Abstract
We have isolated c-Kit+Lin− cells from both human and murine amniotic fluid (AF) and investigated their hematopoietic potential. In vitro, the c-Kit+Lin− population in both species displayed a multilineage hematopoietic potential, as demonstrated by the generation of erythroid, myeloid, and lymphoid cells. In vivo, cells belonging to all 3 hematopoietic lineages were found after primary and secondary transplantation of murine c-Kit+Lin− cells into immunocompromised hosts, thus demonstrating the ability of these cells to self-renew. Gene expression analysis of c-Kit+ cells isolated from murine AF confirmed these results. The presence of cells with similar characteristics in the surrounding amnion indicates the possible origin of AF c-Kit+Lin− cells. This is the first report showing that cells isolated from the AF do have hematopoietic potential; our results support the idea that AF may be a new source of stem cells for therapeutic applications.
Introduction
Defining the embryonic origin of mammalian hematopoietic stem cells (HSCs) is important for several reasons: understanding how specific adult cell lineages develop, uncovering the pathophysiology of inherited diseases of the hematopoietic system, and developing new, HSC-based therapeutic strategies. The process of blood cell development in the mammalian conceptus is particularly complex, as it occurs at least in 3 sites separated in both space and time: the yolk sac (YS), the para-aortic splanchnopleure and aorta-gonad-mesonephros (AGM) region, and the chorioallantoic placenta.1,2 Cells with hematopoietic activity can be further distinguished on the basis of their functional properties, as defined by their ability to give rise to all the blood cell lineages in vitro and/or in vivo in either newborn or adult mice.
In the mouse, the first definitive repopulating HSCs are found at approximately embryonic day (E) 9.0 in the dorsal aorta and in the YS and at approximately E10 in the vitelline, the umbilical arteries, and the placenta.3-10 Given that the circulatory system is already established at E8.0 to E8.25,11 even though not fully functional until E10,12 the question of whether regions other than the dorsal aorta4 represent sites of de novo generation of HSC has, until very recently, been subject to much debate. The work performed by Rhodes et al in embryos lacking a circulatory system now suggests that the placenta may correspond to a true site of HSC generation and expansion.10 These recently published findings still require further investigation to fully define the in vivo functional activity of this hematopoietic site in the absence of circulation because it is well known that prospective hematopoietic cells are influenced by morphogens and factors produced by the immediate environment.1 Strikingly, there are close interactions between the mesoderm of each known hematopoietic site and the endoderm (in the bilaminar YS), trophectoderm (in the placenta), and the dorsal ectoderm and ventral endoderm (in the AGM region).1 These close layers probably provide signaling molecules, including vascular endothelial growth factor, basic fibroblast growth factor, and transforming growth factor-β1, which are essential for the hematopoietic specification of the mesoderm13 via the controlled expression of key hematopoietic transcriptions factors such as Gata2 and Runx1. Indeed, both Gata2- and Runx1/Aml1-deficient mice present a complete absence of definitive HSCs in the AGM, whereas YS hematopoietic progenitors are still present (albeit in low numbers, in the case of the GATA2 deficiency1 ). Indeed, LMO2, GATA2, and SCL were shown to bind to the conserved E regions of the Runx1 locus, inducing its transactivation and the expression of Runx114 transcription factor that marks the onset of definitive hematopoiesis.15,16
Whether human and murine hematopoietic systems have the same extra- and intraembryonic sites is still unknown. In humans, cells with hemogenic potential are observed after approximately 3 to 4 weeks of gestation. Hematopoietic precursor and stem cells with multilineage hematopoietic potential are first detected in the AGM region and then in the YS.17 Even though the multilineage potential of AGM cells has not been tested definitively in vivo, the high similarity between murine and human hematopoietic precursors at equivalent developmental stages argues strongly in favor of HSC status for these cells.
Recently, it has been shown that amniotic fluid (AF) contains a population of stem and progenitor cells able to differentiate into several different tissue-specific lines, at least in vitro.18-21 Moreover, AF-derived CD133+ cells implanted in biodegradable polymers and conditioned in a bioreactor system have been used to generate ex vivo functional heart valves.22 Hence, starting from these observations, we wondered whether hematopoietic precursors and/or stem cells could be detected both in murine and human AF. We isolated cells with hematopoietic phenotypic profiles in both species and performed further molecular and functional characterization. For the murine studies, we performed an in-depth comparison of our candidate hematopoietic population with cells sharing the same phenotype and derived from E12.5 fetal liver (the main site for definitive hematopoiesis at this embryonic stage).23,24 This comparison clearly showed that cells isolated from AF have true hematopoietic potential, both in vitro and in vivo.
Methods
Sampling and cell isolation
C57BL/6 (Ly5.2) green fluorescent protein (GFP)+/− male and C57BL/6 (Ly5.2) GFP−/− female mice were used to obtain both Ly5.2 GFP+/− and Ly5.2 GFP−/− F1 embryos. All experiments and procedures were performed in compliance with the French Ministry of Agriculture regulations for animal experimentation (Act no. 87-847, October 19, 1987; modified May 2001, renewed in June 2008) and were approved by the Ethical Committee of the Hôpital Necker–Enfantes Malades (Paris, France). Embryo age was defined relative to the morning of vaginal plug discovery (E0.5). All dissections were performed under a stereomicroscope (Leica Microsystems, Rueil-Malmaison, France). AF, amnion (Am), and fetal liver (FL) were recovered from the same embryos at embryonic days E9.5, E10.5, E11.5, E12.5, E13.5, E14.5, E15.5, and E19.5. AF samples were harvested carefully by removing the maternal uterine wall to expose amniotic sacs. After isolation of single amniotic sacs, chorion was carefully removed and the placentas were pulled gently apart. Amnion rupture resulted in AF leakage, and AF was harvested with a syringe fitted with a 19-gauge needle. The Am was harvested at the time of surgery, rinsed twice in phosphate-buffered saline (PBS) to avoid blood contamination, dissociated mechanically by aspiration through a 19-gauge needle and treated with 0.12% wt/vol collagenase type I (Sigma-Aldrich, Lyon, France) in PBS supplemented with 10% fetal bovine serum (Invitrogen, Cergy Pontoise, France) for 1.5 hours at 37°C, followed by successive passages through 20-gauge up to 25-gauge needles. The single-cell suspension was then filtered through a 40-μm cell strainer (BD Biosciences, Le Pont de Claix, France). E11.5 to E13.5 FL was also isolated after embryo dissection in Dulbecco modified Eagle medium supplemented with 5% fetal bovine serum. E12.5 embryonic blood (EB) was collected by decapitating fetuses that were allowed to bleed freely with PBS and 1% heparin (Heparine Choay, Paris, France). A single-cell suspension was obtained by mechanical grinding and then filtration through 40-μm cell strainers.
Murine AF-derived c-Kit+ cells were isolated in 2 different ways: (1) using the EasySep Mouse CD117 Selection Cocktail (StemCell Technologies, Grenoble, France) as primary antibodies (Abs), followed by separation with an EasySep phycoerythrin (PE) selection kit (also from StemCell Technologies). In all experiments, only GFP+ cells were taken into account, to avoid any contamination by maternal GFP− cells; (2) using a FACSAria fluorescence-activated cell sorter (FACS) and FACSDiva acquisition software (both from BD Biosciences) to sort the c-Kit+/GFP+/Lin− population after staining with allophycocyanin (APC)–conjugated anti-CD117 monoclonal Abs (mAbs; BD Biosciences) and a combination of Lin PE-conjugated mAbs (containing anti-CD3, CD4, CD8, CD19, B220, Gr1, Mac1, Ter119, and NK1.1 mAbs, all from BD Biosciences). Dead cells were excluded by staining with the Viaprobe (7-amino-actinomycin D [7-AAD]–exclusion assay; BD Biosciences). The same procedure was used to isolate FL- and Am-derived cell populations.
Human AF was collected during routine diagnostic procedures (amniocentesis) from consenting volunteer donors, according to guidelines from the Ethical Committee of the Centre Hospitalier Universitaire Le Kremlin Bicêtre (Kremlin Bicêtre, France). Cord blood samples were harvested on delivery of full-term, healthy pregnancies, after obtaining the mother's written informed consent in accordance with French legislation and ethical guidelines and the Declaration of Helsinki.
C-Kit+ (CD117+) cells were isolated using the FACSAria Sorter and FACSDiva acquisition software to sort c-Kit+Lin− (KL) population stained with an APC-conjugated anti-CD117 mAb (BD Biosciences PharMingen, San Diego, CA) and a combination of Lin PE-conjugated Abs (containing anti-CD3, CD4, CD8, CD13, CD16, CD19, CD20, CD33, CD56, and GlyA [CD235a], all from BD Biosciences PharMingen).
Flow cytometry
Flow cytometry was performed using a FACSCalibur flow cytometer (BD Biosciences) with CellQuest acquisition software (BD Biosciences). Analysis was performed using either CellQuest or FlowJo (TreeStar, Ashland, OR) software. The antibodies used are indicated in Document S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article).
In vitro differentiation protocols
Myeloid and erythroid potentials were assessed by cell culture in a semisolid medium. Between 200 and 2000 cells were gently mixed with 2 mL MethoCult H4230 methylcellulose colony assay medium (StemCell Technologies), supplemented with human recombinant stem cell factor (SCF, 50 ng/mL), granulocyte colony-stimulating factor (20 ng/mL), granulocyte-macrophage colony-stimulating factor (GM-CSF, 20 ng/mL), interleukin-3 (IL-3; 20 ng/mL), IL-6 (20 ng/mL) proteins (R&D Systems, Lille, France), and erythropoietin (epoetin α, 20 U/mL; Eprex, Janssen-Cilag, Issy-les-Moulineaux, France) and cultured for up to 3 weeks in 6-well plates at 37°C in humidified 5% CO2 air. Colonies consisting of at least 50 cells were counted every week and classified according to morphology and color of the colony and the single cells, using an inverted Leica DM1RB microscope (10× magnification). Pictures of colonies were taken with a Leica DC350F camera and processed with LeicaQFluoro software (Leica Microsystems).
B-, NK-, and T-cell differentiation was carried out on a confluent stroma of OP9 cells (ACC 441; DSMZ, Braunschweig, Germany), which were, respectively, transduced or not with either the murine or human ligand of Notch Delta-1.25 Culture medium was (1) for murine B-cell condition, RPMI 1640 (Invitrogen) supplemented with 1% antibiotics, 10% defined fetal calf serum (Perbio Science, Brebieres, France), 5% human AB serum, 50 μM β-mercaptoethanol, and recombinant murine IL-7 (20 ng/mL; kind gift from Cytheris, Issy-les-Moulineaux, France); (2) for human NK-cell differentiation, RPMI 1640 supplemented with 1% antibiotics, 10% defined fetal calf serum and SCF (10 ng/mL), and IL-15 (20 ng/mL; R&D Systems); (3) for murine and human T-cell differentiation, alpha medium with Glutamax I (Invitrogen) supplemented with 1% antibiotics, 20% defined fetal calf serum and either murine recombinant Flt3-L (5 ng/mL) and IL-7 (2 ng/mL) proteins or human recombinant SCF (10 ng/mL), Flt3-L (5 ng/mL), and IL-7 (2 ng/mL) proteins (all from R&D Systems).
Analysis of BrdU incorporation
Pregnant C57BL/6 (Ly5.2) GFP−/− female mice (E12.5) were injected intraperitoneally with 1 mg 5-bromo-2′-deoxyuridine (BrdU; Sigma-Aldrich) twice, with a 2-hour interval between the injections. Cells were harvested 30 minutes after the second injection, as previously described. BrdU labeling was also prolonged up to 24 hours by injecting BrdU again 6 to 8 hours and 14 to 16 hours after the first 2 injections.
Single-cell suspensions were stained with c-Kit APC-conjugated Ab and Lin PE-conjugated Ab. Surface-stained cells were fixed and permeabilized in PBS containing 1% paraformaldehyde and 0.01% Tween 20 for 48 hours at 4°C. The cell suspension was rinsed twice and then incubated with DNase I (GE Healthcare Europe, Saclay, France) at 37°C in a humidified chamber, rinsed again, and then incubated with BrdU fluorescein isothiocyanate–conjugated Ab (BD Biosciences).
Transplantation
Two- to 4-month-old recombination-activating gene 1 (RAG1)−/− C57BL/6 (Ly5.1) mice were given a sublethal 8-Gy dose of gamma-irradiation and injected with 2 × 104 murine AF (mAF) or murine FLKL (mFLKL) cells harvested at E12.5 to E13.5 from GFP+ Ly5.2+ embryos. All the cells were injected via the retro-orbital vein in 150 μL normal salt solution (0.9% NaCl). Flow cytometric analysis of peripheral blood was performed every 4 weeks. Recipients were killed between 16 and 18 weeks after transplantation and hematopoietic organs (bone marrow [BM], spleen, thymus, and lymph nodes) were harvested. For secondary transplantation experiments, BM samples from primary transplanted mice were harvested 18 weeks after injection and directly injected at a ratio of 2 secondary recipients per primary recipient (∼ 20 × 106 cells per recipient). In some cases, Ly5.2+ cells were stained with a peridinin chlorophyll protein–conjugated mAb (BD Biosciences) and sorted using the FACSAria Cell Sorter and FACSDiva acquisition software, before secondary transplantation of 15 × 104 Ly5.2+ cells into 2- to 4-month-old sublethally irradiated RAG1−/− C57BL/6 (Ly5.1) mice. Peripheral blood stainings of injected animals were performed every 4 weeks. Secondary recipients were killed between 10 and 13 weeks after transplantation, and hematopoietic organs were analyzed by flow cytometry.
Gene expression analysis
As previously described,26 cells sorted in diethyl pyrocarbonate–treated PBS were lysed by freezing at −80°C and then heating to 65°C for 2 minutes. After cooling to 4°C, RNA was specifically reverse-transcribed for 1 hour at 37°C by adding 10 μL of a mix containing 12.2 μM of the specific 3′ primer A (Table S1), buffer II 10 times (Applied Biosystems, Courtaboeuf, France), 3.3 mM MgCl2 (Applied Biosystems), 1 mM deoxynucleoside triphosphate (dNTPs; GE Healthcare Europe), 39 units of RNAse block (Stratagene, Amsterdam, The Netherlands), and 11.5 units of murine leukemia virus reverse transcriptase (Applied Biosystems) in a 15-μL reaction volume. The reaction was stopped by incubation at 95°C for 3 minutes.
The cDNAs resulting from the reverse transcription reaction were then amplified. The first round of polymerase chain reaction (PCR) consisted of a denaturation step at 95°C for 10 minutes and 15 cycles of amplification (45 seconds at 95°C, 1 minute at 60°C, and 1 minute 30 seconds at 72°C) with buffer II 10 times, 2 mM MgCl2, 0.2 mM GeneAmp dNTPs, 3 units of AmpliTaq Gold DNA polymerase (all from Applied Biosystems), and 0.015 μM of specific primers A and B (Table S1) in an 85-μL reaction volume.
Each initial PCR product was next amplified separately with 48 cycles of a second PCR (30 seconds at 94°C, 45 seconds at 60°C, and 1 minute at 72°C) in a 20-μL reaction volume containing buffer II 10 times, 2 mM MgCl2, 0.25 mM GeneAmp dNTPs (all Applied Biosystems), 0.5 units of AmpliTaq Gold DNA Polymerase (Applied Biosystems), and 0.25 μM of specific primers A and C (Table S1). PCR products were then resolved on a 2.5% agarose ethidium bromide gel and were sequenced to confirm specificity.
Results
KL cells are present in murine and human AF
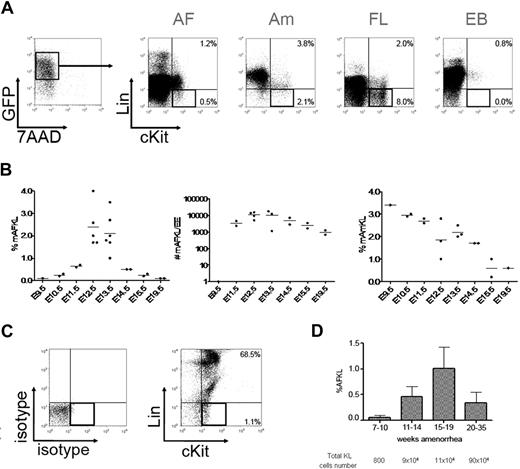
mAF was collected from the offspring of GFP−/− females with GFP+/− males. As the offspring was composed by both GFP− and GFP+ embryos, only GFP+ cells were considered for further analysis to exclude any maternal contamination. GFP+ cells contained viable cells expressing hematopoietic markers (Lin+) and especially those from granulocytic and erythroid lineages. Within the lineage-negative fraction (Lin−; Figure 1A), some c-Kit+ cells were detected in a proportion that varied over the course of gestation (roughly describing a Gaussian curve). However, it must be noted that this calculation underestimates the total number of KL cells in the AF because only GFP+ cells that come from half of the offspring were taken into account. The curve peaked at E12.5 (Figure 1B left panel). The total number of KL cells in the mAF per embryo equivalent rose from 1 at E9.5 to 10 000 at E12.5 and then slowly decreased to 1000 at E19.5 (Figure 1B middle panel). A population of KL cells was also detected in the Am and the FL (Figure 1A); its frequency within the Am steadily decreased from 3.5% of total viable cells at E9.5 to 0.5% before delivery (Figure 1B right panel). At E12.5, EB contained very rare KL cells (0.03%) and was thus not further investigated. To note, FLKL cells can be subdivided into a minor c-Kit low and a major c-Kit high populations, whereas AFKL cells were mainly c-Kit low (Figure 1A). This observation may mirror differences between the 2 environments. The microenvironment, and particularly SCF, found at high doses in the AF27 has been shown to be implicated in the regulation of c-Kit surface expression.28
Developmental kinetics of KL cells in murine AF and Am and in human AF. (A) Murine AF and Am were collected from E9.5 up to E19.5 and analyzed by flow cytometry. Mononuclear cells from AF, Am, FL, and EB were stained with Viaprobe (7-AAD) and a combination of antilineage (Lin) markers and anti–c-Kit antibodies. Lin and c-Kit expression is shown for viable (7-AAD−) GFP+ cells, to exclude any maternal (GFP−) contamination. Numbers represent percentages of cells. We used an inverted Leica DM1RB microscope (10× magnification). Pictures of colonies were taken with a Leica DC350F camera and processed with LeicaQFluoro software (Leica Microsystems). (B) The percentage of AFKL cells as a function of the gestation stage is indicated in the left panel. The middle panel shows the total number of mAFKL cells per embryo equivalent (EE). The right panel indicates the percentage of mAmKL cells among total live cells. Means are represented by bars. (C) Human AF was collected from 7 weeks of amenorrhea up to 35 weeks of amenorrhea. 7-AAD− mononuclear cells were stained with a combination of antilineage (Lin) markers and anti–c-Kit antibodies. Isotype controls are shown in the left panel. (D) The percentage of hAFKL cells relative to total live cells is shown as a mean and SD as a function of the gestation stage. The total number of hAFKL cells estimated for each period (mean AF volume multiplied by mean cellularity of the samples) is indicated below the graph.
Developmental kinetics of KL cells in murine AF and Am and in human AF. (A) Murine AF and Am were collected from E9.5 up to E19.5 and analyzed by flow cytometry. Mononuclear cells from AF, Am, FL, and EB were stained with Viaprobe (7-AAD) and a combination of antilineage (Lin) markers and anti–c-Kit antibodies. Lin and c-Kit expression is shown for viable (7-AAD−) GFP+ cells, to exclude any maternal (GFP−) contamination. Numbers represent percentages of cells. We used an inverted Leica DM1RB microscope (10× magnification). Pictures of colonies were taken with a Leica DC350F camera and processed with LeicaQFluoro software (Leica Microsystems). (B) The percentage of AFKL cells as a function of the gestation stage is indicated in the left panel. The middle panel shows the total number of mAFKL cells per embryo equivalent (EE). The right panel indicates the percentage of mAmKL cells among total live cells. Means are represented by bars. (C) Human AF was collected from 7 weeks of amenorrhea up to 35 weeks of amenorrhea. 7-AAD− mononuclear cells were stained with a combination of antilineage (Lin) markers and anti–c-Kit antibodies. Isotype controls are shown in the left panel. (D) The percentage of hAFKL cells relative to total live cells is shown as a mean and SD as a function of the gestation stage. The total number of hAFKL cells estimated for each period (mean AF volume multiplied by mean cellularity of the samples) is indicated below the graph.
Human AF (hAF) contained a high proportion of similar Lin+ cells (Figure 1C); these were essentially macrophage-granulocyte lineages, together with mastocytes that coexpressed c-Kit, CD9, CD13, and other c-Kit− CD13+ granulocyte precursors. In addition to this large population of Lin+ cells, some KL cells were detected. Similarly to what had been observed in mice, the change in the number of KL cells over the course of gestation was reminiscent of a Gaussian curve. By taking into account the volume and cellularity of AF,29,30 we estimated that the total hAFKL cell number increased up to a peak of 90 × 104 after 20 weeks of amenorrhea (Figure 1D).
Taken as a whole, these results show that KL cells are present in human and murine AF at very early time points in gestation and remain, to a various extent, with a peak at midgestation. In the mouse embryo, KL cells were also found in the Am.
Characterization of murine and human KL cells
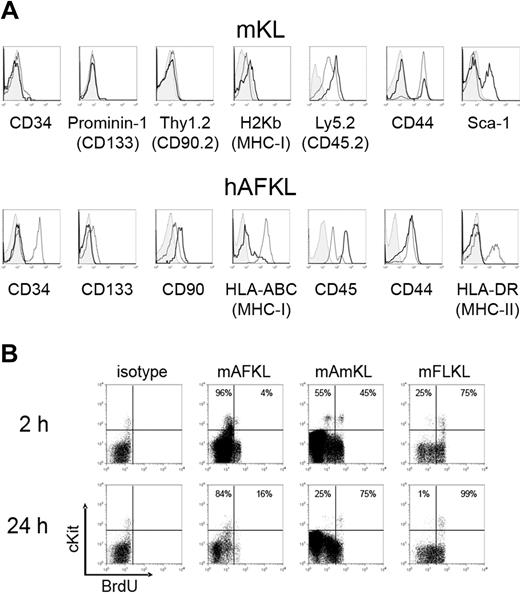
Immunologic phenotyping was performed on KL cells isolated from the murine AF, Am, and FL (Figure 2A top panels; Table S2) and from the human AF and CB (Figure 2A bottom panels; Table S2). Murine AmKL cells exhibited a phenotype fully identical to FLKL cells (data not shown). The phenotypes did not vary over the course of gestation in both species.
Phenotypic characterization of murine and human KL cells. (A) Murine KL cells from the AF (black bold solid line) and FL (gray normal solid line) were stained with antibodies specific for CD34, prominin-1, Thy1.2, H2Kb, Ly5.2, CD44, and Sca-1. Representative histograms for cells at E13.5 are shown. Am is not shown as it overlaps to FL for all the markers analyzed. Human AF (black bold solid line) and CB (gray normal solid line) KL cells were stained with specific mAbs for CD34, CD133, CD90, HLA-ABC, CD45, CD44, and HLA-DR. For both murine and human cells, the control isotype is indicated in light gray. (B) Analyses of murine KL cell-cycling status. Cells from wild-type C57BL/6 embryos were harvested 2 or 24 hours after the first administration of BrdU at E12.5. BrdU incorporation by Lin− cells was detected by fixation, permeabilization, and staining with a fluorescein isothiocyanate–conjugated anti-BrdU Ab. The results of 1 of 2 experiments are shown.
Phenotypic characterization of murine and human KL cells. (A) Murine KL cells from the AF (black bold solid line) and FL (gray normal solid line) were stained with antibodies specific for CD34, prominin-1, Thy1.2, H2Kb, Ly5.2, CD44, and Sca-1. Representative histograms for cells at E13.5 are shown. Am is not shown as it overlaps to FL for all the markers analyzed. Human AF (black bold solid line) and CB (gray normal solid line) KL cells were stained with specific mAbs for CD34, CD133, CD90, HLA-ABC, CD45, CD44, and HLA-DR. For both murine and human cells, the control isotype is indicated in light gray. (B) Analyses of murine KL cell-cycling status. Cells from wild-type C57BL/6 embryos were harvested 2 or 24 hours after the first administration of BrdU at E12.5. BrdU incorporation by Lin− cells was detected by fixation, permeabilization, and staining with a fluorescein isothiocyanate–conjugated anti-BrdU Ab. The results of 1 of 2 experiments are shown.
No differences were detected between mAFKL and mFLKL cells in terms of CD34 (low expression on < 10% cells), prominin-1 (the murine ortholog of CD133; no expression), Thy1.2 (no expression), and major histocompatibility complex class I molecules (low expression). AFKL cells expressed Ly5.2 (CD45.2) at a higher level than FLKL (and AmKL) cells. This difference may be related to the environment and the presence of specific cytokines in the 3 locations, as it has been shown recently that granulocyte colony-stimulating factor, for instance, was able to strongly down-regulate CD45 expression on bone marrow leukocytes while increasing their motility.31 Regarding CD44 expression, as described for c-Kit, 2 populations, 1 CD44− and 1 CD44+, coexisted in the mFL and AFKL populations, the CD44− fraction being minor in the FL, whereas it represented most AFKL cells. The absence of CD44 in most AFKL cells is not surprising, as it mediates cell adhesion; some of the cells may not express this surface marker as they are floating in the AF. Sca-1 was found only on mAFKL cells (∼40%).
Human AFKL cells were compared with CBKL cells. Unlike CBKL cells, which partly expressed CD34, none of the hAFKL cells expressed this marker. Both CBKL and AFKL cells were negative for CD133 and expressed low levels of CD90. A clear difference was observed regarding HLA class I molecules, with the highest level detected in CBKL cells. As in the mouse, the hAFKL cells expressed CD45 at higher levels than CBKL cells, whereas CD44 was present at similar levels in both populations. AFKL cells expressed no HLA-DR, which is present on some CBKL cells. The difference of age and of environment between CB and AF might account for some of the differences observed.
The incorporation of BrdU into replicating DNA was used to label proliferating cells. As shown in Figure 2B, most mAFKL cells were quiescent for at least 24 hours after BrdU injection, whereas the proportion of cycling KL cells in the Am and FL reached 75% and 99%, respectively. These results indicate that mKL cells proliferate much less in the AF than in the Am and FL.
Murine and human KL cells exhibit strong multilineage hematopoietic potential in vitro
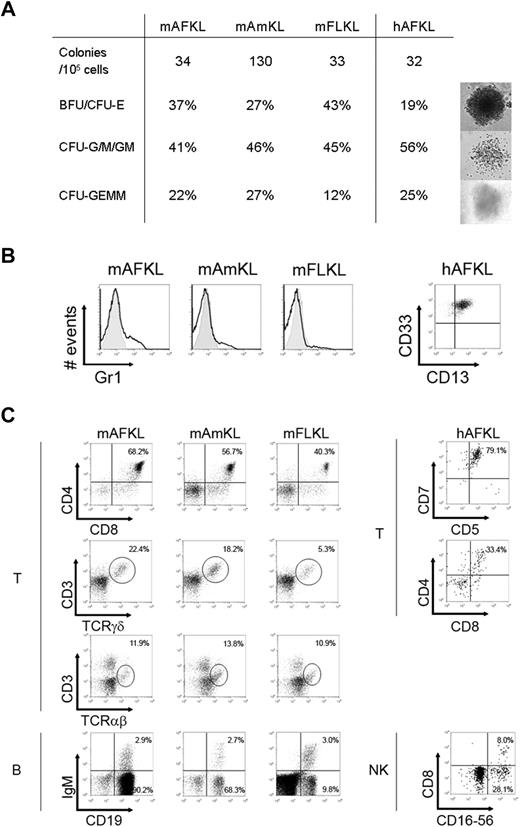
Murine GFP+ KL cells and human KL cells were sorted and cultured in semisolid medium to assess their hematopoietic function (Figure 3A). Murine AF and FLKL cells and human AFKL cells exhibited the same clonogenic potential (∼ 33 per 105 cells, ie, 0.03%), whereas mAmKL cells generated 4-fold more colonies under the same culture conditions. These colonies comprised erythroid colony- and burst-forming units (CFU- or BFU-E) and granulocyte/macrophage CFUs (CFU-G/M/GM; Figure 3A). The proportion of mixed colony-forming unit-granulocyte, erythrocyte, monocyte, megakaryocyte (CFU-GEMM) cells was high in all samples analyzed, thus attesting to the presence of multipotent hematopoietic progenitors. The myeloid differentiation potential of murine and human AFKL cells was further confirmed by liquid cultures (Figure 3B). Human AFKL cells were able to generate CD13+ CD33+ cells very efficiently under such conditions.
In vitro hematopoietic differentiation. (A) Clonogenic potential of murine KL and human AFKL cells. The number of total CFUs obtained 14 days after seeding 105 candidate cells in 7 independent experiments is shown, together with the relative percentages of the different types of colonies generated. Representative images of day 14 mKL cells and hAFKL cell–derived hematopoietic BFU-E, CFU-M, and CFU-GEMM cells (cultured in semisolid methylcellulose-based medium and supplied with hematopoietic cytokines) are shown. (B) Representative results from 1 of 6 myeloid differentiation experiments performed with murine and human KL cells (left and right panels, respectively). Cells were stained with specific mAbs for murine Gr1 myeloid antigen and human CD13 and CD33. (C) Surface phenotype analysis of T- and B-cell differentiation of murine KL cells assessed with OP9-mDelta1 coculture of 5000 candidate cells (left panel). Single-cell suspensions were obtained after 10 days (CD4/CD8), 15 days (CD3/TCRγδ), and 20 days (CD3/TCRαβ) of T-cell differentiation or after 10 days (CD19/IgM) of B-cell differentiation. A forward-scatter/side-scatter gate was set to eliminate stromal cells from the analysis. Only GFP+ 7-AAD− viable cells were taken into account. The results shown are representative of 6 independent experiments performed with donors at various ages of gestation (E11.5, n = 1; E12.5, n = 2; E13.5, n = 1; E14.5, n = 1; E15.5, n = 1; E19.5, n = 1). On the right panels, surface phenotype analysis of T- and NK-cell differentiation of hAFKL (from donors at 16, 18, 19, 21, and 23 weeks of amenorrhea) assessed with OP9-hDelta1 coculture of 5000 candidate cells. Human cells were recovered after 7 days (CD5/CD7), 15 days (CD4/CD8), and 25 days (CD8/CD16-CD56) of coculture. Representative results from 1 of 5 experiments are shown.
In vitro hematopoietic differentiation. (A) Clonogenic potential of murine KL and human AFKL cells. The number of total CFUs obtained 14 days after seeding 105 candidate cells in 7 independent experiments is shown, together with the relative percentages of the different types of colonies generated. Representative images of day 14 mKL cells and hAFKL cell–derived hematopoietic BFU-E, CFU-M, and CFU-GEMM cells (cultured in semisolid methylcellulose-based medium and supplied with hematopoietic cytokines) are shown. (B) Representative results from 1 of 6 myeloid differentiation experiments performed with murine and human KL cells (left and right panels, respectively). Cells were stained with specific mAbs for murine Gr1 myeloid antigen and human CD13 and CD33. (C) Surface phenotype analysis of T- and B-cell differentiation of murine KL cells assessed with OP9-mDelta1 coculture of 5000 candidate cells (left panel). Single-cell suspensions were obtained after 10 days (CD4/CD8), 15 days (CD3/TCRγδ), and 20 days (CD3/TCRαβ) of T-cell differentiation or after 10 days (CD19/IgM) of B-cell differentiation. A forward-scatter/side-scatter gate was set to eliminate stromal cells from the analysis. Only GFP+ 7-AAD− viable cells were taken into account. The results shown are representative of 6 independent experiments performed with donors at various ages of gestation (E11.5, n = 1; E12.5, n = 2; E13.5, n = 1; E14.5, n = 1; E15.5, n = 1; E19.5, n = 1). On the right panels, surface phenotype analysis of T- and NK-cell differentiation of hAFKL (from donors at 16, 18, 19, 21, and 23 weeks of amenorrhea) assessed with OP9-hDelta1 coculture of 5000 candidate cells. Human cells were recovered after 7 days (CD5/CD7), 15 days (CD4/CD8), and 25 days (CD8/CD16-CD56) of coculture. Representative results from 1 of 5 experiments are shown.
We next tested the lymphoid potential of (1) murine KL cells from AF, Am, and FL and (2) human AFKL cells via culture in T-, B-, and NK-promoting conditions. Irrespective of donor age (from E11.5 up to E19.5), murine KL cells from all 3 sites gave rise to CD4+CD8+ double-positive cells and more mature γδ+ and αβ+ T cells within 15 days (Figure 3C top left panels), when cultured on OP9 stromal cells expressing the murine Notch ligand, Delta 1.32 CD19+ cells, some of which displayed cell-surface IgM, were also easily detected in B-cell culture conditions from all the murine sites (Figure 3C bottom left panels). It is noteworthy that mAFKL cells gave rise to B cells with high efficiency, whereas mAmKL cells (like mFLKL cells) consistently yielded fewer CD19+ cells than did the mAFKL cells.
Similarly, human AFKL gave rise to NK cells (ie, CD56+ CD16+, Figure 3C bottom right panel) and T-cell precursors up to the double-positive stage (Figure 3C top right panel).
These results clearly show that both murine and human AFKL cells display an in vitro multilineage hematopoietic potential that is indistinguishable (as far as murine cells are concerned) from the one that characterizes FLKL at the same stage of development.
Murine AFKL cells exhibit strong hematopoietic potential in vivo
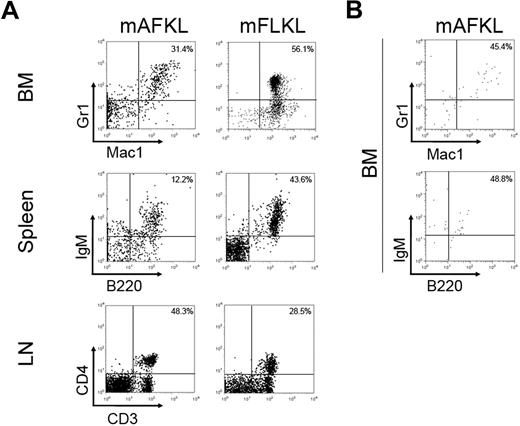
Murine AF and FL KL cells from Ly5.2+ GFP+ embryos were sorted and transplanted into RAG1−/− C57BL/6 (Ly5.1) recipients. We transplanted a mean of 2 × 104 AFKL cells (the amount usually collected from 2 embryos) and, as positive control, 2 × 104 FLKL cells isolated from the same E12.5 or E13.5 embryos. As shown in Table 1 and despite variability in the degree of donor chimerism, engraftment (as demonstrated by the presence of GFP+ Ly5.2+ cells) was observed in all transplanted recipients up to 4 months after transplantation. Regarding the nature of the in vivo differentiated cells, Gr1+ and Mac1+ cells were found in the blood, spleen (data not shown), and BM of all transplanted animals (Table 1; Figure 4A). NK and B cells were detected in most recipients (Table 1; Figure 4A). T cells (both CD4+ and CD8+ CD3+) were detected in the blood (data not shown) and the lymph nodes of all FLKL recipients and 5 of 8 AFKL recipients (Table 1; Figure 4A). These transplantation experiments indicated that KL cells from mAF possess a long-term (4-month) in vivo hematopoietic repopulating capacity similar to the one of FLKL cells.
Summary of reconstitution experiments
| . | mAFKL cells . | mFLKL cells . |
|---|---|---|
| Engraftment | 8/8 | 3/3 |
| Long-term reconstitution | 8/8 | 3/3 |
| Donor chimerism, mean ± SD | 7.2 ± 10.6 | 19.3 ± 13.5 |
| Granulocytes | 8/8 | 3/3 |
| T cells | 5/8 | 3/3 |
| B cells | 7/8 | 3/3 |
| NK cells | 5/7* | 2/2* |
| . | mAFKL cells . | mFLKL cells . |
|---|---|---|
| Engraftment | 8/8 | 3/3 |
| Long-term reconstitution | 8/8 | 3/3 |
| Donor chimerism, mean ± SD | 7.2 ± 10.6 | 19.3 ± 13.5 |
| Granulocytes | 8/8 | 3/3 |
| T cells | 5/8 | 3/3 |
| B cells | 7/8 | 3/3 |
| NK cells | 5/7* | 2/2* |
Engraftment (ie, the proportion of RAG1−/− C57BL/6 [Ly5.1] recipient mice presenting donor-derived cells in the blood 4 weeks after the transfer of E12.5 to E13.5 mAFKL and mFLKL cells) is shown in the first row. Long-term reconstitution was considered to be present when a minimum of 0.5% of donor-derived cells were present in the bone marrow of recipient mice 4 months after transplantation. The proportion of recipient mice presenting myeloid cells (granulocytes) and lymphoid cells of donor origin is indicated for each source of KL cells.
One mouse was not tested for the presence of NK cells.
Hematopoietic reconstitution from mKL cells grafted in RAG1−/− C57BL/6 (Ly5.1) mice. (A) A mean of 2 × 104 AFKL cells sorted from GFP+ Ly5.2+ embryos were transplanted in sublethally irradiated RAG1−/− C57BL/6 (Ly5.1) mice. As a positive control, 2 × 104 FLKL cells were injected in the same model (Table 1). Representative flow cytometry profiles of BM, spleen, and lymph nodes of RAG1−/− C57BL/6 (Ly5.1) transplanted mice 16 weeks after transplantation (demonstrating multilineage reconstitution with B lymphoid, T lymphoid, and myeloid cells) are shown. (B) Representative flow cytometry profiles of BM of RAG1−/− C57BL/6 (Ly5.1) transplanted mice showing multilineage reconstitution with B lymphoid and myeloid cells 16 weeks after secondary transplantation. Dot plots are shown after gating for the GFP+ Ly5.2+ fraction. Two recipients of the 7 transplanted engrafted. These 2 recipients were transplanted with different KL samples: one not sorted (1.5 × 107 cells) and the other sorted for Ly5.2 expression (1.5 × 104 cells) before transplantation.
Hematopoietic reconstitution from mKL cells grafted in RAG1−/− C57BL/6 (Ly5.1) mice. (A) A mean of 2 × 104 AFKL cells sorted from GFP+ Ly5.2+ embryos were transplanted in sublethally irradiated RAG1−/− C57BL/6 (Ly5.1) mice. As a positive control, 2 × 104 FLKL cells were injected in the same model (Table 1). Representative flow cytometry profiles of BM, spleen, and lymph nodes of RAG1−/− C57BL/6 (Ly5.1) transplanted mice 16 weeks after transplantation (demonstrating multilineage reconstitution with B lymphoid, T lymphoid, and myeloid cells) are shown. (B) Representative flow cytometry profiles of BM of RAG1−/− C57BL/6 (Ly5.1) transplanted mice showing multilineage reconstitution with B lymphoid and myeloid cells 16 weeks after secondary transplantation. Dot plots are shown after gating for the GFP+ Ly5.2+ fraction. Two recipients of the 7 transplanted engrafted. These 2 recipients were transplanted with different KL samples: one not sorted (1.5 × 107 cells) and the other sorted for Ly5.2 expression (1.5 × 104 cells) before transplantation.
To test the HSC potential of mAFKL cells, secondary transplantations were performed in 7 recipients. Two of them, which received cells from independent primary transplantation experiments, engrafted. In both cases, donor cells represented approximately 1% of the recipient's total BM cells and consisted of IgM+B220+ and Gr1+Mac1+ cells (Figure 4B). To note, secondary transplantation of mFLKL performed in the same conditions did not result in higher levels of engraftment. These partially successful secondary transplantations confirm the presence of hematopoietic progenitor cells within the mAFKL population and suggest that part of them may possess HSC characteristics.
Gene expression profiling of murine KL cells
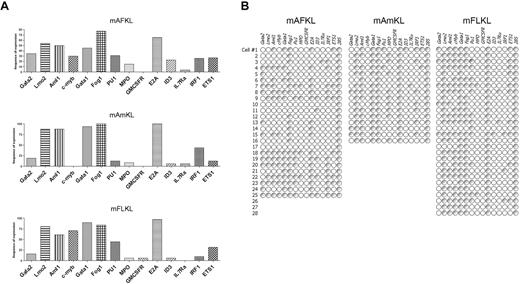
To further characterize the mAF, Am, and FLKL cell populations, we studied the expression of several genes involved in hematopoietic differentiation in single-sorted cells using multiplex reverse-transcribed PCR, as previously described25,26 (Figure 5). Overall, the gene expression profiles of AF, Am, and FLKL cells were similar (Figure 5A). Twenty-eight percent of the AFKL cells coexpressed Gata2 and Lmo2, compared with 18.7% and 14.3% of AmKL and FLKL cells, respectively (Figure 5B). The absence (or low levels) of expression of genes involved in the various differentiation pathways (Pu1, Ets1, Il7ra, Gmcsfr, Id3, MPO) and the coexpression of genes characteristic of HSC (Aml1, Lmo2, Gata2) correlate with the transplantation results and confirm the presence of hematopoietic multipotent cells within the 3 KL cell populations.
Frequency of expression of hematopoietic genes and their coexpression in mFLKL, mAFKL, and mAmKL cells. (A) Single-cell multiplex RT-PCR analyses were performed to investigate the frequency of expression of hematopoietic genes in the 3 cell populations. In all cases 28S was used as an endogenous control. The results are shown as histograms representing the proportion of cells expressing each gene. (B) Single KL cells were sorted and analyzed by multiplex RT-PCR, as described in “Methods.” Each row shows the same (numbered) individual cell. Each column shows a different gene. Empty symbols represent cells not expressing that particular mRNA (< 2 mRNA molecules); gray symbols, positive cells where mRNA levels were not quantified.
Frequency of expression of hematopoietic genes and their coexpression in mFLKL, mAFKL, and mAmKL cells. (A) Single-cell multiplex RT-PCR analyses were performed to investigate the frequency of expression of hematopoietic genes in the 3 cell populations. In all cases 28S was used as an endogenous control. The results are shown as histograms representing the proportion of cells expressing each gene. (B) Single KL cells were sorted and analyzed by multiplex RT-PCR, as described in “Methods.” Each row shows the same (numbered) individual cell. Each column shows a different gene. Empty symbols represent cells not expressing that particular mRNA (< 2 mRNA molecules); gray symbols, positive cells where mRNA levels were not quantified.
Discussion
This is the first paper to describe the presence of cells with a hematopoietic potential in murine and human AF. Indeed, these cells expressed surface markers and genes typically associated with a hematopoietic potential and were able to differentiate all along the hematopoietic pathway. Strikingly, the hematopoietic differentiation results obtained with murine AFKL cells were similar to those seen with KL cells from the FL, which constitutes the major site of fetal hematopoiesis from E10 up until birth.33-35 In the murine system, it is important to note that we fully excluded contamination by maternal cells by gating for paternal GFP expression in the in vitro and in vivo differentiation experiments. Furthermore, contamination by EB was ruled out, not only by the technique used to isolate AF and Am but also by the frequency of KL cells (0.03%) circulating in the embryo. The surface phenotype of murine AFKL and AmKL cells was quite similar to that of FLKL cells. CD45 expression on both human and murine AFKL cells is in favor of their hematopoietic commitment. Sca-1 expression was found in approximately half the AFKL population, as described by others for FL HSCs,36 AGM HSCs,37 and (after E11.5) placental Runx1-LacZ–expressing progenitors.10 Only a small subset of mAFKL cells expressed CD34, as do AGM and placenta HSCs.10,38
Results from BrdU incorporation experiments show that the mAFKL cells were barely proliferating, whereas mAmKL and mFLKL cells were cycling extensively, similarly to what was shown in the recent work published by Rhodes et al for placenta and FL Runx1/LacZ+ cells.10 Cells in the FL and Am divide rapidly because at E12.5 the FL HSC pool is expanding34,35 and the Am is growing in line with fetal dimensions. It is possible that the rare cycling AFKL cells corresponds to hematopoietic progenitors. Further experiments would be required to check this hypothesis. Alternatively, AFKL cells harboring a hematopoietic potential may be quiescent like most AGM HSCs.10 AF contains plenty of mitogens, cytokines (including SCF), growth factors, and proliferation signals,27 but the latter are spread throughout the liquid environment as a whole. The permanent change in molecular signaling may prevent AFKL cells from proliferating and differentiating in situ. Once put in culture, AFKL cells were able to proliferate immediately and at a rate similar to their FL counterparts.
Under appropriate differentiation conditions, murine and human KL cells were able to generate all the blood lineages (ie, myeloid and erythroid colonies), as well as mixed CFU-GEMM and B, NK, and T lymphocytes. However, hAFKL cells only generated immature T-cell precursors, suggesting that the T-cell culture conditions used herein may not be fully appropriate for this population.
Freshly isolated hAFKL cells gave rise to erythroid, myeloid, and lymphoid lineages in in vitro assays but failed to reconstitute the hematopoietic system in irradiated nonobese diabetic/severe combined immunodeficiency mice.
There are several potential explanations for this observation (eg, low numbers of cells injected, transplantation in adult mice, low major histocompatibility complex I expression), and we are now setting up new transplantation conditions to overcome this obstacle. Conversely, cells belonging to all the 3 hematopoietic lineages (ie, lymphoid, myeloid, and erythroid cells) were found in the peripheral blood of sublethally irradiated, RAG1-deficient mice just 4 weeks after the transplantation of small numbers of mAFKL cells and remained stable for the following 4 months. These results confirm the presence of short-term and long-term hematopoietic progenitors in mAF.39 In addition, secondary transplantation of mAFKL partially succeeded, suggesting the presence of true HSCs in the AF. We used a single-cell, multiplexed PCR technique to analyze the expression of several genes known to be involved in either the early or late stages of hematopoietic development. In the present work, we were able to identify an overall pattern of expression which can be considered to be a signature of hematopoietic commitment. Gata2, Lmo2, and Aml1/Runx1 were coexpressed by 28%, 19%, and 14% of the mAFKL, mAmKL, and mFLKL cells, respectively. Most of Gata2/Lmo2/Runx1-expressing KL cells from the 3 locations also expressed Gata1 (a key regulator in the emergence of common myeloid progenitors), whereas others did not express Gata1 and were thus reminiscent of the AGM long-term–repopulating HSCs described by others.16,40-42 Combined Gata1 and Fog expression was also found in 28% of AFKL cells and most Am and FLKL cells, as seen downstream in the erythropoiesis process. Mpo/Pu1 coexpression (which is characteristic of granulocyte/monocyte progenitors) was observed in AFKL and FLKL cells only, whereas half of AmKL cells expressed MPO alone. Interestingly, Cmyb43 was not detected in AmKL cells but was present in most Gata2/Lmo2/Runx1/Gata1-expressing KL cells from the AF and FL. Conversely, lymphoid genes (such as Il7ra and Ets1) were absent or very poorly expressed in all 3 locations, indicating that lymphoid commitment had not yet occurred or that lymphoid progenitors were rare.
Our current results do not contain evidence of functional multipotency at the single-cell level. Nevertheless, the presence of CFU-GEMM after culture in semisolid medium, the engraftment obtained after secondary transplantation, similar to the one obtained with KL cells from the FL, and the coexpression of 3 genes found to be expressed in AGM long-term–repopulating HSCs40 suggest the presence of some HSCs in the murine AF.
The presence of mAFKL cells from E9.5 onward (ie, 2.5 days after Am formation and concomitant with the appearance of multipotent precursors of intraembryonic origin) means that the question of whether these AF cells are intra- or extraembryonically derived cannot yet be answered. Consequently, a direct filiation of AFKL from AmKL cannot be deducted. Nevertheless, the overall gene expression profile suggests that, in contrast to AmKL cells, AFKL cells resemble hematopoietic progenitors from definitive hematopoiesis sites, such as the AGM, placenta, or the umbilical/vitelline arteries.1,10 As far as the AFKL cells' origin is concerned, experiments with specific Cre-Lox mouse models are ongoing.
To the best of our knowledge, this is the first report to demonstrate that murine and human AF contain a multipotent cell population with hematopoietic potential. Although much work remains to be done regarding the characterization of the latter population's in vivo potential and proliferation capacity, our results strongly support the idea that AF may be an excellent source of cells for therapeutic applications.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the Antoine Béclère Hospital (Clamart, France) for providing AF and CB samples, Alain Fischer for fruitful discussion and support, Michel Vekemans for discussions on the possible origin on human AF stem cells, Ana Cumano for fruitful discussions on murine fetal HSCs, Isabelle Godin and Laure Coloumbel for thoughtful comments on the manuscript, and Corinne Garcia and Jérome Mégret for flow cytometry cell sorting.
A.D. was supported by grants from the Fondazione della Città della Speranza, Egide and the Université Italo-Française.
Authorship
Contribution: A.D., P.d.C., D.B., S.E., M.C.-C., and I.A.-S. conceived and designed the experiments; A.D., L.G., D.B., and I.A.-S. performed the experiments; O.P. and R.F. provided hAF samples; L.G. and R.S. performed single-cell PCR on murine cells; S.E. provided Rag1−/− mice; A.D., E.S., S.E., M.C.-C., and I.A.-S. analyzed data; and A.D., M.C.-C., and I.A.-S. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Marina Cavazzana-Calvo, Inserm U768, Hôpital Necker Enfants Malades, 149 rue de Sèvres, F-75743 Paris Cedex 15, France; e-mail: m.cavazzana@nck.aphp.fr.
References
Author notes
*M.C.-C. and I.A.-S. share senior authorship.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal