Abstract
Immunotoxins based on Pseudomonas exotoxin A (PE) are promising anticancer agents that combine a variable fragment (Fv) from an antibody to a tumor-associated antigen with a 38-kDa fragment of PE (PE38). The intoxication pathway of PE immunotoxins involves receptor-mediated internalization and trafficking through endosomes/lysosomes, during which the immunotoxin undergoes important proteolytic processing steps but must otherwise remain intact for eventual transport to the cytosol. We have investigated the proteolytic susceptibility of PE38 immunotoxins to lysosomal proteases and found that cleavage clusters within a limited segment of PE38. We subsequently generated mutants containing deletions in this region using HA22, an anti-CD22 Fv-PE38 immunotoxin currently undergoing clinical trials for B-cell malignancies. One mutant, HA22-LR, lacks all identified cleavage sites, is resistant to lysosomal degradation, and retains excellent biologic activity. HA22-LR killed chronic lymphocytic leukemia cells more potently and uniformly than HA22, suggesting that lysosomal protease digestion may limit immunotoxin efficacy unless the susceptible domain is eliminated. Remarkably, mice tolerated doses of HA22-LR at least 10-fold higher than lethal doses of HA22, and these higher doses exhibited markedly enhanced antitumor activity. We conclude that HA22-LR advances the therapeutic efficacy of HA22 by using an approach that may be applicable to other PE-based immunotoxins.
Introduction
Monoclonal antibodies, either alone or as immunoconjugates linked to other agents, have become valuable therapies for the targeted treatment of cancer. In recent years, several antibody-based therapies have progressed through regulatory approval by the Food and Drug Administration, and it is expected that many more will follow.1 Immunotoxins are a category of immunoconjugate in which antibodies are joined to protein toxins. They exploit the precision of antibodies and the lethality of protein toxins to target and kill cancer cells expressing specific cell surface proteins. Any tumor-associated cell-surface antigen is a potential target for immunotoxins.
A variety of plant, fungal, and bacterial toxins have been adapted for use with immunotoxins, including ricin, diphtheria toxin, and Pseudomonas exotoxin A (PE).2,3 PE-based immunotoxins are currently in clinical trials for the treatment of CD22-expressing lymphomas and leukemias, as well as mesothelin-expressing solid tumors.4,5 A phase 1 trial of the anti-CD22 PE immunotoxin BL22 had a high overall response rate of 81% but was particularly effective against drug-resistant hairy cell leukemia (HCL).6 A phase 1 trial of the anti-CD25 PE immunotoxin LMB-2 showed a 23% response rate in patients with hematologic malignancies refractory to standard chemotherapy.7 A phase 1 trial of the antimesothelin PE immunotoxin SS1P demonstrated minor but encouraging responses for treating solid tumors in patients with mesothelioma or ovarian cancer who had failed standard therapies.5
To convert a toxin into a therapeutic agent, it is necessary to have a detailed understanding of the native toxin. The crystal structure of the 613-residue native PE showed that it is composed of 3 distinct structural regions known as domain I (subdivided into discontinuous domains Ia, residues 1-252, and Ib, residues 365-404), domain II (residues 253-364), and domain III (residues 405-613).8 Cells internalize PE by receptor-mediated endocytosis after an interaction between domain I and the α2-macroglobulin receptor/low-density lipoprotein receptor-related protein 1 (LRP1) or LRP1b.9,10 The internalized toxin traffics through the cell in endocytic vesicles and undergoes several processing steps before crossing the endoplasmic reticulum membrane into the cytosol.11-14 Research has suggested that domain II of PE is involved in the membrane translocation of enzymatic domain III into the cytosol.15,16 Domain III catalyzes the ADP-ribosylation and inactivation of elongation factor 2, which halts protein synthesis and eventually leads to cell death.3 Although the structural boundaries of domain III have been set at residues 405 to 613, functional analyses have shown that domain III requires a segment of domain Ib to retain ADP-ribosylation activity.15,17 The functional domain III is defined by residues 395 to 613 of PE.18
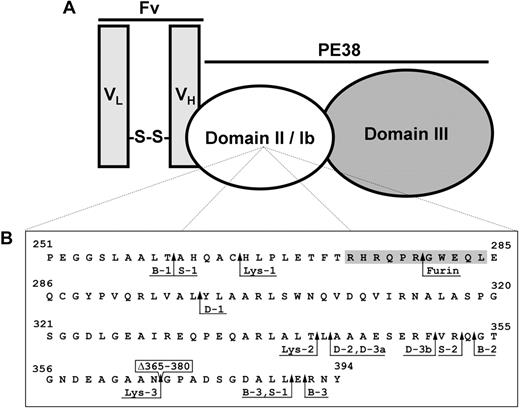
The chief difference between native PE and PE-based immunotoxins is that the variable fragment (Fv) of an antibody replaces domain Ia. This substitution changes the specificity of the toxin and targets it to antigens, such as CD22 or mesothelin. The current generation of PE-based immunotoxins combines the disulfide-linked, 2-chain variable fragment (dsFv) of a monoclonal antibody with PE38, a 38-kDa truncated form of PE. In addition to the removal of residues 1 to 250 of domain Ia, PE38 immunotoxins also lack residues 365 to 384 of domain Ib. Figure 1A illustrates the basic design of a PE38 immunotoxin.
PE-based immunotoxins. (A) The 2-chain disulfide-linked Fv of an antibody targeting a tumor-associated antigen is combined with the PE38 fragment of native PE to create an immunotoxin. (B) PE38 domains II and Ib. The sequences of domain II (residues 251-364) and domain Ib (residues 365-394) from PE38 are shown. Residue numbering is based on the amino acid sequence of native PE. Residues 365 to 380 from native PE (boxed) were deleted in the generation of PE38. Lysosomal protease cleavage sites, determined by N-terminal sequencing of fragments from B3(dsFv)-PE38 digests, are indicated by arrows adjacent to the designation of their corresponding band from SDS-PAGE analysis (Figure 2). Lysosomal protease cleavage sites occur between residues 260-261, 265-266, 297-298, 341-342, 342-343, 351-352, 352-353, 353-354, 364-381, 390-391, and 391-392. The furin cleavage site (279-280) is also indicated. The 11-residue furin-sensitive sequence in domain II from HA22-LR is shaded.
PE-based immunotoxins. (A) The 2-chain disulfide-linked Fv of an antibody targeting a tumor-associated antigen is combined with the PE38 fragment of native PE to create an immunotoxin. (B) PE38 domains II and Ib. The sequences of domain II (residues 251-364) and domain Ib (residues 365-394) from PE38 are shown. Residue numbering is based on the amino acid sequence of native PE. Residues 365 to 380 from native PE (boxed) were deleted in the generation of PE38. Lysosomal protease cleavage sites, determined by N-terminal sequencing of fragments from B3(dsFv)-PE38 digests, are indicated by arrows adjacent to the designation of their corresponding band from SDS-PAGE analysis (Figure 2). Lysosomal protease cleavage sites occur between residues 260-261, 265-266, 297-298, 341-342, 342-343, 351-352, 352-353, 353-354, 364-381, 390-391, and 391-392. The furin cleavage site (279-280) is also indicated. The 11-residue furin-sensitive sequence in domain II from HA22-LR is shaded.
Although the PE38 immunotoxins that have reached clinical trials are comparatively well tolerated at low doses, dose-limiting toxicities have restricted their therapeutic effect. In a phase 1 clinical trial of LMB-2, dose-limiting toxicities more than 40 μg/kg given every other day ×3 consisted of transaminase elevation, diarrhea, cardiomyopathy, and an allergic reaction.7 In a phase 1 clinical trial of immunotoxin SS1P, adverse events of pleuritis, urticaria, and vascular leak syndrome were found to be dose limiting.5 In a phase 1 trial of BL22, dose-limiting toxicities included several cases of hemolytic uremic syndrome and a cytokine release syndrome with systemic vascular leak syndrome.4
Immunotoxins are internalized into cells via target-mediated endocytosis and must reach the cytosol to exert their toxic effect. Because lysosomes are the major degradative pathway for exogenous, internalized macromolecules, immunotoxins must avoid lysosomal degradation on their path to the cytosol.19 Therefore, we decided to determine whether we could produce an immunotoxin resistant to lysosomal degradation by identifying and removing lysosomal protease cleavage sites in the immunotoxin. This report describes the properties of an immunotoxin with deletions that remove cleavage sites. Surprisingly, this immunotoxin retains excellent cytotoxic activity and is also much less toxic to mice. This decrease in toxicity allows much higher doses to be given, with a concomitant increase in antitumor activity.
Methods
Lysosomal preparation of Raji cells
Raji Burkitt's lymphoma cells (1-3 × 108) were harvested, washed twice in cold phosphate-buffered saline (PBS) and once in homogenization buffer (250 mM sucrose, 1 mM ethylenediaminetetraacetic acid), and resuspended in 2 mL of homogenization buffer. Cells in suspension were lysed by nitrogen cavitation using a 45-mL cell disruption bomb (Parr Instrument, Moline, IL), chilled to 4°C, and pressurized with nitrogen gas to 150 to 200 psi for 10 minutes. The disrupted cells were spun at 800g for 10 minutes. The postnuclear supernatant (middle layer) was removed and layered atop an 8.5 mL 27% Percoll solution cushioned on a 1.2-mL layer of 10× homogenization buffer in a 16 × 76 Ultraclear Beckman centrifuge tube and spun at 4°C in a Beckman Type 50 Ti rotor at 36 000g for 1 hour (Beckman Coulter, Fullerton, CA). Fractions from the Percoll gradient were collected and then assayed individually for β-hexosaminidase activity as described.20 The fractions with peak activity were pooled, transferred to 13 × 51 mm thick-walled polycarbonate tubes, and spun at 4°C using a S100 AT4-542 rotor at 200 000g for 30 minutes to remove the Percoll. The supernatant was collected and used to digest immunotoxins.
Lysosomal protease digestion of B3(dsFv)–PE38 and N-terminal sequencing of the fragments
Purified lysosomal proteases cathepsin B, cathepsin D, and cathepsin S (EMD Biosciences, San Diego, CA) or the lysosomal fraction of Raji cells were used to digest the immunotoxin B3(dsFv)-PE38. B3(dsFv)-PE38 (0.2 mg/mL) was incubated either with 5 μg/mL of the purified cathepsin lysosomal proteases (cathepsins B, D, and S) or with 30% (vol/vol) of the lysosomal fraction of Raji cells at 37°C in buffer containing 0.1 M of MES (pH 5.5), 150 mM NaCl, 2 mM DTT, 2 mM ethylenediaminetetraacetic acid, and 0.5% Triton X-100. At time intervals between 0 and 60 hours after the start of incubation, aliquots were removed into Tris-glycine sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer and incubated at 85°C for 5 minutes. Half of each sample was run on a Novex 4% to 20% acrylamide Tris-glycine protein gel (Invitrogen, Carlsbad, CA) and visualized using the Microwave Blue Coomassie blue protein stain (Protiga, Frederick, MD). The remaining sample was fractionated by gel electrophoresis in the same manner and then electroblotted onto polyvinylidene difluoride membrane (ProBlott, Applied Biosystems, Foster City, CA) in 10 mM CAPC (N-Cyclohexyl-3-aminopropanesulfonic acid) buffer (pH 11) using a semidry transfer unit. After blotting, the membrane was briefly rinsed with water, stained with 0.1% Coomassie Blue R-250 in 0.5% acetic acid/40% methanol for 2 minutes, and then destained in 50% methanol in water. Protein bands were excised from the membrane and analyzed using a Procise 494 cLC automated protein sequencer (Applied Biosystems).
Mutations in HA22
Mutations in HA22 were generated using Quikchange site-directed mutagenesis (Stratagene, La Jolla, CA) with mutagenesis primers from Lofstrand Labs (Gaithersburg, MD).
Purification of immunotoxins
Immunotoxins were purified as described,21 except that oxidized, not reduced, glutathione was added to the refolding buffer.
Cell lines
CD22-positive human Burkitt lymphoma cell lines (CA46, Daudi, Raji, and Ramos) were obtained from ATCC (Manassas, VA). The KOPN-8 acute lymphoblastic leukemia (ALL) cell line was obtained from Dr Alan Wayne at the National Cancer Institute (Bethesda, MD). The WSU-chronic lymphocytic leukemia (CLL) cell line, which may actually be a derivative of the REH ALL cell line,22 was obtained from Dr A. Al-Katib (Wayne State University, Detroit, MI). All cell lines were grown at 37°C with 5% CO2 in RPMI 1640 medium supplemented with 10% fetal bovine serum, 2 mM l-glutamine, 1 mM of sodium pyruvate, 100 U penicillin, and 100 μg streptomycin (Invitrogen).
Activity assays
Survival of cells treated with immunotoxins was measured by WST-8 assay using the Cell Counting Kit-8 (Dojindo Molecular Technologies, Gaithersburg, MD) essentially as described.23 Briefly, 104 cells/well were incubated with various concentrations of immunotoxin in a 96-well plate21 for 72 hours, after which 10 μL of the CCK-8 reagent was added to the wells. Plates were incubated until the wells with the maximum absorbance at 450 nm reached values of approximately 1 optical density (OD). Cycloheximide (10 μg/mL final concentration) was used as a control for 100% cell death. Values were normalized between the cycloheximide and PBS/human serum albumin (HSA; 0.2%) controls and fit to a standard 4-parameter sigmoidal equation with a variable slope using the GraphPad Prism (version 2.00; GraphPad Software, San Diego, CA) program to obtain the concentration of immunotoxin at which there was 50% cell death (IC50). Cells from patients with CLL and HCL were assayed as previously described.24 Briefly, leukemia cells were incubated with recombinant immunotoxins for 3 days, then treated with [3H]-leucine to assess protein synthesis inhibition or with WST-1 to assess cell death.
Statistical analysis
The IC50 values from matched pairs of cytotoxicity assays analyzing the effect of HA22 and HA22-LR on the survival of Raji (n = 10), Ramos (n = 3), Daudi (n = 3), CA46 (n = 5), KOPN8 (n = 3), and WSU-CLL (n = 4) cell lines were compared using a paired, 2-tailed t test.
Nonspecific mouse toxicity
Female nude mice (5-6 weeks old, 18-22 g) were intravenously injected with a single dose of 2.0 mg/kg HA22 or HA22-LR ranging from 2.5 to 20 mg/kg in 0.2 mL of PBS containing 0.2% HSA. Mice were observed for 10 days. In another experiment, female nude mice were injected intravenously with 0.2 mL PBS with HSA (0.2%) alone, containing 2.0 mg/kg of HA22, or containing 2.0, 6.0, or 20 mg/kg HA22-LR. Thirty hours after injection, livers and blood samples were collected. Livers were fixed, sectioned, and stained with hematoxylin and eosin (Histoserv, Germantown, MD). Serum was assayed for alanine aminotransferase (ALT) levels (Ani Lytics, Gaithersburg, MD). All procedures involving mice were conducted in accordance with National Institutes of Health guidelines as approved by the Animal Care and Use Committee of the National Cancer Institute.
Pharmacokinetics
Nine female Balb/c mice were injected in the tail vein with 10 μg of HA22 or HA22-LR in 0.2 mL of PBS with 0.2% HSA. Blood samples were taken from 3 separate mice at time intervals of 2, 5, 10, 20, 30, and 60 minutes from the time of injection, and each mouse was bled twice. Groups of 3 mice were bled at time intervals of 2 and 60 minutes, 5 and 30 minutes, or 10 and 20 minutes. Serum was harvested from the blood samples and analyzed by enzyme-linked immunosorbent assay (ELISA)25 in comparison to a standard curve of the corresponding pure immunotoxin to determine the concentration of immunotoxin in the mouse serum.
Mouse xenograft antitumor activity
Forty female severe combined immunodeficiency (SCID) mice were injected subcutaneously with 107 CA46 cells on day 0 as described previously.26 Tumor volume was measured regularly by caliper for the next 6 weeks. When the average tumor size reached approximately 120 mm3, 6 days after implantation, mice were divided into 5 treatment groups of 8 and injected every other day 3 times with 0.2 mL PBS containing 0.2% HSA and either HA22 (0.3 mg/kg) or HA22-LR (1.0. 1.75, or 2.5 mg/kg), or without immunotoxin (PBS/HSA alone). Mice were killed if their tumors exceeded 1000 mm3 or at the end of the 10-week experiment.
Results
Lysosomal protease digestion of immunotoxins
To determine the location of the lysosomal protease cleavage sites within immunotoxins, we required a large quantity of a highly purified immunotoxin. A large stock of B3(dsFv)-PE38, which contains the same PE38 fragment as other immunotoxins but with a different Fv, was available.27 B3(dsFv)-PE38 was incubated either with lysosomal extracts prepared from Raji cells or with purified lysosomal proteases cathepsin B, cathepsin D, or cathepsin S. Aliquots of the reaction were removed at times between 0 and 60 hours, and fragments were separated and visualized by reducing SDS-PAGE (Figure 2).
Cleavage of immunotoxins by lysosomal proteases. The immunotoxin B3(dsFv)-PE38 was incubated with lysosomal extract from Raji cells or with one of the purified lysosomal proteases cathepsin B, cathepsin D, or cathepsin S. Samples of the reaction were removed immediately after the addition of enzyme (0) and at various time intervals up to 60 hours after the start of the incubation, then analyzed by reducing SDS-PAGE. Arrows indicate the VH and VL-PE38 polypeptides that compose the mature immunotoxin and the lysosomal protease cleavage fragments sequenced by Edman degradation.
Cleavage of immunotoxins by lysosomal proteases. The immunotoxin B3(dsFv)-PE38 was incubated with lysosomal extract from Raji cells or with one of the purified lysosomal proteases cathepsin B, cathepsin D, or cathepsin S. Samples of the reaction were removed immediately after the addition of enzyme (0) and at various time intervals up to 60 hours after the start of the incubation, then analyzed by reducing SDS-PAGE. Arrows indicate the VH and VL-PE38 polypeptides that compose the mature immunotoxin and the lysosomal protease cleavage fragments sequenced by Edman degradation.
Each gel shows 2 expected bands at time 0 that correspond to the disulfide-linked polypeptides VL-PE38 and VH, which migrate at approximately 50 kDa and 12 kDa, respectively. Digestion of B3(dsFv)-PE38 with lysosomal extract shows 5 cleavage fragments: 38-kDa (Lys-1), 30-kDa (Lys-2), 27-kDa (Lys-3), 25-kDa (Lys-4), and 23-kDa (Lys-5). Cathepsin B digestion shows 3 fragments: 38-kDa (B-1), 30-kDa (B-2), and 25-kDa (B-3). Cathepsin D digestion shows at least 5 fragments: 36-kDa (D-1), 30-kDa (D-2), 15-kDa (D-3), 14-kDa (D-4), and 13-kDa (D-5). Digestion with cathepsin S shows 4 fragments: 38-kDa (S-1), 30-kDa (S-2), 25-kDa (S-3), and 13-kDa (S-4). The 4 digests contain several fragments that migrate with similar molecular weights, suggesting that the cleavage sites may be similar.
To locate the cleavage sites, the fragments were separated by SDS-PAGE, immobilized by electroblotting, and sequenced using Edman degradation. Arrows in Figure 2 indicate the sequenced fragments. The N-terminal sequences were compared with the sequence of B3(dsFv)-PE38 to determine the locations of the cleavage sites (Figure 1B). The sequences of several fragments correspond to the N-terminus of B3(dsFv)-PE38 VL-PE38 (Lys-4, Lys-5, D-5, and S-4). The remaining fragments are located in domains II or Ib of PE38. No cleavage sites were found in the Fv or PE domain III. Solid black arrows in Figure 1B indicate the locations of the cleavage sites.
Removal of protease-susceptible regions
To make PE38 immunotoxins protease resistant, we needed to eliminate the cleavage sites either through a series of point mutations to prevent protease recognition and cleavage, or through broader deletions to remove the sites entirely. Because there are numerous lysosomal proteases with broad and often overlapping specificity and the observed sites cluster in a limited segment of PE38, we chose the deletion approach.
B3(dsFv)-PE38 is no longer being pursued for therapeutic use, so we chose instead to use another PE38-based immunotoxin, HA22, to carry out the deletions. HA22 is an affinity-optimized, more active variant of the anti-CD22 immunotoxin BL2228 and is currently in clinical trials for the treatment of B-cell malignancies (CLL, HCL, and ALL). A series of deletions removing large segments of domains II and Ib from PE38 were introduced into HA22. The mutant proteins were expressed, purified, and compared with HA22 in vitro using cell survival assays on Raji cells.
Figure 3 indicates the sequence remaining in the mutant proteins and their activities relative to HA22 on Raji cells. Removal of residues 251 to 273 (M1) or 365 to 394 (M2) does not substantially affect immunotoxin activity. Likewise, deleting residues 251 to 273 and 350 to 394, along with changing a free cysteine at position 287 to serine (M3), yields a fully active immunotoxin. The C287S mutation combined with the deletion of residues 350 to 394 and 251 to 280 (M4), which eliminates furin cleavage at Arg279, yields an immunotoxin that is approximately 5-fold less active than HA22. Unexpectedly, a mutant with large deletions that remove most residues and all cleavage sites from domain II and Ib (M5) is still highly active. The M5 mutant retains only an 11-residue sequence (274-284) in domain II containing the furin recognition and Arg279 cleavage site.
HA22 mutants. A series of deletions within domains II and Ib were introduced into the PE38 component of HA22 to eliminate lysosomal protease cleavage sites. These 5 mutant proteins (M1, M2, M3, M4, and M5) are illustrated using an expanded view of domains II and Ib of PE38 to show the extent of the deletions (dotted lines) and the presence of the C287S point mutation. Residue numbering is based on the location of amino acids in native PE. The proteins were subsequently purified and compared with HA22 using an in vitro cytotoxicity assay on Raji cells. The M5 protein was renamed HA22-LR for further analysis. The IC50 (ng/mL) of each mutant relative to the IC50 of HA22 is presented as a mean of at least 3 separate experiments.
HA22 mutants. A series of deletions within domains II and Ib were introduced into the PE38 component of HA22 to eliminate lysosomal protease cleavage sites. These 5 mutant proteins (M1, M2, M3, M4, and M5) are illustrated using an expanded view of domains II and Ib of PE38 to show the extent of the deletions (dotted lines) and the presence of the C287S point mutation. Residue numbering is based on the location of amino acids in native PE. The proteins were subsequently purified and compared with HA22 using an in vitro cytotoxicity assay on Raji cells. The M5 protein was renamed HA22-LR for further analysis. The IC50 (ng/mL) of each mutant relative to the IC50 of HA22 is presented as a mean of at least 3 separate experiments.
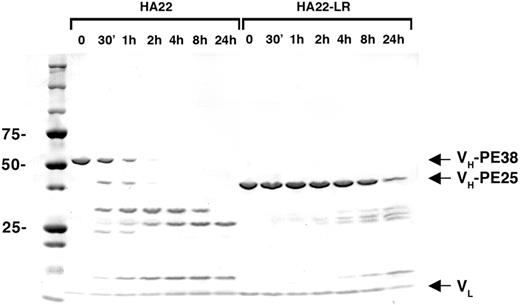
The M5 HA22 mutant is termed HA22-LR to indicate that it is “lysosome resistant.” The purity of HA22-LR is shown in Figure 4. To verify that HA22-LR is resistant to lysosomal degradation, it was treated with lysosomal extracts and examined by SDS-PAGE over 24 hours. Whereas HA22 is largely hydrolyzed into smaller fragments by 30 minutes and completely fragmented after 4 hours, proteolysis of HA22-LR is much slower, with barely detectable hydrolysis at 2 hours and a considerable intact fraction still detectable after 24 hours.
HA22-LR is resistant to digestion with lysosomal extracts. HA22 and HA22-LR were incubated with lysosomal extracts of Raji cells under identical conditions. After the addition of lysosomal extract, samples were removed immediately (0), after 30 minutes, and after 1, 2, 4, 8, and 24 hours, then analyzed by reducing SDS-PAGE. Arrows indicate the VL, VH-PE38 (HA22), and VH-PE25 (HA22-LR) bands that comprise the mature immunotoxins.
HA22-LR is resistant to digestion with lysosomal extracts. HA22 and HA22-LR were incubated with lysosomal extracts of Raji cells under identical conditions. After the addition of lysosomal extract, samples were removed immediately (0), after 30 minutes, and after 1, 2, 4, 8, and 24 hours, then analyzed by reducing SDS-PAGE. Arrows indicate the VL, VH-PE38 (HA22), and VH-PE25 (HA22-LR) bands that comprise the mature immunotoxins.
The activity of HA22-LR was investigated with a cell survival assay on additional CD22-positive tumor cell lines and compared with HA22 using a paired, 2-tailed t test between the resulting IC50 values (Table 1). HA22-LR had activity indistinguishable from HA22 on the Ramos (n = 3), CA46 (n = 5), and Daudi (n = 3) lymphoma cell lines but had significant differences against the WSU-CLL cell line (212% activity, P = .01, n = 4), the KOPN-8 ALL cell line (22% activity, P = .01, n = 3), and the Raji cell line (49%, P = .001, n = 10). As a negative control, we probed the ability of an antimesothelin immunotoxin (SS1P)29 to kill Raji cells, which lack mesothelin expression. Neither SS1P nor SS1P-LR demonstrated a significant cytotoxic effect on Raji cells at 1 μg/mL (data not shown). Although there is some variability in the activity of HA22-LR, we conclude that HA22-LR and HA22 have generally similar activities on CD22-positive cell lines.
Activity of HA22 and HA22-LR on six CD22-positive cell lines
| Cell line . | IC50 ± SE (ng/mL) . | Relative activity . | |
|---|---|---|---|
| HA22 . | HA22-LR . | ||
| CA46 (n = 5) | 0.30 ± 0.08 | 0.26 ± 0.06 | 1.15 |
| Daudi (n = 3) | 0.27 ± 0.04 | 0.24 ± 0.04 | 1.12 |
| Ramos (n = 3) | 1.62 ± 0.28 | 1.78 ± 0.15 | 0.91 |
| Raji* (n = 10) | 0.36 ± 0.04 | 0.73 ± 0.09 | 0.49 |
| KOPN-8* (n = 3) | 0.10 ± 0.02 | 0.45 ± 0.05 | 0.22 |
| WSU-CLL* (n = 4) | 2.50 ± 0.53 | 1.18 ± 0.34 | 2.12 |
| Cell line . | IC50 ± SE (ng/mL) . | Relative activity . | |
|---|---|---|---|
| HA22 . | HA22-LR . | ||
| CA46 (n = 5) | 0.30 ± 0.08 | 0.26 ± 0.06 | 1.15 |
| Daudi (n = 3) | 0.27 ± 0.04 | 0.24 ± 0.04 | 1.12 |
| Ramos (n = 3) | 1.62 ± 0.28 | 1.78 ± 0.15 | 0.91 |
| Raji* (n = 10) | 0.36 ± 0.04 | 0.73 ± 0.09 | 0.49 |
| KOPN-8* (n = 3) | 0.10 ± 0.02 | 0.45 ± 0.05 | 0.22 |
| WSU-CLL* (n = 4) | 2.50 ± 0.53 | 1.18 ± 0.34 | 2.12 |
Significant difference (P < .05 in a paired, 2-tailed t test) between the IC50 values of HA22 and HA22-LR.
Cytotoxicity toward freshly obtained malignant cells
To determine whether the new immunotoxin would also kill cells obtained directly from patients, we analyzed cells from 5 patients with CLL and 3 with HCL. As shown in Table 2, activity was observed for all patient cell populations tested with HA22-LR. In CLL, malignant cells from all 5 patients were more sensitive to HA22-LR than to HA22, by a median of over 17-fold (P = .009, Wilcoxon). IC50 values for the inhibition of protein synthesis ranged from less than 1 to 5.6 ng/mL. HA22-LR inhibited protein synthesis by 55% at 1 ng/mL in cells from patient CLL 2 (IC50 < 1 ng/mL; data not shown). Assays for cell death in CLL patient cells also showed more sensitivity to HA22-LR than to HA22. Whereas the IC50 values of HA22 in CLL patient cells varied widely from 8 to more than 1000 ng/mL, IC50s of HA22-LR varied by less than 10-fold. In HCL, HA22-LR was generally less active than HA22 with respect to protein synthesis inhibition. Assays for cell death in 2 of the 3 HCL patient cell populations showed similar findings. In summary, HA22-LR was highly cytotoxic toward CD22-positive CLL and HCL cells, but among CLL cells, which displayed variable sensitivity toward HA22, the cytotoxicity of HA22-LR was significantly more potent and more uniform.
Cytotoxicity of HA22 and HA22-LR toward freshly obtained CLL and HCL cells
| . | IC50 ± SD (ng/mL) . | Relative activity . | Assay type . | |
|---|---|---|---|---|
| HA22 . | HA22-LR . | |||
| CLL 1 | > 1000 | 4.7 ± 0.54 | > 210 | Protein synthesis |
| CLL 1 | 55 ± 12.8 | 3.4 ± 0.53 | 16.2 | Cell death |
| CLL 2 | 16.8 ± 1.05 | < 1 | > 16.8 | Protein synthesis |
| CLL 2 | 10.1 ± 0.48 | 1.32 ± 0.164 | 7.65 | Cell death |
| CLL 3 | 8.1 ± 2.1 | 3.9 ± 0.50 | 2.07 | Protein synthesis |
| CLL 4 | 290 ± 167 | 5.6 ± 1.10 | 51.8 | Protein synthesis |
| CLL 5 | 8.0 ± 1.51 | 3.7 ± 0.27 | 2.16 | Protein synthesis |
| HCL 1 | 5.2 ± 0.37 | 5.9 ± 1.03 | 0.88 | Protein synthesis |
| HCL 2 | 0.177 ± 0.0062 | 1.25 ± 0.24 | 0.14 | Protein synthesis |
| HCL 2 | 0.165 ± 0.0098 | 2.0 ± 0.39 | 0.08 | Cell death |
| HCL 3 | 1.76 ± 0.51 | < 1 | > 1.76 | Protein synthesis |
| HCL 3 | 2.1 ± 0.51 | 1.51 ± 0.29 | 1.39 | Cell death |
| . | IC50 ± SD (ng/mL) . | Relative activity . | Assay type . | |
|---|---|---|---|---|
| HA22 . | HA22-LR . | |||
| CLL 1 | > 1000 | 4.7 ± 0.54 | > 210 | Protein synthesis |
| CLL 1 | 55 ± 12.8 | 3.4 ± 0.53 | 16.2 | Cell death |
| CLL 2 | 16.8 ± 1.05 | < 1 | > 16.8 | Protein synthesis |
| CLL 2 | 10.1 ± 0.48 | 1.32 ± 0.164 | 7.65 | Cell death |
| CLL 3 | 8.1 ± 2.1 | 3.9 ± 0.50 | 2.07 | Protein synthesis |
| CLL 4 | 290 ± 167 | 5.6 ± 1.10 | 51.8 | Protein synthesis |
| CLL 5 | 8.0 ± 1.51 | 3.7 ± 0.27 | 2.16 | Protein synthesis |
| HCL 1 | 5.2 ± 0.37 | 5.9 ± 1.03 | 0.88 | Protein synthesis |
| HCL 2 | 0.177 ± 0.0062 | 1.25 ± 0.24 | 0.14 | Protein synthesis |
| HCL 2 | 0.165 ± 0.0098 | 2.0 ± 0.39 | 0.08 | Cell death |
| HCL 3 | 1.76 ± 0.51 | < 1 | > 1.76 | Protein synthesis |
| HCL 3 | 2.1 ± 0.51 | 1.51 ± 0.29 | 1.39 | Cell death |
Mouse toxicity studies
Immunotoxins, such as HA22 and LMB-2, do not react with mouse CD22. When injected into mice, the animals die of nonspecific liver toxicity.30 To assess nonspecific toxicity, nude mice were injected intravenously with a single dose of HA22-LR ranging from 2.5 to 20 mg/kg and observed for 10 days. No deaths were observed through the 20-mg/kg dose level (Table 3). Higher doses were not evaluated. In marked contrast and consistent with previous data,25 a 2.0-mg/kg dose of HA22 produced death in 100% (5 of 5) of mice. The intravenous single-dose LD50 of HA22-LR is greater than 20 mg/kg, indicating a decrease in nonspecific toxicity of more than 10-fold relative to HA22.
Nonspecific toxicity of HA22-LR
| Immunotoxin dose, mg/kg . | Dead/total mice . |
|---|---|
| HA22 | |
| 2.0 | 5/5 |
| HA22-LR | |
| 2.5 | 0/12 |
| 5.0 | 0/4 |
| 10 | 0/10 |
| 20 | 0/10 |
| Immunotoxin dose, mg/kg . | Dead/total mice . |
|---|---|
| HA22 | |
| 2.0 | 5/5 |
| HA22-LR | |
| 2.5 | 0/12 |
| 5.0 | 0/4 |
| 10 | 0/10 |
| 20 | 0/10 |
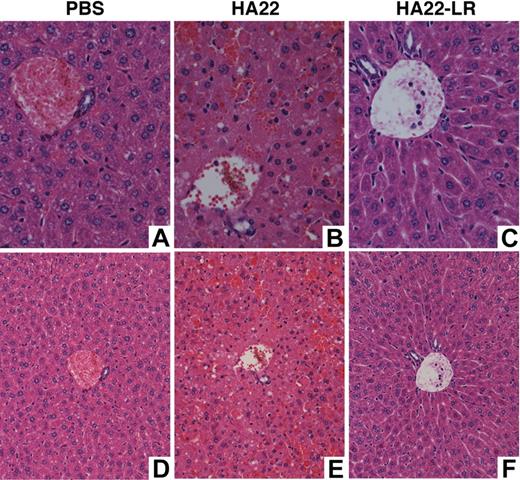
To evaluate the liver toxicity of HA22-LR, we examined liver histology and ALT serum levels in mice 30 hours after intravenous treatment with HA22 or HA22-LR. ALT levels in mice treated with PBS or HA22-LR at 2.0, 6.0, or 20 mg/kg fell within the limits of the reference range (24-140 U/L), but the mouse treated with 2.0 mg/kg HA22 had serum ALT levels at 5238 U/L, indicative of severe liver damage. Figure 5 shows representative liver sections from mice treated with PBS, 2.0 mg/kg HA22, or 20 mg/kg HA22-LR. The sections from both the PBS-treated mouse and the HA22-LR-treated mouse show no liver damage. Livers from mice treated with HA22-LR at 2.0 and 6.0 mg/kg also appear normal (data not shown). The liver section from the HA22-treated mouse shows massive hepatic necrosis.
Liver sections. Nude mice were treated intravenously with PBS, 2 mg/kg of HA22, or 20 mg/kg of HA22-LR. After 30 hours, livers were removed, fixed in formalin, sectioned, and stained with hematoxylin and eosin. Panel A (original magnification ×400) and panel D (original magnification ×200) show a representative section of the PBS-treated mouse. Panel B (original magnification ×400) and panel E (original magnification ×200) show a representative section of the HA22-treated mouse. There are many individual necrotic hepatocytes in various stages of cell death as well as the loss of hepatocytes and replacement with blood in sinusoids. Panel C (original magnification ×400) and panel F (original magnification ×200) show a representative section of the HA22-LR-treated mouse. Slides were viewed with an Olympus BX41 microscope. Images were acquired using an Olympus DP41 camera with its own Olympus DP Controller software. Photoshop CS3 was used to produce the composite.
Liver sections. Nude mice were treated intravenously with PBS, 2 mg/kg of HA22, or 20 mg/kg of HA22-LR. After 30 hours, livers were removed, fixed in formalin, sectioned, and stained with hematoxylin and eosin. Panel A (original magnification ×400) and panel D (original magnification ×200) show a representative section of the PBS-treated mouse. Panel B (original magnification ×400) and panel E (original magnification ×200) show a representative section of the HA22-treated mouse. There are many individual necrotic hepatocytes in various stages of cell death as well as the loss of hepatocytes and replacement with blood in sinusoids. Panel C (original magnification ×400) and panel F (original magnification ×200) show a representative section of the HA22-LR-treated mouse. Slides were viewed with an Olympus BX41 microscope. Images were acquired using an Olympus DP41 camera with its own Olympus DP Controller software. Photoshop CS3 was used to produce the composite.
Mouse pharmacokinetics
Balb/c mice were injected with 10 μg of either HA22 or HA22-LR and bled at intervals between 2 and 60 minutes. The concentration of immunotoxin in mouse serum was measured by ELISA. Data were fit to a single exponential decay function (Figure 6). The half-life (t1/2) of HA22 was 14.6 minutes (k = 0.047), whereas the half-life of HA22-LR was 7.8 minutes (k = 0.089).
Pharmacokinetics of HA22-LR. Balb/c mice were injected intravenously with 10 μg of either HA22 (□) or HA22-LR (○) and bled at several intervals between 2 and 60 minutes from the time of injection. The concentration of immunotoxin in the serum at the various intervals was determined by ELISA and fit to a single exponential decay function. The corresponding half-life (t1/2) is indicated. Each point is the concentration of immunotoxin in the serum of one mouse, and the concentration at each time interval was determined from at least 2 different mice.
Pharmacokinetics of HA22-LR. Balb/c mice were injected intravenously with 10 μg of either HA22 (□) or HA22-LR (○) and bled at several intervals between 2 and 60 minutes from the time of injection. The concentration of immunotoxin in the serum at the various intervals was determined by ELISA and fit to a single exponential decay function. The corresponding half-life (t1/2) is indicated. Each point is the concentration of immunotoxin in the serum of one mouse, and the concentration at each time interval was determined from at least 2 different mice.
Mouse xenograft antitumor activity
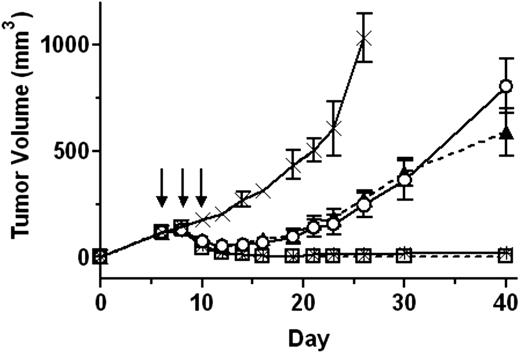
The low animal toxicity and in vitro activity of HA22-LR encouraged us to evaluate its efficacy using a mouse xenograft tumor model. SCID mice with CA46 xenograft tumors averaging approximately 120 mm3 were treated intravenously every other day 3 times with PBS, 0.3 mg/kg HA22, or HA22-LR at doses of 1.0, 1.75, or 2.5 mg/kg. Tumor size was measured regularly for up to 40 days (Figure 7) and observed visually for 10 weeks.
HA22-LR has potent antitumor activity. SCID mice with CA46 xenograft tumors were treated every other day 3 times (on days 6, 8, and 10) intravenously with PBS (×; solid line), 0.3 mg/kg HA22 (○; solid line), or HA22-LR at 1.0 (▲; dashed line), 1.75 (□; solid line), or 2.5 (*; dashed line) mg/kg. Arrows indicate days when treatment was administered. Tumor size was measured over the course of 40 days. Points represent the mean tumor size of all mice in the treatment group. Error bars show the 95% confidence interval of each mean value.
HA22-LR has potent antitumor activity. SCID mice with CA46 xenograft tumors were treated every other day 3 times (on days 6, 8, and 10) intravenously with PBS (×; solid line), 0.3 mg/kg HA22 (○; solid line), or HA22-LR at 1.0 (▲; dashed line), 1.75 (□; solid line), or 2.5 (*; dashed line) mg/kg. Arrows indicate days when treatment was administered. Tumor size was measured over the course of 40 days. Points represent the mean tumor size of all mice in the treatment group. Error bars show the 95% confidence interval of each mean value.
The tumors of PBS-treated mice rapidly grew to an average size greater than 1000 mm3 on day 26. Mice treated on days 6, 8, and 10 with 0.3 mg/kg HA22, the maximum dose that can be given to mice every other day 3 times without toxicity, caused regressions that brought the average tumor size to a minimum of 52 mm3 on day 12. By day 21, all of the tumors had resumed rapid growth.
The tumor response to the 1.0-mg/kg dose of HA22-LR was nearly identical to 0.3 mg/kg HA22, but 1.75 mg/kg HA22-LR was much more effective. On day 14, 5 of 8 mice treated with 1.75 mg/kg of HA22-LR had undetectable tumors that remained imperceptible for the duration of the study. The 3 of 8 remaining tumors initially shrunk but grew to an average size of 54 mm3 on day 40. The 2.5-mg/kg dose of HA22-LR demonstrated a remarkable antitumor activity. In 7 of 8 mice, the tumors completely disappeared by day 14 and had not returned by 10 weeks. The one remaining tumor diminished to 10 mm3 on day 14 but grew to 30 mm3 on day 40. We conclude that the low animal toxicity of HA22-LR allows larger doses of immunotoxin to be given safely, which dramatically enhances its antitumor activity.
Discussion
Here we use lysosomal protease cleavage analysis to identify protease-susceptible sites in an immunotoxin. Deletion of these sites produced a smaller immunotoxin, HA22-LR, that maintains excellent cytotoxic activity on CD22-positive cell lines and on cells directly isolated from patients with HCL and CLL. In addition, HA22-LR was considerably less toxic to mice, demonstrating a greater than 10-fold reduction in nonspecific toxicity. Previous studies in mice have shown that HA22 has a single-dose LD50 of 1.33 mg/kg.25 In this paper, we show that a single intravenous dose of 2.0 mg/kg of HA22 killed 5 of 5 mice, but doses of HA22-LR up to 20 mg/kg did not kill any of the injected mice, and the histology of the liver was normal. This large decrease in animal toxicity allowed us to administer much higher treatment doses, which led to greatly enhanced antitumor activity.
The nonspecific toxicity of immunotoxins in mice is primarily the result of liver damage,31-33 and toxicity in patients is also partly the result of hepatic toxicity.4-7 Mouse liver toxicity to LMB-2, and by extension all PE38 immunotoxins, is associated with the accumulation of the immunotoxin in Kupffer cells in the liver, which leads to the localized release of tumor necrosis factor-α and severe hepatotoxicity.30 The low nonspecific toxicity of HA22-LR indicates that it lacks elements in HA22, presumably the segments removed from domains II and Ib, responsible for uptake by Kupffer cell and/or stimulation of tumor necrosis factor-α release. The removed segments, however, are not essential for anti-CD22 targeted toxicity because HA22-LR retains antitumor activity similar to HA22.
Another factor that may contribute to the difference in nonspecific toxicity is the difference in the half-lives of HA22 and HA22-LR (Figure 6), which itself is probably the result of more efficient filtration and removal of HA22-LR (51.0 kDa) than HA22 (63.3 kDa) by glomeruli in the kidney.34 The 2-fold difference in half-life alone, however, is insufficient to explain the more than 10-fold difference in nonspecific toxicity. Previous efforts to reduce the nonspecific toxicity of immunotoxins have demonstrated that lowering the isoelectric point (pI) of the Fv in the immunotoxins LMB-2, B3(dsFv)-PE38, or SS1P decreases their nonspecific toxicity approximately 2- to 3-fold in mice.32,33 This observation does not account for the difference between HA22 and HA22-LR because the 2 constructs have an identical Fv and the pI of HA22-LR is slightly increased relative to the pI of HA22 (pIHA22 = 5.26 and pIHA22-LR = 5.63). In addition, the 2- to 3-fold difference in toxicity observed for this strategy is also much smaller than the more than 10-fold difference between HA22 and HA22-LR.
To produce the HA22-LR immunotoxin, we identified and removed lysosomal protease cleavage sites within PE38. We studied the digestion of the immunotoxin B3(dsFv)-PE38 with both lysosomal extracts and the individual cathepsins B, D, and S, which have been implicated in antigen processing.35-39 We found that lysosomal protease cleavage of immunotoxins is concentrated within domains II and Ib of the PE38 toxin fragment. Prior work with native PE has shown that domain Ib is highly susceptible to limited proteolysis with chymotrypsin, Staphylococcal serine proteinase, pepsin A, and subtilisin,40 confirming that domain Ib is easily accessible to proteases. Our results also show that domain II in PE38 is also protease accessible whereas domain III is less easily cleaved, probably because of a more compact, stable structure.
We used the information from the cleavage analysis to produce a series of deletions in the HA22 immunotoxin that eventually removed most of domains II and Ib, leaving only a short stretch of 11 amino acids from domain II (Figure 3). This 11-residue fragment is composed of the amino acid sequence RHRQPRGWEQL and contains a furin protease cleavage site that is important for intracellular processing and activation of the native toxin.11,16 The new immunotoxin, which we have termed HA22-LR to emphasize its enhanced resistance to lysosomal proteases, is composed of an anti-CD22 dsFv attached to a 25-kDa fragment of PE (PE25) containing the 11-residue fragment from domain II and all of domain III. When tested on several CD22-expressing cell lines, the activity of HA22-LR was similar to the HA22 immunotoxin from which it was derived.
Previous research has shown that domain Ib is not essential for the activity of PE immunotoxins.17,18,41-43 Domain II, however, has been proposed to play a key role in membrane translocation during PE intoxication.15,43-46 Our results suggest that a major component of the translocation activity of domain II may be localized to a short stretch of residues around the furin cleavage site. Both our data showing a 5-fold decrease in the activity of the M4 mutant, which eliminates the furin cleavage site, and previous work16 indicate that furin cleavage plays an important role in the cytotoxicity of PE. An additional possibility is that the resistance of HA22-LR to lysosomal degradation may compensate for any loss of translocation activity by allowing HA22-LR to survive longer within the cell. The cell surface targets of immunotoxins and the targeted cell type may also influence their intracellular trafficking and access to the cytosol. Future studies will investigate these possibilities.
We found that HA22-LR had similar or slightly less cytotoxicity compared with HA22 on cells with high CD22 expression, including CD22-positive cells lines and fresh HCL patient cells. However, its cytotoxicity on CLL patient cells was more potent and more uniform than HA22. The reason for this is unknown, but we speculate that it derives from the resistance of HA22-LR to lysosomal degradation, leading to longer intracellular survival relative to HA22. It is doubtful that the enhanced activity in CLL patient cells is because HA22-LR simply survives longer than HA22 in the media during the 3-day incubation as experiments have shown that HA22 has excellent stability in serum and in cell culture medium (data not shown). It is possible that lysosomal protease digestion is a major mechanism of immunotoxin resistance for CLL cells and that the HA22-LR molecule overcomes this resistance. Lysosomal protease digestion would also be present in cells with high CD22 expression but may be treatment-limiting only in CLL, where CD22 expression is low and the relatively small number of internalized molecules limits immunotoxin activity. In addition, the activity of HA22-LR in CLL is very similar to that observed for HA22 in HCL, suggesting that HA22-LR should be developed further as potential treatment for this disease.
In addition to nonspecific toxicities, another important factor limiting the utility of immunotoxins is the development of antibodies that react with the toxin and neutralize its activity.47 We have recently described a mutant immunotoxin, HA22-8X, that is significantly less immunogenic in mice because many of the B-cell epitopes have been removed. Most of the remaining B-cell epitopes in HA22-8X are located in the regions of domain II and domain Ib of PE. Coincidentally, these regions have been removed from HA22-LR. We anticipate that combining the mutations in both these molecules will produce an immunotoxin that is even less immunogenic; such studies have been initiated.
In conclusion, HA22-LR has several advantages over HA22 that may also be applicable to other PE immunotoxins, but HA22-LR appears especially promising for the treatment of CLL. One major advantage of HA22-LR is that its nonspecific toxicity in mice is more than 10-fold lower than HA22. Although we cannot be certain how this will translate into human toxicity, the use of HA22-LR may help to prevent treatment-related side effects and allow patients to receive higher doses for a better therapeutic outcome. In addition, the deletions used to generate HA22-LR eliminate known antibody epitopes and should help to limit the generation of neutralizing antibodies, allowing more treatment cycles to be given to patients. Relative to HA22, HA22-LR also has greatly enhanced, more uniform activity against patient-derived CLL cells and generally similar activity on CD22-positive cell lines and HCL patient cells. For these reasons, HA22-LR represents an important advance in immunotoxin development.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Mark Raffeld (Department of Pathology, Center for Cancer Research, National Cancer Institute) for taking the photographs of the liver sections and evaluating the slides.
This work was supported in part by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research, in part under a PRAT Fellowship (J.E.W.) by the National Institute of General Medical Sciences, and in part with federal funds from the National Cancer Institute, National Institutes of Health (contract N01-CO-12 400).
National Institutes of Health
Authorship
Contribution: J.E.W. designed and performed research, analyzed and interpreted data, and wrote the manuscript; L.X., O.C., and I.M. performed research; R.J.K. analyzed and interpreted data; and D.J.F. and I.P. conceived the research and guided its design, analysis, and interpretation.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
Correspondence: Ira Pastan, Laboratory of Molecular Biology, National Cancer Institute, 37 Convent Dr, Rm 5106, Bethesda, MD 20892-4264; e-mail: pastani@mail.nih.gov.
References
Author notes
*D.J.F. and I.P. are co-senior authors.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal