large vessels.10 Hence, a potential explanation for the absence of spontaneous bleeds (including joint bleeds) in this study may be that continuous expression of FVIIa offers a “protective” and localized hemostatic effect, particularly in the microcirculation. Surprisingly, despite the lack of appreciable changes in the hemostatic parameters and cFVIIa levels in the treated HB dog after low-dose AAV8-cFVIIa infusion, we only observed 3 nonspontaneous bleeds within the first 8 months after vector administration in an observation period of 34 months. Similar results in HA dogs receiving a low vector dose would be expected given the similarity of these 2 hemophilia models in response to rhFVIIa infusions.12 The fact that AAV-mediated, low vector dose FVIIa gene transfer may offer some clinical benefit certainly warrants further investigation in the dog model because it will be safe and may have potential application in prophylaxis. Such experiments in dogs, which are currently underway, will help define the lowest expression levels that result in a measurable clinical improvement.
An important observation in hemophilic mice transgenic for mFVIIa was the premature mortality of mice continuously expressing mFVIIa at levels more than 2 μg/mL.10 Because the dog model of hemophilia has been a good predictor of efficacy of hemophilia treatments, it was therefore pertinent in this study, where FVIIa is continuously expressed, to carefully examine the potential for thromboembolic disease, DIC, and hepatotoxicity. Levels of TAT and D-dimer throughout the study were within the normal range of HA/HB dogs, even in dogs that received the highest vector dose (HA-2-J57, HA-3-E66), suggesting that continuous expression of FVIIa at the levels reported here does not result in a procoagulant state. This was in good agreement with the partial correction of their in vitro hemostatic parameters. The possibility of systemic consumptive coagulation was evaluated using platelet counts, D-dimer and fibrinogen levels, biomarkers used for the diagnosis of DIC,39 all of which were negative. Lastly, monitoring liver enzymes did not reveal any evidence of hepatotoxicity and demonstrated, for the first time, that AAV8 vector doses up to 2.6 E15 vector genomes are well tolerated in hemophilic dogs.
An important observation of the current study is that the level of expression required for efficacy is higher in comparison to similar AAV-based, liver-directed approaches for hemophilia using cFVIII or cFIX, where phenotypic improvement was observed with as little as 4% to 9% of cFVIII (4-20 ng/mL,33,40 assuming 100-200 ng/mL is 100%) or 5% of cFIX (∼ 250 ng/mL,22 assuming 5 μg/mL is 100%). Consequently, the AAV8 vector doses used here were higher than used for AAV8-cFVIII (1E13 vector genomes/kg40 ) or AAV8-cFIX (5.25E13 vector genomes/kg41 ). However, this is not surprising; recombinant FVIIa has a substantially shorter half-life (∼ 2.5 hours42 ) than either recombinant FVIII (∼12 hours43 ) or FIX (∼ 19 hours44 ) and pharmacologic levels required for hemostatic efficacy are higher (as shown by bolus dosing of rhFVIIa compared with recombinant FVIII or FIX). Clearly, a strategy to reduce the effective vector dose may be desirable and is currently underway, using a combination of improved AAV vectors45 as well as optimization of guanine-cytosine content, cis-regulatory elements, and codon usage.46 Moreover, complementary to improvements on the DNA level, changes on the protein level may also be implemented using high-activity variants of FVIIa.47,48
In conclusion, our data demonstrate that liver-directed, AAV8-mediated gene transfer of cFVIIa in HA and HB dogs is safe in the short and medium term and results in a marked and sustained phenotypic improvement. Although conventional gene transfer for hemophilia is based on delivery of FVIII or FIX for therapeutic expression, this is the first proof-of-concept delivery of a gene-based FVIII/FIX bypassing agent in a large animal model of hemophilia. This approach has potential applications not only for hemophilia gene therapy but also for platelet disorders, FVII deficiency, or prophylaxis in congenital hemophilia complicated by inhibitors, as has been reported for rhFVIIa.8
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Ulla Hedner (Novo Nordisk), Dr Sriram Krishnaswamy, and Dr Rodney M. Camire at the Children's Hospital of Philadelphia for helpful discussions.
This work was supported by the Howard Hughes Medical Institute (K.A.H.) and the National Institutes of Health (NIH, Bethesda, MD; grants U01 HL66948 and P01 HL074124, K.A.H.; HL063098, T.C.N.; and RR02512, U.G.).
National Institutes of Health
Authorship
Contribution: P.M. designed and performed research, analyzed data, and wrote the paper; E.R. performed research and analyzed data; M.N.A. and H.D.D. performed research; U.G. has worked on FVII-deficient dogs and provided the canine FVII-deficient plasma; S.Z. provided the AAV vector preparation; E.M. and A.D. performed research; M.E. provided reagents and assisted in the development of the cFVIIa antigen assay; T.C.N. performed research and analyzed data; and K.A.H. designed research and wrote the paper.
Conflict-of-interest disclosure: M.E. is an employee of Novo Nordisk A/S. The remaining authors declare no competing financial interests.
Correspondence: Katherine A. High, William H. Bennett Professor of Pediatrics, Investigator, Howard Hughes Medical Institute, Children's Hospital of Philadelphia, 3615 Civic Center Blvd, 302 Abramson Research Center, Philadelphia, PA 19104; e-mail: high@email.chop.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal