To the editor:
Type 1 hemochromatosis is generally due to homozygous p.C282Y mutation in HFE.1,2 We report the case of a young woman with a classical hemochromatosis phenotype due to a homozygous deletion in the 6p chromosome region containing HFE.
The proband is a 29-year-old woman of Sardinian (Italian) origin. At the age of 27, serum iron was 33.2 μmol/L (186 μg/dL), serum ferritin 3146 pmol/L (1400 μg/L), and serum alanine aminotransferase (s-ALT) 70 U/L. Diagnosis of hemochromatosis was confirmed at subsequent analyses including magnetic resonance. A test for HFE mutations (NLM, Settala, Italy) gave no signal. There were no causes of secondary iron overload. She began phlebotomies every 2 weeks and after 3.5 g of iron removal serum ferritin decreased to 422 pmol/L (188 μg/L), s-ALT normalized (23 U/L), whereas serum iron did not change (36 μmol/L [201 μg/dL]), indicating she was not yet iron-depleted. Nevertheless, therapy was stopped, and after 3 months serum ferritin raised to 1229 pmol/L (547 μg/L). She came to our attention in June 2008. She denied family consanguinity up to the fifth generation. Her grandparents originated from different regions of Sardinia.
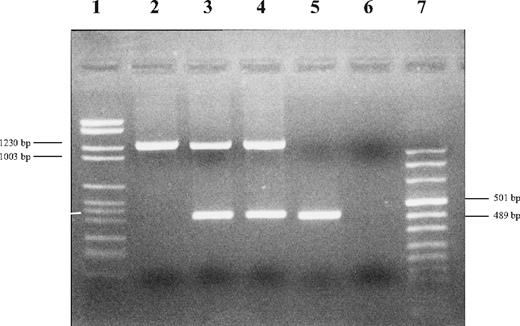
After obtaining informed consent, we analyzed her DNA sample for pC282Y and pH63D by real-time polymerase chain reaction (PCR; Celbio, Milano, Italy), confirming the absence of signal and suggesting a deletion in HFE-containing region. By analyzing several polymorphic markers centromeric and telomeric to HFE, we roughly define the upstream and downstream limits at 26 181 749 and 26 225 235, respectively. Meanwhile, Le Gac et al3 reported a very similar rearrangement in a different woman from Sardinia. By using their primers (gap-F and gap-R) and those for HFE exon 5,4 we confirmed that the proband was homozygous and her parents heterozygous for a gross deletion (Figure 1). To better mark deletion boundaries, we designed an internal forward primer and confirmed, by sequencing, that the proband and her parents had the same deletion [Chr6 g.(26 175 442)_g.(26 208 186)del] reported by Le Gac et al.3
Electrophoresis of PCR products of proband, parents and control. Upper bands correspond to PCR products obtained by using gap-F and gap-R primers designed by Le Gac et al.3 Lower bands correspond to HFE exon 5. Lane 1: DNA molecular marker II; lane 2: proband; lane 3: father; lane 4: mother; lane 5: control; lane 6: negative; and lane 7: DNA molecular marker VIII (Roche Diagnostics, Mannheim, Germany).
Electrophoresis of PCR products of proband, parents and control. Upper bands correspond to PCR products obtained by using gap-F and gap-R primers designed by Le Gac et al.3 Lower bands correspond to HFE exon 5. Lane 1: DNA molecular marker II; lane 2: proband; lane 3: father; lane 4: mother; lane 5: control; lane 6: negative; and lane 7: DNA molecular marker VIII (Roche Diagnostics, Mannheim, Germany).
The patient we describe in the present report showed a more marked iron overload than that described by the French group, as indicated by the higher serum ferritin and iron removed despite her younger age, supporting the role of modifier factors in modulating phenotype expression in HFE-related hemochromatosis.5 The absence of close consanguinity within the present family and, as far as we could assess, with the case reported by LeGac et al,3 suggest that this deletion is present at the population level in Sardinia and may derive from a common ancestor. Sardinian population is an ancient genetic isolate, and the few data available indicate that p.C282Y mutation is very rare or even absent.6 Thus, it is possible that the deletion might be the most common cause of hemochromatosis in this geographic area. However, considering the mechanism of deletion, it cannot be excluded it can result from multiple mutational events and explain other hemochromatosis cases.
Authorship
Acknowledgments: We thank Giorgio Casati for technical assistance in sequencing.
This work was supported in part by the Italian Ministry of University and Research (MIUR) FIRB 2003 (Rome, Italy) and by the Association for the Study of Hemochromatosis and Iron Overload Diseases (ONLUS; Monza, Italy).
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Alberto Piperno, Department of Clinical Medicine and Prevention, via Cadore, 48, University of Milano-Bicocca, Monza, Italy 20052; e-mail: alberto.piperno@unimib.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal