Abstract
The activation of Toll-like receptor 9 (TLR9) expressed within B cells is associated with enhanced humoral immunity. However the role of TLR9 in the stimulation of B-cell responses, and more specifically in shaping the outcome of B-cell differentiation, remains unclear. Here, we observed that immunization with the TLR9 agonist CpG linked to protein antigen gave rise to enhanced production of antigen-specific class-switched antibodies in vivo. Unlike dendritic cells, B cells are unable to acquire these conjugates by macropinocytosis and instead depend on uptake through a signaling-competent B-cell receptor (BCR), provided the overall BCR-antigen avidity exceeds a defined threshold. The resultant stimulation of intrinsic TLR9 leads to enhanced antigen-specific B-cell proliferation and differentiation to form extrafollicular plasma cells. Thus, the direct conjugation of antigen and CpG reveals a mechanism that may operate during the initiation of primary immune responses, and may prove useful as a strategy for the design of adjuvants suitable for vaccinations.
Introduction
B lymphocytes comprise a critical component of the adaptive immune system, producing antibodies necessary for both immediate and long-lived protection from pathogenic infection. B cells encounter antigen (Ag) within specialized tissues, such as the spleen or lymph nodes, known as secondary lymphoid organs. After Ag-induced activation, B cells can follow 2 distinct pathways of differentiation. In extrafollicular (EF) regions of the secondary lymphoid organs, including the medullary cords of lymph nodes or the red pulp of the spleen, activated B cells give rise to short-lived plasma cells (PCs) responsible for the rapid production of low-affinity antibodies.1 Alternatively, activated B cells can form germinal centers (GCs) where they undergo affinity maturation, resulting in differentiation into either long-lived PCs, which maintain the immune response by secreting high-affinity antibodies, or into long-lasting memory cells.2-4 Though at this stage the precise combination of factors responsible for determining the outcome of B-cell differentiation remains unclear, it has been proposed that Ag binding strength can influence the fate of activated B cells. As such, high-affinity Ag favors differentiation into EF PCs, while lower affinity Ag induces the preferential entry of B cells into GCs.5
B cells recognize and respond to Ag through surface B-cell receptors (BCRs) that possess a remarkably wide range of potential affinities.6-9 The BCR is a heterotrimeric complex containing a membrane immunoglobulin (Ig) responsible for extracellular Ag binding, together with an Igα/β sheath required for mediating intracellular signaling through its immunoreceptor tyrosine-based activation motifs (ITAMs).10 Engagement of the BCR by specific Ag induces signaling that results in a variety of cellular processes,11 including the internalization of accumulated Ag. The subsequent presentation of processed antigenic peptide-loaded major histocompatibility complex-II (MHC-II) molecules on the surface of the B-cell membrane stimulates the recruitment of specific helper CD4+ T cells, required for the full activation of B cells.12,13 As the process of BCR-mediated internalization provides a mechanism whereby Ag can access intracellular compartments, it would seem likely that several other receptors expressed within the B cell could potentially participate in shaping the outcome of B-cell activation in a similar manner.
In addition to the BCR, B cells express germline encoded Toll-like receptors (TLRs) more usually associated with innate immune cell function.14 The specificity of TLRs for pathogen-associated ligands is dependent on either the restricted expression of the ligand within bacteria or viruses, or the atypical cellular location of more universally expressed ligands.15 The expression of TLR9 in humans is restricted to plasmacytoid dendritic cells (pDCs) and B cells.16,17 While the majority of TLR9 appears to be concentrated in the endoplasmic reticulum,18,19 TLR9 has also been observed within small intracellular vesicles in resting primary B cells.20 Interestingly, in contrast to the full-length version that is located in the ER, processed TLR9 that is found in endolysomes was very recently demonstrated to be functional in mouse dendritic cells (DCs) and macrophages.21 Stimulation of TLR9 by hypomethylated CpG motifs derived from microbial DNA initiates an intracellular MyD88-mediated signaling pathway resulting in the release of proinflammatory cytokines,22 and promoting B-cell proliferation and antibody production.23 Initially, the direct stimulation of TLR9 within B cells was implicated in contributing to the development and pathogenesis of autoimmune diseases, such as systemic lupus erythematosus.24,25 In line with this, it has been shown that autoreactive B cells can be activated after dual ligation of BCR and TLR9.17,24-26 Furthermore, these activated auto-reactive B cells differentiate into EF PCs capable of mediating the production of autoantibodies of IgG2 isotypes.27,28 More recently, several studies have identified an important role for TLR9 stimulation during B-cell differentiation.29-31 However, the significance and role of B cell–intrinsic TLR9 stimulation during the development of primary immune responses remains controversial.32,33
Previous investigations into the role of TLR9 in the development of B-cell responses have predominantly used soluble oligonucleotides containing hypomethylated CpG motifs as stimulatory ligands.27,30,31 These soluble ligands enter cells through nonspecific uptake mechanisms,34 and thereby result in nondiscriminatory and widespread stimulation of B cells. Here, we have developed a strategy for directly conjugating CpG and Ag, in an effort to confer specificity on the immunostimulation mediated by CpG. We observed that these Ag-CpG conjugates are indeed capable of specifically enhancing B-cell proliferation and differentiation to form PCs both in vitro and in vivo. In addition, we have shown that B-cell uptake of these Ag-CpG conjugates is dependent on exceeding a threshold of Ag avidity sufficient to stimulate BCR-mediated internalization. Furthermore, we have demonstrated that the amount of TLR9 ligand determines the extent of differentiation to form PCs induced by Ag-CpG conjugates. As these Ag-CpG–containing particulates resemble the form in which B cells encounter TLR9 ligands in physiologically relevant situations, this mechanism of B-cell intrinsic TLR9 stimulation likely represents the means of humoral immune stimulation in vivo.
Methods
General reagents
Hen egg lysozyme (HEL) and ovalbumin (OVA) were from Sigma-Aldrich (St Louis, MO); chicken-γ-globulin (CγG) was from Jackson ImmunoResearch (West Grove, PA); carboxyfluorescein succinimidyl ester (CFSE) was from Invitrogen (Carlsbad, CA); 5′ biotinylated CpG 1668 was from Sigma-Genosys (St Louis, MO); and recombinant interleukin-6 (IL-6) was from BD Biosciences (San Jose, CA). HELRD, HELK, HELKD, and HELRKD were described previously.35 We purchased 0.13-μm streptavidin-coated microspheres from Bangs Laboratories (Fishers, IN) and 0.2-μm FluoSpheres neutravidin microspheres from Invitrogen.
Antibodies
Monoclonal antibodies against mouse Ags: anti–CD138-PE (clone 281-2) and -biotin; anti-CD45.2–PerCP–Cy5.5 (10G); anti–IL-6 (MP5-20F3); anti–IL-6–biotin (MP5-32C11); anti-CD16/32 (2.4G2); anti-IgMa–biotin (DS-1); anti-IgG1–biotin (A85-1); anti-IgG2b–biotin (R12-3); anti-IgG3–biotin (R40-82); and anti-CD45R/B220 (RA3-6B2; all BD Biosciences). Monoclonal anti–HEL F10 has been described.36 Polyclonal anti–mouse-IgG–horseradish peroxidase (HRP; Pierce, Rockford, IL); monoclonal anti–β-actin (AC-15) and monoclonal anti–chicken egg albumin (OVA-14; both Sigma-Aldrich); anti-phospho–p38 MAP kinase (Thr180/Tyr182) and anti-p38 MAP kinase antibody (both Cell Signaling, Danvers, MA). The polyclonal antibodies against mouse Ags: anti-IgM–biotin; anti-IgG–biotin; anti-IgG2c–biotin; anti-IgM; anti-IgG; anti-IgG1; anti-IgG2b; anti-IgG2c, and anti-IgG3 (all Southern Biotech, Birmingham, AL). Anti–chicken lysozyme (United States Biological, Marblehead, MA), Alexa Fluor 488 goat anti–mouse IgG (H+L) and Alexa Fluor 546 goat anti–rat IgG (H+L) (both Invitrogen).
Mice
MD4; MD4 TLR9−/−22; MD4 MyD88−/−37 ; HyHEL1038 ; IgM/βY>L; and IgM/β39 mice were bred and maintained on C57BL/6 (Charles River, Wilmington, MA) background at the animal facility of Cancer Research UK. The Cancer Research UK Animal Ethics Committee and the United Kingdom Home Office approved all experiments.
Microsphere coating
Streptavidin-coated microspheres were washed before addition of biotinylated CpG and/or biotinylated Ag (OVA, HEL, or CγG) and resuspended in phosphate-buffered saline (PBS). To generate microspheres coated with HEL mutants at various densities, biotinylated anti–HEL F10 and/or biotinylated CγG was used for initial coating, as described previously.36
B-cell purification, labeling, and culture
Splenic B cells were enriched by negative selection to more than 99% purity using a B-cell purification kit (Miltenyi Biotec, Bergisch Gladbach, Germany) and labeled with 2μM CFSE. B cells were cultured in RPMI 1640 media (Invitrogen) supplemented with 10% fetal calf serum (FCS; PAA Labs, Pasching, Austria), 50 μM β-mercaptoethanol (Sigma-Aldrich), 25 mM Hepes (Invitrogen), and 10 units/mL penicillin/streptomycin (Invitrogen). A total of 106 CFSE-labeled B cells stimulated with 1 μL particulates were harvested after a 72-hour incubation to assess proliferation and differentiation.
DC purification and culture
Bone marrow–derived DCs were generated by culturing precursors from mice femurs in the media described above supplemented with recombinant granulocyte macrophage–colony stimulating factor (GM-CSF; R&D Systems, Minneapolis, MN). After 5 days, DCs were enriched to more than 99% purity using CD11c+ microbeads (Miltenyi Biotec).
Adoptive transfer and immunizations
Aliquots of 1 to 5 × 106 CFSE-labeled MD4 or HyHEL10 B cells together with 1 to 10 μL particulates containing HEL and/or CpG were adoptively transferred by tail-vein injection into wild-type (WT) C57BL/6 mice. Mice were immunized intraperitoneally with 1 to 10 μL particulates coated with OVA or CγG (as Ag) and/or CpG.
Flow cytometry
Detection of proliferation and differentiation to form PCs in the spleen was based on a method described previously.40 Briefly, single-cell suspensions of the spleen were prepared and samples were blocked with purified anti-CD16/32. HEL-specific B cells were detected with HEL followed with anti–HEL F10–Alexa Fluor 647. PCs were identified through their binding to anti–CD138-PE. To detect intracellular HEL binding, B cells were fixed in paraformaldehyde (PFA) and permeablized using 0.1% saponin. The extent of cellular particulate uptake stimulated with 1 μL/106 cells fluorescent particulates was assessed by flow cytometry after 3 hours.
Immunologic assays and immunohistochemistry
Enzyme-linked immunosorbet assays (ELISAs) were used to quantify the production of sera antigen-specific IgMa and IgG, and IL-6, in a manner similar to that described previously.36 Enzyme-linked immunosorbent spot (ELISPOT) plates coated with 100 μg/mL HEL or CγG in PBS were blocked and incubated with splenocytes as described36 before development with either anti-IgG–HRP or anti-IgMa–biotin. Immunohistochemistry of splenic sections was performed as described previously.36
Results
Direct linkage of CpG to Ag enhances specific antibody responses after in vivo immunization
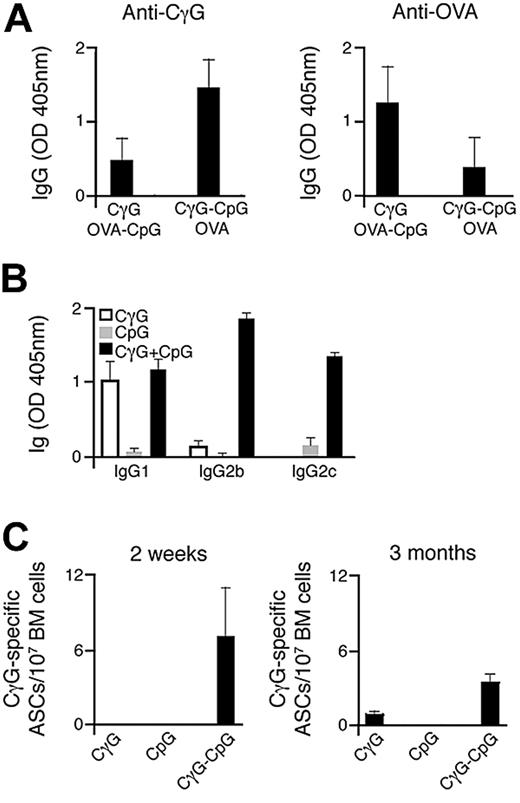
We initially sought to investigate the impact that direct conjugation of Ag and CpG has on humoral immune responses in vivo. To achieve this, we have designed an approach involving the direct conjugation of both Ag and CpG onto streptavidin polystyrene microspheres, comparable in diameter to that of a typical viral pathogen. The successful generation of particulate conjugates, after coating with biotinylated Ag and/or CpG, was confirmed by flow cytometry (Figure S1A, available on the Blood website; see the Supplemental Materials link at the top of the online article). C57BL/6 mice were immunized simultaneously with 2 particulate Ags, CγG, and ovalbumin (OVA). For each group of mice immunized, one of the particulate Ags was conjugated with CpG. The production of Ag-specific IgG antibodies was measured by ELISA 14 days after particulate administration. As shown in Figure 1A, selective enhancement in Ag-specific antibody titers after immunization was observed for the particulate Ag carrying conjugated CpG (Ag-CpG). This enhanced response to particulate Ag-CpG was accompanied by the production of class-switched Ag-specific antibodies, predominantly of the IgG2b and IgG2c isotypes (Figure 1B). Interestingly, antibodies of the IgG2 isotype are particularly effective mediators of immune responses associated with virus neutralization,41 and their production has been associated with TLR9 stimulation.27,29 Furthermore, we observed Ag-specific antibody-secreting cells (ASCs) within the bone marrow 3 months after single-dose immunization with particulate CγG-CpG (Figure 1C).
Immunization with particulate Ag-CpG enhances specific antibody responses and promotes class switching to IgG2 isotypes. (A) C57BL/6 mice (3 mice per group) were immunized once with either 1 μL OVA-CpG–coated particles with 1 μL CγG-coated particles or 1 μL CγG-CpG particles with 1 μL OVA-coated particles. Serum CγG- (left panel) and OVA-specific IgG (right panel) were measured 14 days after immunization. Data are representative of 2 independent experiments. (B,C) C57BL/6 mice were immunized with 10 μL of either particulate CγG alone, particulate CpG alone or particulate CγG-CpG. (B) CγG-specific IgG subtypes were determined by ELISA 14 days after immunization; (C) ELISPOTs were used to quantify the number of bone marrow CγG-specific IgG secreting ASCs 14 days and 3 months after immunization. Values represent the mean (± SD) from triplicate samples.
Immunization with particulate Ag-CpG enhances specific antibody responses and promotes class switching to IgG2 isotypes. (A) C57BL/6 mice (3 mice per group) were immunized once with either 1 μL OVA-CpG–coated particles with 1 μL CγG-coated particles or 1 μL CγG-CpG particles with 1 μL OVA-coated particles. Serum CγG- (left panel) and OVA-specific IgG (right panel) were measured 14 days after immunization. Data are representative of 2 independent experiments. (B,C) C57BL/6 mice were immunized with 10 μL of either particulate CγG alone, particulate CpG alone or particulate CγG-CpG. (B) CγG-specific IgG subtypes were determined by ELISA 14 days after immunization; (C) ELISPOTs were used to quantify the number of bone marrow CγG-specific IgG secreting ASCs 14 days and 3 months after immunization. Values represent the mean (± SD) from triplicate samples.
Thus, we have demonstrated that immunization with particulates directly linked to both Ag and CpG enhances Ag-specific antibody titers and induces class-switching mainly to the IgG2 subtype in vivo.
BCR-mediated uptake of Ag-CpG conjugates is required to stimulate TLR-dependent differentiation into PCs in vitro
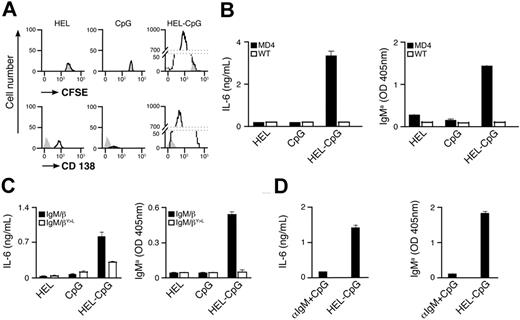
To elucidate the mechanism underlying the enhancement in specific antibody titers, we have used transgenic MD4 B cells expressing BCR specific for HEL.42 CFSE-labeled MD4 B cells were stimulated with particles containing HEL (as Ag) and/or CpG in vitro (Figure S1B). Three days after stimulation, flow cytometry was used to monitor B-cell proliferation and differentiation to form PCs by dilution of CFSE and up-regulation of CD138 expression, respectively. Extensive proliferation of MD4 B cells, together with differentiation to form PCs, was observed after stimulation with particulate HEL-CpG (Figure 2A). These B-cell responses correlated with secretion of both IL-6 and HEL-specific IgMa (Figure 2B), in line with previous reports.23 Similar results were obtained with particulates of various sizes, provided their diameter did not exceed 0.5 μm (data not shown). Interestingly, no proliferation or differentiation was observed upon stimulation with particulates containing either HEL or CpG alone (Figure 2A,B).
BCR-mediated uptake of particulate Ag-CpG gives rise to B-cell proliferation and PC differentiation in vitro. (A,B) CFSE-labeled B cells were stimulated with 1 μL particulate HEL alone, particulate CpG alone, or particulate HEL-CpG for 72 hours. (A) Flow cytometry was used to measure proliferation by CFSE dilution (top panel) and CD138 up-regulation (bottom panel) in stimulated (black line) and unstimulated (gray filled) MD4 B cells; (B) IL-6 (left panel) and IgMa (right panel) secretion by WT and MD4 B cells were determined by ELISA. (C) MD4 B cells expressing the chimeric BCRs IgM/β or IgM/βY<L were stimulated with 1 μL particulate HEL alone, particulate CpG alone, or particulate HEL-CpG for 72 hours. IL-6 (left panel) and IgMa (right panel) secretion were determined by ELISA. (D) MD4 B cells were stimulated with either a mixture of 1 μL particulate CpG and 5 μg soluble anti-IgM (αIgM + CpG) or 1 μL particulate HEL-CpG (HEL-CpG) for 72 hours. IL-6 (left panel) and IgMa (right panel) secretion were determined by ELISA. Values represent the mean (± SD) from triplicate samples.
BCR-mediated uptake of particulate Ag-CpG gives rise to B-cell proliferation and PC differentiation in vitro. (A,B) CFSE-labeled B cells were stimulated with 1 μL particulate HEL alone, particulate CpG alone, or particulate HEL-CpG for 72 hours. (A) Flow cytometry was used to measure proliferation by CFSE dilution (top panel) and CD138 up-regulation (bottom panel) in stimulated (black line) and unstimulated (gray filled) MD4 B cells; (B) IL-6 (left panel) and IgMa (right panel) secretion by WT and MD4 B cells were determined by ELISA. (C) MD4 B cells expressing the chimeric BCRs IgM/β or IgM/βY<L were stimulated with 1 μL particulate HEL alone, particulate CpG alone, or particulate HEL-CpG for 72 hours. IL-6 (left panel) and IgMa (right panel) secretion were determined by ELISA. (D) MD4 B cells were stimulated with either a mixture of 1 μL particulate CpG and 5 μg soluble anti-IgM (αIgM + CpG) or 1 μL particulate HEL-CpG (HEL-CpG) for 72 hours. IL-6 (left panel) and IgMa (right panel) secretion were determined by ELISA. Values represent the mean (± SD) from triplicate samples.
To identify the critical features of the particulates that enable stimulation of B-cell responses, we used flow cytometry to detect cellular uptake of fluorescently labeled particulates. While B cells were able to take up particulate HEL-CpG, we observed that they could not capture particulate CpG alone (Figure S1C). Thus, particulates containing CpG alone cannot stimulate nonspecific B-cell responses in the same manner as has been observed for soluble CpG (Figure 2A,B). The failure of particulate CpG to enter B cells is not due to a general exclusion of these particulates from cells, as the uptake of CpG by dendritic cells is not impaired by conjugation to particulates (Figure S1C). However, we observed that Ag on the particulate enables entry into the B cell (Figure S1C), suggesting that the BCR is involved in the mechanism used to allow the entry of these conjugates into B cells.
In an effort to substantiate the involvement of the BCR in the uptake of CpG particulates, we have used B cells expressing a chimeric signaling–deficient BCR (IgM/βY>L). The IgM/βY>L BCR retains high affinity for HEL but contains mutated β-chain ITAMs, rendering the BCR unable to transmit intracellular signals and, therefore, unable to mediate efficient Ag internalization.39 As shown in Figure 2C, a signaling-competent BCR is required, as B-cell differentiation is severely impaired in cells expressing the IgM/βY>L BCR. Importantly, B cells expressing the chimeric IgM/β BCR with or without mutated ITAMs exhibit comparable responses to stimulation with soluble CpG (data not shown).
BCR signaling has been shown to enhance nonspecific endocytosis and theoretically, therefore, could facilitate indirect particulate Ag-CpG B-cell entry.43 To exclude this possibility, we stimulated MD4 B cells with a mixture containing soluble anti-IgM, to initiate BCR signaling, together with particulate CpG. As expected, this treatment did not give rise to B-cell differentiation (Figure 2D), suggesting that particulate Ag-CpG enters the B cell directly via BCR-mediated internalization. This is in line with our previous observations showing enhanced antibody production by particulate CpG in an Ag-specific manner (Figure 1A).
Importantly, though particulate HEL alone can be taken up through the BCR (Figure S1C), it was unable to stimulate B-cell proliferation and differentiation (Figure 2A,B). This demonstrates the absolute requirement for CpG in mediating B-cell responses to particulate HEL-CpG. To verify that these responses were dependent on TLR9 activation, we have generated TLR9−/− and MyD88−/− MD4 mice. As expected, both TLR9−/− and MyD88−/− MD4 B cells were unable to proliferate and differentiate in response to stimulation with particulate HEL-CpG (Figure 3A,B; Figure S1D), demonstrating that TLR9 was indeed essential for these processes. Furthermore, stimulation with particulate HEL-CpG gave rise to more sustained phosphorylation of p38 compared with either particulate HEL or CpG alone (Figure S1E).
B-cell proliferation and differentiation in response to particulate Ag-CpG is dependent on stimulation of TLR9 in vitro. (A,B) MD4 and (A) MD4 TLR9−/− or (B) MD4 MyD88−/− B cells were stimulated with 1 μL particulate HEL alone, particulate CpG alone or particulate HEL-CpG for 72 hours. IL-6 (left panels) and IgMa (right panels) secretion were determined by ELISA. Values represent the mean (± SD) from triplicate samples.
B-cell proliferation and differentiation in response to particulate Ag-CpG is dependent on stimulation of TLR9 in vitro. (A,B) MD4 and (A) MD4 TLR9−/− or (B) MD4 MyD88−/− B cells were stimulated with 1 μL particulate HEL alone, particulate CpG alone or particulate HEL-CpG for 72 hours. IL-6 (left panels) and IgMa (right panels) secretion were determined by ELISA. Values represent the mean (± SD) from triplicate samples.
Taken together, we have demonstrated that a signaling-competent BCR mediates the uptake of particulate Ag-CpG necessary for stimulation of intracellular TLR9. Thus, the sequential stimulation of BCR and TLR9 by particulate Ag-CpG initiates B-cell proliferation and differentiation to form PCs.
A tight Ag-BCR avidity threshold regulates Ag-CpG–stimulated B-cell activation in vitro
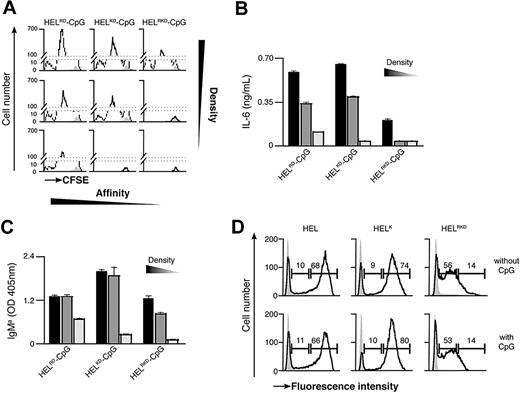
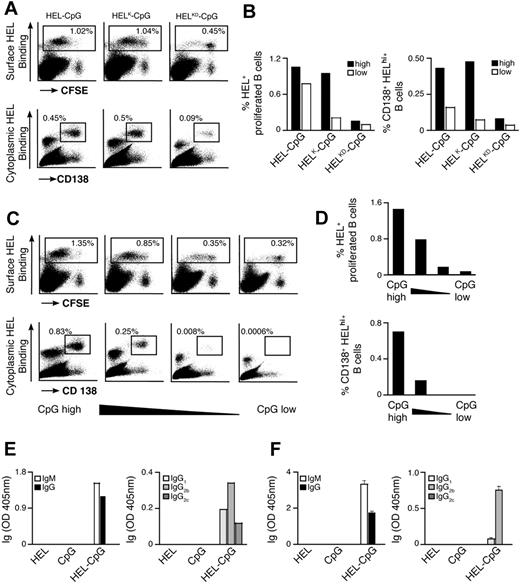
Previous observations have suggested that the binding strength of the Ag-BCR interaction influences the outcome of B-cell differentiation.5 As antigen recognition by the BCR is required for particulate Ag-CpG to stimulate B-cell responses, we examined the influence of Ag avidity, in terms of both affinity and density, on TLR9-dependent proliferation and differentiation in vitro. To address this, we used several previously described recombinant HEL mutants that bind the BCR with diminished affinity35 : WT HEL (Ka 2 × 1010 M−1); HELRD (Ka 7.9 × 108 M−1); HELK (Ka 8.7 × 106 M−1); HELKD (Ka 4.0 × 106 M−1); and HELRKD (Ka 0.8 × 106 M−1). The various HEL proteins were immobilized to the particulates through the biotinylated monoclonal anti-HEL F10 as a bridge to ensure comparable coating densities.
Decreasing the affinity of HEL by approximately 5000-fold (Ka from 2 × 1010 to 4.0 × 106 M−1) had little impact on either B-cell proliferation or IL-6 production when Ag was present on the particulate CpG at high density (Figure 4A-C; Figure S2A,B). However, a further 5-fold reduction in HEL affinity (to Ka 0.8 × 106 M−1) dramatically reduced the capacity of the CpG particulates to stimulate B-cell activation (Figure 4A,B). It is, therefore, apparent that a minimum threshold of Ag affinity must be surpassed to trigger BCR-mediated Ag internalization, associated B-cell proliferation and IL-6 secretion. Observing such a threshold in Ag affinity suggests that the response induced by particulate Ag-CpG is dependent on the amount of particles taken up by the B cells. To corroborate this hypothesis, we used fluorescent particles to compare the capacity of B cells to acquire the different HEL conjugates. As shown in Figure 4D, above the affinity threshold the amount of particles taken up was comparable throughout a wide range of affinities (from Ka 2 × 1010 to 4.0 × 106 M−1). In contrast, below this threshold we observed a marked reduction in the amount of particles acquired by the B cells. Therefore, it is evident that above a tight threshold, the affinity of Ag for the BCR influences the amount of Ag-CpG particulates internalized.
BCR-mediated uptake of Ag-CpG conjugates is regulated by the avidity of the Ag-BCR interaction in vitro. (A-C) CFSE-labeled MD4 B cells were stimulated with 1 μL particulates coated with CpG together with HELRD either (left panels), HELKD (middle panels), or HELRKD (right panels) at high (top panels), intermediate (middle panels), or low density (bottom panels). B-cell proliferation and differentiation were measured 72 hours after stimulation. (A) CFSE dilution in stimulated (black line) or unstimulated (filled gray) MD4 B cells was measured by flow cytometry. (B) IL-6 and (C) IgMa secretion were measured by ELISA. (D) MD4 B cells were stimulated with 1 μL fluorescent particulates left either uncoated (filled gray) or coated with HEL, HELK, or HELRKD in the absence (top panels) or presence (bottom panels) of CpG. Flow cytometry was used to assess binding of particulates: gates shown indicate the percentage of live cells binding intermediate levels (left gate) and high levels (right gate) of particulates. Values represent the mean (± SD) from triplicate samples.
BCR-mediated uptake of Ag-CpG conjugates is regulated by the avidity of the Ag-BCR interaction in vitro. (A-C) CFSE-labeled MD4 B cells were stimulated with 1 μL particulates coated with CpG together with HELRD either (left panels), HELKD (middle panels), or HELRKD (right panels) at high (top panels), intermediate (middle panels), or low density (bottom panels). B-cell proliferation and differentiation were measured 72 hours after stimulation. (A) CFSE dilution in stimulated (black line) or unstimulated (filled gray) MD4 B cells was measured by flow cytometry. (B) IL-6 and (C) IgMa secretion were measured by ELISA. (D) MD4 B cells were stimulated with 1 μL fluorescent particulates left either uncoated (filled gray) or coated with HEL, HELK, or HELRKD in the absence (top panels) or presence (bottom panels) of CpG. Flow cytometry was used to assess binding of particulates: gates shown indicate the percentage of live cells binding intermediate levels (left gate) and high levels (right gate) of particulates. Values represent the mean (± SD) from triplicate samples.
As the binding strength of the Ag-BCR interaction is dependent on the avidity of Ag seen by the BCR, we postulated that further discrimination of stimulatory capacity might be observed by reducing the density of HEL on particulates. To generate particulates containing reduced densities of HEL, we included an irrelevant biotinylated protein during the initial coating phase to compete with biotinylated F10 for binding to the streptavidin microspheres (Figure S2C). As expected, stimulation with CpG particulates containing reduced densities of the various HEL proteins gave rise to lesser amounts of B-cell proliferation and IL-6 secretion (Figure 4A,B). A 2-fold reduction in the density of HELRKD on CpG-containing particulates yielded them unable to stimulate B-cell proliferation and IL-6 production (Figure 4A,B). Similarly, a 4-fold reduction in the density of the higher affinity HELRD protein also rendered CpG-containing particulates unable to stimulate B-cell responses (Figure 4A,B). Interestingly, the avidity threshold of the BCR-Ag interaction to induce IgMa secretion appears lower than that required for stimulation of proliferation and IL-6 production (Figure 4C). It is, therefore, likely that Ag must be present at a sufficient avidity to induce the minimum degree of BCR clustering required for internalization of particulate Ag-CpG. Thus, even a low-affinity Ag present at sufficiently high density might surpass the threshold required to enable BCR-mediated internalization and associated stimulation of B-cell responses.
Thus, we have demonstrated the importance of Ag avidity in dictating the extent of TLR9-mediated B-cell proliferation and differentiation in vitro. We suggest, therefore, that the overall strength of the interaction between BCR and Ag must exceed a defined threshold to allow for efficient of uptake of particulates and subsequent TLR9 stimulation by particulate CpG.
The extent of B-cell differentiation by particulate Ag-CpG is determined by the strength of TLR9 stimulation in vitro
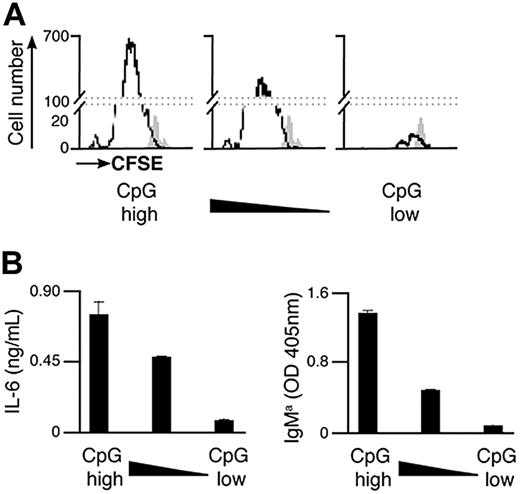
As the total amount of particulate Ag-CpG taken up by B cells is dependent on the overall avidity of the Ag-BCR interaction, it is likely that the CpG component of the particulate impacts the extent of B-cell activation induced. We therefore sought to investigate the effect of varying the amount of CpG conjugated to particulate HEL on the B-cell responses induced. To achieve this, particulates were coated with different amounts of CpG but the same overall amount of HEL to ensure constant Ag avidity and thus BCR-mediated entry to the cell. As shown in Figure 5, a reduction in the amount of CpG present on the particles correlated directly with a decrease in B-cell proliferation and secretion of IL-6 and IgMa. As such, particulate HEL containing the largest amount of conjugated CpG stimulated differentiation to form PCs to the greatest extent. However, B-cell differentiation was scarcely detectable after stimulation with particulates containing a 10-fold reduction in the amount of CpG conjugated.
Amount of CpG present on the Ag-containing particulates modulates the extent of B-cell proliferation and differentiation to form PCs in vitro. (A,B) CFSE-labeled B cells were stimulated with 1 μL particulate HEL conjugated with various densities of CpG. The density of CpG is represented from highest to lowest on moving from left to right. Proliferation and differentiation of MD4 B cells were measured 72 hours after stimulation. (A) Flow cytometry was used to measure CFSE dilution in stimulated (black line) and unstimulated (filled gray) MD4 cells. (B) IL-6 (left panel) and IgMa (right panel) secretion were assessed by ELISA.
Amount of CpG present on the Ag-containing particulates modulates the extent of B-cell proliferation and differentiation to form PCs in vitro. (A,B) CFSE-labeled B cells were stimulated with 1 μL particulate HEL conjugated with various densities of CpG. The density of CpG is represented from highest to lowest on moving from left to right. Proliferation and differentiation of MD4 B cells were measured 72 hours after stimulation. (A) Flow cytometry was used to measure CFSE dilution in stimulated (black line) and unstimulated (filled gray) MD4 cells. (B) IL-6 (left panel) and IgMa (right panel) secretion were assessed by ELISA.
It is therefore evident that the amount of CpG acquired by B cells is critical for the generation of PCs in response to particulate Ag-CpG, indicating that the extent of signaling through TLR9 is important in determining the outcome of B-cell differentiation.
Particulate HEL-CpG stimulates differentiation to form EF PC in vivo
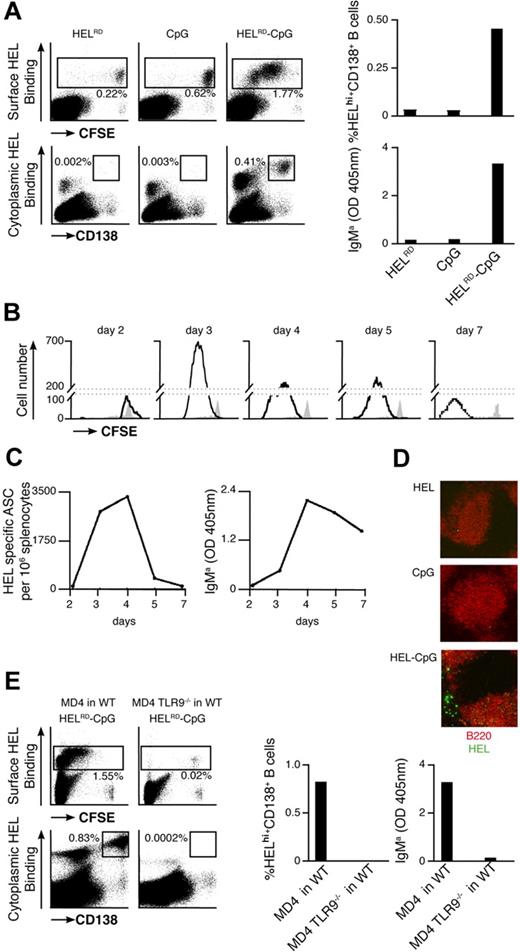
We were keen to ascertain whether our in vitro observations were representative of B-cell responses to Ag-CpG particulates in vivo. To assess this, CFSE-labeled MD4 B cells and particles containing HEL and/or CpG were coadministered to WT recipient mice. The proliferation of MD4 splenic B cells was assessed by CFSE dilution at indicated time points after adoptive transfer. In addition, the presence of CD138+ intracellular HEL+ MD4 splenic PCs was quantified using multicolor flow cytometry.
Extensive proliferation of HEL-specific B cells was observed upon coinjection of MD4 B cells and particulate HELRD-CpG (Figure 6A left panel). This proliferation reached a maximum 3 days after stimulation (Figure 6B) and was coincident with the formation of PCs and the appearance of HEL-specific IgMa in the serum (Figure 6A right panels; Figure 6C). This CD138+ PC population appeared short-lived in nature, as their number peaked around 3 to 4 days after stimulation. In line with this, similar kinetics were observed for the population of HEL-specific PCs formed in the EF region of the spleen (Figure 6D). As TLR9−/− MD4 B cells were unable to respond to stimulation with particulate HEL-CpG after their adoptive transfer into WT recipient mice, B-cell proliferation and differentiation were dependent on TLR9 (Figure 6E). Furthermore, these responses were not detected on stimulation of WT MD4 B cells with either particulate CpG or HEL alone (Figure 6A). As such, and in agreement with our in vitro observations, the sequential engagement of BCR and TLR9 is required to stimulate robust B-cell responses in vivo. Thus, we have demonstrated that particulate HEL-CpG stimulates the TLR9-mediated proliferation and differentiation of B cells to short-lived EF PCs in vivo.
Particulate Ag-CpG promotes B-cell proliferation and differentiation to form short-lived EF PCs in vivo. (A-D) CFSE-labeled MD4 B cells were adoptively transferred into C57BL/6 mice and challenged with 10 μL particulates containing either HELRD alone, CpG alone, or HELRD-CpG. (A) Four days after transfer, flow cytometry was used to measure CFSE dilution (top left panels) and CD138 up-regulation (bottom left panels) in HEL-binding cells in the spleen of recipient mice. The percentage of MD4 PCs (HEL intracellularhi, CD138+) present (top right panel) is shown as a proportion of total splenocytes, and serum HEL-specific IgMa (bottom right panel) was measured by ELISA. (B) Flow cytometry was used to measure CFSE dilution in HEL-binding cells in the spleens of recipient mice in stimulated (black line) and unstimulated (filled gray) MD4 cells at the indicated times after challenge. (C) ELISPOTs (left panel) were used to detect splenic HEL-specific ASCs, and ELISAs (right panel) were used to measure serum HEL-specific IgMa. (D) After 5 days, immunofluorescence microscopy (Zeiss LSM 510 meter; Carl Zeiss MicroImaging, Gottingen, Germany) was used to detect HEL-binding cells (HEL–Alexa 488) and splenic follicular B cells (anti-B220–Alexa 543) in cryosections mounted in Fluoromount-G (Southern Biotech). Images were acquired using Zeiss LSM 510 version 3.2 software and processed in ImageJ version 1.41 (http://rsb.info.nih.gov/ij/). (E) CFSE-labeled MD4 B cells or MD4 TLR9−/− B cells were adoptively transferred into C57BL/6 mice, and challenged with 10 μL HELRD-CpG particulates. Four days after the transfer flow cytometry was used to measure CFSE dilution (top left panels) and CD138 (bottom left panels) in HEL-binding cells in the spleen of recipient mice. The percentage of MD4 PCs (HEL intracellularhi, CD138+) present (middle panel) is shown as a proportion of total splenocytes; serum HEL-specific IgMa (right panel) was measured by ELISA.
Particulate Ag-CpG promotes B-cell proliferation and differentiation to form short-lived EF PCs in vivo. (A-D) CFSE-labeled MD4 B cells were adoptively transferred into C57BL/6 mice and challenged with 10 μL particulates containing either HELRD alone, CpG alone, or HELRD-CpG. (A) Four days after transfer, flow cytometry was used to measure CFSE dilution (top left panels) and CD138 up-regulation (bottom left panels) in HEL-binding cells in the spleen of recipient mice. The percentage of MD4 PCs (HEL intracellularhi, CD138+) present (top right panel) is shown as a proportion of total splenocytes, and serum HEL-specific IgMa (bottom right panel) was measured by ELISA. (B) Flow cytometry was used to measure CFSE dilution in HEL-binding cells in the spleens of recipient mice in stimulated (black line) and unstimulated (filled gray) MD4 cells at the indicated times after challenge. (C) ELISPOTs (left panel) were used to detect splenic HEL-specific ASCs, and ELISAs (right panel) were used to measure serum HEL-specific IgMa. (D) After 5 days, immunofluorescence microscopy (Zeiss LSM 510 meter; Carl Zeiss MicroImaging, Gottingen, Germany) was used to detect HEL-binding cells (HEL–Alexa 488) and splenic follicular B cells (anti-B220–Alexa 543) in cryosections mounted in Fluoromount-G (Southern Biotech). Images were acquired using Zeiss LSM 510 version 3.2 software and processed in ImageJ version 1.41 (http://rsb.info.nih.gov/ij/). (E) CFSE-labeled MD4 B cells or MD4 TLR9−/− B cells were adoptively transferred into C57BL/6 mice, and challenged with 10 μL HELRD-CpG particulates. Four days after the transfer flow cytometry was used to measure CFSE dilution (top left panels) and CD138 (bottom left panels) in HEL-binding cells in the spleen of recipient mice. The percentage of MD4 PCs (HEL intracellularhi, CD138+) present (middle panel) is shown as a proportion of total splenocytes; serum HEL-specific IgMa (right panel) was measured by ELISA.
Analogous to the in vitro findings, we observed a threshold in the strength of the BCR-Ag interaction requires to be surpassed to induce B-cell activation in vivo (Figure 7A). As such, a greater than 2000-fold reduction in HEL affinity (from Ka 2 × 1010 to 8.7 × 106 M−1) does not diminish B-cell responses stimulated. However, a further 2-fold decrease in Ag affinity leads to severely impaired B-cell proliferation and differentiation after stimulation by HEL-CpG particulates. Furthermore, particulates coated with a lower density of HEL continue to enable B-cell proliferation and differentiation, albeit at slightly reduced levels. In contrast, particulates containing a low density of HELK are unable to yield significant B-cell responses. Thus, provided a lower affinity Ag is present at sufficient density, particulates can be used to stimulate B-cell proliferation and differentiation in vivo (Figure 7B). These observations demonstrate the requirement for a threshold of BCR binding strength to be surpassed before TLR9-mediated stimulation of B-cell responses to particulate Ag-CpG in vivo.
Avidity of the Ag-BCR interaction and TLR9 signaling strength modulate the extent of PC formation in vivo. (A,B) CFSE-labeled MD4 B cells were adoptively transferred into C57BL/6 mice and challenged with 10 μL particulates containing CpG and either HEL (left panels), HELK (middle panels), or HELKD (right panels) at high or low densities. (A) After 4 days, flow cytometry was used to measure: CFSE dilution (top panels) and CD138 up-regulation (bottom panels) in HEL-binding cells in the spleen of recipient mice. (B) Flow cytometry was used to quantify the percentage of live HEL binding B cells that have undergone proliferation (left panel) and the percentage of MD4 PCs (HEL intracellularhi, CD138+; right panel) as a proportion of total splenocytes. (C,D) CFSE-labeled MD4 B cells were adoptively transferred into C57BL/6 mice and challenged with 10 μL particulates containing HEL and various densities of CpG. The density of CpG is represented from highest to lowest moving from left to right. (C) After 4 days, flow cytometry was used to measure: CFSE dilution (top panels) and CD138 up-regulation (bottom panels) in HEL-binding cells in the spleen of recipient mice. (D) Flow cytometry was used to quantify the percentage of live HEL binding B cells that have undergone proliferation (top panel), and the percentage of MD4 PCs (HEL intracellularhi, CD138+; bottom panel) as a proportion of total splenocytes. (E) HyHEL10 B cells were adoptively transferred into C57BL/6 mice and challenged with 10 μL particulates coated with HEL alone, CpG alone, or HEL-CpG. After 7 days, ELISAs were used to measure the levels of HEL-specific Igs in the serum of recipient mice. IgM and IgG (left panel) and IgG subtypes IgG1, IgG2b, and IgG2c (right panel). (F) HyHEL10 B cells were stimulated with 1 μL particulate HEL alone, particulate CpG alone, or particulate HEL-CpG for 7 days. ELISAs were used to measure the levels of HEL-specific Igs secreted into the culture medium as described in panel E. Values represent the mean (± SD) from triplicate samples.
Avidity of the Ag-BCR interaction and TLR9 signaling strength modulate the extent of PC formation in vivo. (A,B) CFSE-labeled MD4 B cells were adoptively transferred into C57BL/6 mice and challenged with 10 μL particulates containing CpG and either HEL (left panels), HELK (middle panels), or HELKD (right panels) at high or low densities. (A) After 4 days, flow cytometry was used to measure: CFSE dilution (top panels) and CD138 up-regulation (bottom panels) in HEL-binding cells in the spleen of recipient mice. (B) Flow cytometry was used to quantify the percentage of live HEL binding B cells that have undergone proliferation (left panel) and the percentage of MD4 PCs (HEL intracellularhi, CD138+; right panel) as a proportion of total splenocytes. (C,D) CFSE-labeled MD4 B cells were adoptively transferred into C57BL/6 mice and challenged with 10 μL particulates containing HEL and various densities of CpG. The density of CpG is represented from highest to lowest moving from left to right. (C) After 4 days, flow cytometry was used to measure: CFSE dilution (top panels) and CD138 up-regulation (bottom panels) in HEL-binding cells in the spleen of recipient mice. (D) Flow cytometry was used to quantify the percentage of live HEL binding B cells that have undergone proliferation (top panel), and the percentage of MD4 PCs (HEL intracellularhi, CD138+; bottom panel) as a proportion of total splenocytes. (E) HyHEL10 B cells were adoptively transferred into C57BL/6 mice and challenged with 10 μL particulates coated with HEL alone, CpG alone, or HEL-CpG. After 7 days, ELISAs were used to measure the levels of HEL-specific Igs in the serum of recipient mice. IgM and IgG (left panel) and IgG subtypes IgG1, IgG2b, and IgG2c (right panel). (F) HyHEL10 B cells were stimulated with 1 μL particulate HEL alone, particulate CpG alone, or particulate HEL-CpG for 7 days. ELISAs were used to measure the levels of HEL-specific Igs secreted into the culture medium as described in panel E. Values represent the mean (± SD) from triplicate samples.
Finally, we were keen to establish the impact of varying the amount of TLR9 stimulation on the outcome of B-cell differentiation in vivo. As shown in Figure 7C and D, the extent of B-cell proliferation and formation of HEL-specific PCs was dependent directly on the density of CpG conjugated with the particulate HEL. Hence, as in vitro, the amount of TLR9 stimulation is important in determining B-cell responses in vivo and could potentially be useful as a mechanism for fine-tuning the outcome of stimulation with Ag-CpG particulates.
Taken together, it is evident that after BCR-mediated internalization, particulate Ag-CpG conjugates stimulate B-cell proliferation and differentiation to form EF PCs in vivo, therefore establishing the physiological significance of our in vitro observations.
Particulate HEL-CpG stimulates the production of Ag-specific class-switched antibodies
Our initial observations indicated that immunization of mice with particulate Ag-CpG led to the production of antigen-specific class-switched antibodies. However, as the transgenic MD4 B cells used in our investigations thus far are unable to undergo class-switch, we have used an alternative transgenic model system to further investigate this phenomenon. This transgenic mouse system yields B cells expressing the high-affinity HyHEL10 BCR and able to undergo class-switch recombination.
HyHEL10 B cells adoptively transferred into a WT recipient underwent extensive proliferation and differentiation to form PCs in response to particulate HEL-CpG, in a manner similar to that observed for MD4 B cells (data not shown). Analysis of the isotype of antibodies secreted revealed class-switch recombination occurred after stimulation with particulate HEL-CpG, resulting in the production of IgG predominantly of the IgG2 subtype (Figure 7E). Importantly, as a similar pattern of antibody isotypes was detected after simulation of HyHEL10 B cells alone in culture, the class-switch we observed in vivo was likely to be independent of CD4+ T-cell help (Figure 7F). These findings are consistent with our observations from the original immunizations performed (Figure 1).
Thus, it is evident that, after BCR-mediated internalization, particulate Ag-CpG stimulates not only B-cell proliferation and differentiation to form short-lived PCs, but also class-switch recombination to the IgG2 isotype.
Discussion
The TLR9 agonist CpG has the capacity to stimulate a plethora of responses associated with activation of both the innate and adaptive branches of the immune system. Here, we have established that the direct conjugation of CpG with Ag gives rise to enhanced and specific B-cell responses. This study involved developing a strategy to generate particulates with both Ag and CpG immobilized on the surface to enable their uptake by B cells through the BCR. We observed this receptor-mediated uptake is characterized by a tightly regulated avidity threshold and results in delivery of CpG to TLR9 intrinsic to the B cell in an Ag-specific manner. Importantly, particulate CpG alone is prohibited from using nonspecific means of entering B cells, rendering particulate Ag-CpG highly selective in its capacity to stimulate TLR9-mediated responses. Furthermore, we have shown that after BCR-mediated uptake, TLR9 engagement triggers a dramatic enhancement in B-cell proliferation and formation of short-lived EF PCs.
Several previous investigations into the impact of TLR9 stimulation on B-cell behavior have used soluble CpG. Such studies report that stimulation of TLR9 leads to the enhanced proliferation of B cells and differentiation to form PCs capable of producing isotype-switched antibodies.27,30,31 Here, we observed that particulate CpG, unlike soluble CpG, cannot enter B cells via nonspecific fluid-phase pinocytosis. In contrast, the conjugation of CpG to a particulate did not prevent its uptake by dedicated phagocytes such as DCs. These phagocytes retain the capacity to acquire particulates potentially through pattern recognition receptors associated with innate immune cell function. Interestingly, this mode of internalization might represent one of the mechanisms alluded to in a recent investigation into the differential impact of immunostimulation with various forms of CpG.44 In contrast, B cells require the presence of an Ag-specific and signaling-competent BCR to enable efficient uptake of particulate Ag-CpG. The necessity of the BCR in the internalization of immune complexes containing TLR9-stimulatory ligands has been demonstrated during the development of autoimmune diseases.24,25
In this study, we have demonstrated that the avidity of the Ag-BCR interaction influences the outcome of B-cell activation after stimulation with particulate Ag-CpG. Early investigations of T cell–dependent B-cell responses introduced the notion that the decision of activated B cells to differentiate into either PCs or GCs is a stochastic process.45,46 However, 2 more recent studies have used a variety of Ag and BCR affinities to investigate the impact of the overall BCR-Ag avidity on the outcome of B-cell differentiation.5,47 As Ags that induced greater signaling through the BCR preferentially drive B cells to become EF PCs, Brink's group has proposed an elegant model whereby the signaling strength of the BCR-Ag interaction controls the outcome of B-cell differentiation.5 Here, above a defined threshold, we observed a correlation between the avidity of the Ag-BCR interaction, the internalization of particulates, and the amount of differentiation to form EF PCs. Thus, it appears that after internalization, TLR stimulation may override the BCR-dependent signaling to determine the outcome of B-cell differentiation. Furthermore, a similar mechanism may underlie previous observations after stimulation with (4-hydroxy-3-nitrophenyl) acetyl (NP) as Ag in the presence of adjuvant.47 We, therefore, suggest that the results presented here are not contrary, but rather complementary, to that of the previous Ag-BCR avidity studies, in that, as anticipated by the Brink study,5 the differentiation of B cells is controlled through a combination of factors. The concept of combinatorial signaling functioning to shape the outcome of B-cell activation has been suggested previously.31 Indeed, the authors demonstrated that the sustained survival and differentiation of naive human B cells required engagement of the BCR with Ag, the availability of T-cell help, and signaling through the TLR system.
Here, we have shown that, provided the avidity threshold required for BCR-mediated internalization is met, stimulation of intracellular TLR9 by particulate Ag-CpG influences the outcome of B-cell differentiation. Furthermore, using an adoptive transfer strategy, we have shown conclusively that B-cell proliferation and differentiation to form short-lived PCs is dependent on stimulation of intrinsic TLR9 in vivo. As such, increasing the extent of TLR9-mediated stimulation, by increasing the amount of CpG conjugated to the particulate Ag, enhances the generation of EF PCs in a quantitative manner. Our observations are in agreement with previous investigations that have identified a critical role for intrinsic TLR-mediated signaling in the differentiation of activated B cells.29,31 Moreover, it has been demonstrated very recently that TLR-mediated signaling, but not CD4+ T-cell help, is absolutely required for the activation and EF development of auto-reactive B cells.48 Interestingly, and in support of our observations, it has been found that TLR9-mediated signaling can be dissociated temporally from initial stimulation of the BCR.31 The ability of TLR-mediated signaling to override BCR-dependent signals and stimulate the production of EF PCs is likely to play an important role during the early stages of the immune response through the rapid production of first-wave protective antibodies.
After BCR-mediated uptake, we observed that particulate Ag-CpG gains access to stimulate TLR9 within the B cell. Previous studies have demonstrated that BCR stimulation results in formation of an intracellular complex formed by the fusion of many endosomal-like vesicles.49 This complex is the site where internalized receptors become localized, and appears similar to the autophagosome-like compartment rich in TLR9 observed after BCR stimulation.20 The functional significance of directed localization of endocytosed BCR together with associated Ag was first appreciated through the demonstration that newly synthesized MHC-II molecules were also located within these endosomal compartments.50 Accordingly, BCR-mediated internalization facilitates processing and efficient loading of Ag onto MHC-II for subsequent presentation to specific CD4+ helper T cells necessary for full B-cell activation.12,13,51 Furthermore, it has been demonstrated that the MHC-like molecule CD1d acquires lipidic Ags, such as αGalCer, within endosomal compartments before its surface presentation to iNKT cells.51,52 A similar mechanism of BCR-mediated internalization was required for the stimulation of specific iNKT-mediated B-cell proliferation and differentiation to EF PCs in response to particulate Ag-αGalCer.36 These observations taken together implicate endosomal or endosomal-like compartments as sites critical for the coordination of intracellular communications that ultimately govern the outcome of cellular processes such as differentiation.
We demonstrate here the selective and controlled stimulation of Ag-specific humoral immune responses through the use of particulate Ag-CpG conjugates. As these particulates provide a means of directing the immunostimulatory capacity of CpG to a specific population of cells, they could prove of enormous value as effective adjuvants in the future design of successful vaccination strategies. As such, the use of CpG in this particulate form would be expected to guard against the development of autoimmune diseases associated with nonspecific TLR9-stimulation17,24-26 and lymphoid follicle destruction53 associated with repeated administration of soluble CpG. In addition, through variation of their precise composition, particulate Ag-CpG could be used to offer intricate control of the immune responses stimulated on immunization. Indeed, inclusion of multiple Ags within the particulate CpG conjugates could potentially allow for more effective induction of protective immune responses. However, design of an effective long-term vaccination strategy would also require the establishment of long-lived memory B cells, and this remains an important issue to be addressed in the further development of these particulates. Furthermore, these particulates may also prove useful to direct Epstein-Barr virus preferentially to antigen-specific B cells for the selective production of monoclonal antibodies from immortalized memory B cells.54
In summary, we have developed an approach for the direct conjugation of Ag and the immunostimulant CpG on the surface of a particulate. These particulates gain selective entry into Ag-specific B cells through BCR-mediated endocytosis, allowing engagement of intracellular TLR9. Stimulation with these particulates results in enhanced B-cell proliferation and differentiation to form EF PCs competent to secrete Ag-specific class-switched antibodies in vivo. Investigations using these particulates are useful not only in elucidating principles concerning the involvement of TLR9 during the development of the primary immune responses, but also potentially in advancement of Ag-specific immunostimulants required in vaccinations.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Shizuo Akira, Caetano Reis e Sousa, and Jason Cyster for the kind provision of the TLR9−/−, MyD88−/−, and HyHEL10 mice; Caetano Reis e Sousa and Patricia Barral for helpful advice and discussions; and the members of Lymphocyte Interaction Laboratory, and in particular Naomi Harwood, for critical reading of the manuscript.
This work was funded by Cancer Research UK (London, United Kingdom).
Authorship
Contribution: J.E.-D. designed the research, performed the experiments, analyzed results, and prepared the manuscript; and F.D.B. designed the research, analyzed results, and prepared the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Facundo D. Batista, Lymphocyte Interaction Laboratory, Cancer Research UK London Research Institute, Lincoln's Inn Fields Laboratories, 44 Lincoln's Inn Fields, London WC2A 3PX, United Kingdom; e-mail: facundo.batista@cancer.org.uk.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal