Abstract
Alloreactive natural killer (NK) cells are an important influence on hematopoietic stem cell transplantation (HSCT) outcome. In HLA-mismatched HSCT, alloreactivity occurs when licensed donor NK cells expressing inhibitory killer Ig-like receptors (KIR) for donor MHC class I ligands recognize the lack of the class I ligands in the mismatched recipient (“missing self”). Studies in HLA-matched HSCT, however, have also demonstrated improved outcome in patients lacking class I ligands for donor inhibitory KIR (“missing ligand”), indicating that classically nonlicensed donor NK cells expressing KIR for non-self MHC class I ligands may exhibit functional competence in HSCT. We examined NK function in 16 recipients of T cell–depleted allografts from HLA-identical or KIR-ligand matched donors after myeloablative therapy. After HSCT, nonlicensed NK cells expressing inhibitory KIR for non-self class I exhibit robust intracellular IFN-γ and cytotoxic response to target cells lacking cognate ligand, gradually becoming tolerized to self by day 100. These findings could not be correlated with cytokine environment or phenotypic markers of NK development, nor could they be attributed to non-KIR receptors such as CD94/NKG2A. These findings confirm that NK alloreactivity can occur in HLA-matched HSCT, where tolerance to self is either acquired by the stem cell–derived NK cell after exiting the bone marrow or where tolerance to self can be temporarily overcome.
Introduction
Natural killer (NK) cells are bone marrow–derived lymphocytes capable of mediating early innate immune responses to viral infections and recognition of transformed malignant cells and are regulated by quantitative differences in activating and inhibitory signals.1,2 Inhibitory signals are mediated through major histocompatibility complex (MHC) class I engagement of inhibitory receptors.3-6 In humans, inhibitory NK receptors with specificity for the human MHC (human leukocyte antigen or HLA) proteins include the lectin-like CD94:NKG2A heterodimer, which recognizes complexes of HLA-E bound to peptides from the leader sequences of other class I molecules; the immunoglobulin-like transcript 2 (ILT2), whose recognition of a relatively conserved region in the class I molecule provides broad class I specificity; and the polymorphic killer immunoglobulin-like receptors (KIR), whose members' recognition of the HLA-C allotypes with asparagine 80 (HLA-Cw3 and related alleles, or group C1 alleles), HLA-C allotypes with lysine 80 (HLA-Cw4 and related alleles, or group C2 alleles), and HLA-A and HLA-B allotypes with the Bw4 epitope confer narrower specificity.6-8 The functional relationship between KIR and its MHC class I ligand transcends a simple receptor-ligand arrangement where ligand engagement triggers downstream inhibitory signaling. Instead, KIR-HLA interactions are the foundation for NK function and tolerance to self: human NK cells expressing inhibitory KIR specific for self–HLA class I are preferentially endowed with functional competence and effector function; conversely, cells expressing inhibitory KIR for which they lack the cognate class I ligand are rendered hyporesponsive.9-11 In this way, mature NK cells achieve tolerance to self, responding only when encountering cells lacking self–MHC class I ligands (“missing self”).
The clinical significance of missing self is most evident in the allogeneic hematopoietic stem cell transplantation (HSCT) setting, where NK cells are the first lymphocyte subset to reconstitute the peripheral blood after transplantation and are thought to mediate suppression of graft-versus-host disease (GVHD),12,13 promotion of bone marrow engraftment,14 and mediation of a graft-versus-leukemia effect.12,13,15-17 Through its antileukemic effects, donor NK alloreactivity therefore plays a significant role in HSCT outcome by decreasing leukemia relapse and ultimately increasing survival. How NK cells in HSCT behave through KIR recognition of target cell HLA has been the subject of interest, with evidence supporting missing self alloreactivity, relevant only to HLA-mismatched, KIR ligand–incompatible HSCT.13,18 However, several studies in HLA-matched HSCT have demonstrated an improved outcome in patients lacking class I ligand for donor inhibitory KIR. In contrast to missing self, this suggests that NK cells can break tolerance and function according to a less restrictive “missing HLA ligand” mechanism.19-22
We propose that in HSCT, these 2 mechanisms need not be mutually exclusive. Using 6-color staining and flow cytometric analysis of intracellular interferon-γ (IFN-γ) production and CD107 mobilization to target cell panels, we evaluated the effector response of inhibitory KIR-expressing NK from 16 patients receiving T cell–depleted stem cell allografts from HLA-compatible, KIR ligand-compatible donors. Assessment of NK function at serial time points after HSCT reveals a period after HSCT when NK cells have not yet acquired tolerance to self and become activated according to “missing ligand.” Specifically, we find that NK cells with inhibitory KIR for non-self HLA (“nonlicensed” NK cells) exhibit effector function early post-HSCT, and NK cells with inhibitory KIR for self-HLA (“licensed” NK cells) are hyperresponsive. Thus, while the mature NK cell in a healthy person achieves tolerance by preferentially endowing functional competence to inhibitory KIR specific for self-HLA (missing self), the NK cell after HSCT achieves self-tolerance only after transitioning from a state where all inhibitory KIR receptors are capable of recognizing lack of ligand (missing ligand). These results indicate that NK alloreactivity in HSCT is driven by both missing self and missing ligand mechanisms and that HLA-identical HSCT recipients can benefit from NK alloreactivity early post-HSCT, due to lack of HLA ligand for donor inhibitory KIR.
Methods
Patient samples
Sixteen patients with acute myelogenous leukemia (AML), chronic myelogenous leukemia (CML), acute lymphoblastic leukemia (ALL), or myelodysplastic syndrome (MDS), who underwent T cell–depleted hematopoietic stem cell transplantation (TCD-HSCT) from an HLA-identical sibling or an HLA-compatible unrelated donor between May 2003 and April 2007, were included in this study. All donor-recipient pairs were HLA-compatible and KIR-ligand matched. Patient and transplant characteristics are detailed in Table 1, and donor KIR and HLA typing are listed in Table 2. All patients in this study underwent myeloablative conditioning. Ten patients received conditioning with fludarabine or cyclophosphamide, thiotepa, and total body irradiation (TBI). Five patients received conditioning with busulfan, melphalan, and fludarabine. Five patients received allografts from an unrelated donor, and 10 received allografts from a sibling. Bone marrow (n = 2) and peripheral blood stem cells (PBSCs) were used (n = 14). No post-HSCT immunosuppression was given to the patients. The median CD34+ cell dose was 7.6 × 106 CD34+/kg.
HSCT patient and allograft characteristics
| Patient no. . | Age, y . | Disease . | Donor . | HLA-match . | PBSC versus BM . | ATG or anti-CD52 . | TCD method . |
|---|---|---|---|---|---|---|---|
| 1 | 31 | MDS | URD | 9/10 C | PBSC | Yes | CD34+ selection |
| 2 | 46 | AML | Sibling | 10/10 | PBSC | No | CD34+ selection |
| 3 | 8 | AML | Sibling | 10/10 | BM | No | SBA-E-BM |
| 4 | 19 | Pre-B ALL | URD | 10/10 | PBSC | Yes | CD34+ selection |
| 5 | 54 | AML | URD | 9/10 DQ | PBSC | Yes | CD34+ selection |
| 6 | 5 | AML | URD | 8/10 A,C | BM | Yes | SBA-E-BM |
| 7 | 55 | CML | Sibling | 10/10 | PBSC | No | CD34+ selection |
| 8 | 58 | CML | Sibling | 10/10 | PBSC | No | CD34+ selection |
| 9 | 56 | AML | URD | 9/10 B | PBSC | Yes | CD34+ selection |
| 10 | 15 | AML | Sibling | 10/10 | PBSC | No | CD34+ selection |
| 11 | 48 | AML | Sibling | 10/10 | PBSC | No | CD34+ selection |
| 12 | 29 | AML | Sibling | 10/10 | PBSC | No | CD34+ selection |
| 13 | 67 | AML | Sibling | 10/10 | PBSC | Yes | CD34+ selection |
| 14 | 59 | MDS | Sibling | 10/10 | PBSC | Yes | CD34+ selection |
| 15 | 38 | AML | Sibling | 10/10 | PBSC | Yes | CD34+ selection |
| 16 | 59 | AML | Sibling | 10/10 | PBSC | Yes | CD34+ selection |
| Patient no. . | Age, y . | Disease . | Donor . | HLA-match . | PBSC versus BM . | ATG or anti-CD52 . | TCD method . |
|---|---|---|---|---|---|---|---|
| 1 | 31 | MDS | URD | 9/10 C | PBSC | Yes | CD34+ selection |
| 2 | 46 | AML | Sibling | 10/10 | PBSC | No | CD34+ selection |
| 3 | 8 | AML | Sibling | 10/10 | BM | No | SBA-E-BM |
| 4 | 19 | Pre-B ALL | URD | 10/10 | PBSC | Yes | CD34+ selection |
| 5 | 54 | AML | URD | 9/10 DQ | PBSC | Yes | CD34+ selection |
| 6 | 5 | AML | URD | 8/10 A,C | BM | Yes | SBA-E-BM |
| 7 | 55 | CML | Sibling | 10/10 | PBSC | No | CD34+ selection |
| 8 | 58 | CML | Sibling | 10/10 | PBSC | No | CD34+ selection |
| 9 | 56 | AML | URD | 9/10 B | PBSC | Yes | CD34+ selection |
| 10 | 15 | AML | Sibling | 10/10 | PBSC | No | CD34+ selection |
| 11 | 48 | AML | Sibling | 10/10 | PBSC | No | CD34+ selection |
| 12 | 29 | AML | Sibling | 10/10 | PBSC | No | CD34+ selection |
| 13 | 67 | AML | Sibling | 10/10 | PBSC | Yes | CD34+ selection |
| 14 | 59 | MDS | Sibling | 10/10 | PBSC | Yes | CD34+ selection |
| 15 | 38 | AML | Sibling | 10/10 | PBSC | Yes | CD34+ selection |
| 16 | 59 | AML | Sibling | 10/10 | PBSC | Yes | CD34+ selection |
SBA-E-BM indicates T-cell depletion by bone marrow soybean agglutination with sheep red blood cell (RBC) rosetting; URD, unrelated donor; PBSC, peripheral blood stem cell collection; BM, bone marrow; AML, acute myelogenous leukemia; CML, chronic myelogenous leukemia; MDS, myelodysplastic syndrome; and ALL, acute lymphoblastic leukemia.
KIR and HLA genotypes for HSCT donors
| Patient no. . | Donor KIR genotype . | Donor HLA KIR ligands . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Inhibitory . | Activating . | HLA-Bw . | HLA-C . | |||||||||
| 2DL1 . | 2DL2 . | 2DL3 . | 3DL1 . | 2DS1 . | 2DS2 . | 2DS3 . | 2DS4 . | 2DS5 . | 3DS1 . | |||
| 1 | + | + | + | + | + | + | + | Bw6/Bw6 | C2/C2 | |||
| 2 | + | + | + | + | + | + | + | Bw6/Bw6 | C1/C1 | |||
| 3 | + | + | + | + | + | + | Bw6/Bw6 | C1/C2 | ||||
| 4 | + | + | + | + | + | Bw4/Bw6 | C1/C2 | |||||
| 5 | + | + | + | + | + | + | + | Bw4/Bw4 | C2/C2 | |||
| 6 | + | + | + | + | + | + | + | Bw6/Bw6 | C1/C2 | |||
| 7 | + | + | + | + | Bw4/Bw6 | C1/C2 | ||||||
| 8 | + | + | + | + | Bw6/Bw6 | C1/C2 | ||||||
| 9 | + | + | + | + | Bw4/Bw6 | C1/C1 | ||||||
| 10 | + | + | + | + | + | + | Bw4/Bw6 | C1/C2 | ||||
| 11 | + | + | + | + | + | + | + | Bw4/Bw6 | C1/C1 | |||
| 12 | + | + | + | + | + | + | + | + | + | + | Bw4/Bw6* | C2/C2 |
| 13 | + | + | + | + | + | + | + | + | Bw4/Bw6 | C1/C2 | ||
| 14 | + | + | + | + | + | + | + | + | + | Bw4/Bw4 | C1/C1 | |
| 15 | + | + | + | + | + | + | + | Bw4/Bw6* | C1/C2 | |||
| 16 | + | + | + | + | + | + | + | Bw6/Bw6 | C1/C1 | |||
| Patient no. . | Donor KIR genotype . | Donor HLA KIR ligands . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Inhibitory . | Activating . | HLA-Bw . | HLA-C . | |||||||||
| 2DL1 . | 2DL2 . | 2DL3 . | 3DL1 . | 2DS1 . | 2DS2 . | 2DS3 . | 2DS4 . | 2DS5 . | 3DS1 . | |||
| 1 | + | + | + | + | + | + | + | Bw6/Bw6 | C2/C2 | |||
| 2 | + | + | + | + | + | + | + | Bw6/Bw6 | C1/C1 | |||
| 3 | + | + | + | + | + | + | Bw6/Bw6 | C1/C2 | ||||
| 4 | + | + | + | + | + | Bw4/Bw6 | C1/C2 | |||||
| 5 | + | + | + | + | + | + | + | Bw4/Bw4 | C2/C2 | |||
| 6 | + | + | + | + | + | + | + | Bw6/Bw6 | C1/C2 | |||
| 7 | + | + | + | + | Bw4/Bw6 | C1/C2 | ||||||
| 8 | + | + | + | + | Bw6/Bw6 | C1/C2 | ||||||
| 9 | + | + | + | + | Bw4/Bw6 | C1/C1 | ||||||
| 10 | + | + | + | + | + | + | Bw4/Bw6 | C1/C2 | ||||
| 11 | + | + | + | + | + | + | + | Bw4/Bw6 | C1/C1 | |||
| 12 | + | + | + | + | + | + | + | + | + | + | Bw4/Bw6* | C2/C2 |
| 13 | + | + | + | + | + | + | + | + | Bw4/Bw6 | C1/C2 | ||
| 14 | + | + | + | + | + | + | + | + | + | Bw4/Bw4 | C1/C1 | |
| 15 | + | + | + | + | + | + | + | Bw4/Bw6* | C1/C2 | |||
| 16 | + | + | + | + | + | + | + | Bw6/Bw6 | C1/C1 | |||
+ indicates the presence of the gene in each person.
Individuals with Bw4 epitopes exclusively contributed by HLA-A antigens.
The use of patient samples for this study was approved by the institutional review board of Memorial Sloan-Kettering Cancer Center. Samples were collected, and written informed consent was obtained from the patients or their legal guardians in accordance with the Declaration of Helsinki. Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll/Hypaque density gradient centrifugation and cryopreserved in liquid nitrogen. Genomic DNA was prepared from 5 × 106 PBMCs using the Puregene DNA Isolation kit (Gentra Systems, Minneapolis, MN) or the QIAamp DNA Blood Mini kit (QIAGEN, Valencia, CA) according to the manufacturer's instructions.
KIR and HLA genotyping
Previously published primers were used for the detection of the KIR genes 2DS1, 2DS2, 2DS3, 2DS4, 2DS5, 2DL1, 2DL2, 2DL3, 2DL4, 3DS1, 3DL1, 3DL2, 3DL3, and pseudogenes 3DP1 and 2DP1.23 HLA genotyping was performed by high-resolution typing by the American Red Cross HLA Laboratory (Philadelphia, PA).
Target cells and culture conditions
The HLA class I–negative 721.221 parent cell line, 721.221 transfectants expressing the HLA-Bw4 ligand (HLA-B4403), and the HLA-Bw6 epitope (HLA-B3502) were kindly provided by Dr Bo Dupont (Sloan-Kettering Institute, New York, NY). The 721.221 transfectant expressing the HLA-Cw3 ligand (HLA-Cw0304) and the 721.221 transfectant expressing the HLA-Cw4 ligand (HLA-Cw0401) were generated in Dr Peter Parham's laboratory (Stanford University, Stanford, CA) and used with his permission. These and the HL-60 cell line were cultured in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum, 100 μg/mL l-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin. The target cell line PhALL3.1 was derived from a patient with Philadelphia chromosome–positive acute lymphoblastic leukemia (kindly provided by Dr Renier Brentjens, Memorial Sloan-Kettering Cancer Center, New York, NY) and was cultured in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum, 100 μg/mL l-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin, 1% nonessential amino acids, 1% sodium pyruvate, 1% N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (Hepes), and 1% α-mercaptoethanol. The KASUMI-1 cell line was cultured in RPMI 1640 medium supplemented with 20% heat-inactivated fetal bovine serum, 100 μg/mL l-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin. All cell lines were incubated at 37°C, 5% CO2. Class I molecule expression was monitored weekly using G46-2.6–fluorescein isothiocyanate (FITC; anti–human HLA-A, B, C antibody). Cryopreserved PBMCs from a patient with 80% circulating acute biphenotypic leukemic blasts (BPL-1) were cultured in Dulbecco modified Eagle medium (DMEM) medium with 5% human serum for 24 hours before use.
Single-cell analysis by flow cytometry for KIR and intracellular IFN-γ
Single-cell analyses of NK function within phenotypically discrete NK groups were performed as previously desribed.10 Phenotyping for surface expression of CD16 and CD117(c-kit) was determined using anti–CD16-APC-Cy7(3G8) and anti–CD117-PE(YB5.B8) (BD Biosciences, San Jose, CA). Each analysis was performed in triplicate on each sample on 2 separate occasions.
CD107 mobilization assay
CD107, a marker of intracytoplasmic cytolytic granules, was used as an indicator of effector cell degranulation.24-26 FITC-CD107a monoclonal antibody (mAb; BD Biosciences) was added to the NK effector and 721.221 target cell mixtures for a 5- to 6-hour incubation. FITC+ cells were then gated by flow cytometry, revealing cells that have undergone granule-mediated cytotoxicity. GolgiStop was added for a final concentration of 6 μg/mL during the assay to prevent the acidification of the endosomal compartment, which could alter the fluorescence of internalized CD107:FITC-CD107 mAb complexes.
Assay of cytokines
Blood plasma samples were stored at −80°C. Plasma concentrations of tumor necrosis factor-α (TNF-α), IFN-γ, interleukin-2 (IL-2), IL-12, IL-15, and IL-6 were investigated in duplicate using an enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems, Minneapolis, MN). The sensitivities of TNF-α, IFN-γ, IL-2, IL-15, IL-6, and IL-12 were 0.5, 15.6, 31.2, 3.9, 0.156, and 0.785 pg/mL, respectively. ELISAs were performed according to the manufacturer's instruction.
Results
To determine whether the restrictions of NK licensing are altered after transplantation resulting in missing ligand-driven NK alloreactivity, we examined NK function among 16 TCD-HSCT donor-recipient pairs. Among the donors, 3 donors were homozygous for KIR haplotype-A, which contains the genes for inhibitory KIR receptors KIR2DL3, KIR2DL1, and KIR3DL1, and at most, one gene for an activating KIR, KIR2DS4. Other donors had at least one gene for the activating KIR2DS1, KIR2DS2, KIR2DS3, KIR2DS5, or KIR3DS1. All donors exhibited unique HLA genotypes composed of various combinations of KIR ligands as indicated in Table 2. Two donors had HLA-A antigens with the Bw4 epitope.
Post-HSCT NK repertoire reconstitution has been well-described phenotypically,27,28 but functional studies are critical to the understanding of NK behavior in HSCT. While comparison of post-HSCT NK response with donor NK response on a population basis to the HLA class I–negative target cell 721.221 reveals a slight decrease in NK function early post-HSCT (P = .06, Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article), the population-based study does not scrutinize at the level of the single cell any receptor-driven NK reactivity responsible for missing self or missing ligand behavior. We therefore used multicolor flow cytometry to restrict our analysis to NK populations exclusively expressing one receptor, allowing the evaluation of NK function mediated by a single inhibitory KIR, which could be defined as licensing or nonlicensing based on the HLA genotype of the person. Thus, even within a single person, licensed NK cells exclusively expressing inhibitory KIR for self-HLA class I ligands (S-KIR) could be compared with nonlicensed NK cells exclusively expressing inhibitory KIR for non-self HLA class I ligands (NS-KIR). CD94/NKG2A-expressing NK cells were also examined and compared with the KIR-expressing populations for effector function. NK populations from the donor and from the patient before and after transplantation at serial time points were evaluated at the single-cell level and compared for effector function and class I–mediated inhibition.
NK cells expressing inhibitory KIR for self-HLA are hyperresponsive in the immediate posttransplantation period
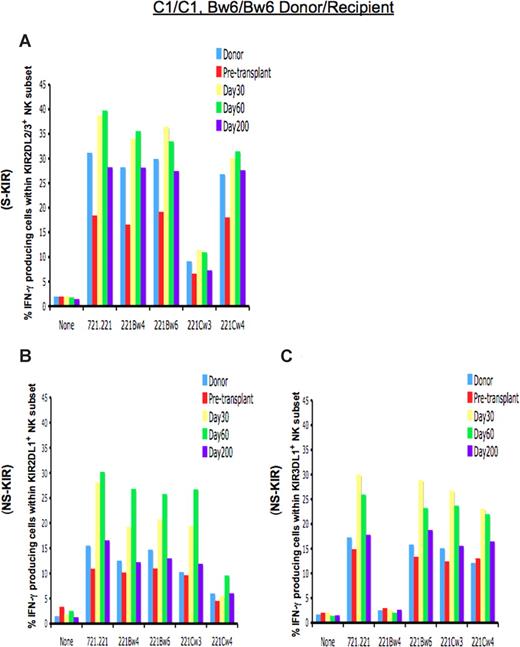
Figure 1A demonstrates the response of 2DL3-expressing cells in a C1/C1 homozygous patient who received a TCD allograft from an HLA-identical donor. Predicted to be licensed for functional competence, KIR2DL3-expressing NK cells from the donor and HSCT recipient demonstrated functional competence, with a significant proportion of the cells elaborating IFN-γ when challenged with the HLA-negative cell line 721.221 or 721.221 transfectants missing self class I expression. The cells were appropriately inhibited by the presence of the HLA-C1 ligand in the 721.221/Cw3 transfectant. On day +30 after TCD allograft infusion, the NK-cell population exclusively expressing KIR2DL3 was hyperresponsive, with higher proportions of NK cells expressing IFN-γ when challenged with target cells lacking the cognate ligand. This hyperresponsiveness normalized and approached donor levels by day +200.
Function of NK cells expressing inhibitory KIR is altered immediately after TCD-HSCT. Shown is the percentage of IFN-γ–producing cells among KIR-expressing NK populations from the HLA-C1/C1, Bw6/Bw6 donor, the HLA-identical patient (patient no. 2) before transplantation, and the patient at day +30, day +60, and day +200 after HSCT after coincubation with the indicated target cells. (A) NK cells exclusively expressing the inhibitory KIR for self-HLA class I (S-KIR) KIR2DL3 after HSCT are hyperresponsive compared with the donor and patient pre-HSCT, normalizing to donor level by day +200, and reactivity is inhibited upon challenge with the cognate class I ligand HLA-Cw3. NK cells exclusively expressing the inhibitory KIR for the non-self HLA class I (NS-KIR) KIR2DL1 (B) or KIR3DL1 (C) are functionally competent compared with the donor and patient pre-HSCT, reacting to lack of ligand. Reactivity is inhibited upon challenge with the cognate class I ligands HLA-Cw4 or Bw4, respectively.
Function of NK cells expressing inhibitory KIR is altered immediately after TCD-HSCT. Shown is the percentage of IFN-γ–producing cells among KIR-expressing NK populations from the HLA-C1/C1, Bw6/Bw6 donor, the HLA-identical patient (patient no. 2) before transplantation, and the patient at day +30, day +60, and day +200 after HSCT after coincubation with the indicated target cells. (A) NK cells exclusively expressing the inhibitory KIR for self-HLA class I (S-KIR) KIR2DL3 after HSCT are hyperresponsive compared with the donor and patient pre-HSCT, normalizing to donor level by day +200, and reactivity is inhibited upon challenge with the cognate class I ligand HLA-Cw3. NK cells exclusively expressing the inhibitory KIR for the non-self HLA class I (NS-KIR) KIR2DL1 (B) or KIR3DL1 (C) are functionally competent compared with the donor and patient pre-HSCT, reacting to lack of ligand. Reactivity is inhibited upon challenge with the cognate class I ligands HLA-Cw4 or Bw4, respectively.
NK cells expressing inhibitory KIR for non-self HLA have functional capacity immediately after HSCT
We sought to determine whether there is a similar period of altered function in the NK populations expressing inhibitory KIR for which the patient lacks the class I ligand (non-self HLA), a population normally predicted to be nonlicensed and hyporesponsive. In a C1/C1 person, NK cells expressing the NS-KIR KIR2DL1 would not be expected to exhibit effector function, because the cognate C2 ligand is lacking in that person. Indeed, in the C1/C1 donor and patient before HSCT, NK cells expressing KIR2DL1 were hyporesponsive (Figure 1B), with only 10% to 15.5% of cells expressing IFN-γ to a target cell lacking class I expression. In contrast, post-HSCT NK cells expressing the NS-KIR KIR2DL1 displayed functional competence, exhibiting approximately a 2-fold higher response to lack of ligand (missing ligand) compared with similar cells in the donor. These cells were silenced only when coincubated with a target cell expressing the cognate ligand HLA-Cw4. The effector response was KIR ligand-specific, as coincubation with target transfectants expressing noncognate class I proteins (HLA-Cw3 or HLA-Bw4) did not inhibit response. The class I ligand specificity of inhibition further indicates that the higher effector response was not due to contribution from other inhibitory receptors, such as CD94/NKG2A, which are not class I–specific. This unexpectedly licensed phenotype among NK cells expressing the non-self KIR2DL1 was not permanent, as these cells became less responsive thereafter, normalizing to donor levels by day +200 and achieving tolerance to self (Figure 1B).
The alteration in licensing was also seen for KIR3DL1, the receptor for the HLA-Bw4 ligand. The same patient and his donor displayed an HLA-Bw6/Bw6 genotype and therefore lacked the HLA-Bw4 ligand. Lack of the HLA-Bw4 ligand renders the cognate KIR3DL1 a non-self receptor, leading to a nonlicensed phenotype and hyporesponsiveness in NK cells expressing KIR3DL1 in the donor and the patient pre-HSCT (Figure 1C). After HSCT, however, KIR3DL1-expressing NK cells demonstrated functional competence, exhibiting higher IFN-γ response to target cells lacking the HLA-Bw4 ligand. The heightened response was abrogated only by the Bw4 ligand, indicating that the KIR3DL1 receptor is responsible for the functional competence. Again, the licensed phenotype waned over the ensuing weeks after HSCT, and NK cells expressing the NS-KIR3DL1 gradually adopted a donor phenotype, becoming hyporesponsive by day +200 (Figure 1C).
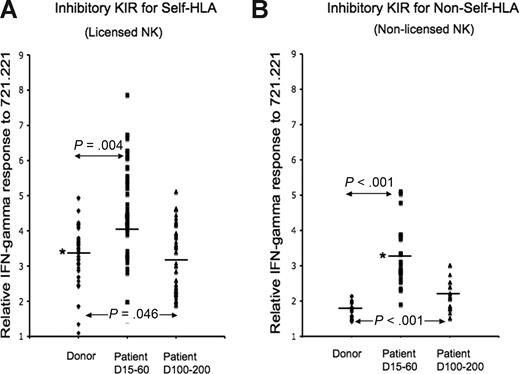
These findings were not unique to 1 patient. We compared NK IFN-γ response to target cells from 16 donors and recipient patients at multiple post-HSCT time points. Because the absolute measurement of IFN-γ response can vary from person to person, we examined the ratio of IFN-γ response to background (IFN-γ production from KIR− NK cells), thereby allowing aggregate analysis of all 16 donor-recipient sets. Within each patient and in aggregate, NK cells exclusively expressing inhibitory KIR for self-class I molecules consistently displayed a higher response to 721.221 target cells lacking class I expression at day 15 through 60 after HSCT (P = .004, Figure 2), including KIR3DL1 cells from 2 patients whose Bw4 epitope was contributed solely by the HLA-A antigen HLA-A2402 (Figure S2). NK response normalized to donor levels after day +100 (Figure 2A). To a more significant degree, however, NK cells exclusively expressing inhibitory KIR for non-self class I molecules displayed higher response to 721.221 in the immediate post-HSCT period (P < .001), decreasing but still remaining higher than donor level by day 100 to 200 (P < .001; Figure 2B). Consistent with the findings in Figure 1, in all cases, NK cells expressing KIR for non-self HLA responded to target cells lacking the cognate ligand and were inhibited only by target cells expressing the cognate ligand. It is noteworthy that in the first 3 months after HSCT, the effector response in NK cells expressing KIR for non-self, cells that would normally be considered nonlicensed, matched in magnitude the steady-state response of licensed cells in the healthy donors.
Aggregate NK function from 16 TCD-HSCT donor-recipient pairs. IFN-γ response to class I–negative 721.221 target cells was calculated relative to baseline for each inhibitory KIR in each of 16 TCD-HSCT donor-recipient pairs by the formula (KIR+IFN-γ+/KIR+)/(KIR−IFN-γ+/KIR−). The relative response of NK cells exclusively expressing specific inhibitory KIR was compared among the allograft donor, the patient at day +15 to 60, and the patient at days +100 to +200 after HSCT. (A) NK cells exclusively expressing inhibitory KIR to self-HLA from patients at days +15 to +60 are hyperresponsive compared with their donors (P = .004), normalizing to donor levels after day +100. (B) NK cells exclusively expressing inhibitory KIR to non-self HLA from patients at days +15 to +60 after HSCT are functionally competent (P < .001) compared with the donor, decreasing but still higher than donor by days +100 to +200 (P < .001). The paired Student t test was performed to compute the P value for these comparisons. NK cells expressing KIR for non-self HLA from days +15 to +60 NK cells are comparable in effector response to steady-state healthy donor NK cells expressing KIR for self-HLA (*).
Aggregate NK function from 16 TCD-HSCT donor-recipient pairs. IFN-γ response to class I–negative 721.221 target cells was calculated relative to baseline for each inhibitory KIR in each of 16 TCD-HSCT donor-recipient pairs by the formula (KIR+IFN-γ+/KIR+)/(KIR−IFN-γ+/KIR−). The relative response of NK cells exclusively expressing specific inhibitory KIR was compared among the allograft donor, the patient at day +15 to 60, and the patient at days +100 to +200 after HSCT. (A) NK cells exclusively expressing inhibitory KIR to self-HLA from patients at days +15 to +60 are hyperresponsive compared with their donors (P = .004), normalizing to donor levels after day +100. (B) NK cells exclusively expressing inhibitory KIR to non-self HLA from patients at days +15 to +60 after HSCT are functionally competent (P < .001) compared with the donor, decreasing but still higher than donor by days +100 to +200 (P < .001). The paired Student t test was performed to compute the P value for these comparisons. NK cells expressing KIR for non-self HLA from days +15 to +60 NK cells are comparable in effector response to steady-state healthy donor NK cells expressing KIR for self-HLA (*).
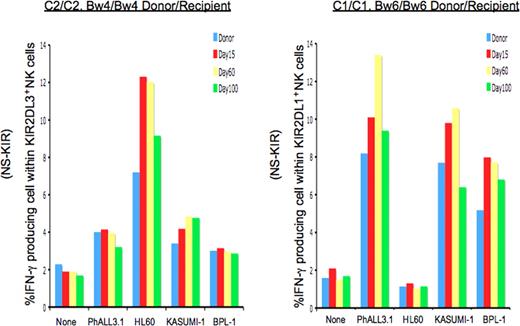
Extending the analysis to more clinically relevant target cells, we examined nonlicensed NK response to AML and ALL leukemic cell lines, as well as to leukemic blasts from a patient with biphenotypic acute leukemia. As shown in Figure 3, post-HSCT NK cells from 2 patients were challenged with target leukemia cells with diverse HLA genotypes. While not as dramatic as with the 721.221 target cells, the results with the myeloid and lymphoid leukemic targets are consistent: after HSCT, NK cells expressing inhibitory KIR for non-self display elevated effector function against leukemic targets lacking cognate class I ligand (missing ligand) compared with the donor control. The same NK cells were not activated by target cells expressing the relevant class I ligand.
Nonlicensed NK cells expressing inhibitory KIR for non-self class I after TCD-HSCT are activated in response to leukemic cell lines and primary leukemic blasts lacking cognate ligands. Percentages of IFN-γ–producing cells among nonlicensed NK cells exclusively expressing the inhibitory KIR for non-self HLA class I ligand (NS-KIR) after activation by leukemic targets are shown for 2 patients. Targets include the patient-derived ALL cell line PhALL3.1 (HLA-C1/C1; Bw6/Bw6), the AML cell lines HL60 (HLA-C2/C2; Bw4/Bw6) and KASUMI-1 (HLA-C1/C1; Bw4/Bw6), and primary biphenotypic leukemic blasts PBL-1 (HLA-C1/C1; Bw4/Bw4). (A) Compared with the donor control, NK cells exclusively expressing NS-KIR KIR2DL3 from the HLA-C2/C2, Bw4/Bw4 post-HSCT patient (patient no. 5) are specifically activated against HL60, which lacks the cognate HLA-C1 ligand. (B) NK cells exclusively expressing NS-KIR KIR2DL1 from the HLA-C1/C1, Bw4/Bw4 post-HSCT recipient (patient no. 16) are activated against PhALL3.1, KASUMI-1, and PBL-1, which lack the cognate HLA-C2 ligand.
Nonlicensed NK cells expressing inhibitory KIR for non-self class I after TCD-HSCT are activated in response to leukemic cell lines and primary leukemic blasts lacking cognate ligands. Percentages of IFN-γ–producing cells among nonlicensed NK cells exclusively expressing the inhibitory KIR for non-self HLA class I ligand (NS-KIR) after activation by leukemic targets are shown for 2 patients. Targets include the patient-derived ALL cell line PhALL3.1 (HLA-C1/C1; Bw6/Bw6), the AML cell lines HL60 (HLA-C2/C2; Bw4/Bw6) and KASUMI-1 (HLA-C1/C1; Bw4/Bw6), and primary biphenotypic leukemic blasts PBL-1 (HLA-C1/C1; Bw4/Bw4). (A) Compared with the donor control, NK cells exclusively expressing NS-KIR KIR2DL3 from the HLA-C2/C2, Bw4/Bw4 post-HSCT patient (patient no. 5) are specifically activated against HL60, which lacks the cognate HLA-C1 ligand. (B) NK cells exclusively expressing NS-KIR KIR2DL1 from the HLA-C1/C1, Bw4/Bw4 post-HSCT recipient (patient no. 16) are activated against PhALL3.1, KASUMI-1, and PBL-1, which lack the cognate HLA-C2 ligand.
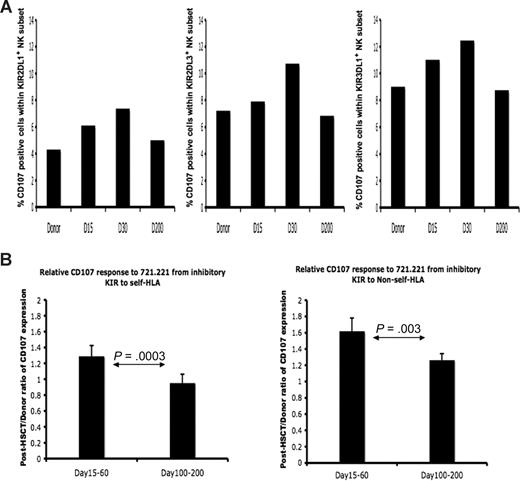
Because NK-cell cytokine secretion and cytotoxic function do not always correlate precisely, we also examined NK cytotoxic function from donors and posttransplantation patients at different time points. Multicolor flow cytometry was used to measure cell-surface mobilization of CD107, an indicator of effector cell degranulation and cytotoxicity. Figure 4A shows CD107 staining for KIR-expressing subsets from patient no. 9, whose HLA genotyping was HLA-C1/C1, -Bw4/Bw6. Post-HSCT NK cells expressing the NS-KIR2DL1 achieved a response comparable with the steady-state NK cells in the donor expressing the S-KIR2DL3 or KIR3DL1. Although all KIR-expressing populations displayed increased response, aggregate data from 3 separate donor-recipient pairs demonstrates that the increased cytotoxic response was higher in the NK populations expressing KIR for non-self HLA (Figure 4B). As was seen for intracellular IFN-γ, the cytotoxic response approached donor levels by day +200, achieving a phenotype of tolerance to self HLA class I.
Licensed and nonlicensed NK-cell degranulation after HSCT. Donor and post-HSCT patient PBMCs (patients 1, 8, 9) were incubated with 721.221 target cells and measured for CD107a expression. Degranulation was assessed as the percentage of CD107a+ cells within NK populations expressing specific KIR. (A) Percentage of CD107a-positive cells within the NK cells exclusively expressing NS-KIR KIR2DL1 (left), S-KIR KIR2DL3 (middle), and S-KIR KIR3DL1 (right) from the healthy HLA-C1/C1, Bw4/Bw6 HSCT donor (no. 9), the HLA-C1/C1, Bw4/Bw6 patient at day +15, day +30, and day +200 after HSCT. Findings are representative of studies performed in 3 donor-recipient pairs. (B) The post-HSCT/pre-HSCT ratio of CD107a expression was compared in the patient at days +15 to +60 and days +100 to +200 after HSCT (n = 3). The paired Student t test was performed to compute the P value for these comparisons.
Licensed and nonlicensed NK-cell degranulation after HSCT. Donor and post-HSCT patient PBMCs (patients 1, 8, 9) were incubated with 721.221 target cells and measured for CD107a expression. Degranulation was assessed as the percentage of CD107a+ cells within NK populations expressing specific KIR. (A) Percentage of CD107a-positive cells within the NK cells exclusively expressing NS-KIR KIR2DL1 (left), S-KIR KIR2DL3 (middle), and S-KIR KIR3DL1 (right) from the healthy HLA-C1/C1, Bw4/Bw6 HSCT donor (no. 9), the HLA-C1/C1, Bw4/Bw6 patient at day +15, day +30, and day +200 after HSCT. Findings are representative of studies performed in 3 donor-recipient pairs. (B) The post-HSCT/pre-HSCT ratio of CD107a expression was compared in the patient at days +15 to +60 and days +100 to +200 after HSCT (n = 3). The paired Student t test was performed to compute the P value for these comparisons.
Inhibitory KIR expression after HSCT is not equivalent among KIRs
Our data suggest that NK function after HSCT is altered such that normally hyporesponsive NK cells expressing KIR for non-self HLA display functional capacity early post-HSCT, reacting to missing ligand. Because the relevance of this finding is limited to the KIR-expressing NK population, it was important to document if KIR expression after HSCT is substantially altered. We therefore evaluated receptor expression among NK cells taken from all 16 patients after HSCT and compared them to donor expression. Overall KIR expression in the early post-HSCT NK repertoire was not significantly changed compared with the donor repertoire, with an average of 53.8% of NK cells expressing KIR by day +30. However, at day +100, NK cells experienced a lag in KIR2DL1 expression, whereas KIR2DL3 and KIR3DL1 were expressed at levels higher than in the donors (Table 3). There was no association between the delay in KIR2DL1 expression and its status as a self-HLA receptor or non-self HLA receptor within the patient. Among KIR+ cells, apart from the delay in 2DL1 expression, there were no differences in KIR expression or KIR repertoire compared with healthy donors. Other notable differences in phenotypic repertoire were the increased percentage of CD94/NKG2A+ cells in the first 200 days after HSCT (> 70%). Contrary to steady-state NK repertoires in healthy humans, there was no discernible bias in NK expression of inhibitory KIR for self-HLA over KIR for non-self HLA among NK cells early post-HSCT.
Inhibitory receptor expression post-HSCT
| . | Ratio R/D . | ||
|---|---|---|---|
| D30 . | D60 . | D100 . | |
| KIR2DL1 | 0.28 ± 0.18 | 0.46 ± 0.29 | 0.59 ± 0.44 |
| KIR2DL2/3 | 1.26 ± 0.50 | 1.40 ± 0.37 | 1.57 ± 1.03 |
| KIR3DL1 | 1.31 ± 0.85 | 1.60 ± 0.57 | 1.49 ± 0.89 |
| . | Ratio R/D . | ||
|---|---|---|---|
| D30 . | D60 . | D100 . | |
| KIR2DL1 | 0.28 ± 0.18 | 0.46 ± 0.29 | 0.59 ± 0.44 |
| KIR2DL2/3 | 1.26 ± 0.50 | 1.40 ± 0.37 | 1.57 ± 1.03 |
| KIR3DL1 | 1.31 ± 0.85 | 1.60 ± 0.57 | 1.49 ± 0.89 |
R indicates the percentage of NK cells positive for the inhibitory receptor in the recipient at days +30, +60, and +100 after HSCT; D, the percentage of NK cells positive for the inhibitory receptor in the donor; and Ratio R/D, the ratio calculated for each recipient/donor pair (n=16).
CD94/NKG2A is not responsible for breaking NK tolerance to self after HSCT
To exclude the possibility that the missing ligand effector response among NK cells expressing KIR for non-self MHC is due to CD94/NKG2A, we simultaneously stained cells for CD94/NKG2A and KIR and analyzed the functional response for KIR−NKG2A+ NK cells. Contrary to the KIR+ cell populations, the KIR−NKG2A+ population at day +30 after HSCT exhibited a significantly poorer response to a class I–negative target cell compared with similar populations in the donor and patient pre-HSCT (P = .046, Figure S3). By day +200 after HSCT, the functional responsiveness of the KIR−NKG2A+ population increased and approached donor response levels.
Although nearly all KIR+ NK cells coexpress CD94/NKG2A immediately after transplantation, by day +100 to 200 after HSCT, more than 30% of KIR+ NK cells are CD94/NKG2A− and can be examined separately for effector function. Among the CD94/NKG2A− NK population, cells expressing inhibitory KIR for non-self HLA in patients at day 100 to 200 after transplantation still display a significantly higher effector response compared with the donor control (Figure 5), which could be abrogated by cognate ligand (data not shown). In contrast, by this later post-HSCT time point, we found that CD94/NKG2A− NK cells expressing inhibitory KIR for self-HLA responded below donor response levels. By simultaneously staining for KIR and CD94/NKG2A and examining NK subsets separately, we conclude that CD94/NKG2A does not contribute to the observed missing ligand effector function in NK cells expressing KIR for non-self.
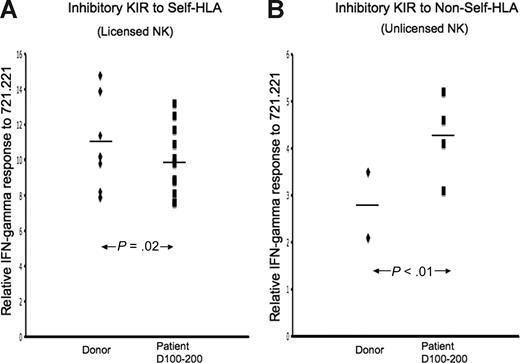
Unlicensed KIR+NKG2A− NK cells after HSCT demonstrate functional competence. IFN-γ response to class I–negative 721.221 target cells was calculated relative to baseline for each inhibitory KIR in each of 3 TCD-HSCT donor-recipient pairs (nos. 10, 12, and 14) by the formula (NKG2A−KIR+IFN-γ+/NKG2A−KIR+)/(NKG2A−KIR−IFN-γ+/NKG2A−KIR−). The relative response of NK cells exclusively expressing specific inhibitory KIR was compared between the allograft donor and the patient at days +100 to +200 after HSCT. (A) CD94/NKG2A-negative NK cells exclusively expressing inhibitory KIR to self-HLA from patients at days +100 to +200 are slightly hyporesponsive compared with their donors (P = .02). (B) CD94/NKG2A-negative NK cells exclusively expressing inhibitory KIR to non-self HLA from patients demonstrate higher response compared with the donor (P < .01). The paired Student t test was performed to compute the P value for these comparisons.
Unlicensed KIR+NKG2A− NK cells after HSCT demonstrate functional competence. IFN-γ response to class I–negative 721.221 target cells was calculated relative to baseline for each inhibitory KIR in each of 3 TCD-HSCT donor-recipient pairs (nos. 10, 12, and 14) by the formula (NKG2A−KIR+IFN-γ+/NKG2A−KIR+)/(NKG2A−KIR−IFN-γ+/NKG2A−KIR−). The relative response of NK cells exclusively expressing specific inhibitory KIR was compared between the allograft donor and the patient at days +100 to +200 after HSCT. (A) CD94/NKG2A-negative NK cells exclusively expressing inhibitory KIR to self-HLA from patients at days +100 to +200 are slightly hyporesponsive compared with their donors (P = .02). (B) CD94/NKG2A-negative NK cells exclusively expressing inhibitory KIR to non-self HLA from patients demonstrate higher response compared with the donor (P < .01). The paired Student t test was performed to compute the P value for these comparisons.
Majority of KIR+ NK cells belong to the CD56dim NK subset
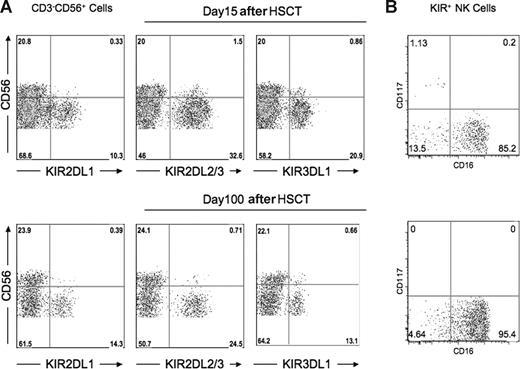
Previous reports have shown that compared with donors, the proportion of CD56bright NK cells is increased in recipients after HSCT, with most of the NK cells demonstrating an immature phenotype CD56bright or CD56dimKIR−CD16−CD117−/+.29,30 It was therefore possible that the unexpected functional competence among NK cells expressing KIR for non-self HLA and the heightened effector function of NK cells expressing KIR for self-HLA could be associated with an immature phenotype, fading with progressive development outside the bone marrow. Among the healthy donors, the percentage of CD56bright NK cells averaged 9.9%. After transplantation, however, the overall percentage of CD56bright NK cells increased to an average of 22.5%, with one patient exhibiting a population as high as 51%. Importantly, however, more than 95% of KIR+ NK cells in post-HSCT recipients exhibited CD56dim expression (Figure 6A). Further phenotype analysis showed that among KIR+ cells, 85.2% were CD56dim CD16+CD117− at the early time points after HSCT (days 15-60), increasing to 95.4% thereafter (Figure 6B).
Majority of post-HSCT KIR+ NK cells belong to the CD56dim NK subset. PBMC preparations from the patient at day +15 after HSCT and day +100 after HSCT were stained with monoclonal antibodies specific for CD3, CD56, KIR2DL1, KIR2DL3, KIR3DL1, CD16, and CD117. (A) Flow cytometric staining gated on CD3−CD56+ NK cells demonstrates that the overwhelming majority of KIR+ cells are CD56dim at both time points. Data are representative of analysis from 15 patients. (B) Flow cytometric staining gated on CD3−CD56+ KIR+ cells shows the majority of cells at both time points are CD117−CD16+. Data are representative of analysis from 6 patients.
Majority of post-HSCT KIR+ NK cells belong to the CD56dim NK subset. PBMC preparations from the patient at day +15 after HSCT and day +100 after HSCT were stained with monoclonal antibodies specific for CD3, CD56, KIR2DL1, KIR2DL3, KIR3DL1, CD16, and CD117. (A) Flow cytometric staining gated on CD3−CD56+ NK cells demonstrates that the overwhelming majority of KIR+ cells are CD56dim at both time points. Data are representative of analysis from 15 patients. (B) Flow cytometric staining gated on CD3−CD56+ KIR+ cells shows the majority of cells at both time points are CD117−CD16+. Data are representative of analysis from 6 patients.
Posttransplantation plasma cytokine levels are not associated with changes in NK-effector function
GVHD, infection, and conditioning for HSCT can be associated with increases in serum levels of inflammatory cytokines,31-34 whose altered levels might influence NK function and licensing.35-37 To investigate whether the alteration of NK response in the early stage after HSCT was associated with increased plasma cytokine levels in the patient, we measured the levels of TNF-α, IFN-γ, IL-2, IL-15, IL-6, and IL-12 by ELISA assay at multiple time points after HSCT. We detected no significant differences for the cytokine levels between the early time points (days 15-60) and late time points (days 100-200) after HSCT (data not shown).
Discussion
Immune reconstitution after allogeneic HSCT provides a unique in vivo environment in which to study NK tolerance. By performing studies on samples from patients receiving a TCD allograft, we were more likely to demonstrate KIR-driven NK effects without interference from T-cell effects on NK function.38,39 We found that the functional response of the aggregate NK population after HSCT was not significantly changed from the overall response of the donor NK repertoire, underscoring the importance of examining function of discrete NK subsets. Analysis at the single cell level permits a more accurate assessment of NK alloreactivity that would otherwise not be detected if functional response were measured simply at the population level.
Our results from 16 TCD-HSCT donor-recipient pairs show that while NK cells expressing inhibitory KIR for self-HLA class I ligands are hyperresponsive early post-HSCT, NK cells expressing inhibitory KIR recognizing non-self HLA class I ligands unexpectedly have functional capacity immediately after HSCT, becoming activated upon encountering target cells lacking ligand for the inhibitory KIR. Thus, NK cells early posttransplantation do not behave strictly according to the tenets of NK licensing where only NK cells expressing inhibitory KIR for self-HLA class I molecules are functionally competent. Instead, these findings support the possibility that NK cells become activated upon recognition of lack of class I ligand for any inhibitory KIR (missing ligand) in the first 3 to 6 months after HSCT, restricting themselves gradually to a tolerized state where they become activated only upon recognition of lack of self-class I ligands (missing self).
For the first time, these findings explain previous observations that AML and MDS patients receiving allografts from HLA-matched donors experience lower relapse and higher survival if the patient lacks the class I ligands for the donor's inhibitory KIR,19-22 clinical correlations that seemed improbable given the immunogenetic rules governing NK licensing. Our results suggest that after HSCT, there is a period during which NK cells acquire tolerance to self through progressive anergy of NK cells expressing inhibitory KIR for non-self HLA; before acquisition of tolerance, alloreactivity due to lack of KIR ligand in the host is most evident. We have demonstrated alloreactivity according to missing ligand in leukemic target cells, but it is likely that in vivo NK alloreactivity occurs against hematopoietic cells in general. Further supporting a model that tolerance is acquired after HSCT is the observation that in haploidentical transplants with KIR ligand incompatibility, alloreactive donor NK clones are most frequently isolated in the first 3 months after HSCT, becoming increasingly rare thereafter and unidentifiable by 1 year, implying that even NK cells missing self can acquire tolerance to their new immunogenetic milieu.16
These findings could not be correlated with current phenotype-based models of NK development. In NK development, expression of the inhibitory receptor CD94/NKG2A is followed by KIR expression,40-42 and other laboratories have reported increased expression of CD94/NKG2A on the NK cells in the early post-HSCT period.29,30 Although CD94/NKG2A can confer low-level effector function to NK cells in a healthy person,10 the early increased expression of CD94/NKG2A after HSCT could not be responsible for the effector function of NK cells expressing KIR for non-self class I ligands: the response of these cells could only be specifically inhibited by cognate ligands for KIR; post-HSCT NK cells exclusively expressing CD94/NKG2A as their inhibitory receptor expressed a markedly hyporesponsive phenotype; and missing ligand function was clearly identified in the NKG2A− KIR+ population. Taken together, these findings lead us to conclude that the NK function after HSCT is inherent to KIR expression and not to CD94/NKG2A expression.
We found that KIR+NKG2A− cells can first be detected around day 75 after HSCT, consistent with a recent study by Vago et al.43 The study's authors, however, described a general hyporesponsiveness of the entire NK population when challenged with leukemic targets, which we did not observe. In addition, contrary to our findings, they reported post-HSCT cells expressing a single KIR to be hyporesponsive in the months after HSCT compared with the same population in the donor. This inconsistency with our findings may be due to the presence in their study of T cells added back to the patients, which may have altered NK development or function.
NK-cell development in lymphoid tissue spans 5 phenotypically different stages, where fully mature and functional NK cells are CD56dimCD16+KIR+ and/or NKG2A+.40 Phenotypic analysis of the specific NK cells in our study demonstrated the overwhelming majority of the KIR+ NK cells after HSCT belonged to the mature CD56dim CD16+CD117− phenotype, implying that circulating NK cells acquiring tolerance are phenotypically mature.
If acquisition of tolerance to self is not inherent to NK development, post-HSCT missing ligand behavior may be due to environmental factors leading to a more activated state and disruption of the rules of licensing. Cytokines elaborated during the conditioning regimen and early after HSCT may affect the differentiation and peripheral homeostasis of NK cells. While we found that IL-6 and TNF-α were slightly increased early post-HSCT compared with late post-HSCT, the levels were not statistically different. We were therefore unable to correlate differences in cytokine levels with alterations in NK licensing, as has been observed by other groups in mice, where most recently it was shown that viral infection and inflammation could break NK tolerance to missing self.35-37
Similarly, the natural cytotoxicity receptors (NCRs; NKp30, NKp44, and NKp46) are capable of activating NK cells,44 and there is a direct correlation between the surface density of expressed NCR and the ability of NK cells to kill tumor cells.45,46 While we observed that NKp30 and NKp46 expression was elevated early post-HSCT, whether NCR expression can be correlated with NK licensing requires further investigation.
By minimizing T-cell interference of NK alloreactivity and development,38,39 T-cell depletion of the allograft provides the optimal setting in which to detect in vivo alterations in NK licensing. Because the functional repertoire is defined by the phenotypic repertoire, aberrations in KIR expression may affect NK alloreactivity. Regardless of whether the inhibitory KIR was for self or non-self class I ligand, we found KIR2DL3 and KIR3DL1 to be expressed at higher levels after HSCT compared with the donors, and a comparative delay in KIR2DL1 expression, recapitulating the in vitro observation made in the development of CD34+ umbilical cord stem cells to KIR-expressing NK cells.47
HLA-A antigens presenting the Bw4 epitope recognized by KIR3DL1 have recently been shown not only to protect targets from lysis by KIR3DL1-expressing NK cells but also to confer functional competence to KIR3DL1-expressing cells.7,8 Of the 16 patients in this study, 2 could be characterized as having the Bw4 epitope presented exclusively by an HLA-A antigen. Interestingly, 3DL1-expressing cells from these 2 patients exhibited functional capacity, although not to the same degree as 3DL1-expressing cells from patients with Bw4 presented by HLA-B antigens. Nevertheless, the data support the inclusion of HLA-A Bw4 epitopes in future analyses of KIR/HLA interactions and clinical outcomes in HSCT.
These studies have important implications for our current understanding of NK alloreactivity in HSCT. Identification of NK alloreactivity according to missing ligand in HLA-identical HSCT argues against the deliberate selection of HLA-nonidentical donors over HLA-identical donors to engender missing-self alloreactivity in efforts to achieve an improved clinical outcome. Direct comparisons between the impact of NK allorecognition according to missing self and missing ligand have yielded mixed results, but these have either relied on correlations with KIR/HLA or with isolation of alloreactive NK clones.20,48 Comparisons of single-cell functional analyses of the KIR-expressing repertoires among patients receiving KIR-ligand matched or mismatched allografts may therefore be a more informative approach. Finally, our findings support the possibility that NK cells may behave according to missing ligand in other clinical settings such as autologous HSCT, which has applicability to both hematologic and solid maligancies.49 Large studies of KIR/HLA combinations therefore should be undertaken in autologous HSCT patient populations, where identification of a missing ligand effect could be useful as a prognostic marker.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We gratefully acknowledge the Memorial Sloan-Kettering Cancer Center (MSKCC) Pediatric and Adult Allogeneic Bone Marrow Transplantation Services for their contribution of donor-recipient samples for this study; Dr Marcel van den Brink for the use of his flow cytometry equipment; Drs Bo Dupont and Peter Parham for the use of their 721.221 transfectants; and Dr Renier Brentjens for the use of PhALL3.1.
This study was supported in part by The Marrow Foundation (Minneapolis, MN) and National Institutes of Health (NIH, Bethesda, MD) grant nos. HL070053, CA23766, and HL088134.
National Institutes of Health
Authorship
Contribution: J.Y. designed the project, performed the studies, and prepared the manuscript; J.M.V. contributed clinical data collection; X.-R.L. contributed flow cytometric analysis of post-HSCT NK phenotype; J.P. assisted functional analysis; R.S.H. performed KIR genotyping; R.J.O. contributed donor-recipient pairs for analysis; and K.C.H. designed the project and prepared the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Katharine C. Hsu, Adult Allogeneic Bone Marrow Transplantation Service, Department of Medicine, Box 336, Memorial Sloan-Kettering Cancer Center, 1275 York Ave, New York, NY; e-mail: hsuk@mskcc.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal